Abstract
Objectives
Affect identification accuracy paradigms have increasingly been utilized to understand psychiatric illness including Bipolar Disorder (BD) and Major Depressive Disorder (MDD). This investigation focused on perceptual accuracy in affect identification in both visual and auditory domains among patients with BD, relative to Healthy Controls (HC) and patients with MDD. Demographic and clinical variables, in addition to medications were also investigated.
Methods
The visual Facial Emotion Perception Test (FEPT) and auditory Emotional Perception Test (EPT) were administered to adults with BD (n = 119) and MDD (n = 78) as well as HC (n = 66).
Results
Performance on the FEPT was significantly stronger than on the EPT irrespective of group. Performance on the EPT did not significantly differentiate the groups. On the FEPT, BD samples had the greatest difficulty relative to HC in identification of sad and fearful faces. BD participants also had greater difficulty identifying sad faces relative to MDD participants though not after controlling for severity of illness factors. For the BD (but not MDD) sample several clinical variables were also correlated with FEPT performance.
Conclusions
The findings suggest that disruptions in identification of negative emotions such as sadness and fear may be a characteristic trait of BD. However, this effect may be moderated by greater illness severity found in our BD sample.
Keywords: Bipolar Disorder, Major Depressive Disorder, Affect perception
1. Introduction
Difficulties in emotion processing, or affect perception, are potential risk factors in the development of psychiatric illness but paradoxically may also be a result of the illness. Despite a large number of neuroimaging studies investigating emotion processing deficits among patients with mood disorders, less is known about specific performance alterations on affective processing tasks associated with Bipolar Disorder (BD). Further, it is unclear whether disruptions in perceptual accuracy of specific emotions are moderated by current symptoms of depression. Finally, it is unknown whether emotion processing decrements are influenced by perception modality (i.e., auditory or visual). Investigation of performance differences between patients diagnosed with BD, Major Depressive Disorder (MDD), and controls, accounting for modality and valence, will inform the development of further neuroimaging and behavioral research into the pathophysiology of the mood disorders, as well as approaches to diagnosis and treatment.
1.1. Auditory and visual emotion processing
In both auditory and visual domains, processing of emotion involves both perception and recognition of the meaning embedded in affective stimuli (Adolphs et al., 1994). Adolphs (2002) suggested that recognizing emotions from auditory prosody cues are more difficult than from facial expressions. In support of this, some models suggest that picture–word categorization activates sensory features and affective content simultaneously, whereas words first activate lexical concepts and only later access affective features (De Houwer and Hermans, 1994; Viswanathan and Childers, 2003). Other investigators have found that emotional pictures are processed more rapidly than are emotion words (De Houwer and Hermans, 1994). It is further suggested that processing pictures rapidly activate both semantic and affective networks. In contrast, verbal material must pass through lexical cognitive systems before being assessed for salience and affective properties. The implication of slower processing speed and the distinct neuroanatomy for visual and auditory stimulus processing is that there need not necessarily be processing impairment in multiple modalities if the problem occurs earlier within a particular sensory network.
Exploration of the neuroanatomical pathways involved in emotion processing offer insight into the specific processing of different emotional stimuli. Recognition of auditory emotion prosody has been found to involve right frontoparietal regions with possible basal ganglia involvement (Hornak et al., 1996; Breitenstein et al., 1998; Buchanan et al., 2000; Adolphs et al., 2002). Processing auditory emotional material not involving prosody is noted to involve orbital prefrontal areas (Blood et al., 1999; Frey et al., 2000). Adolphs (2002) suggests that although recognizing emotions from auditory prosody cues are globally more difficult than from facial expressions, this effect is more pronounced for certain emotions like disgust. The implication from these and similar data is that not only does the brain process affective stimuli in discrete anatomical pathways depending on sensory modality but also affect category (fear, disgust, etc.).
1.2. Individual emotions
Further evidence linking distinct neural circuitry to distinct emotional material is provided by double dissociation studies utilizing facial emotion perception tasks. One prior study demonstrated that individuals with amygdala lesions are impaired in recognizing fear relative to perception of emotions such as disgust (Adolphs et al., 1994). Conversely, individuals with Huntington’s disease have been reported to show deficits in perception of disgust relative to fear (Sprengelmeyer et al., 1996), which is supported by evidence of activation in the basal ganglia following presentation of disgust expressions in Healthy Controls (HC) (Phillips et al., 1997).
