Abstract
Selective-breeding of house mice for increased voluntary wheel-running has resulted in multiple physiological and behavioral changes. Characterizing these differences may lead to experimental models that can elucidate factors involved in human diseases and disorders associated with physical inactivity, or potentially treated by physical activity, such as diabetes, obesity, and depression. Herein, we present ethological data for adult males from a line of mice that has been selectively bred for high levels of voluntary wheel-running and from a non-selected control line, housed with or without wheels. Additionally, we present concentrations of central monoamines in limbic, striatal, and midbrain regions. We monitored wheel-running for 8 weeks, and observed home-cage behavior during the last 5 weeks of the study. Mice from the selected line accumulated more revolutions per day than controls due to increased speed and duration of running. Selected mice exhibited more active behaviors than controls, regardless of wheel access, and exhibited less inactivity and grooming than controls. Selective-breeding also influenced the longitudinal patterns of behavior. We found statistically significant differences in monoamine concentrations and associated metabolites in brain regions that influence exercise and motivational state. These results suggest underlying neurochemical differences between selected and control lines that may influence the observed differences in behavior. Our results bolster the argument that selected mice can provide a useful model of human psychological and physiological diseases and disorders.
Keywords: Artificial selection, Behavior, Dopamine, Motivation, Serotonin, Wheel-running
1. Introduction
Physical activity is central to the health and survival of an organism (Bouchard et al., 1994a, 1994b; Feder et al., 2010; Garland and Carter, 1994; Koch and Britton, 2001), and is influenced by an individual’s propensity and ability to engage in exercise (Garland et al., 2011b). Selective-breeding experiments with laboratory mice (Swallow et al., 1998a) and rats (Koch and Britton, 2001) have demonstrated the heritability of both propensity and ability to exercise, and studies using each of these selective-breeding paradigms have provided evidence supporting a positive intrinsic (i.e., genetic) relationship between these traits (Swallow et al., 1998b; Waters et al., 2008b). Interestingly, several similar correlated responses have emerged in populations of mice selectively bred for increased voluntary wheel-running and rats selectively bred for treadmill endurance. These include parallel increases in voluntary wheel-running and treadmill endurance (Meek et al., 2009; Waters et al., 2008b), increased intermittency of wheel-running (Girard et al., 2001; Waters et al., 2008b), reduced body mass and body fat (Meek et al., 2009; Nehrenberg et al., 2009; Noland et al., 2007; Swallow et al., 1999, 2001), altered mitochondrial and glycolytic enzyme levels (Houle-Leroy et al., 2000;Walsh et al., 2006), alteredmuscle fiber phenotype (Guderley et al., 2006; Howlett et al., 2003), and changes in central monoamine activity (Mathes et al., 2010; Rhodes et al., 2005; Waters et al., 2008a). Some inconsistent correlated responses have also been reported, including increased baseline plasma corticosterone concentrations in selected mice of both sexes (Girard and Garland, 2002; Malisch et al., 2007, 2009), but not in selected female rats (Waters, 2007). Taken together, these selective-breeding programs demonstrate the intrinsic nature of exercise traits, and reveal some associations of exercise with other physiological and psychological traits.
The monoamine neurotransmitters dopamine (DA), norepinephrine (NE), and serotonin (5-hydroxytryptamine; 5-HT) play a role in mediating a wide range of behaviors, including adaptive and maladaptive responses to both appetitive (Koob, 1992, 2008) and noxious (Serafine et al., 2012) stimuli, aberrant behaviors associated with psychosis (Henn, 2011), and physical exercise (Dishman et al., 2006). A number of brain systems associated with physical activity utilize these neurotransmitters (Dishman, 2006, 2006; White-Welkley et al., 1996), and manipulating these neural systems can directly influence physical activity (Gainetdinov et al., 1999; Izenwasser et al., 1999; Uceyler et al., 2010, Rhodes et al., 2005). Reciprocally, physical exercise impacts central DA (Dishman et al., 2006), 5-HT (Greenwood et al., 2003, 2005) andNE (Dishman et al., 2006; Greenwood et al., 2005) systems. Given this close relationship, selective-breeding for traits that influence physical activity will likely impact these systems (Garland et al., 2011b).
The relationship between stress-related mental disorders, exercise, and central monoamines has major clinical importance. Animal studies demonstrate a strong ameliorative effect of voluntary exercise (such as wheel-running) on the long-term impact of intense and chronic stress exposure, and a wealth of evidence points toward monoamine systems being involved in this effect of exercise (reviewed in Novak et al. (2012)). As well, exercise is successfully utilized in clinical practice to treat stress-related mental disorders in humans (Blumenthal et al., 2007; Dunn et al., 2002, 2005). Thus, animals that differ in their intrinsic exercise abilities and habits will be important tools in advancing our understanding of the role that exercise can play in therapies for these mental disorders.
This utility is demonstrated in mice selectively bred for increased voluntary wheel-running activity. These animals exhibit heightened responses to both DA reuptake inhibitors (Rhodes et al., 2001), and DA type one (D1) receptor antagonists (Rhodes and Garland, 2003); both these classes of drugs are commonly used to treat stress related psychological disorders. Furthermore, selected mice exhibit dampened wheel-running elicited increases in c-fos expression in DA terminal regions including the dorsal striatum and the medial-frontal and entorhinal cortices compared to controls (Rhodes et al., 2003). Less evidence is available concerning the effects of this selection paradigm on central NE or 5-HT systems, both of which are important targets for pharmacological therapeutics of stress related disorders (Mutlu et al., 2012; Porter and Bell, 1999). However, mice selected for high levels of wheel-running are more sensitive to Ritalin (methylphenidate), a psychostimulant that affects both dopaminergic and noradrenergic activity. The effects of fluoxetine, which enhances synaptic serotonin by inhibiting reuptake, are unaffected by selection for increased wheel-running (Rhodes et al., 2001; Rhodes and Garland, 2003).
In the current study, we build upon data that demonstrate altered brain function in mice selectively bred for voluntary wheel-running (Keeney et al., 2012; Rhodes and Garland, 2003; Rhodes et al., 2003, 2005), and characterize these changes in terms of their behavioral consequences. This study provides insight into the ethological effects of this selective-breeding paradigm (see also Bronikowski et al., 2001; Careau et al., 2012; Carter et al., 2000; Jonas et al., 2010a; Koteja et al., 1999), and more generally contributes to an understanding of the relationships between central neural systems and behavioral output. We housed adult males from both a selected line and a nonselected control line individually, in cages either with or without a running-wheel present. Following 8 weeks in these experimental conditions, we analyzed brains for monoamines and associated metabolites in monoamine cell body and terminal areas associated with motivation, stress response, and physical activity to elucidate possible effects of selective-breeding and voluntary wheel-running on these systems. We assayed the behavior of all animals via scan sampling for the same reasons. Our results present behavioral effects of selection for increased wheel-running, and provide evidence of neurochemical changes that may explain these differences.
