Abstract
Hypercholesterolemia is a major cardiovascular risk factor that increases the incidence of atherosclerotic diseases in adults, although the association is less well established in the elderly. The role of statins is well characterized for the reduction of myocardial infarction incidence or death in individuals with a history or high risk of cardiovascular diseases, regardless of age. Therapeutic measures recommended to prevent cardiovascular diseases and to reduce cholesterol levels in the elderly, such as lifestyle changes and lipid-lowering drugs, particularly statins, are based on studies conducted in younger adults. This narrative review aims to summarize the main observational studies and randomized clinical trials that have studied the relationship between cholesterol and cardiovascular diseases and the potential benefits and drawbacks of statins use in elderly patients.
Keywords: hypercholesterolemia, cardiovascular disease, coronary artery disease, stroke, peripheral arterial disease, aged
Cholesterol is a biological molecule that is essential to cell membrane structure and function and to hormone and vitamin synthesis in mammals. Certain genetic disorders can affect its metabolism and give rise to diseases that can occur in the very young. However, its main importance lies in its role in atherosclerosis, a degenerative process that affects medium and large caliber arteries and is responsible for the majority of cardiovascular diseases (CVD), the leading cause of death worldwide [World Health Statistics 2008. www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdf].
The relationship between cholesterol and atherosclerosis is primarily characterized by a rise in plasma concentration [1], although other mechanisms can also intervene. The process begins at an early age with an accumulation of cholesterol esters in the intimal layer of the arteries, which produces a lesion known as a fatty streak [2]. As this process continues over time, it leads to more advanced lesions: atherosclerotic plaque [3]. The growth of this plaque towards the lumen of the vessel and/or ruptures with subsequent thrombosis lead to ischemic disease, which can present as coronary heart disease (CHD), ischemic stroke or peripheral artery disease (PAD).
In addition to increased plasma cholesterol levels, other risk factors such as smoking, hypertension and diabetes, can contribute to the development of atherosclerosis through phenomena that increase endothelial permeability, inflammation, oxidation and coagulation [4, 5].
The majority of cardiovascular disease cases and deaths occur in the elderly (>65 years) and very elderly (> 80 years) [6], perhaps due to increased exposure to these harmful agents over time (Figure 1) [7]. The over-65 population constitutes about 17% and 14% of the European and U.S. populations, respectively, in 2010 and is increasing worldwide [www.ec.europa.eu/Eurostat and www.census.gov]. The increase in aging population and predominant Western lifestyles, which are also being adopted in developing countries, combines to produce higher population levels of cholesterol and atherogenic dyslipidemia; the result is a growing increase in the incidence of cardiovascular disease and death. However, age-adjusted mortality in younger populations makes it appear that there is a downward trend in many developing countries [8].
Figure 1.
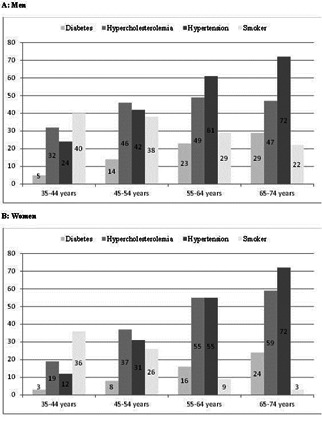
Cardiovascular risk factors prevalence (%) by age and sex. Pooled analysis with individual data from 28,887 participants of 11 population-based Spanish studies [7].
In this article, our aim is to review the epidemiological and clinical evidence that supports the relationship between hypercholesterolemia and CVD in the elderly and very elderly. We will focus on the characteristics of lipid metabolism disorders, their association with CVD risk, and the potential benefits of lifestyle changes and pharmacological therapies in older adults.
Lipid metabolism and age
Plasma cholesterol originates in three ways: from the intestinal absorption of food, mainly of animal origin; from bile salts secreted by the liver and subsequently reabsorbed by the intestine; and from cellular synthesis, primarily of hepatic origin [9]. The body’s capacity for intestinal absorption and synthesis is determined genetically and adapts to the needs of the organism.
Cholesterol is transported in the plasma by lipoproteins. These macromolecules are composed of cholesterol esters, fatty acid esters (triglycerides) and a number of polar lipids and proteins (apoproteins) that provide the necessary solubility for cholesterol transport in the plasma and the key to its metabolism, respectively. Cholesterol therefore shares its plasma transport with other fats, such as fatty acids, whose main function is energy storage. The different composition of each component determines the physical/chemical properties of the lipoproteins. The most common classification is based on density, which determines the presence of chylomicrons, very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) [10].
Total plasma cholesterol is determined by the provision of cholesterol from VLDL, IDL, LDL and HDL. LDL transport most plasma cholesterol (about 60%). These are responsible for conveying cholesterol from the liver to the peripheral tissues and depositing it in the intimal layer of the arteries under certain circumstances, thereby starting the atherosclerotic process. HDL can remove excess cholesterol from cells, including macrophages loaded with cholesterol in atherosclerotic lesions, and transport this surplus to the liver. They transport about 30% of plasma cholesterol. The balance of both lipoproteins determines the onset, progression and complications of atherosclerotic plaque and therefore disease.
The concentration of plasma cholesterol increases with age from puberty until 45 to 55 years of age in men, then decreases. In women, it continues to increase until about 10 years later, after which it declines in the last decades of life (Figure 2). The decreases can be explained by a reduction in LDL synthesis due to decreased liver function, but may also result from a survival bias in subjects with lower levels. Compared to LDL cholesterol, HDL levels fluctuate less, especially in men; studies in post-menopausal women have reported variable fluctuations [11–20].
Figure 2.
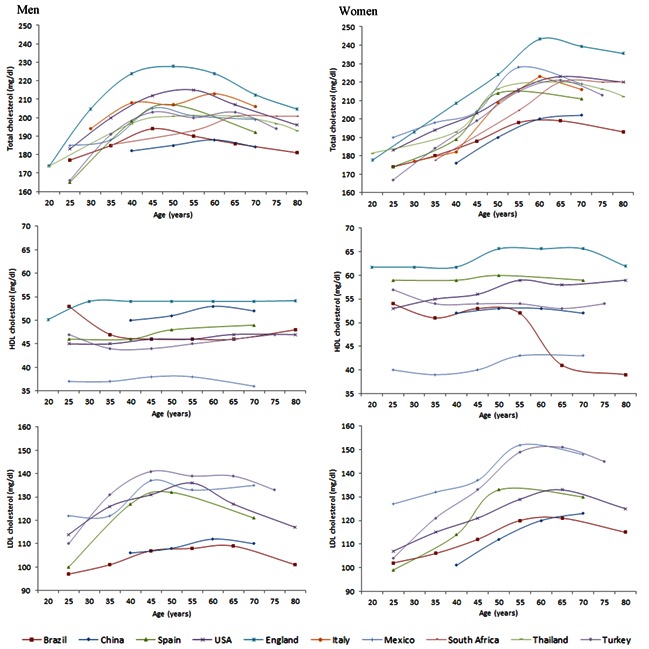
Total cholesterol, high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) in different countries of the world by age and sex [11–20].
The factors that determine plasma cholesterol concentration are varied and depend on the specific circumstances of the elderly patient. They include diet, exercise, and metabolic disorders:
Diet
Diets rich in cholesterol and saturated and trans fats increase the concentration of LDL cholesterol [21]. Certain intraluminal conditions in the intestine, such as increased transit time or the cholesterol content of bile, can also increase LDL concentration [9]. Both factors are present in the elderly.
Exercise
Regular physical exercise does not reduce total cholesterol and LDL if there is no weight loss, although it is effective in increasing HDL cholesterol and reducing triglycerides [22]. It also causes a change in the phenotypic expression of LDL and decreases the amount of small LDLs, which are more atherogenic. A high proportion of the elderly have a sedentary lifestyle [23]. The reasons cited for this inactivity were poor health, pain, obesity, fatigue or negative prior experiences, such as falls and injuries [24].