A meta-analysis by Phan et al. (2002) reviewed 55 positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) activation studies that investigated response to specific emotional stimuli among healthy volunteers. Their findings suggest that fear induction was strongly associated with activation in the amygdala, sadness with activation in the subgenual cingulate, and happiness and disgust with activation in the basal ganglia. Activation of the medial prefrontal cortex was not specific to any one emotion. Rather, the prefrontal cortex appears to be involved in the voluntary control and/or suppression of emotions and emotional displays (Beauregard, et al., 2001; Ochsner et al., 2002; Woolley et al., 2004).
Thus the extant literature suggests that emotion processing in the brain takes place in distributed networks with both generalized and specific functions. How the functioning of these systems may be disrupted among psychiatric populations remains unclear, and further understanding would aid in the differentiation of their distinct pathophysiological mechanisms.
1.3. Emotion processing in psychiatric illness
Emotion processing inaccuracies and biases in MDD have been demonstrated in studies of perception of facial expressions (Langenecker et al., 2005; Deldin et al., 2001). Langenecker et al. (2005) reported that healthy adult women were more likely to incorrectly identify an expression as happy (positive bias) compared to women with depression, though biases in response to neutral stimuli were equivalent between the groups.
Other groups have shown a general pattern of impairment in MDD in the recognition of positive facial expressions (Suslow et al., 2001; Surguladze et al., 2004) that may persist even following treatment. In contrast, other studies have found that adults with MDD were more likely than healthy adults to classify sad faces incorrectly and were no different from healthy adults in identifying neutrally posed expressions (Mikhailova et al., 1996). Still others have found no evidence that depressed adults perform more poorly than healthy individuals in recognizing any particular type of emotional expression (Persad and Polivy, 1993). Thus there has been substantial variability in the findings across studies comparing HC and MDD on tasks of affect perception, and comparisons of MDD and BD are at present underrepresented in the literature. There have also been a number of fMRI studies investigating neural correlates of facial emotion processing among individuals with mood disorder. Individuals with MDD have been shown to have increased response within the left amygdala to fearful (Sheline et al., 2001) and sad facial stimuli (Fu et al., 2004).
For the purpose of our investigation we examined performance in tasks of affect recognition in a sample of BD individuals and compared their performance to both HC as well as a sample of individuals with MDD. Some studies on emotion processing in BD suggest that emotion perception accuracy is mood dependent, such that a bias toward negative emotions exists during the depressed phase of illness (George et al., 1998) and a positive emotional bias is observed during mania and hypomania (Vuilleumier et al., 1998; Lembke and Ketter, 2002). Still, another study found that in the manic phase of BD, subjects made no errors in recognition of happy faces but increased errors in recognizing fear, disgust, anger, surprise, and sadness (Lembke and Ketter, 2002). These authors also found that overall, individuals in a manic phase of illness performed worse than HC and euthymic individuals with bipolar I and bipolar II disorder. Another study found that there was no difference in facial emotion processing in euthymic patients with BD and HC, suggesting that deficits in emotion perception are illness-phase dependent rather than underlying trait-based deficit (Venn et al., 2004).
With this in mind, it might be assumed that affect identification difficulties in persons with BD would be similar to those observed among individuals with MDD after accounting for depressive symptoms. To our knowledge this phenomenon has not been directly investigated, though a previous study reported that adults with BD generally show greater difficulty with affect identification than do adults with MDD (Langenecker et al., 2005).