2. Results
2.1. Computer-based wheel-running analyses
As expected, selected mice ran significantly farther than control mice across the 56 days of the study (12,811+259 versus 4247 ± 167 m/day [mean ±SEM]; F1,21=14.928, P<0.001; Fig. 1A). The increased total running distance of selected mice resulted from both an increase in running duration (minutes run per day; F1,21=24.548, P<0.001; Fig. 1B) and average running speed (revolutions per min; F1,21=6.320, P<0.020; Fig. 1C). The maximum speed, indexed as the highest number of revolutions in a single minute throughout the study, was also significantly higher for selected mice (F1,21=4.989, P<0.0037; data not shown).
Fig. 1.
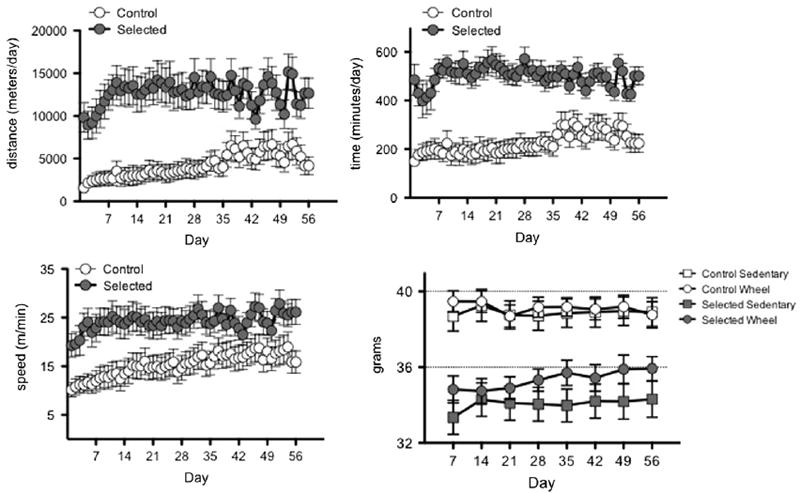
Wheel-running activity (distance, time, speed) and body mass of mice from selected and control lines over eight weeks, beginning at 96.5+1.4 days (mean+S.E.M.). Graphs illustrate daily means±SEM (n=12 selected and 12 control mice). Selected mice ran further than controls due to both increased duration and speed of wheel-running. Control mice consistently weighed more than selected mice throughout the study; presence of a running-wheel did not significantly affect body mass (means±SEM).
2.2. Body mass
Selected mice weighed significantly less than control mice throughout the study (34.34±0.202 g versus 38.71±0.174 g; F1,50=33.659, P<0.001; Fig. 1). Presence of a running-wheel did not statistically affect body mass (F1,50=0.736, P=0.395) and did not interact with selection line (F1,50=0.722, P=0.399).
2.3. Scan sampling behavioral assessment
For simplicity, we treat the times of day (0:900, 15:00, 21:00 h) during which we sampled behavior as independent, and analyze them separately. We present behavioral differences resulting from selective-breeding, as well as any effects that interact with this factor.
2.3.1. 09:00–10:00 h
We observed no significant effects of selection on behavior during the 09:00 time period.
2.3.2. 15:00–16:00 h
Selection significantly affected behavior during the mid-dark (15:00) phase (F6,45=9.196, P<0.001). Animals from the selected line exhibited higher levels of active behavior P<0.001; Fig. 2A), and decreased grooming P<0.033; Fig. 2B), inactive P<0.003; Fig. 2C), and sleeping P<0.001; Fig. 2D) behaviors at this time. Selection and wheel access interacted (Line × Wheel; F6,45=6.141, P<0.001) to affect consummatory behavior (F1,50=14.227, P<0.001; Fig. 2E) at this observation period. In the sedentary condition, control mice exhibited more consummatory behavior than selected mice P<0.003). As well, control mice decreased their consummatory behavior when housed with a running-wheel P<0.001), while selected animals trended toward an increase in consummatory behavior when housed with a runningwheel P<0.044).
Fig. 2.
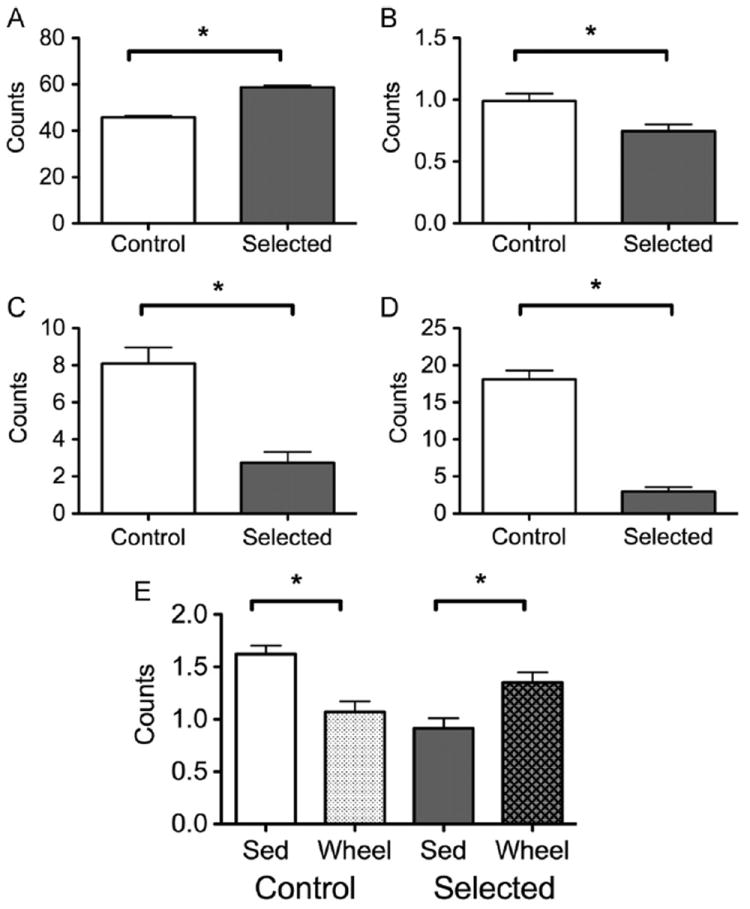
Observed behavior at 15:00. All mice from the selected line exhibited significantly more active (A) behavior, and less grooming (B), inactive (C), and sleeping (D) behavior than controls. Presence of a wheel affected the consummatory (E) behavior of these lines differently, eliciting a decrease in controls and an increase in selected animals over the course of the study [“Sed” indicates sedentary mice, housed without wheels]. *–P<0.05.
