Abstract
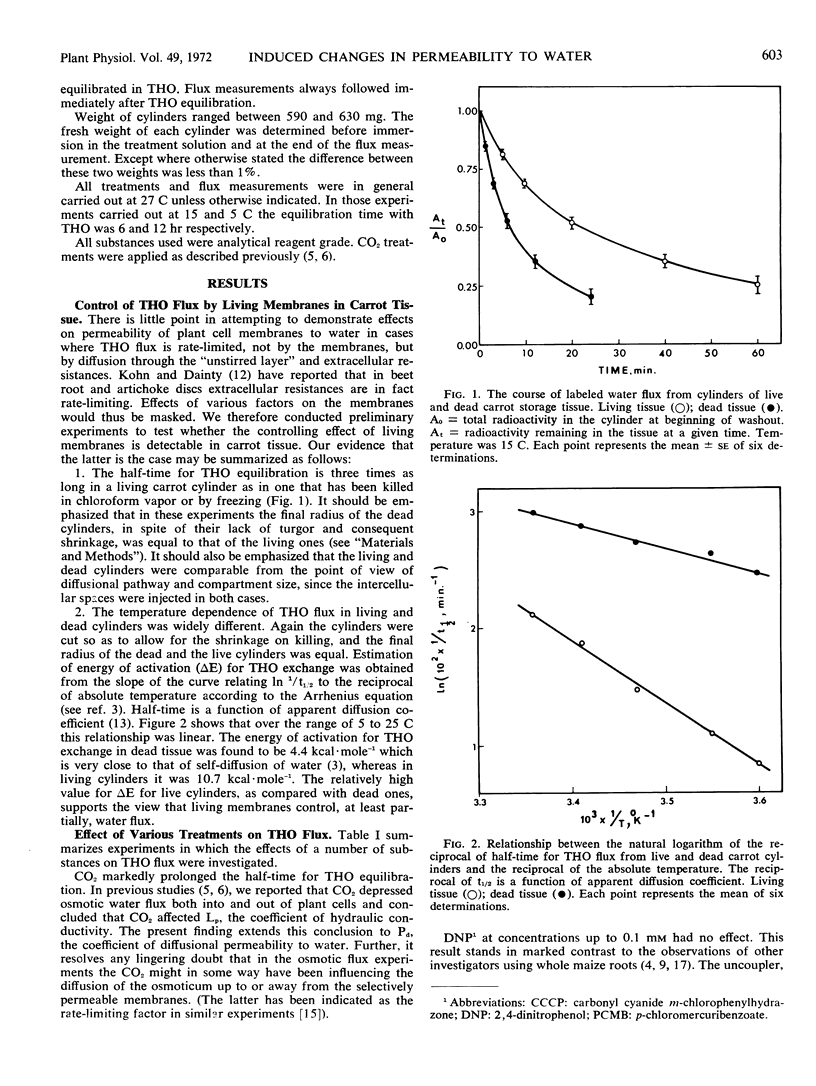
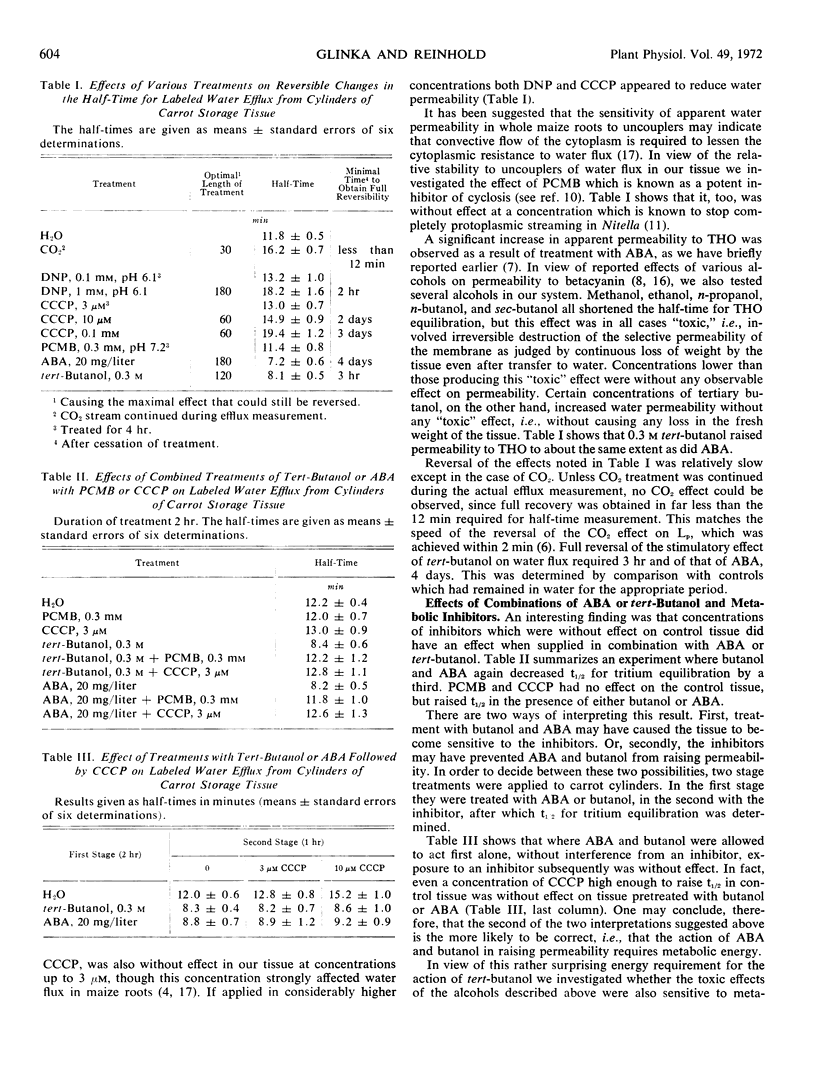
The half-time for THO equilibration was three times longer for a living carrot (Daucus carota L.) cylinder than for a dead one. Furthermore, the energy of activation of THO flux was more than twice as high for the living cylinder. Passage through living membranes thus constitutes a rate-limiting step for THO flux in carrot tissue.
CO2 increased the half-time (t½) for THO equilibration. Treatment with abscisic acid or with tertiary butanol decreased t½. In neither case was the selective permeability of the membrane destroyed.
p-Chloromercuribenzoate and carbonyl cyanide m-chlorophenylhydrazone, if supplied together with abscisic acid or tertiary butanol, abolished their action. If abscisic acid or butanol was first allowed to act alone, its effect was stable to subsequent treatment with the inhibitors. p-Chloromercuribenzoate and carbonyl cyanide m-chlorophenylhydrazone at concentrations at which they affected abscisic acid and butanol action, did not influence THO flux in control tissue. At considerably higher concentrations, however, 2, 4-dinitrophenol and carbonyl cyanide m-chlorophenylhydrazone raised t½. The CO2 effect was very rapidly reversible. Full reversal of the butanol effect required 3 hours, and that of abscisic acid required 4 days.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt D. H., Green J. W. The sodium permeability of butanol-treated erythrocytes. The role of calcium. Biochim Biophys Acta. 1971 Jan 5;225(1):46–55. doi: 10.1016/0005-2736(71)90282-3. [DOI] [PubMed] [Google Scholar]