2. Hypotheses
Our primary interest was to investigate affect processing by sensory modality in BD. To that end we compared emotion processing performance between patients with BD and HC. We also included a second level of comparison of emotion processing between BD and MDD in auditory and visual emotion processing while controlling for the severity of depression symptoms. In keeping with the existing literature reviewed above, it was expected that all participants would perform less well on the auditory as compared to the visual emotion processing task (Adolphs, 2002; De Houwer and Hermans, 1994; Viswanathan and Childers, 2003). Nevertheless, in both visual and auditory domains it was expected that BD would perform more poorly than HC or MDD participants due to an overall increased illness burden and potentially a greater disruption in the function of emotion processing circuitry. Based on the variability observed in the prior literature we did not pose specific hypotheses regarding individual emotional stimuli, and these were examined in post-hoc analyses. Nevertheless, as there is evidence in the literature of a negative emotion bias in BD and MDD commensurate with depression symptomatology, we expected depression severity to be positively correlated with increased accuracy for negative emotions in the auditory and visual domains. The relationship between clinical variables, medication classes, and affect processing accuracy were conducted in post-hoc analyses where significant main effects of group were found.
3. Methods
3.1. Participants
Data were collected from four separate protocols investigating the pathophysiology of BD and MDD. Each study was approved by Institutional Review Board at the University of Michigan. Participants from each study were assessed with a structured clinical interview (i.e., Structured Clinical Interview for DSM-IV) (SCID-IV; First et al., 2002); or Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994; Langenecker et al., 2009) to determine diagnosis. The DIGS assesses the most severe episode for each category. Current substance abuse or dependence in the last 5 years were exclusionary criteria, as was a history of schizophrenia or schizoaffective disorder, intelligence quotient (IQ) less than 80, or medical illness associated with increased rates of depression including, but not limited to, neoplastic disease, endocrinopathies, or cerebrovascular disease. Inclusion criteria included individuals with a history of BD or MDD that had required treatment. Severity of depression symptoms, but not presence of current depressive episode, was assessed in all participants using the Hamilton Rating Scale for Depression (HAM-D) 17-item test. The Young Mania Rating Scale (YMRS) assessed the severity of manic symptoms, but not presence of manic episode, in the BD sample only. No BD participant scored above eight on the YMRS. A score above eight is considered to be in the clinically significant range of severity on the YMRS.
There were 66 HC, 78 cases of MDD, and 119 cases of BD. Illness severity markers and demographic variables included age at onset of first psychiatric symptoms (AAO), chronicity, number of inpatient hospitalizations, mania and hypomania AAO, average number of manic and hypomanic episodes per year, depression AAO, and average number of depressive episodes per year (see Table 1). It should be noted that the AAO variable captures the first depressive or the first manic episode, whichever is first. Thus, a discrepancy between first AAO and depression AAO, or mania AAO is seen for some BD individuals. The percent of individuals in the BD and MDD groups taking different medication classes is presented in Table 2.
Table 1.
Demographic characteristics of HC, MDD, and BD groups.
| HC (n = 66) | BD (n = 119) | Major depression (n = 78) | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Sex (% male) | 35.80% | 33.33% | 30.80% |
| Age (years) | 37.2 (13.2) | 37 (11.8) | 38.9 (12.5) |
| Education (years) | 15.9 (2.2) | 15.4 (2.4) | 15.7 (2.5) |
| HAM-Da | 1.2 (2.0) | 8.8 (5.9) | 14.5 (8.0) |
| Age at onsetb | n/a | 16.9 (7.3) | 25.06 (12.3) |
| Chronicityc | n/a | 20.0 (12.2) | 15.9 (12.4) |
| Inpatient Hosp.d | n/a | 2.9 (3.8) | .5 (1.2) |
| Mania AAO | n/a | 20.3 (11.9) | n/a |
| Manic number of episodes | n/a | 6.7 (14.8) | n/a |
| Hypomania AAO | n/a | 14.6 (12.5) | n/a |
| Hypomania number of episodes | n/a | 28.8 (65.4) | n/a |
| Depression AAOe | n/a | 18.3 (8.6) | 25.06 (12.3) |
| Depression number of episodesf | n/a | 23.48 (56.1) | 4.74 (5.7) |
Note: there were no significant differences in age, education, or sex distribution across groups at p < .05.
HAM-D between groups, F(2, 271) = 87.9, p < .001, MDD > BD > HC.
Age at onset = age of onset for first mood episode; t(180) = 5.5, p < .001, MDD > BD.
Chronicity (years ill) F(1, 178) = 4.7, p = .031.
Number of inpatient hospitalizations; between groups, F(1, 174) = 23.3, p < .001, BD > MDD.