Our repeated-measures analysis revealed effects of selection on the longitudinal patterns (Line × Week; F24,27=2.070, P=0.035) of grooming (F3.49,174.50=6.618,P<0.001; Fig. 3A) and inactive (F3.56,177.82=4.540,P=0.002; Fig. 3B) behaviors. During our first observation period (week 4), control mice exhibited a strong trend toward more grooming behavior than selected animals (P=0.042); however, by the final observation period, selected animals exhibited more grooming behavior than controls (P=0.015) due to both an increase in grooming by selected animals and a decrease in grooming by control animals. Regarding inactive behavior, all animals exhibited similar levels during the first observation period; control animals increased levels of this behavior in later periods, resulting in significantly higher levels of inactive behavior in control animals compared to selected animals (P=0.003).
Fig. 3.
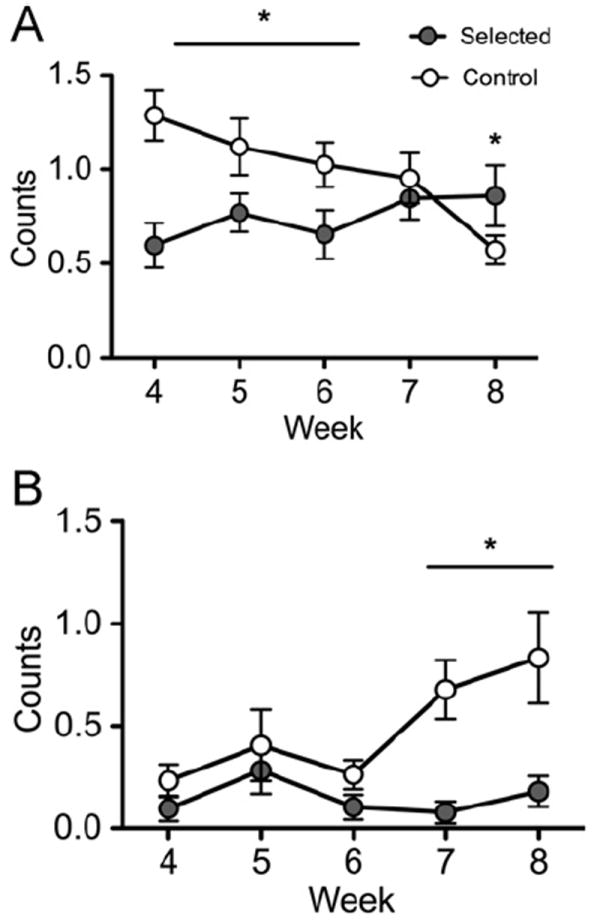
Longitudinal behavioral patterns at 15:00. Selection influenced the longitudinal pattern of grooming (A) and inactive (B) behaviors. Initially, control animals exhibited more grooming than selected animals; however, by the end of the study selected animals exhibited higher levels of grooming than controls, due to a decrease in grooming in controls, and increased grooming in selected animals. During the first three observation weeks, all animals exhibited very low levels of activity. Control animals increased the expression of inactive behavior, exhibiting significantly higher levels of inactivity than selected animals in the final 2 weeks of the experiment.
2.3.3. 21:00–22:00 h
Selection for increased wheel-running significantly affected behavior during the 21:00 observation period (F=6,45=12.433, P=0.001), resulting in increased active behavior (P=0.001; Fig. 4A), and decreased grooming (P=0.019; Fig. 4B), inactive (P=0.028; Fig. 4C) and sleeping (P=0.001; Fig. 4D) behaviors. We also observed an interaction effect of selection and wheel access (Line × Wheel; F=6,45=5.039, P=0.001), affecting active (F1,50=5.771, P=0.020; Fig. 4E), sleeping (F1,50=4.145, P<0.047; Fig. 4F) and rearing (F1,50=5.712, P=0.021; Fig. 4G) behaviors. These interactions break down as follows. While the presence of a running-wheel increased levels of active behavior in both lines of animals, these changes were more pronounced in selected animals. The presence of a running-wheel depressed levels of sleeping in selected animals P<0.001), but had no effect in control animals (P=0.347). Selected animals exhibited more rearing behavior than control animals in sedentary cages (P=0.029); however, the presence of a running-wheel diminished levels of rearing in both lines, resulting in equivalent rearing behavior in all mice (P=0.282).
Fig. 4.
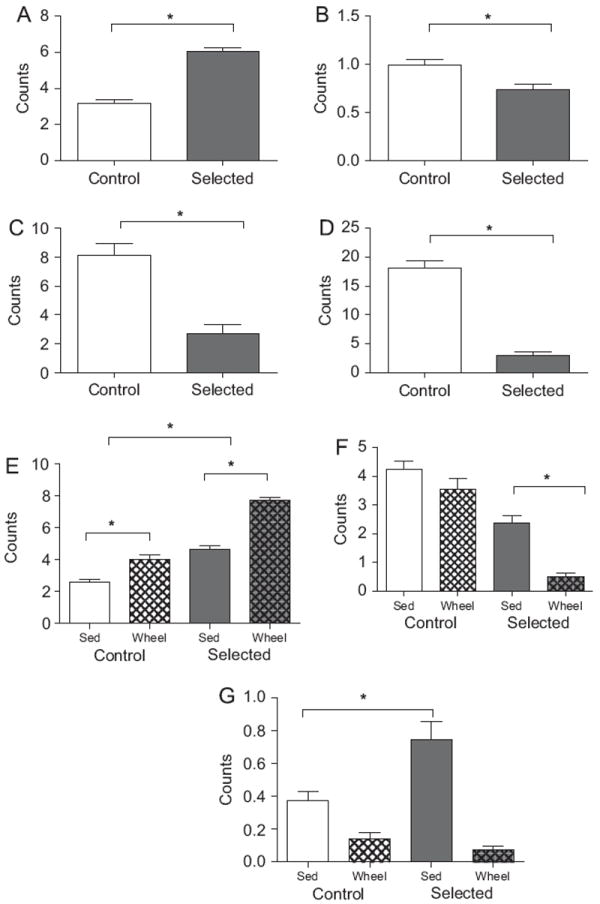
Observed behavior at 21:00. During the 21:00 observation period, selected animals exhibited more active (A) behavior, and less grooming (B), inactive (C), and sleeping (D) behavior than controls. Presence of a running-wheel interacted with selection history to affect active (E), sleeping (F), and rearing (G) behaviors. Presence of a running-wheel increased active behavior more dramatically in selected animals than in controls. Presence of a running-wheel significantly decreased sleeping only in selected animals. Sedentary selected animals exhibited more rearing than sedentary control animals; presence of a wheel decreased rearing in both populations, and statistically abolished this difference.