Genetic metabolic disorders
The synthesis and catabolism of cholesterol is genetically determined. Errors in the genes can increase plasma cholesterol concentration. The best known of these errors affect recognition of the LDL’s liver receptors, giving rise to familial hypercholesterolemia, which results in very high levels of cholesterol and premature CHD. In the majority of subjects, however, significantly high plasma cholesterol is a result of the interaction between unhealthy lifestyle habits (diets rich in animal fats) or concomitant diseases (diabetes mellitus, hypothyroidism, nephropathy, etc.) and other genetic disorders, such as polygenic hypercholesterolemia, familial combined hyperlipidemia, dysbetalipoproteinemia, and other lesser known disorders leading to late clinical manifestations [21, 25].
Lipid metabolism and comorbidity
Various disease processes are conducive to the onset of hypercholesterolemia, atherogenic forms of LDL, or an imbalance between pro- and anti-atherogenic lipoproteins. Many of these are more frequently present in the elderly and seem to have a common pathophysiological substrate, such as a lipoprotein lipase defect responsible for the catabolism of VLDL [25], among other mechanisms.
Obesity, metabolic syndrome and type-2 diabetes mellitus
Increased fat tissue (especially perivisceral fat), physical inactivity and insulin resistance are associated with a number of carbohydrate and lipid metabolism disorders, such as metabolic syndrome and diabetes mellitus, which increase the risk of atherogenesis [26, 27]. While this metabolic situation may only slightly increase total and LDL cholesterol concentrations, it increases VLDL cholesterol and triglycerides, reduces HDL cholesterol, and causes phenotypically smaller LDLs, making them more permeable and easier oxidation [28]. The primary mechanism for these changes is likely to be triggered by a massive arrival to the liver of free fatty acids (FFA) from the perivisceral fat, often caused by hypertrophia of the adipocytes and/or a lack of insulin anti-lipolytic action. FFA stimulates the hepatic synthesis and secretion of VDLD (triglycerides-rich lipoproteins). Also, the reduced activity of the lipoprotein lipase secondary to insulin resistance is likely to contribute to the increased VLDL concentration. The VLDL interacts with the circulating LDL and HDL, exchanging triglycerides for cholesterol esters through cholesteryl ester transfer protein (CETP). Upon arrival to the liver, these lipoproteins lose the triglycerides by means of the hepatic lipase, which has increased capacity in individuals with insulin resistance and increased FFA concentrations. The result is the synthesis of small LDL particles and a decrease in the HDL concentration [28]. This type of lipid disorder is known as diabetic or atherogenic dyslipidemia and is closely associated with aging [29]. In the Spanish population, obesity in those older than 65 years has a prevalence of around 35% [7], in some areas exceeding 50% [31]. Prevalence of metabolic syndrome, or the association of abdominal obesity with other risk factors, was higher than 40% [31] and of diabetes above 25% [7].
Hypothyroidism
Hypothyroidism is a common disease in the elderly (1–10%), especially in women [32]. The most frequent lipid disorder associated with hypothyroidism is increased total cholesterol and LDL due to limited synthesis of the LDL hepatic receptor, which decreases the plasmatic clearance. Other less frequent lipid disorders are increased levels of triglycerides, HDL cholesterol or lipoprotein (a).
Thyroid hormones have a key role in lipid metabolism due to their influence on the activity of several enzymes. For instance, the decreased activity of 3-hidroxy-3-methyl-glutaryl CoA observed in individuals with hypothyroidism may explain the poor response to lipid-lowering treatment, the decreased activity of CETP and hepatic lipase is responsible for the increase in HDL, and of the lipoprotein lipase that decreases the clearance of triglycerides-rich lipoproteins [33, 34].
The association between hypothyroidism and atherosclerotic disease has also been reported [35] even for subclinical forms [36] in those younger than 80. Thyroid stimulating hormone (TSH) screening in the elderly with hypercholesterolemia and treatment with L-thyroxine in the clinical forms is therefore a priority, because controlled clinical trials provide no evidence of subclinical forms. It is also advisable to screen for and treat hypothyroidism before starting statin therapy, as an increased risk of side effects has been reported [21].
Nephropathy
Chronic kidney disease increases with age. The National Health and Nutrition Examination Survey (NHANES 1988–1994 and 1999–2006) reported a prevalence of renal failure, defined as a glomerular filtration rate <60 ml/min/1.73 m2, of 7.5%, 26.5% and 51.1% in the age ranges of 60–69, 70–79 and 80+, respectively [37]. Chronic renal disease is associated with increased incidence of dyslipidemia [38] and premature CVD [39], which constitutes its main cause of death. The pattern and magnitude of dyslipidemia depend on the degree of renal failure, the presence of proteinuria, and the kind of treatment selected for terminal renal failure (peritoneal dialysis or hemodialysis). The typical dyslipidemia pattern is increased VLDL and IDL and a decrease in HDL. Total and LDL cholesterol can also be increased in the event of proteinuria or peritoneal dialysis [38]. Elderly patients experience an age-associated process of glomerular filtration rate reduction, which has been defined as a benign deterioration, but for very elderly patients represents an increased risk of cardiovascular disease and death, as well as functional and cognitive deterioration and frailty [37]. Moreover, renal glomerulus can suffer from the consequences of organ damage caused by other risk factors such as hypertension and diabetes, which are more common in elderly patients in the form of nephrosclerosis or diabetic nephropathy, the leading causes of end-stage renal disease and dialysis. The presence of renal failure also increases the risk of myopathy in patients being treated with statins in combination with fibrates. It is therefore advisable to make an assessment of renal function in elderly patients to ensure that the dyslipidemia is not renal in origin and to assess the most appropriate lipid-lowering therapy [21].
Drugs
Several commonly used drugs in the elderly population can alter the lipid profile in terms of increasing total and LDL cholesterol and triglycerides and/or reducing HDL cholesterol. It has been reported that thiazide and loop diuretics increase LDL and reduce HDL cholesterol, although this effect is negligible with currently recommended doses. Beta-blockers without intrinsic sympathomimetic activity or α-blocking properties tend to lower HDL cholesterol and increase triglycerides. Currently, however, these side effects are not a contraindication for their use. Some drugs with hormonal action reduce HDL cholesterol and are often used in elderly patients. These include tamoxifen, with its antiestrogenic action for treating breast cancer, and androgen, sometimes used by elderly men. Immunosuppressants, such as cyclosporine, increase total and LDL cholesterol, and require statins for their control, but in low doses because statins inhibit catabolism. New atypical antipsychotics, commonly used for behavioral disorders, can also increase triglycerides and reduce HDL, probably due to the weight gain associated with these drugs [33].
Hypercholesterolemia as a cardiovascular risk factor in the elderly
The lipid theory of atherosclerosis was first proposed in the mid-nineteenth century, but it was not until a hundred years later, around 1960, when it was definitively confirmed for CHD. The magnitude of the association between high cholesterol levels and cardiovascular risk was first observed in ecological studies, then tested in animal models and later confirmed in large observational and interventional studies [40]. In this section, we review the burden of cardiovascular disease, the prevalence of hypercholesterolemia and the major observational studies that examine the relationship between cholesterol and the various forms of CVD in the elderly.
Burden of cardiovascular disease in the over-65 population
Most cases of CVD are found in the elderly population, According to U.S. population records, 864,480 people died of these diseases in 2005 [41], 82% of them over 65 [42]. In terms of morbidity, almost half of the 80 million Americans having some form of CVD were over 60 [41]. Moreover, the projections of CHD cases and deaths in the coming decades are alarming [43–45]. The United States expects a 26% increase in incidence, 47% in prevalence and 56% in mortality caused by CHD between 2010 and 2040, mainly due to the progressive aging of the population [43], with a 41% increase in costs associated with this disease. In China, in excess of 7.8 million coronary events (69% increase) and 3.4 million deaths from CHD (64% increase) are projected for the decade 2020–2029 compared to 2000–2009 [44]. Finally, the projections for the Spanish population indicate that by 2049 that 60% of acute coronary syndrome cases will occur in people over 75 years of age [45].