Depression age at onset F(1, 135) = 6.8, p = .010 BD < MDD.
Depression number of episodes F(1, 164) = 5.1, p = .025 BD > MDD.
Table 2.
Medication classification percent by diagnostic group.
| Lithium % | AEDa % | Sedatives % | Anti-psychotics % | Stimulantsb % | Anti-depressantsb % | |
|---|---|---|---|---|---|---|
| BD | 34.1 | 55.8 | 30.2 | 41.9 | 8.5 | 44.9 |
| MDD | 0 | 5.1 | 9 | 6.4 | 2.6 | 54.3 |
AED = Anti-pileptic drug.
All medication classes were significantly different between groups at p < .001 except stimulants and anti-depressants which were statistically equivalent between BD and MDD groups.
3.2. Measures
Participants were administered the Emotion Perception Test (EPT) (Green and Allen, 1997), which utilizes auditory stimuli representative of five affect labels: happy, sad, fearful, angry, and neutral. The task consists of auditory presentation of 45 short sentences in 4–5 sec presentation windows. Participants were then given 4 sec to select one of the five affect labels to which it belongs. The Facial Emotion Perception Test (FEPT; Rapport et al., 2002; Langenecker et al., 2005) was also administered and consisted of 52 facial affective stimuli using happy, sad, angry, fearful and neutral faces. There are happy, sad, angry and fearful choices for this task, but there is not a “neutral” choice option. In this way the task includes an embedded measure to evoke response bias. Response bias was not selected as an outcome measure of interest in the present study. Stimuli were presented for 300 msec and then replaced by a masked stimulus for 100 msec. Participants then had a 2600 msec response window. The total percent correct on each task served as an overall metric of affect perception (Table 3). Accuracy for individual emotions was also rendered as performance indices. The FEPT and EPT accuracy were compared to confirm prior reports of greater difficulty in accuracy for auditory relative to visual emotion identification. Consistent with prior literature (Adolphs, 2002; De Houwer and Hermans, 1994; Viswanathan and Childers, 2003), percent correct performance on affect recognition in the auditory domain [M = 78%, standard deviation (SD) = 9.1%] was significantly worse for the entire sample compared to the visually presented FEPT stimuli [M = 85.2%, SD = 10.5%; t(261) = 10.9, p < .001]. Performance levels between the two tasks were significantly correlated (r = .41, p < .001).
Table 3.
Percent accuracy on FEPT.
| Overall Accuracy M (SD) |
Sada,b M (SD) |
Feara M (SD) |
Angry M (SD) |
Happy M (SD) |
|
|---|---|---|---|---|---|
| HC | 87.3 (8.6) | 80.1 (16.8) | 86.4 (12.6) | 86.2 (12.9) | 96.4 (6.6) |
| BD | 84.1 (11.1) | 75.0 (14.9) | 81.4 (17.6) | 84.4 (17.0) | 95.1 (8.0) |
| MDD | 86.5 (10.8) | 81.1 (15.0) | 85.4 (16.9) | 85.5 (19.9) | 96.3 (6.1) |
Fear: HC > BD F(1, 193) = 5.35, p = .022; Sad: HC > BD F(1, 193) = 4.9, p = .028.
Sad: MDD > BD F(1, 202) = 9.4, p = .002. Non-significant after controlling for # inpatient hospitalizations and medication load.
Individuals in the BD and MDD sample were taking a range of medications across a range of broad classes. We adopted a protocol often seen in the literature to assess total medication load. Anti-depressant, anxiolytic, mood stabilizer, and anti-psychotic medications were coded as absent = 0, low = 1, or high = 2 based on previously employed methods to convert each medication to a standardized dose (Hassel et al., 2008; Almeida et al., 2008; and Sackheim, 2001.) Anti-psychotics were converted into chlorpromazine dose equivalents (Davis and Chen, 2004). Following Hassel’s et al. (2008) methodology we generated a composite measure of total medication load by summing all individual medication codes for each individual medication within categories for each BD and MDD participant.