We observed selection effects on the longitudinal patterns of behavior during the 21:00 observation period (Line × Week; F24,27=2.842, P=0.005). These effects were observed in the pattern of inactive behavior (F3.46,136.37=6.691, P<0.001; Fig. 5) throughout the study. During the initial observation periods, selected and control animals exhibited similar levels of inactive behavior (P=0.911). Selected animals increased their inactive behavior during the later observation periods, resulting in significantly higher levels of inactive behavior in selected animals compared to control animals during this final observation period (P=0.001).
Fig. 5.
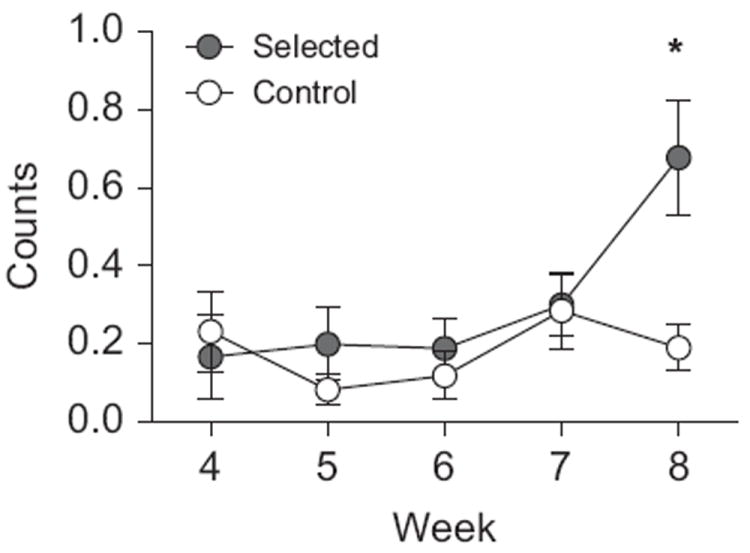
Longitudinal patterns of behavior at 21:00. Selection influenced the longitudinal pattern of inactive behavior. All animals exhibited low levels of inactivity during the initial weeks of observation. Selected animals increased this behavior during the final week of the study, exhibiting significantly more inactivity at this time than controls.
2.3.4. Wheel-running behavior
We observed similar wheel-running behavior for selected and control mice during the 09:00 observation period (P=0.581; Fig. 6A). During the 15:00 time period, we observed more wheel-running in selected animals than in controls (F1,22=17.39, P<0.001; Fig. 6B). A similar effect of selection was observed during the 21:00 time period (F1,22=30.75, P<0.001; Fig. 6C).
Fig. 6.
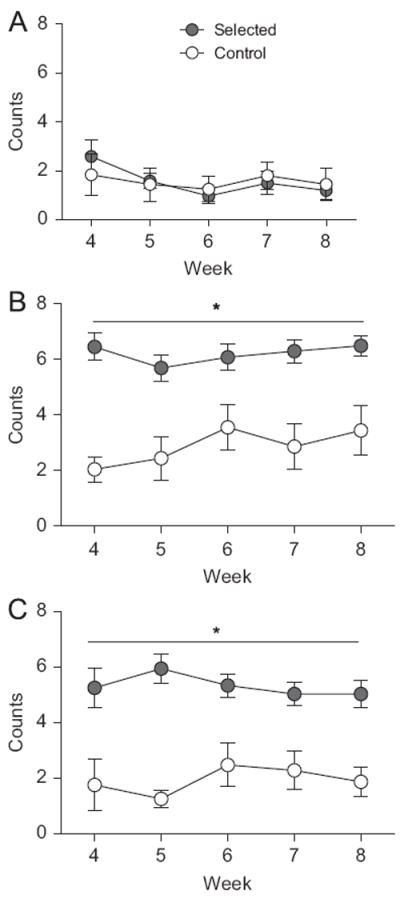
Visually observed wheel-running for mice from control and selected lines. We observed selected mice engaging in more wheel-running than controls during the 15:00 (B) and 21:00 (C) observation periods, but not during the 09:00 (A) period.
2.4. Monoamine concentrations in brain nuclei—all animals
Table 1 presents means, standard errors, and significance levels for all brain regions and neurotransmitters. Emphasis in the following sections is placed on comparisons that were statistically significant, based on two-way ANOVAs, for the effects of selection history, after controlling for multiple comparisons (i.e., P<0.0004).
TABLE 1.
Monoamine concentrations and ratios measured from microdissected brain regions for mice from the selected and control lines.