Prevalence of hypercholesterolemia and age
Epidemiological studies conducted in different geographical areas have shown a high prevalence of hypercholesterolemia in later life [11–16, 20] (Table 1). Western countries have the highest prevalence, consistent with the higher incidence of cardiovascular disease and death.
Table 1.
Prevalence of hypercholesterolemia in elderly from different countries of the world by sex [11–16, 20]
| Sex and country | Total cholesterol | |
|---|---|---|
| Women | ≥200 mg/dl* | ≥240 mg/dl** |
| China, 65–74 yrs | 47.8% | 18.2% |
| Spain, ≥65 yrs | 77.6% | 45.8% |
| England, ≥65 yrs | 91.7% | 54.6% |
| Mexico, ≥60 yrs | 44.2% | -- |
| South Africa, ≥60 yrs | 58.0% | -- |
| Thailand, ≥60 yrs | -- | 10.8% |
| Turkey, ≥70 yrs | 58.5% | 23.8% |
| Men | ≥200 mg/dl* | ≥240 mg/dl** |
| China, 65–74 yrs | 30.6% | 7.5% |
| Spain, ≥65 yrs | 52.5% | 23.1% |
| England, ≥65 yrs | 81.9% | 40.4% |
| Mexico, ≥60 yrs | 56.9% | -- |
| South Africa, ≥60 yrs | 78.7% | -- |
| Thailand, ≥60 yrs | -- | 18.4% |
| Turkey, ≥70 yrs | 42.5% | 17.0% |
193 mg/dl in England and South Africa;
250 mg/dl in England
Hypercholesterolemia and ischemic heart disease
The Prospective Studies Collaboration meta-analysis, which brought together 61 mainly European and American cohort studies, concluded that a lower total cholesterol plasma concentration of 1 mmol/L (39 mg/dL) was associated with a lower risk of mortality from CHD (hazard ratio: 0.83, 95% CI: 0.81–0.85) in individuals of both sexes from 70 to 89 years of age [46]. This association was continuous, with no risk thresholds [49, 50]. However, cohort analysis of elderly individuals has produced mixed results in the degree of association between cholesterol levels and CHD [48–53]. In the follow-up of a cohort with an average age of 80+ (63% with a history of CHD) before statins were used, increases of 10 mg/dL in total cholesterol levels augmented the risk of developing coronary events by 1.12 [48]. Moreover, analysis of the Kaiser Permanente Heart Study and Framingham Heart Study cohorts showed significant associations between cholesterol levels and the risk of CHD mortality in individuals with and without a history of CVD [49–51]. However, in the EPESE cohort, consisting of individuals with an average age of 79 years, there was no significant association between total cholesterol levels ≥ 240 mg/dL and CHD incidence and mortality, or all-cause mortality [52]. In an analysis of this same cohort, Corti et al [53] allude to the need for additional adjustment for frailty indicators (blood iron concentration and levels of albumin) because this was a highly vulnerable population. In fact, the analysis adjusted for these frailty indicators did show that ischemic heart disease mortality -- and higher all-cause mortality -- was significantly higher in individuals with total cholesterol levels ≥240 mg/dL. In subsequent studies this association is more complex, with increased risk of mortality in the elderly with low total cholesterol levels [54–59]. In fact, Honolulu Heart Program researchers confirmed these results after repeating the adjusted models proposed by Corti et al in the EPESE cohort [54]. It is now believed that the association between total cholesterol and mortality has a J-shaped curve in the elderly. It is possible that low cholesterol levels in older people are an indicator of comorbidity and frailty [59–61] and increased risk of mortality.
In addition, a progressive decline has been reported in the relative risk that total cholesterol confers on CHD mortality in the elderly [62,63], which probably masks the decrease in HDL cholesterol [46]. The protective effect of this cholesterol fraction has been known for years [50, 64, 65]. A decrease of 10 mg/dL of HDL cholesterol is associated with an increased likelihood of coronary events in men by a factor of 1.70 and in women by 1.95, while an increase of 10 mg/dL of LDL cholesterol increased it by 1.28 [48]. The effect of reduced levels of HDL cholesterol on coronary risk would be greater than an increase in LDL cholesterol levels in the elderly [66].
Hypercholesterolemia and stroke
Strokes are a leading cause of death in older people. A third of ischemic stroke cases are diagnosed in individuals over the age of 80, greatly increasing short-term mortality in this age group [67]. The most significant risk factors in the development of strokes are age, sex, systolic blood pressure, diabetes, smoking, left ventricular hypertrophy, heart failure and atrial fibrillation [68]. Of all of these, arterial hypertension is the one with higher relative and attributable risk. The role of hypercholesterolemia in the development of strokes is, however, disputed [69,70].
The Prospective Studies Collaboration meta-analysis [46] reports a negative or insignificant association in subjects aged 70 or over between total cholesterol and mortality due to global or ischemic strokes, and low levels of total cholesterol was associated with an increase in mortality due to hemorrhagic strokes between the ages of 70 and 80. Also observed was an inverse association between total cholesterol levels and the severity of ischemic strokes [71–74]. For example, the results of the Copenhagen Stroke Study, which included hospital patients diagnosed with strokes, showed that higher levels of total cholesterol were associated to a lesser extent with cerebral infarction, and therefore, the event was less serious and mortality due to this cause was lower [74]. The authors suggest that this could be a reason for the lower mortality observed in elderly individuals with high levels of total cholesterol [54–58]. Various studies in elderly people have shown how elevated levels of HDL cholesterol can protect against the development of ischemic strokes [70–77].
Hypercholesterolemia and peripheral artery disease
PAD is closely associated with age, with estimated prevalence three times greater when asymptomatic cases are considered [78,79], conferring a marked risk of patients presenting with other CVD [80,81]. The role of hypercholesterolemia in this disease is disputed, as prospective studies in the general and elderly population have variously reported no association, or a role only for some forms of disease or certain population groups [82–85]. In the Framingham study, which had a 36-year follow-up, total cholesterol ≥240 mg/dL in people over 65 did not confer a significantly increased risk of PAD, except in men younger than 65 with a relative risk (RR) of 2.0 [82]. In a prospective Dutch study of 2,589 subjects with an average age of 64 and 7.2-year mean follow-up, hypercholesterolemia showed no significant predictive value for all clinical forms of PAD, although asymptomatic forms were predicted (odds ratio (OR): 1.6, 95% CI: 1.1–2.5) [83]. Other studies that found no association include the Quebec Cardiovascular Study with respect to the onset of intermittent claudication [84] and the DESIR study of 3,805 French subjects under the age of 65 with a 6-year follow-up [85].
Some prospective studies, however, did report a relationship [86–88]. The 25-year Health Professional Follow-up Study, which included almost 44,000 men of working age, showed that the diagnosis of hypercholesterolemia was associated with the incidence of PAD, defined as both symptomatic and asymptomatic forms of the disease, conferring a population attributable risk of 17% (95% CI: 7%–26%), lower than the hypertension estimate of 41% (95% CI: 31%–50%) and smoking 44% (95% CI: 33%–53%) and slightly higher than diabetes 14% (95% CI: 10%–19%), which was much less prevalent [86]. Another study reported this association in middle age [87] and one for patients over 65 detected that only total cholesterol worsened the evolution of the ankle-brachial index [88]. The reasons that have been advanced for this lack of association have included the use by many patients of lipid-lowering drug treatment, which delays PAD progression [82] as has been shown in clinical trials [89, 90], or simply being a reflection of the less relevant role of hypercholesterolemia in the development of lower limb ischemia compared to other vascular beds and the lack of joint assessment with HDL cholesterol [82, 91]. In the Framingham study, the total cholesterol/HDL ratio showed differences in the Kaplan-Meier curves for the occurrence of PAD in men and women between the ages of 45 and 84 [82]. In the Women’s Health Study, HDL cholesterol significantly decreased the risk of PAD [91]. Cross-sectional studies have found reduced levels of HDL cholesterol in elderly people with PAD [76, 92, 93], as in the Offspring cohort of the Framingham study, with an average age of 60, in which only the HDL cholesterol value showed association when adjusted for the other variables [92]. However, to further complicate the situation in the DESIR study, the elevated levels of HDL cholesterol behaved as a risk factor for PAD. The authors attributed this increased risk to the possible effect of the functionality of the different subtypes of HDL cholesterol [94].