3.3. Statistical analyses
The initial statistical procedure was to carry out an omnibus t-test comparing performance on EPT and FEPT for the entire sample. The BD sample was of primary interest and thus their performance in accuracy for individual emotions was first compared to HC and then to MDD groups in individual multiple analysis of variance (MANOVA) and multiple analysis of covariance (MANCOVA) respectively. Dependent variables for all analyses included general accuracy on the EPT and FEPT as well as indices of individual emotion accuracy (i.e., sad, fear, angry, happy).
To reduce the variability in performance contributed by current depression symptoms, we controlled for HAM-D score as a covariate in the MANCOVA between BD and MDD. We elected to carry out separate analyses comparing BD to HC, from BD to MDD since the HAM-D with limited variability was not an appropriate covariate for a healthy sample (see Miller and Chapman, 2001 for assumptions of ANCOVA). Carrying out a separate MANCOVA between the MDD and BD samples also allowed further post-hoc analyses controlling for clinical severity indices that would not have been appropriate for the HC sample.
Correlational analyses between clinical variables such as medication load, depression severity, illness onset, chronicity, number of hospitalizations, etc. were compared with performance indices in post-hoc analyses when main effect of group analyses were significant. In the case of continuously coded variables Pearson correlation coefficients were reported. Spearman rank correlation was reported for total medication load.
4. Results
4.1. Demographics
There were no significant differences in age or education between groups. Chi-square analysis revealed no difference in sex ratios by group x2(2) = .416, p = .82. Age, sex, and education data for all groups are presented in Table 1. BD and MDD individuals differed significantly in the number of symptoms of depression they were currently experiencing. Specifically, the MDD group reported an overall higher average HAM-D score (M = 14.5, SD = 8.0) compared to the BD group (M = 8.8, SD = 5.9), F(1, 205) = 33.5, p < .001 (Table 1). The BD sample had a younger age at onset of mood episode, which averaged 16.9 years versus MDD average of 23.4 years, F(1, 199) = 21.9, p < .001. BD patients had a longer course of illness F(1, 178) = 4.7, p = .031, greater number of inpatient hospitalizations F(1, 174) = 23.3, p < .001, and greater number of episodes of depression per year F(1, 164) = 5.1, p = .025. There was also a significant difference in medication load between the BD sample (M = 2.74) and the MDD sample (M = .40), t(184) = −7.2, p < .001. Generally, performance on the FEPT and EPT did not differ between males and females within HC, MDD, and BD samples. However, in one exception in the eight analyses, females with MDD were found to be more accurate for angry visual stimuli compared to males with MDD F(1, 63) = 6.55, p = .013.
4.2. Comparison of BD with HC subjects by stimulus modality and affect identification accuracy
The first MANOVA tested the hypothesis that patients with BD exhibit emotion identification difficulties in auditory and visual domains. Comparison of HC to patients with BD on visual emotion processing revealed significant decrements in BD patients for overall accuracy on the FEPT F(1, 193) = 5.23, p = .023, η2 = .026. This was primarily driven by group differences for fear F(1, 193)=5.35, p = .022, η2 = .027 and sad F(1, 193)=4.9, p = .028, η2 = .025 accuracy. There were no group differences for happy or anger accuracy in the visual domain between these two groups (Fig. 1). There were no differences between HC and BD in performance on the auditory EPT.
Fig. 1.
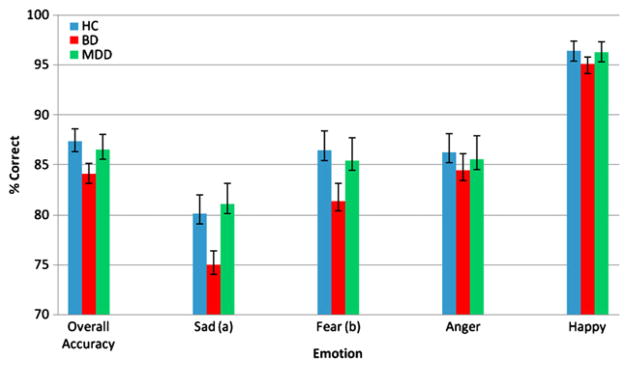
Percent accuracy on facial emotion processing task. Emotion category accuracy by diagnosis (a) = HC >BD F(1, 193) = 5.39, p = .022. (b) = HC > BD F(1, 193) = 4.9, p = .028.