| NE | EPI | DA | DOPAC | 5HIAA | 5HT | DOPAC/DA | 5HIAA/5HT | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CeA | Control | Sedentary | 18.4 ± 2.84 | P Values | 0.4 ± 0.07 | P Values | 20.7 ± 2.08 | P Values | 7.3 ± 0.70 | P Values | 14.2 ± 0.82 | P Values | 28.9 ± 0.93 | P Values | 0.4 ± 0.03 | P Values | 0.5 ± 0.02 | P Values | ||||||||
| Wheel | 14.9 ± 1.11 | Wheel | 0.26602 | 0.6 ± 0.20 | Wheel | 0.12738 | 21.3 ± 4.65 | Wheel | 0.55262 | 7.4 ± 1.17 | Wheel | 0.64849 | 13.3 ± 1.44 | Wheel | 0.65161 | 28.9 ± 2.01 | Wheel | 0.97017 | 0.4 ± 0.03 | Wheel | 0.77843 | 0.5 ± 0.03 | Wheel | 0.77612 | ||
| Selected | Sedentary | 12.5 ± 1.06 | Selection | 0.02247 | 0.5 ± 0.15 | Selection | 0.51325 | 22.4 ± 4.69 | Selection | 0.42842 | 6.0 ± 0.77 | Selection | 0.48291 | 13.2 ± 1.14 | Selection | 0.56901 | 29.5 ± 1.18 | Selection | 0.82497 | 0.3 ± 0.04 | Selection | 0.06331 | 0.4 ± 0.03 | Selection | 0.55147 | |
| Wheel | 11.7 ± 0.47 | Selection × Wheel | 0.47976 | 0.7 ± 0.20 | Selection × Wheel | 0.74610 | 28.2 ± 9.42 | Selection × Wheel | 0.62578 | 7.1 ± 1.85 | Selection × Wheel | 0.65311 | 13.0 ± 1.21 | Selection × Wheel | 0.78278 | 29.3 ± 4.32 | Selection × Wheel | 0.95848 | 0.3 ± 0.03 | Selection × Wheel | 0.67090 | 0.5 ± 0.04 | Selection × Wheel | 0.41611 | ||
| NaccSh | Control | Sedentary | 2.6 ± 0.25 | P Values | 0.4 ± 0.06 | P Values | 327.3 ± 11.89 | P Values | 48.0 ± 2.13 | P Values | 10.0 ± 0.32 | P Values | 16.7 ± 0.54 | P Values | 0.1 ± 0.01 | P Values | 0.6 ± 0.02 | P Values | ||||||||
| Wheel | 2.4 ± 0.31 | Wheel | 0.66491 | 0.4 ± 0.06 | Wheel | 0.92945 | 318.0 ± 15.20 | Wheel | 0.14364 | 48.1 ± 3.89 | Wheel | 0.77019 | 8.3 ± 1.05 | Wheel | 0.43062 | 14.2 ± 1.85 | Wheel | 0.91690 | 0.2 ± 0.01 | Wheel | 0.31095 | 0.6 ± 0.08 | Wheel | 0.66962 | ||
| Selected | Sedentary | 2.2 ± 0.33 | Selection | 0.26434 | 0.6 ± 0.12 | Selection | 0.41443 | 330.3 ± 29.13 | Selection | 0.29997 | 45.0 ± 2.41 | Selection | 0.04321 | 8.6 ± 0.63 | Selection | 0.00626 | 15.0 ± 1.02 | Selection | 0.84490 | 0.1 ± 0.02 | Selection | 0.23140 | 0.6 ± 0.05 | Selection | 0.08619 | |
| Wheel | 2.5 ± 0.34 | Selection × Wheel | 0.07014 | 0.5 ± 0.11 | Selection × Wheel | 0.97553 | 329.8 ± 20.16 | Selection × Wheel | 0.53514 | 38.7 ± 2.16 | Selection × Wheel | 0.13487 | 8.7 ± 0.43 | Selection × Wheel | 0.32461 | 14.5 ± 0.93 | Selection × Wheel | 0.06931 | 0.1 ± 0.00 | Selection × Wheel | 0.34556 | 0.6 ± 0.06 | Selection × Wheel | 0.22145 | ||
| dICPu | Control | Sedentary | 0.5 ± 0.14 | P Values | 1.9 ± 0.21 | P Values | 387.0 ± 10.56 | P Values | 65.1 ± 4.20 | P Values | 6.8 ± 0.32 | P Values | 8.3 ± 0.26 | P Values | 0.2 ± 0.01 | P Values | 0.8 ± 0.03 | P Values | ||||||||
| Wheel | 0.6 ± 0.15 | Wheel | 0.14141 | 1.8 ± 0.26 | Wheel | 0.52215 | 388.4 ± 16.06 | Wheel | 0.90538 | 71.5 ± 3.17 | Wheel | 0.70368 | 7.0 ± 0.29 | Wheel | 0.63515 | 7.6 ± 0.54 | Wheel | 0.03604 | 0.2 ± 0.01 | Wheel | 0.83981 | 0.9 ± 0.05 | Wheel | 0.27553 | ||
| Selected | Sedentary | 0.5 ± 0.08 | Selection | 0.30083 | 2.0 ± 0.21 | Selection | 0.72055 | 436.4 ± 15.89 | Selection | 0.00362 | 66.7 ± 10.60 | Selection | 0.26381 | 6.0 ± 0.46 | Selection | 0.00354 | 6.7 ± 0.40 | Selection | 0.00011 | 0.2 ± 0.02 | Selection | 0.00988 | 0.9 ± 0.10 | Selection | 0.35958 | |
| Wheel | 1.2 ± 0.51 | Selection × Wheel | 0.31279 | 1.8 ± 0.11 | Selection × Wheel | 0.77354 | 431.6 ± 14.81 | Selection × Wheel | 0.82370 | 55.2 ± 3.21 | Selection × Wheel | 0.17744 | 5.5 ± 0.24 | Selection × Wheel | 0.24913 | 5.8 ± 0.13 | Selection × Wheel | 0.78875 | 0.1 ± 0.01 | Selection × Wheel | 0.13364 | 0.9 ± 0.05 | Selection × Wheel | 0.41081 | ||
| PVN | Control | Sedentary | 49.5 ± 5.58 | P Values | 1.9 ± 0.34 | P Values | 6.5 ± 0.68 | P Values | 4.1 ± 0.28 | P Values | 13.8 ± 0.71 | P Values | 25.8 ± 0.87 | P Values | 0.7 ± 0.05 | P Values | 0.5 ± 0.02 | P Values | ||||||||
| Wheel | 58.2 ± 4.05 | Wheel | 0.10823 | 1.3 ± 0.15 | Wheel | 0.00882 | 5.6 ± 0.77 | Wheel | 0.58849 | 3.9 ± 0.50 | Wheel | 0.20437 | 13.2 ± 0.61 | Wheel | 0.64880 | 24.3 ± 1.05 | Wheel | 0.61406 | 0.7 ± 0.08 | Wheel | 0.86450 | 0.5 ± 0.02 | Wheel | 0.33553 | ||
| Selected | Sedentary | 73.5 ± 7.18 | Selection | 0.71480 | 1.6 ± 0.30 | Selection | 0.14308 | 5.9 ± 0.81 | Selection | 0.86969 | 3.8 ± 0.53 | Selection | 0.12860 | 11.7 ± 1.07 | Selection | 0.26820 | 24.7 ± 1.18 | Selection | 0.87955 | 0.6 ± 0.06 | Selection | 0.33185 | 0.5 ± 0.04 | Selection | 0.20894 | |