Treatment of hypercholesterolemia in the elderly
Lipid-lowering therapy in elderly patients has attracted growing interest in recent years due to the progressive increase in this population and increased survival of those who already have some form of CVD. The exclusion of elderly patients from the first major clinical trials resulted in a lack of evidence as to whether it was possible to obtain the same benefits and with the same security as in the younger population. Moreover, the lack of a unanimous characterization of the elderly patient and the high comorbidity has also hampered comparison between studies and the drawing of conclusions on these aspects.
Changes in lifestyle
Recommendations for the prevention of CVD are based on smoking sensation, a lower-fat diet in terms of quantity and quality, and regular physical exercise [1, 21]. These recommendations are generally derived from studies of the younger population. There is no abundant evidence of the benefit to elderly patients. Smoking cessation is the most cost-efficient way to reduce the risk of CVD and death, including elderly patients [95]. Different diets have shown effectiveness in reducing total cholesterol, LDL cholesterol and triglycerides and increasing HDL cholesterol [96–98]. Most of these are based on a reduction of total saturated fat content, replaced by mono and polyunsaturated fats, as well as other dietary changes. Some studies indicate a lower risk of cardiovascular disease and death in primary [99,100] and secondary [96, 101] prevention, with the amount of ω-3 fatty acids from fish or supplements as an associated beneficial factor. Moreover, it is known that low-calorie weight-reduction diets, generally at the expense of a reduction of total fat, improve the lipid profile in obese patients [102]. There is no evidence, however, to suggest that these diets improve the lipid profile and provide a clinical benefit in the form of preventing cardiovascular events or increasing survival in the elderly, so caution is recommended when indicating diets that can lead to poor nutrition in this age group.
Regular physical exercise improves the lipid profile in the general population, but these effects in the elderly have not been clearly demonstrated [103–109]. In a study of individuals aged between 45 and 65, the amount and intensity of exercise was significant in terms of HDL and triglyceride levels, as well as favorable changes in the size of the lipoproteins. Some meta-analyses in adults, including the elderly, also found reductions in total and LDL cholesterol in terms of aerobic exercise and resistance [104–106]. These benefits, however, have not been demonstrated in the elderly population [107,108]. Exercise lowers the risk of death in a wide variety of clinical situations, but can increase risk in patients with prior CVD or the elderly [109], so initial medical supervision is advisable if the patient previously had a sedentary lifestyle.
The benefits of statin therapy
Although various drugs can lower plasma cholesterol, the bulk of the evidence in preventing cardiovascular morbidity and mortality comes from statins, as there are no clinical trials of other drugs in this population group. We review this evidence in terms of primary and secondary prevention (Table 2):
Table 2.
Baseline characteristics of major statin studies in primary and secondary prevention of cardiovascular events
| Study | Type of prevention | Number of patients | Patients over 65 years | Drug/doses | Follow up (median) | Beneficial outcomes in the intervention group* | Observations |
|---|---|---|---|---|---|---|---|
| AFCAPS/TexCAPS[110] | Primary | 6605 | 22 % | Lovastatina 20–40 mg vs placebo | 5.2 yrs | MI, UA and death | |
| ASCOT-LLA [111] | Primary | 10305 | 64 % over 60 yrs | Atorvastatina 10 mg vs placebo | 3.3 yrs | MI and CV death | Stopped prematurely |
| JUPITER [112,113] | Primary | 17802 | 32% over 70 yrs | Rosuvastatina 20 mg vs placebo | 1.9 yrs | MI, stroke, death, revascularization and hospitalization. | Stopped prematurely |
| CHS [114] | Primary | 1914 | 100 % | Various | 7.3 yrs | MI, stroke, coronary death and all cause of mortality | Only elderly people, not randomized |
| 4S [116,117] | Secondary | 4444 | 23 % | Simvastatina 20–40 mg vs placebo | 5.4 yrs | All-cause death, CV events or revascularization | |
| CARE [118,119] | Secondary | 4159 | 31 % | Pravastatina 40 mg vs placebo | 5 yrs | Death, major coronary events and stroke. | |
| LIPID [120, 121] | Secondary | 9014 | 39 % | Pravastatina 40 mg vs placebo | 6.1 yrs and 8 yrs | CV death and overall mortality | |
| HPS [122] | Secondary | 20536 | 28% over 70 yrs | Simvastatin 40 mg vs placebo | 5 years | All- cause and coronary death, major CV events and revascularization | |
| PROSPER [123] | Primary and secondary | 5804 | 100 % over 70 yrs | Pravastatina 40 mg vs. placebo | 3.2 years | MI, stroke or CV death. | Only elderly patients. TIA was reduced but not stroke |
| SPARCL [124] | Secondary | 4731 | Non available | Atorvastatina 80 mg vs. placebo | 4.9 years | Non fatal or fatal stroke | Increase of hemorrhagic stroke |
More detailed explanation in the text
MI: Myocardial Infarction, UA: unstable angina, CV: Cardiovascular, TIA: Transient ischemic attack
Primary prevention: No randomized primary prevention trials have focused exclusively on the elderly population; we will review the main studies that included the over-65 population. The AFCAPS/TexCAPS clinical trial [110] included 6,005 men and women, of whom 22% were older than 64 (65–73 years), and had a 5.2-year follow-up. Compared to a placebo, lovastatin in daily doses of 20–40 mg decreased the incidence of non-fatal myocardial infarction, unstable angina or sudden cardiac death by 37% overall and 30% in the elderly. The ASCOT-LLA trial [111] was stopped prematurely (3.3 years) due to the benefits observed with the use of 10 mg of atorvastatin vs placebo in subjects with normal cholesterol levels and no history of CHD and at least 3 cardiovascular risk factors. Atorvastatin reduced the primary endpoint (non-fatal MI and fatal CHD events of 1.9 vs. 3% p=0.0005). From the sample of 10,305 patients, 6,570 were older than 60 and, in this subgroup, the RR reduction was similar in individuals who were younger and older than 60 (34% vs. 36%). The JUPITER trial [112] also had to be prematurely stopped when significant differences were observed in favor of the daily use of 20 mg of rosuvastatinvs placebo. This study included 17,802 subjects with LDL cholesterol below 130 mg/dL and high-sensitivity PCR over 2.0 mg/L. The primary endpoint, consisting of cardiovascular death, myocardial infarction, stroke, revascularization and hospitalization for unstable angina, decreased significantly. Further analysis of the 5,695 patients older than 70 years [113] also showed a significant decrease in the primary endpoint in this population, but not in all-cause mortality. In 2002, the only clinical trial on primary prevention in the elderly (65+), Cardiovascular Health Study, was published but was not randomized [114]; it included 1,250 men and 664 women, with a 7.2-year mean follow-up. Statin use significantly decreased the combined point of myocardial infarction, stroke and death from CHD (16.7 vs. 20.4% p<0.001). It also decreased all-cause mortality and the results were similar in the over-75 subgroup. A meta-analysis of primary prevention trials with statins [115], which recruited more than 70,000 subjects, determined that benefits were obtained in all-cause mortality and major coronary and cerebrovascular events in the over 65 population, but these did not reach significance.