4.3. Comparison of BD with MDD by stimulus modality and affect identification accuracy
For the visual domain, the MDD group performed significantly better than the BD group in accuracy for sad stimuli (MAN-COVA, with HAM-D as covariate), F(1, 202) = 9.4, p = .002, η2 = .04 (Fig. 1). MANCOVA with HAM-D as covariate, comparing BD and MDD revealed no significant group differences in affect perception accuracy in the auditory domain. HAM-D score itself was not significantly associated with any of the FEPT or EPT measures (all ps < .05).
4.4. Post-hoc relationship of clinical variables with visual emotion accuracy in the BD sample
All correlational procedures were conducted as two-tailed tests with an alpha of .05. For the BD sample several clinical variables correlated with one or more FEPT indices. The number of inpatient psychiatric hospitalizations was negatively correlated with overall performance on the FEPT, r = −.189, sad r = −.189, and angry r = −.196 (all ps < .05). The number of manic episodes was negatively correlated with overall performance on the FEPT r = −.223, p = .017 and happy accuracy r = −.395, p < .001. Accuracy for happy stimuli was also negatively correlated with hypomania AAO, r = −.326, p < .001, and number of reported hypomania episodes, r = −.264, p = .004. AAO of depression and number of depressive episodes were not significantly correlated with FEPT performance for the BD sample.
4.5. Post-hoc analysis of medication effects on visual emotion accuracy in the MDD and BD samples
We used Spearman rank correlation to examine the relationship between medication load and clinical severity indices and performance on FEPT. Medication load was not distributed evenly across the two groups, with much higher loads in the BD group t(184) = −7.2, p < .001. Therefore, we had to determine whether medication load was negatively or positively related to performance in each group separately. In the MDD sample total medication load was correlated with HAM-D score rs = −.361, p = .003. Total medication load also correlated with number of inpatient hospitalizations rs = .358, p = .008 in the MDD sample. For the BD sample total medication load was correlated significantly with HAM-D score rs = .330, p < .001 and with mania AAO rs = .214, p = .025. Medication load was neither correlated with other clinical and severity variables, nor was it correlated with FEPT, or EPT dependent for the BD or MDD samples.
5. Discussion
The present findings support the hypothesis that emotion perception decrements may exist in BD relative to HC in the visual domain. This was particularly evident in accuracy for identification of sad and fearful expressions. The BD sample also demonstrated difficulty in emotion processing in the visual domain compared to a sample of participants with MDD. However, in this case the discrepancy was limited to accuracy for sad facial stimuli, which had no relationship to current reported depressive symptomatology. We also explored relationship between clinical variables and performance on the FEPT in the BD sample. A number of clinical variables, including number of manic episodes, AAO of hypomania, and number of psychiatric hospitalizations were related to performance on the FEPT.
Though previous literature proposes that emotion processing deficits may be selectively impaired in BD, our investigation suggests that this is moderated by illness burden reflected in the number of severe episodes requiring hospitalization as well as a closely correlated medication load. The findings from this investigation suggest that relative difficulty in the identification of sadness may be a unique feature of BD that is potentially due to latent increased illness burden or severity within the BD sample. Another possibility is that decreased accuracy in facial emotion processing represents a unique deficit in the phenotypic expression of BD that is confounded by the typically faster recurrence of illness in those patients. That is, there may be an underlying inefficiency in areas of the brain related to processing negative emotions and sadness such as the subcallosal cingulate and medial prefrontal cortex (Deldin et al., 2001). This would be supported by prior models that propose left-approach and right-withdrawal related biobehavioral and affective systems (Davidson, 1998). That is, our data suggest that there may be a deficit in withdrawal related (ex. fear & sad) but not approach related emotional stimuli in bipolar individuals. This could relate to recent findings of right lateralized white matter disruption in BD but not in MDD (Versace et al., 2010). This would make sense since the BD group is the only one in our study with a propensity for manic mood states. Gender analyses were not a main focus of the current investigation though we did note that females with MDD showed significantly better accuracy for angry stimuli compared to males with MDD. This is consistent with prior work showing that when errors are made on an emotion perception task, females are likely to show a bias for labeling sad and fearful stimuli as angry (Wright et al., 2009). It is therefore possible that our finding indicates an increased accuracy for angry stimuli in females by way of an overall general tendency to respond to negative emotional stimuli as angry. Other gender differences in FEPT performance within HC and BD groups were non-significant in the current study.