| Wheel | 39.6 ± 11.98 | Selection × Wheel | 0.00964 | 0.7 ± 0.22 | Selection × Wheel | 0.55284 | 5.9 ± 1.42 | Selection × Wheel | 0.60585 | 2.9 ± 0.47 | Selection × Wheel | 0.48386 | 13.1 ± 1.27 | Selection × Wheel | 0.30717 | 25.0 ± 1.23 | Selection × Wheel | 0.40752 | 0.6 ± 0.11 | Selection × Wheel | 0.41989 | 0.5 ± 0.04 | Selection × Wheel | 0.56156 | ||
| NaccC | Control | Sedentary | 28.3 ± 3.93 | P Values | 0.9 ± 0.31 | P Values | 157.8 ± 7.94 | P Values | 61.0 ± 2.98 | P Values | 15.1 ± 0.41 | P Values | 20.5 ± 1.87 | P Values | 0.4 ± 0.02 | P Values | 0.8 ± 0.08 | P Values | ||||||||
| Wheel | 20.2 ± 2.98 | Wheel | 0.98581 | 0.9 ± 0.29 | Wheel | 0.59156 | 177.9 ± 10.13 | Wheel | 0.81404 | 69.4 ± 5.29 | Wheel | 0.25511 | 13.8 ± 1.07 | Wheel | 0.21841 | 16.8 ± 2.16 | Wheel | 0.19327 | 0.4 ± 0.02 | Wheel | 0.34431 | 0.9 ± 0.05 | Wheel | 0.74380 | ||
| Selected | Sedentary | 17.8 ± 2.63 | Selection | 0.66998 | 0.7 ± 0.29 | Selection | 0.29511 | 153.7 ± 10.96 | Selection | 0.71051 | 58.3 ± 6.19 | Selection | 0.03019 | 12.1 ± 0.50 | Selection | 0.40815 | 17.0 ± 1.57 | Selection | 0.52422 | 0.4 ± 0.04 | Selection | 0.08827 | 0.7 ± 0.07 | Selection | 0.94692 | |
| Wheel | 22.8 ± 3.61 | Selection × Wheel | 0.46209 | 0.7 ± 0.26 | Selection × Wheel | 0.48449 | 161.9 ± 7.80 | Selection × Wheel | 0.82396 | 52.6 ± 2.79 | Selection × Wheel | 0.24497 | 12.3 ± 1.00 | Selection × Wheel | 0.16940 | 21.1 ± 2.62 | Selection × Wheel | 0.41057 | 0.3 ± 0.02 | Selection × Wheel | 0.16475 | 0.6 ± 0.07 | Selection × Wheel | 0.73834 | ||
| MeA | Control | Sedentary | 12.4 ± 0.40 | P Values | 1.5 ± 0.33 | P Values | 0.7 ± 0.17 | P Values | 1.3 ± 0.18 | P Values | 8.5 ± 0.33 | P Values | 12.1 ± 0.49 | P Values | 3.1 ± 0.97 | P Values | 0.7 ± 0.04 | P Values | ||||||||
| Wheel | 14.6 ± 1.91 | Wheel | 0.04889 | 1.1 ± 0.46 | Wheel | 0.90902 | 1.2 ± 0.28 | Wheel | 0.04687 | 1.9 ± 0.14 | Wheel | 0.10097 | 8.9 ± 0.63 | Wheel | 0.09407 | 11.7 ± 1.22 | Wheel | 0.89426 | 2.0 ± 0.41 | Wheel | 0.29631 | 0.8 ± 0.11 | Wheel | 0.58139 | ||
| Selected | Sedentary | 11.2 ± 0.71 | Selection | 0.41291 | 0.7 ± 0.10 | Selection | 0.20437 | 0.7 ± 0.23 | Selection | 0.71431 | 1.6 ± 0.22 | Selection | 0.83114 | 7.1 ± 0.43 | Selection | 0.07377 | 10.4 ± 0.51 | Selection | 0.14980 | 36.9 ± 31.84 | Selection | 0.31722 | 0.7 ± 0.02 | Selection | 0.13968 | |
| Wheel | 13.9 ± 1.62 | Selection × Wheel | 0.84608 | 1.1 ± 0.27 | Selection × Wheel | 0.26031 | 1.1 ± 0.30 | Selection × Wheel | 0.71431 | 1.7 ± 0.17 | Selection × Wheel | 0.13816 | 8.4 ± 0.62 | Selection × Wheel | 0.33486 | 13.6 ± 1.47 | Selection × Wheel | 0.07394 | 2.3 ± 0.85 | Selection × Wheel | 0.32558 | 0.7 ± 0.07 | Selection × Wheel | 0.33566 | ||
| CA1 | Control | Sedentary | 5.1 ± 0.29 | P Values | 0.4 ± 0.14 | P Values | 0.2 ± 0.04 | P Values | 0.2 ± 0.03 | P Values | 5.7 ± 0.31 | P Values | 5.4 ± 0.34 | P Values | 47.9 ± 46.63 | P Values | 1.1 ± 0.05 | P Values | ||||||||
| Wheel | 4.5 ± 0.47 | Wheel | 0.31294 | 0.4 ± 0.16 | Wheel | 0.31294 | 0.2 ± 0.03 | Wheel | 0.72506 | 0.3 ± 0.05 | Wheel | 0.65427 | 4.8 ± 0.34 | Wheel | 0.11773 | 4.6 ± 0.43 | Wheel | 0.13737 | 2.4 ± 0.92 | Wheel | 0.42004 | 1.1 ± 0.04 | Wheel | 0.79080 | ||
| Selected | Sedentary | 6.5 ± 1.96 | Selection | 0.72967 | 0.4 ± 0.17 | Selection | 0.25332 | 0.2 ± 0.04 | Selection | 0.41681 | 0.2 ± 0.05 | Selection | 0.05929 | 4.5 ± 0.43 | Selection | 0.01940 | 4.6 ± 0.50 | Selection | 0.14005 | 1.5 ± 0.49 | Selection | 0.40164 | 1.0 ± 0.07 | Selection | 0.50047 | |
| Wheel | 3.9 ± 0.37 | Selection × Wheel | 0.38024 | 0.8 ± 0.24 | Selection × Wheel | 0.30467 | 0.2 ± 0.02 | Selection × Wheel | 0.88446 | 0.1 ± 0.03 | Selection × Wheel | 0.04809 | 4.2 ± 0.27 | Selection × Wheel | 0.48402 | 4.1 ± 0.33 | Selection × Wheel | 0.72602 | 0.7 ± 0.13 | Selection × Wheel | 0.43605 | 1.0 ± 0.07 | Selection × Wheel | 0.81468 | ||
| CA3 | Control | Sedentary | 21.3 ± 1.07 | P Values | 0.3 ± 0.07 | P Values | 1.0 ± 0.39 | P Values | 0.7 ± 0.16 | P Values | 12.7 ± 0.88 | P Values | 12.6 ± 2.88 | P Values | 1.1 ± 0.26 | P Values | 1.2 ± 0.14 | P Values | ||||||||
| Wheel | 22.2 ± 2.46 | Wheel | 0.94964 | 0.3 ± 0.07 | Wheel | 0.31978 | 0.7 ± 0.09 | Wheel | 0.59752 | 0.7 ± 0.05 | Wheel | 0.30480 | 11.1 ± 0.52 | Wheel | 0.12047 | 11.2 ± 1.09 | Wheel | 0.47981 | 1.1 ± 0.20 | Wheel | 1.0 ± 0.06 | Wheel | 0.56657 | |||