Secondary prevention: The first clinical trial to demonstrate the effectiveness of statins in the prevention of CVD was 4 S [116], which randomized 4,444 individuals with a history of CHD to receive simvastatin (20–40 mg daily) or placebo. At start-up, 1,021 individuals were between 65 and 70 and were the subject of a specific analysis compared to younger participants [117]. Their reduction of the endpoint (all-cause death, cardiovascular and major cardiovascular events or revascularization) had a RR similar to the younger population, but twice the reduction in the absolute risk of death from all causes and coronary causes was twice. Later, the CARE study [118] randomized 4,159 individuals of both sexes between the ages of 21 and 75 with a history of myocardial infarction to receive 40 mg of pravastatin daily vs placebo. After an average of 5 years of follow-up, pravastatin significantly reduced all of the major coronary events by around 35%. Further analysis of 1,283 subjects aged 65 and over [119] showed a significant decrease in the RR of major coronary events, by 32% (28.1 vs 19.7%, p<0.001), of coronary death by 45% (10.3 vs 5.8%, p=0.004) and stroke by 40% (7.3 vs 4.5%, p=0.03) in the pravastatin group. In 1998, the LIPID study results were published [120], in which 9,014 patients with previous myocardial infarction were randomized to receive 40 mg of pravastatin or placebo, and showed a significant reduction in death from CHD (12.3 vs 15.9% p<0.001). The 8-year follow-up of a subgroup of 3,514 patients older than 65 at the beginning of the study showed a significant reduction in all-cause mortality, CHD death, fatal and non-fatal infarction and the need for myocardial revascularization [121]. The largest study was the Heart Protection Study [122] with 20,536 individuals of both sexes (5,806 aged 70–80) with previous myocardial infarction, other cardiovascular arterial disease or no coronary disease, randomized to simvastatin 40 mg or placebo with a 5-year follow- up. Simvastatin significantly reduced cardiovascular and all-cause mortality (12.9 vs. 14.7% p = 0.0003). In the older group, major cardiovascular events were reduced by simvastatin, 23% in people aged 65–69 and 18% in those aged 70–80 at study entry, similar to patients younger than 65 years (24%). The HPS was the first study to show benefits in women, the elderly, and patients with diabetes. The PROSPER study [123] was the only trial that included only elderly patients. This randomized study included 5,802 men and women up to the age of 70 and was designed specifically to evaluate the efficacy of statins in the elderly. Patients received pravastatin 40 mg daily or placebo. At 3.2 years, pravastatin significantly decreased the primary end-point: non-fatal myocardial infarction, stroke or CHD death by 15% compared to placebo. The risk of stroke was unaffected but transient ischemic attack was reduced by 25%. The SPARCL study [124] aimed to determine whether statins (atorvastatin 80 mg daily) reduced the risk of stroke after a recent stroke or transient ischemic attack (TIA). The study included 4,731 patients with an average age of 63 who had either stroke or TIA within one to six months prior to study entry and had no known CHD. The 5-year absolute reduction in the primary end point (first non-fatal or fatal stroke), was 16% but the reduction of major cardiovascular events risk was 35%. The overall mortality rate was similar in both groups but there was a small increase in the incidence of hemorrhagic stroke in the atorvastatin group.
Several meta-analyses have also been published. The Cholesterol Treatment Trialists’ (CTT) Collaboration [125] included 90,056 participants from 14 randomized trials with 34,982 patients over 65 and 7,304 over 75. Statin therapy decreased the incidence of major cardiovascular events, stroke and coronary revascularization in a 5-year follow-up. The relative risk of CHD death, major coronary events and major vascular events per mmol/L of LDL cholesterol reduction was reduced by 22% vs 17%, 26% vs 19% and 22% vs 19% in the ≤65-year-old group compared to the >65-year-old group, respectively. In a new meta-analysis of this same group [126] of 26 randomized clinical trials with 170,000 participants, the RR reduction of major cardiovascular events per mmol/L of LDL cholesterol reduction with statins in one year was 22% in patients under 65, 22% between 65 and 74, and 16% in patients 75 or older, all of which were significant. Roberts et al [127] conducted a meta-analysis of 51,351 older adults to determine the effect of statins on major cardiovascular events including stroke, concluding that statins significantly reduced all-cause and CHD mortality and the risk of stroke and myocardial infarction in the elderly.
Safe use in the elderly
Statin therapy is generally safe and well tolerated. Factors that have been associated with an increased risk of the onset of adverse effects are age, small body size, female sex, previous renal or hepatic dysfunction, alcohol abuse and hypothyroidism [21]. The main side effects associated with its use are myopathy, hepatitis, diabetes, cancer and hemorrhagic stroke. Myopathy can manifest itself as myalgia or muscle weakness (present in 5%–10% of patients in the clinical practice), but is defined by elevated creatine kinase at least 5 times the upper limit of normal (ULN) and has a low incidence < 1/1000 of the cases treated. In the only study of the elderly [123], there was similar incidence of myalgia in the groups receiving pravastatin versus placebo and no cases of elevated creatine kinase more than 10 times ULN or rhabdomyolysis, the main and most serious complication. In the meta-analysis comparing high versus low doses of statins, there was an increased risk of rhabdomyolysis, mainly with 80 mg of simvastatin daily [126]. Cases of rhabdomyolysis are also more common with concomitant use of fibrates, particularly gemfibrozil [128]. Elevated levels of hepatic enzymes are also common (0.5–2%), are dose-dependent, and usually subside with dose suspension or reduction. In the PROSPER study [123], there was only one case of elevated transaminases of more than three times ULN. In this same study, the risk of cancer increased in the pravastatin group by 25% (p= 0.020). WOSCOP 10-year follow-up studies, also with pravastatin, did not observe this increased risk [129], even in the main meta-analyses [115, 126], although safety in the elderly population was not specifically studied. The increased RR of 1.66 (95% CI: 1.08–2.55) for hemorrhagic strokes comes particularly from the SPARCL trial [124] of atorvastatin 80 mg in patients with prior stroke. Its authors have linked it with initially low numbers of LDL or with a history of hemorrhagic stroke. The CTT meta-analysis [126] specifically studied this risk and found an insignificant increase. In the most recent meta-analyses [130, 131], which studied the overall safety of statin use, no increased risk of cancer, rhabdomyolysis or high creatine kinase was observed. There was an increased risk of diabetes (OR: 1.09, 95% CI: 1.02–1.16), elevated AST (OR: 1.12, 95% CI: 1.02 – 1.22) and ALT (OR: 1.30, 95% CI: 1.13 – 1.50) [133]. In the analysis that was differentiated by the various molecules, lovastatin was associated with an increase in AST, simvastatin with an increase in ALT, and rosuvastatin with increased diabetes [131]. None of these studies specifically analyzed the elderly population.
Clinical guideline recommendations
Recently published European guidelines [21] recommended treating the total burden of CVD in the population and stated that “primary prevention in the elderly should not differ from that of younger subjects”. In brief, treatment with statins in the elderly with CVD is recommended in the same way as for younger patients (Class 1 Level B). The guideline included a recommendation to start lipid-lowering medication at a low dose in the elderly (Class I level C). Finally, for elderly people without CVD, particularly if at least one CV risk factor exists, the recommendation for statin use is weak (Class IIb Level B). The NCEP III guidelines recommended specific lipid goals based on a person’s overall risk of CHD: the higher the risk, the lower the goal; this recommendation included the elderly population [132]. At the same time, if the suggested cholesterol level cannot be achieved with the use of statins, the guidelines recommend adding nicotinic acid or ezetimibe to the therapeutic regimen and, in patients with high risk and hypertriglyceridemia or low HDL cholesterol, a fibrate or nicotinic acid combined with a statin as well, although there are no studies of the effect on morbidity and mortality in elderly patients.
Recommendations for very elderly patients
There is not available enough evidence in the use of statin in the very elderly patients (80 and over), but we have to take into account that this is a high risk group who could benefit significantly from lipid-lowering therapy to reduce cardiovascular events [21]. Until we have enough evidence, clinical judgment should guide decision-making.