It is of particular interest to further investigate the role that processing negative emotions have in the course of BD since the stress diathesis model suggests that psychosocial stressors play a role in both onset and course of BD (Miklowitz et al., 1988; Bidzinska, 1984; Glassner and Haldipur, 1983). Prior work (Langenecker et al., 2005) found that a global emotion processing factor differentiated between adults with BD in the depressed phase and healthy adults; however, the present findings suggest that this effect was likely driven primarily by impairments in visual rather than auditory affect perceptual accuracy. Based upon these data, future investigations utilizing neuroimaging of visual emotion processing in BD may clarify the potential endophenotypic characteristics of the illness. Further, gathering data on brain–behavior relationships would represent a more proximal linkage to possible genetic mechanisms over purely behavioral data.
Consistent with our hypothesis and prior literature (Adolphs, 2002; Viswanathan and Childers, 2003), the present findings indicate that individuals across diagnostic conditions performed better when identifying affective stimuli in a visual modality, specifically faces (FEPT), as compared to verbalized (EPT) affective content. This robust finding was evident irrespective of diagnostic group, and is consistent with theories of slower activation in lexical emotion concept networks than visual recognition networks (De Houwer and Hermans, 1994; Viswanathan and Childers, 2003). Given the consistency of this finding, visual emotion processing may represent a more innate cognitive process than decoding emotion from auditory stimuli. However, it is also possible that in our study the EPT produced lower overall scores simply by virtue of its metric. For example, the FEPT and EPT differ in stimuli presentation time (300 msec vs a full spoken sentence). In this study and others thus far, it remains unclear whether differences seen in affect perception reflect underlying neurobiological and/or neuropsychological mechanisms or are partially confounded by measurement artifacts. Future research on affect perception in psychiatric illness would benefit from additional studies utilizing visual/auditory measurement instruments alone, those integrating visual and auditory stimuli, and that are co-normed or otherwise verified as psychometrically symmetrical, as well as including neuroendocrine and structural imaging correlates.
One limitation to the present study was heterogeneity between the diagnostic groups in terms of medications and illness severity. Nevertheless, these are likely latent characteristics of the phenomenon studied and were thoroughly evaluated in secondary analyses. As with all cross-sectional studies, it is unclear if the potential differences inherent in the diagnostic groups, or phase of illness at presentation potentially affect the findings. For example, it is possible that individuals with fewer current symptoms of depression are representative of a less virulent form of illness than those who are currently depressed. Our method of controlling for current symptoms of depression may not adequately control for the potential sources of between group variability in illness severity. Future studies may benefit from the inclusion of attention monitoring and/or regulation measures so as to discern the relative contribution from these factors in emotion perception paradigms. Finally, future research employing a longitudinal design that follows patients with MDD and BD from depressed to remitted phases could best address these issues.