| Selected | Sedentary | 18.1 ± 1.28 | Selection | 0.04660 | 0.4 ± 0.05 | Selection | 0.31978 | 0.7 ± 0.20 | Selection | 0.64701 | 0.6 ± 0.09 | Selection | 0.05077 | 10.2 ± 0.70 | Selection | 0.01421 | 10.2 ± 0.69 | Selection | 0.23667 | 1.2 ± 0.34 | Selection | 0.74466 | 1.0 ± 0.07 | Selection | 0.52713 | |
| Wheel | 17.0 ± 3.16 | Selection × Wheel | 0.62358 | 0.3 ± 0.07 | Selection × Wheel | 0.37031 | 0.6 ± 0.13 | Selection × Wheel | 0.97184 | 0.4 ± 0.07 | Selection × Wheel | 0.42178 | 8.9 ± 1.28 | Selection × Wheel | 0.83055 | 8.8 ± 1.60 | Selection × Wheel | 0.98573 | 0.8 ± 0.21 | Selection × Wheel | 0.57338 | 1.1 ± 0.09 | Selection × Wheel | 0.35478 | ||
| dRN | Control | Sedentary | 80.0 ± 5.52 | P Values | 1.3 ± 0.44 | P Values | 4.7 ± 0.37 | P Values | 3.8 ± 0.27 | P Values | 22.0 ± 1.40 | P Values | 27.6 ± 2.59 | P Values | 0.8 ± 0.04 | P Values | 0.8 ± 0.03 | P Values | ||||||||
| Wheel | 65.4 ± 4.60 | Wheel | 0.08605 | 1.3 ± 0.29 | Wheel | 0.94768 | 4.3 ± 0.45 | Wheel | 0.28988 | 4.0 ± 0.45 | Wheel | 0.75762 | 17.5 ± 0.51 | Wheel | 0.01458 | 22.9 ± 1.48 | Wheel | 0.18285 | 1.0 ± 0.12 | Wheel | 0.77637 | 0.8 ± 0.05 | Wheel | 0.43660 | ||
| Selected | Sedentary | 57.2 ± 3.93 | Selection | 0.00261 | 1.1 ± 0.54 | Selection | 0.94768 | 3.0 ± 0.22 | Selection | 0.00040 | 2.9 ± 0.22 | Selection | 0.00507 | 16.5 ± 0.86 | Selection | 0.00177 | 20.8 ± 1.56 | Selection | 0.02257 | 1.0 ± 0.09 | Selection | 0.52060 | 0.8 ± 0.05 | Selection | 0.93243 | |
| Wheel | 53.3 ± 5.97 | Selection × Wheel | 0.31035 | 1.5 ± 0.40 | Selection × Wheel | 0.60464 | 3.2 ± 0.09 | Selection × Wheel | 0.51763 | 2.9 ± 0.36 | Selection × Wheel | 0.88644 | 15.7 ± 0.64 | Selection × Wheel | 0.08724 | 20.1 ± 1.13 | Selection × Wheel | 0.31807 | 0.9 ± 0.11 | Selection × Wheel | 0.21340 | 0.8 ± 0.03 | Selection × Wheel | 0.91823 | ||
| LC | Control | Sedentary | 40.7 ± 6.75 | P Values | 2.8 ± 2.58 | P Values | 3.7 ± 0.83 | P Values | 4.9 ± 0.99 | P Values | 9.8 ± 0.90 | P Values | 13.6 ± 2.10 | P Values | 1.6 ± 0.29 | P Values | 1.3 ± 0.66 | P Values | ||||||||
| Wheel | 30.3 ± 9.08 | Wheel | 0.76624 | 2.0 ± 1.61 | Wheel | 0.36581 | 3.2 ± 1.68 | Wheel | 0.98850 | 4.1 ± 0.93 | Wheel | 0.77593 | 10.2 ± 2.75 | Wheel | 0.70123 | 16.6 ± 3.71 | Wheel | 0.20810 | 2.2 ± 0.56 | Wheel | 0.36203 | 0.6 ± 0.13 | Wheel | 0.33242 | ||
| Selected | Sedentary | 38.1 ± 6.60 | Selection | 0.51888 | 1.0 ± 0.20 | Selection | 0.36581 | 2.7 ± 0.29 | Selection | 0.55602 | 3.8 ± 0.50 | Selection | 0.51059 | 9.0 ± 0.57 | Selection | 0.71810 | 16.2 ± 2.07 | Selection | 0.26508 | 1.4 ± 0.08 | Selection | 0.13336 | 0.6 ± 0.05 | Selection | 0.36640 | |
| Wheel | 43.6 ± 10.78 | Selection × Wheel | 0.33704 | 0.5 ± 0.29 | Selection × Wheel | 0.92440 | 3.1 ± 0.76 | Selection × Wheel | 0.66812 | 4.1 ± 0.95 | Selection × Wheel | 0.56571 | 9.8 ± 1.88 | Selection × Wheel | 0.90737 | 19.9 ± 2.02 | Selection × Wheel | 0.88748 | 1.4 ± 0.15 | Selection × Wheel | 0.37052 | 0.5 ± 0.08 | Selection × Wheel | 0.45500 | ||
| SNc | Control | Sedentary | 32.7 ± 4.67 | P Values | 0.2 ± 0.05 | P Values | 19.8 ± 2.43 | P Values | 13.2 ± 1.11 | P Values | 16.7 ± 0.99 | P Values | 20.4 ± 1.77 | P Values | 0.7 ± 0.05 | P Values | 0.9 ± 0.07 | P Values | ||||||||
| Wheel | 30.6 ± 2.62 | Wheel | 0.14792 | 0.6 ± 0.06 | Wheel | 0.88565 | 18.6 ± 1.53 | Wheel | 0.54463 | 12.4 ± 0.45 | Wheel | 0.92175 | 15.7 ± 0.82 | Wheel | 0.34229 | 21.2 ± 1.91 | Wheel | 0.68829 | 0.7 ± 0.05 | Wheel | 0.68353 | 0.8 ± 0.06 | Wheel | 0.32381 | ||
| Selected | Sedentary | 34.9 ± 4.18 | Selection | 0.67450 | 0.3 ± 0.09 | Selection | 0.88565 | 14.4 ± 0.58 | Selection | 0.21840 | 8.1 ± 0.53 | Selection | 0.00004 | 12.8 ± 0.83 | Selection | 0.00028 | 18.4 ± 1.06 | Selection | 0.26072 | 0.6 ± 0.04 | Selection | 0.00996 | 0.7 ± 0.05 | Selection | 0.06740 | |
| Wheel | 25.0 ± 3.10 | Selection × Wheel | 0.33906 | 0.5 ± 0.15 | Selection × Wheel | 0.28901 | 18.3 ± 3.23 | Selection × Wheel | 0.27748 | 9.2 ± 0.68 | Selection × Wheel | 0.24792 | 12.1 ± 0.72 | Selection × Wheel | 0.85462 | 19.0 ± 2.47 | Selection × Wheel | 0.95911 | 0.5 ± 0.06 | Selection × Wheel | 0.87839 | 0.7 ± 0.06 | Selection × Wheel | 0.67043 | ||