Conclusions
Hypercholesterolemia is highly prevalent in the elderly population because of its association with environmental and genetic factors and comorbidity. The majority of cardiovascular disease and death is also concentrated in this population. The link between elevated plasma cholesterol and CHD has been well demonstrated in this age group, while evidence is lacking to confirm a link with ischemic stroke and PAD. Statin therapy is effective in secondary prevention of CHD in the elderly; for stroke prevention, the balance between risks and benefits has yet to be clearly established. Regarding PAD, not enough studies have been done to show the benefits with respect to disease progression and prevention of amputations. There is also a lack of evidence on the usefulness of statins in primary prevention, especially after the age of 80. Statin therapy in the elderly is safe if the recommendations for this population are followed, although side effects may occur more frequently than in the younger population. Further intervention studies are necessary to determine the role of statins in the prevention of strokes and PAD, especially in the older population.
Acknowledgments
We appreciate the revision of the English text by Elaine Lilly, Ph.D., of Writer’s First Aid. Dr. Grau was funded by grants from Health Institute Carlos III-FEDER, Spain (Red HERACLES RD06/0009) and (Miguel Servet CP12/03287).
Reference
- 1.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (ATP III) Third report of the National Cholesterol Education Program (NCEP). Final report. Circulation. 2002;106:3143–21. [PubMed] [Google Scholar]
- 2.Stary HC, Chandler B, Glagov S, Guyton JR, Insull W, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–56. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arterioclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcome: Part I: Pathophysiology and clinical trial evidence (Risk factors through stable coronary disease) Circulation. 2006;114:2850–70. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis: an inflammatory disease. N Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistic-2012 Update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grau M, Elosua R, Cabrera de Leon A, Guembe MJ, Baena-Diez, Vega-Alonso T, et al. Cardiovascular risk factors in Spain in the first decade of the 21st century, a pooled analysis with individual data from 11 population-based studies: the DARIOS study. Rev Esp Cardiol. 2011;64:295–304. doi: 10.1016/j.recesp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, Chatenoud L, Bertoccio P, Lucchini F, Negri E, La Vecchia C. Mortality from cardiovascular and cerebrovascular disease in Europe and others areas of the world: an update. Eur J Cardiovasc Prev Rehabil. 2009;16:333–50. doi: 10.1097/HJR.0b013e328325d67d. [DOI] [PubMed] [Google Scholar]
- 9.Wang DQ-H, Cohen DE. Ballantyne. Lipidología Clínica. Barcelona: J&C Ediciones Medicas SL; 2011. Absorción y excreción del colesterol y otros esteroles; pp. 26–44. (Spanish Edition of Clinical Lipidology 2009) [Google Scholar]
- 10.Pownall HJ, Gotto AM. Ballantyne. Lipidología Clínica. Barcelona: J&C Ediciones Medicas SL; 2011. Metabolismo de las lipoproteínas plasmáticas humanas; pp. 1–10. (Spanish Edition of Ballantyne. Clinical Lipidology 2009) [Google Scholar]
- 11.He J, Gu D, Reynolds K, Wu X, Muntner P, Zhao J, et al. Inter ASIA Collaborative Group. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation. 2004;110:405–11. doi: 10.1161/01.CIR.0000136583.52681.0D. [DOI] [PubMed] [Google Scholar]
- 12.Guallar-Castillón P, Gil-Montero M, León-Muñoz LM, Graciani A, Bayán-Bravo A, Taboada JM, et al. Magnitude and management of hypercholesterolemia in the adult population of Spain, 2008–2010: The ENRICA Study. Rev Esp Cardiol. 2012;65:551–8. doi: 10.1016/j.recesp.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Primatesta P, Poulter NR. Levels of dyslipidaemia and improvement in its management in England: results from the Health Survey for England 2003. Clin Endocrinol (Oxf) 2006;64:292–8. doi: 10.1111/j.1365-2265.2006.02459.x. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Salinas CA, Gómez-Pérez FJ, Rull J, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52:S44–53. doi: 10.1590/s0036-36342010000700008. [DOI] [PubMed] [Google Scholar]
- 15.Khonputsa P, Veerman JL, Vos T, Aekplakorn W, Bertram M, Abbott-Klafter J, et al. Joint prevalence and control of hypercholesterolemia and hypertension in Thailand: third national health examination survey. Asia Pac J Public Health. 2012;24:185–94. doi: 10.1177/1010539510377651. [DOI] [PubMed] [Google Scholar]
- 16.Erem C, Hacihasanoglu A, Deger O, Kocak M, Topbas M. Prevalence of dyslipidemia and associated risk factors among Turkish adults: Trabzon lipid study. Endocrine. 2008;34:36–51. doi: 10.1007/s12020-008-9100-z. [DOI] [PubMed] [Google Scholar]
- 17.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 18.de Souza LJ, Souto Filho JT, de Souza TF, Reis AF, Gicovate Neto C, Bastos DA, et al. Prevalence of dyslipidemia and risk factors in Campos dos Goytacazes, in the Brazilian state of Rio de Janeiro. Arq Bras Cardiol. 2003;81:249–64. doi: 10.1590/s0066-782x2003001100005. [DOI] [PubMed] [Google Scholar]
- 19.Capuano V, Bambacaro A, D’Arminio T, Del Regno B, Dantonio V, Lanzara C. Changes in total serum cholesterol for cardiovascular disease in a Mediterranean area, 1989–1999. Eur J Epidemiol. 2003;18:27–32. doi: 10.1023/a:1022533117139. [DOI] [PubMed] [Google Scholar]
- 20.Norman R, Bradshaw D, Steyn K, Gaziano T, South African Comparative Risk Assessment Collaborating Group Estimating the burden of disease attributable to high cholesterol in South Africa in 2000. S Afr Med J. 2007;97:708–15. [PubMed] [Google Scholar]
- 21.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund, et al. The task force for the management of dyslipidaemia of the European Society of Cardiology and the European Atherosclerosis Society. ESC/EAS Guidelines for the management of dyslipidaemia. Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 22.Church TS, Lavie CJ. Ballantyne. Lipidología Clínica. Barcelona: J&C Ediciones Medicas SL; 2011. Ejercicio y lípidos; pp. 232–9. (Spanish Edition of Ballantyne. Clinical Lipidology 2009) [Google Scholar]
- 23.Healy GN, Clark BK, Winkler EAH, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med. 2011;41:216–27. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallinen J, Leinonen R, Hirvensalo M, Lyyra TM, Heikkinen E, Rantanen T. Perceived constraints on physical exercise, among obese and non-obese older people. Prev Med. 2009;49:506–10. doi: 10.1016/j.ypmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Brunzell JD. Ballantyne. Lipidología Clínica. Barcelona: J&C Ediciones Medicas SL; 2011. Dislipemiagenética; pp. 71–84. (Spanish Edition of Ballantyne Clinical Lipidology 2009) [Google Scholar]
- 26.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 27.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginsberg HN, Zhang Y-L, Hernanadez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–40. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Mathus-Vliegen EM. Obesity management task force of the European Association for the Study of Obesity. Obes Facts. 2012;5:460–83. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 30.Félix-Redondo FJ, Baena-Diez JM, Grau M, Tormo MA, Fernández-Bergés D. Prevalence of obesity and cardiovascular risk in the general population of a health area in Extremadura (Spain) The Hermex study Endocrinol Nutr. 2012;59:160–8. doi: 10.1016/j.endonu.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Bergés S, Cabrera de León A, Sanz H, Elosua R, Guembe MJ, Alzamora M, Vega-Alonso T, et al. Metabolic syndrome in Spain: prevalence and coronary risk associated with harmonized definition and WHO proposal. DARIOS study. Rev Esp Cardiol. 2012;63:241–8. doi: 10.1016/j.recesp.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Bensenor IM, Olmos RD, Lotufo PA. Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging. 2012;7:97–111. doi: 10.2147/CIA.S23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone NJ. Ballantyne. Lipidología Clínica. Barcelona: J&C Ediciones Medicas SL; 2011. Evaluación clínica para detectar causas genéticas y secundarias de dislipemia; pp. 144–54. (Spanish Edition of Ballantyne. Clinical Lipidology 2009) [Google Scholar]
- 34.Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids. 2011;2011:575840. doi: 10.1155/2011/575840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappola AR, Ladenson PW. Hypothyroidism and aterosclerosis. J Clin Endocrinol Metab. 2003;88:2438–44. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 36.Rodondi N, den Elzen WO, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–74. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci. 2012;67:1379–86. doi: 10.1093/gerona/gls173. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard SS. Impact of dyslipidaemia in end-stage renal disease. J Am Soc Nephrol. 2003;14:S315–30. doi: 10.1097/01.asn.0000081698.10331.83. [DOI] [PubMed] [Google Scholar]