Acknowledgments
We acknowledge funding from the Heinz C. Prechter Bipolar Research Fund (MGM and support for HML, BDH, LMF, MPS, EFHS, MMK, SAL) and KL2 Career Development Award (RR024987, SAL), K23 Award (MH074459, SAL and support for HML, BDH), PO1 MH 42251 (HA, JKZ, EAY), National Alliance for Research in Schizophrenia and Depression awards (JKZ, SAL) and KO5 MH 01931(EAY), MO1 RR00042 (General Clinical Research Center), Rachel Upjohn Clinical Scholars Awards (SAL, SLW), internal support from the Depression and Neuropsychology Sections of the Department of Psychiatry, University of Michigan Medical Center, and the Department of Psychology.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinions in Neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs RH, Damasio H, Tranel D. Neural systems for recognition of emotional prosody: A 3-D lesion study. Emotion. 2002;2(1):23–51. doi: 10.1037/1528-3542.2.1.23. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Almeida JRC, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: Signal effects of gender and trait anxiety. Psychiatry Research: Neuroimaging. 2008;171(2009):54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. The Journal of Neuroscience. 2001;21(18):1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidzinska EJ. Stress factors in affective diseases. British Journal of Psychiatry. 1984;144:161–166. doi: 10.1192/bjp.144.2.161. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nature Neuroscience. 1999;2(4):382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Daum I, Ackermann H. Emotional processing following cortical and subcortical brain damage: Contribution of the frontostriatal circuitry. Behavioral Neurology. 1998;11(1):29–42. doi: 10.1155/1998/579029. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, et al. Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Cognitive Brain Research. 2000;9(3):227–238. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N. Dose response and dose equivalency of antipsychotics. Journal of Clinical Psychopharmacology. 2004;24(2):192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]
- De Houwer J, Hermans D. Differences in the affective processing of words and pictures. Cognition and Emotion. 1994;8(1):1–20. [Google Scholar]
- Deldin P, Keller J, Gergen JA, Miller GA. Cognitive bias and emotion in neuropsychological models of depression. Cognition and Emotion. 2001;15(6):787. [Google Scholar]
- First Michael B, Spitzer Robert L, Gibbon Miriam Williams, et al. Biometrics Research. New York: New York State Psychiatric Institute; Nov, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) [Google Scholar]
- Frey S, Kostopoulos P, Petrides M. Orbitofrontal involvement in the processing of unpleasant auditory information. European Journal of Neuroscience. 2000;12(10):3709–3712. doi: 10.1046/j.1460-9568.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- George MS, Huggins T, Mcdermut W, Parekh PI, Rubinow D, Post RM. Abnormal facial emotion recognition in depression: Serial testing in an ultra-rapid-cycling patient. Behavior Modification. 1998;22(2):192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- Glassner B, Haldipur CV. Life events and early and late onset of bipolar disorder. American Journal of Psychiatry. 1983;140(2):215–217. doi: 10.1176/ajp.140.2.215. [DOI] [PubMed] [Google Scholar]
- Green PW, Allen LM. The Emotional Perception Test. Durham, NC: CogniSyst, Inc; 1997. [Google Scholar]
- Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: No associations with psychotropic medication load. Bipolar Disorders. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34(4):247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27(3):320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: Cognitive phenotypes in bipolar disorder. Journal of Affective Disorders. 2009;122(3):285–293. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. American Journal of Psychiatry. 2002;159(2):302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biological Psychiatry. 1996;40(8):697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, Mintz J. Family factors and the course of bipolar affective disorder. Archives of General Psychiatry. 1988;45(3):225–231. doi: 10.1001/archpsyc.1988.01800270033004. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Persad SM, Polivy J. Differences between depressed and non-depressed individuals in the recognition of and response to facial emotional cues. Journal of Abnormal Psychology. 1993;102(3):358–368. doi: 10.1037//0021-843x.102.3.358. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Friedman SR, Tzelepis A, Van Voorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16(1):102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- Sackheim H. The definition and meaning of treatment resistant depression. Journal of Clinical Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust: Perception of faces and emotions in Huntington’s disease. Brain. 1996;119(5):1647–1665. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Philips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Perceptual and Motor Skills. 2001;92:857–868. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- Venn HR, Gray JM, Montagne B, Murray LK, Burt DM, Frigerio E, et al. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disorders. 2004;6(4):286–293. doi: 10.1111/j.1399-5618.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JRC, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biological Psychiatry. 2010;68:560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Childers TM. An enquiry into the process of categorization of pictures and words. Perceptual and Motor Skills. 2003;96(1):267–287. doi: 10.2466/pms.2003.96.1.267. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Ghika-Schmid F, Bogousslavsky J, Assal G, Regli F. Persistent recurrence of hypomania and prosopoaffective agnosia in a patient with right thalamic infarct. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11(1):40–44. [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Werner K, Rankin KP, Ekman P, Levenson RW, et al. The autonomic and behavioral profile of emotional dysregulation. Neurology. 2004;63(9):1740–1743. doi: 10.1212/01.wnl.0000143054.54412.5b. [DOI] [PubMed] [Google Scholar]
- Wright SL, Langenecker SAL, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, et al. Gender-specific disruptions in emotion processing in younger adults with depression. Depression and Anxiety. 2009;26(2):182–189. doi: 10.1002/da.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]