- Glinka Z. Abscisic Acid raises the permeability of plant cells to water. Plant Physiol. 1971 Jul;48(1):103–105. doi: 10.1104/pp.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Rapid Changes in Permeability of Cell Membranes to Water Brought About by Carbon Dioxide & Oxygen. Plant Physiol. 1962 Jul;37(4):481–486. doi: 10.1104/pp.37.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z., Reinhold L. Reversible Changes in the Hydraulic Permeability of Plant Cell Membranes. Plant Physiol. 1964 Nov;39(6):1043–1050. doi: 10.1104/pp.39.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effect of sterols on the permeability of alcohol-treated red beet tissue. Plant Physiol. 1968 Apr;43(4):484–488. doi: 10.1104/pp.43.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHIMOTO U., AKABORI H. Protoplasmic streaming of an internodal cell of Nitella flexilis; its correlation with electric stimulus. J Gen Physiol. 1959 Jul 20;42(6):1167–1183. doi: 10.1085/jgp.42.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEES G. C., WEATHERLEY P. E. The mechanism of water absorption by roots. II. The role of hydrostatic pressure gradients across the cortex. Proc R Soc Lond B Biol Sci. 1957 Dec 3;147(928):381–391. doi: 10.1098/rspb.1957.0057. [DOI] [PubMed] [Google Scholar]
- Philip J. R. Osmosis and Diffusion in Tissue: Half-times and Internal Gradients. Plant Physiol. 1958 Jul;33(4):275–278. doi: 10.1104/pp.33.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Halpern L. A. THE EFFECT OF BRANCHING AT C-1 ON THE BIOLOGICAL ACTIVITY OF ALCOHOLS. Proc Natl Acad Sci U S A. 1964 May;51(5):765–768. doi: 10.1073/pnas.51.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley J. T. Radial Exchange of Labeled Water in Intact Maize Roots. Plant Physiol. 1965 Jul;40(4):711–717. doi: 10.1104/pp.40.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]