- 39.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for the development of cardiovascular disease: A statement from the American Heart Association council on kidney in cardiovascular disease, high blood pressure research, clinical cardiology and epidemiology and prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol. 2008;61:299–310. [PubMed] [Google Scholar]
- 41.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 42.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25:563–77. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827–33.e5. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran A, Zhao D, Gu D, Coxson P, Chen CS, Cheng J, et al. The future impact of population growth and aging on coronary heart disease in China: projections from the Coronary Heart Disease Policy Model-China. BMC Public Health. 2008;8:394. doi: 10.1186/1471-2458-8-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degano IR, Elosua R, Marrugat J. Epidemiology of acute coronary syndromes in Spain: estimation of the number of cases and trends from 2005 to 2049. Rev Esp Cardiol. 2012 doi: 10.1016/j.rec.2013.01.018. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 47.Martin MJ, Hulley SB, Browner WS, Kuller LH, Wentworth D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet. 1986;2:933–6. doi: 10.1016/s0140-6736(86)90597-0. [DOI] [PubMed] [Google Scholar]
- 48.Aronow WS, Ahn C. Risk factors for new coronary events in a large cohort of very elderly patients with and without coronary artery disease. Am J Cardiol. 1996;77:864–6. doi: 10.1016/S0002-9149(97)89183-7. [DOI] [PubMed] [Google Scholar]
- 49.Rubin SM, Sidney S, Black DM, Browner WS, Hulley SB, Cummings SR. High blood cholesterol in elderly men and the excess risk for coronary heart disease. Ann Intern Med. 1990;113:916–20. doi: 10.7326/0003-4819-113-12-916. [DOI] [PubMed] [Google Scholar]
- 50.Castelli WP, Wilson PW, Levy D, Anderson K. Cardiovascular risk factors in the elderly. Am J Cardiol. 1989;63:12H–19H. doi: 10.1016/0002-9149(89)90110-0. [DOI] [PubMed] [Google Scholar]
- 51.Wong ND, Wilson PW, Kannel WB. Serum cholesterol as a prognostic factor after myocardial infarction: the Framingham Study. Ann Intern Med. 1991;115:687–93. doi: 10.7326/0003-4819-115-9-687. [DOI] [PubMed] [Google Scholar]
- 52.Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272:1335–40. [PubMed] [Google Scholar]
- 53.Corti MC, Guralnik JM, Salive ME, Harris T, Ferrucci L, Glynn RJ, et al. Clarifying the direct relation between total cholesterol levels and death from coronary heart disease in older persons. Ann Intern Med. 1997;126:753–60. doi: 10.7326/0003-4819-126-10-199705150-00001. [DOI] [PubMed] [Google Scholar]
- 54.Schatz IJ, Masaki K, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358:351–5. doi: 10.1016/S0140-6736(01)05553-2. [DOI] [PubMed] [Google Scholar]
- 55.Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–26. doi: 10.1111/j.1532-5415.2005.53106.x. [DOI] [PubMed] [Google Scholar]
- 56.Brescianini S, Maggi S, Farchi G, Mariotti S, Di Carlo A, Baldereschi M, ILSA Group Low total cholesterol and increased risk of dying: are low levels clinical warning signs in the elderly? Results from the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2003;51:991–6. doi: 10.1046/j.1365-2389.2003.51313.x. [DOI] [PubMed] [Google Scholar]
- 57.Li JZ, Chen ML, Wang S, Dong J, Zeng P, Hou LW. A long-term follow-up study of serum lipid levels and coronary heart disease in the elderly. Chin Med J (Engl) 2004;117:163–7. [PubMed] [Google Scholar]
- 58.Casiglia E, Mazza A, Tikhonoff V, Scarpa R, Schiavon L, Pessina AC. Total cholesterol and mortality in the elderly. J Intern Med. 2003;254:353–62. doi: 10.1046/j.1365-2796.2003.01200.x. [DOI] [PubMed] [Google Scholar]
- 59.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–23. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 60.Reuben DB, Ix JH, Greendale GA, Seeman TE. The predictive value of combined hypoalbuminemia and hypocholesterolemia in high functioning community-dwelling older persons: Mac Arthur Studies of Successful Aging. J Am Geriatr Soc. 1999;47:402–6. doi: 10.1111/j.1532-5415.1999.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 61.Aronow WS, Frishman WH. Management of hypercholesterolemia in older persons for the prevention of cardiovascular disease. Cardiol Rev. 2010;18:132–40. doi: 10.1097/CRD.0b013e3181c29571. [DOI] [PubMed] [Google Scholar]
- 62.Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153:1065–73. [PubMed] [Google Scholar]
- 63.Kannel WB, D’Agostino RB, Sullivan L, Wilson PW. Concept and usefulness of cardiovascular risk profiles. Am Heart J. 2004;148:16–26. doi: 10.1016/j.ahj.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 65.Zimetbaum P, Frishman WH, Ooi WL, Derman MP, Aronson M, Gidez LI, et al. Plasma lipids and lipoproteins and the incidence of cardiovascular disease in the very elderly. The Bronx Aging Study. Arterioscler Thromb. 1992;12:416–23. doi: 10.1161/01.atv.12.4.416. [DOI] [PubMed] [Google Scholar]
- 66.Weverling-Rijnsburger AW, Jonkers IJ, van Exel E, Gussekloo J, Westendorp RG. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch Intern Med. 2003;163:1549–54. doi: 10.1001/archinte.163.13.1549. [DOI] [PubMed] [Google Scholar]
- 67.Marini C, Baldassarre M, Russo T, De Santis F, Sacco S, Ciancarelli I, et al. Burden of first-ever ischemic stroke in the oldest old: evidence from a population-based study. Neurology. 2004;62:77–81. doi: 10.1212/01.wnl.0000101461.61501.65. [DOI] [PubMed] [Google Scholar]
- 68.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 69.O’Donell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. INTERSTROKE Investigators Risk factors for ischemic and intracerebral haemorraghic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 70.Asplund K, Karvanen J, Giampaoli S, Jousilahti P, Niemelä M, Broda G, et al. Relative risks for stroke by age, sex and population based on follow-up of 18 European population in the MORGAM Project. Stroke. 2009;40:2319–26. doi: 10.1161/STROKEAHA.109.547869. [DOI] [PubMed] [Google Scholar]
- 71.Dyker AG, Weir CJ, Lees KR. Influence of cholesterol on survival after stroke: retrospective study. BMJ. 1997;314:1584–8. doi: 10.1136/bmj.314.7094.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vauthey C, de Freitas GR, van Melle G, Devuyst G, Bogousslavsky J. Better outcome after stroke with higher serum cholesterol levels. Neurology. 2000;54:1944–9. doi: 10.1212/wnl.54.10.1944. [DOI] [PubMed] [Google Scholar]
- 73.Zuliani G, Cherubini A, Atti AR, Ble A, Vavalle C, Di Todaron F, et al. Low cholesterol levels are associated with short-term mortality in older patients with ischemic strokes. J Gerontol A Biol Sci Med. 2004;59:293–7. doi: 10.1093/gerona/59.3.m293. [DOI] [PubMed] [Google Scholar]
- 74.Olsen TS, Christiensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol level are associated with less severe strokes and lower all-cause mortality: ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38:2646–51. doi: 10.1161/STROKEAHA.107.490292. [DOI] [PubMed] [Google Scholar]
- 75.Bihari-Varga M, Székely J, Gruber E. Plasma high density lipoproteins in coronary, cerebral and peripheral vascular disease. The influence of various risk factors. Atherosclerosis. 1981;40:337–45. doi: 10.1016/0021-9150(81)90144-1. [DOI] [PubMed] [Google Scholar]
- 76.Aronow WS, Ahn C. Correlation of serum lipids with the presence or absence of atherothrombotic brain infarction and peripheral arterial disease in 1,834 men and women aged > or = 62 years. Am J Cardiol. 1994;73:995–7. doi: 10.1016/0002-9149(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 77.Milionis HJ, Liberopoulus E, Goudevenos J, Bairaktari ET, Seferiadis K, Elisaf MS. Risk factors for first-ever acute ischemic non-embolic stroke in the elderly individuals. Int J Cardiol. 2005;99:269–75. doi: 10.1016/j.ijcard.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Ramos R, Quesada M, Solanas P, Subirana I, Sala J, Vila J, et al. REGICOR investigators Prevalence of symptomatic and asymptomatic peripheral arterial disease and the value of the ankle-brachial index to stratify cardiovascular risk. Eur J Vasc Endovasc Surg. 2009;38:305–11. doi: 10.1016/j.ejvs.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Félix-Redondo FJ, Fernández-Bergés D, Grau M, Baena-Diez JM, Mostaza JM, Vila J. Prevalence and clinical characteristics of peripheral arterial disease in the study population Hermex. Rev Esp Cardiol. 2012;65:726–33. doi: 10.1016/j.recesp.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 81.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Eng J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 82.Kanell WB. Risk factors for atherosclerosic cardiovascular outcomes in different arterial territories. J Cardiovasc Risk. 1994;1:333–9. [PubMed] [Google Scholar]
- 83.Hooi JD, Kester ADM, Stoffers HEJH, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–72. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 84.Dagenais GR, Maurice S, Robitaille NM, Gingras S, Lupien PJ. Intermittent claudication in Quebec men from 1974–1986: The Quebec Cardiovascular Study. Clin Invest Med. 1991;14:93–100. [PubMed] [Google Scholar]
- 85.Tapp RJ, Balkau B, Shaw JE, Valensi P, Cailleau M, Eschwege E, DESIR Study Group Association of glucose metabolism, smoking and cardiovascular risk factors with incident peripheral arterial disease: the DESIR study. Atherosclerosis. 2007;190:84–9. doi: 10.1016/j.atherosclerosis.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 86.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–7. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Goldbourt U. Epidemiology of intermittent claudication in middle age men. Am J Epidemiol. 1994;140:418–30. doi: 10.1093/oxfordjournals.aje.a117264. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AM, Polak JF, et al. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med. 2005;163:1896–902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 89.Pedersen TR, Kjekshus J, Pyörälä K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S) Am J Cardiol. 1998;81:333–5. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 90.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 91.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–31. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 92.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of PAD in the Framingham Offspring Study. Am Heart J. 2002;143:961–5. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 93.Pomrehn P, Duncan B, Weissfeld L, Wallace RB, Barnes R, Heiss G, et al. The association of dyslipoproteinemia with symptoms and signs of peripheral arterial disease. The Lipid Research Clinics Program Prevalence Study. Circulation. 1986;73:I100–7. [PubMed] [Google Scholar]
- 94.Skilton MR, Chin-Dusting JP, Dart AM, Brazionis L, Lantieri O, O’Dea K, et al. DESIR Study Group Metabolic health, obesity and 9-year incidence of peripheral arterial disease: the D.E.S.I.R. study. Atherosclerosis. 2011;216:471–6. doi: 10.1016/j.atherosclerosis.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 95.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–28. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Lorgeril M, Salen P, Martin Jl, Monjaud I, Delaye J, Memelle N. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction: Final Report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 97.Vicent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964–71. doi: 10.1093/ajcn/82.5.964. [DOI] [PubMed] [Google Scholar]
- 98.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvadó L, Ruiz-Gutierrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann Intern Med. 2006 doi: 10.7326/0003-4819-145-1-200607040-00004. 145-1-11. [DOI] [PubMed] [Google Scholar]
- 99.Hu FB, Bronner L, Willet WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 100.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willet WC. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–16. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 101.Gruppo Italiano per lo Studio della sopravivenza nell’infarto miocárdico Dietary supplementation with n-3 poluunsaturated acids and vitamin E after myocardial infarction. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 102.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on bloods lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–8. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 103.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Eng J Med. 2003;347:1483–92. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 104.Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: a meta-analysis of randomized controlled trials. J Mens Health Gend. 2006;3:61–70. doi: 10.1016/j.jmhg.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelley GA, Kelley KS, Tran ZVU. Aerobic exercise and lipids and lipoproteinas in women: A meta-analysis of randomized controlled trials. J Womens Health. 2004;13:1148–64. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. Preventive Medicine. 2009;48:9–19. doi: 10.1016/j.ypmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 107.Fonong T, Toth MJ, Ades PA, Katzel LI, Calles-Escandon J, Poehlman ET. Relationship between physical activity and HDL-Cholesterol in healthy older men and women: a cross-sectional and exercise intervention study. Atherosclerosis. 1996;127:177–183. doi: 10.1016/s0021-9150(96)05950-3. [DOI] [PubMed] [Google Scholar]
- 108.Boardley D, Fahlman M, Topp R, Morgan AL, McNevin The impact of exercise training on blood lipids in older adults. Am J Geriatr Cardiol. 2007;16:30–35. doi: 10.1111/j.1076-7460.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- 109.Kokkinos P. Physical activity, health benefits and mortality risk. ISRN Cardiol. 2012;2012:718789. doi: 10.5402/2012/718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 111.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower –than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 112.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vacular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 113.Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010;152:488–96. doi: 10.1059/0003-4819-152-8-201004200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemaitre RN, Psaty BM, Heckbert SR, Kronmal RA, Newman AB, Burke GL. Therapy with hydroxymethylglutaryl coenzyme A reductase inhibitors (statins) and associated risk of incident cardiovascular events in older adults: evidence from the Cardiovascular Health Study. Arch Intern Med. 2002;162:1395–400. doi: 10.1001/archinte.162.12.1395. [DOI] [PubMed] [Google Scholar]
- 115.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RGJ, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomized controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scandinavian Simvastatin Survival Study group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 117.Miettinen TA, Pyorala K, Olsson AG, Musliner TA, Cook TJ, Faergeman O, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S) Circulation. 1997;96:4211–8. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 118.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Coles TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 119.Lewis SJ, Moye LA, Sacks FM, Johnstone DE, Timmis G, Mitchell J, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–9. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 120.The Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 121.The LIPID Study Group Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: The LIPID trial follow–up. Lancet. 2002;359:1379–87. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 122.Heart Protection Study Collaborative Group MCR/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckey BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 124.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. [Google Scholar]
- 125.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Cholesterol Treatment Trialists’(CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 126.Cholesterol Treatment Trialists’ Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roberts CG, Guallar E, Rodriguez A. Efficacy and safety of statin monotherapy in older adults: a meta-analysis. J Gerontol Biol Sci Med Sci. 2007;62:879–87. doi: 10.1093/gerona/62.8.879. [DOI] [PubMed] [Google Scholar]
- 128.Franssen R, Vergeer M, Stroes ES, Kastelein JJ. Combination statin-fibrate therapy: safety aspects. Diabetes Obes Metab. 2009;11:89–94. doi: 10.1111/j.1463-1326.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 129.Ford I, Murray H, Packard CJ, Shepherd J, MacFarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Eng J Med. 2007;357:1477–86. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 130.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–24. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 131.Alberton M, Wu P, Druyts E, Briel M, Mills EJ. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145–157. doi: 10.1093/qjmed/hcr158. [DOI] [PubMed] [Google Scholar]
- 132.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ, Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]