| VTA | Control | Sedentary | 29.4 ± 3.88 | P Values | 1.3 ± 0.35 | P Values | 28.7 ± 5.06 | P Values | 19.9 ± 1.90 | P Values | 16.1 ± 1.15 | P Values | 22.7 ± 2.18 | P Values | 0.8 ± 0.09 | P Values | 0.7 ± 0.06 | P Values | ||||||||
| Wheel | 27.7 ± 2.11 | Wheel | 0.12998 | 2.4 ± 0.42 | Wheel | 0.83385 | 30.6 ± 4.37 | Wheel | 0.09070 | 21.2 ± 1.98 | Wheel | 0.11054 | 15.2 ± 1.46 | Wheel | 0.65142 | 20.7 ± 2.40 | Wheel | 0.68478 | 0.7 ± 0.05 | Wheel | 0.86517 | 0.7 ± 0.03 | Wheel | 0.21543 | ||
| Selected | Sedentary | 21.2 ± 1.18 | Selection | 0.90488 | 1.6 ± 0.29 | Selection | 0.83385 | 11.5 ± 1.91 | Selection | 0.14631 | 10.3 ± 1.45 | Selection | 0.00508 | 11.7 ± 0.62 | Selection | 0.82742 | 19.0 ± 1.42 | Selection | 0.01001 | 0.9 ± 0.07 | Selection | 0.03941 | 0.6 ± 0.07 | Selection | 0.32216 | |
| Wheel | 36.9 ± 8.13 | Selection × Wheel | 0.06298 | 2.3 ± 0.64 | Selection × Wheel | 0.68422 | 30.1 ± 10.20 | Selection × Wheel | 0.16144 | 16.6 ± 3.60 | Selection × Wheel | 0.29554 | 13.5 ± 0.98 | Selection × Wheel | 0.20664 | 23.3 ± 3.56 | Selection × Wheel | 0.21138 | 0.8 ± 0.15 | Selection × Wheel | 0.73663 | 0.6 ± 0.04 | Selection × Wheel | 0.56940 | ||
Values are presented as means ± SE. P values from two-way ANOVAs for individual brain regions are presented in each cell. pFDR was used to adjust for multiple comparisons, and P values <0.0004 are considered statistically significant and are in inverse color (see Section 5).
2.4.1. Dopamine (DA) and the dopamine metabolite dihydroxyphenylacetic acid (DOPAC)
Concentrations of DA were lower in the dorsal raphe nucleus (t13=12.789, P=0.0004) of selected mice compared to controls. Selected animals also exhibited lower concentrations of DOPAC in the substantia nigra (t13=25.988, P=0.0003) compared to controls.
2.4.2. Serotonin (5-HT) and the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA)
Selected mice had lower concentrations of 5-HT in the dorsal striatum (t13=21.663, P=0.0001) and lower concentrations of 5-HIAA in the substantia nigra (t13=18.339, P=0.0003).
3. Discussion
Although female mice of these selected lines demonstrate higher levels of voluntary wheel-running than males (Garland et al., 2011a), and thus may exhibit exaggerated effects of selection, we chose to include only males in this study due to the large scope of the experiment (long term recording of multiple modes of behavior coupled with analyses of multiple neurotransmitters in multiple brain regions). The addition of female mice would add the complexity of another sex (in each of the 4 groups), as well as a necessity to account for estrous stage. This complexity is best left to a subsequent study designed specifically to compare results from female mice to the findings and interpretations herein.
3.1. Wheel-running behavior
Mice selectively bred for high voluntary wheel-running ran an average of three times further than control mice during the study (Fig. 1A). This increase in distance was accomplished by both an increase in the running speed and running duration (Fig. 1B and C). The relative levels of running distance and running speeds in selected and control mice are comparable to those found in numerous previous studies (Garland et al., 2002, 2011a; Keeney et al., 2012; Swallow et al., 1998a).
3.2. Body mass
As previously reported, selected mice weighed less than controls (Garland et al., 2002, 2011a; Girard et al., 2001; Swallow et al., 1999); this difference was observed regardless of whether or not animals were housed with running-wheels (Fig. 1D). Therefore, increased wheel-running does not account for the body mass difference between control and selected lines (Swallow et al., 1999). Behavioral assessment revealed some differences that could contribute to the difference in body mass, including more mid-dark phase inactive behavior in control mice, as well as higher levels of consummatory behaviors in sedentary control mice compared to selected mice (Fig. 2). These differences in behavior, together with intrinsic physiological differences, such as elevated circulating corticosterone levels (Girard and Garland, 2002; Malisch et al., 2007), could influence the observed divergence in body mass between selected and control animals.
3.3. Behavior
Our ethogram data add to behavioral results presented in previous reports (Bronikowski et al., 2001; Careau et al., 2012; Jonas et al., 2010a; Koteja et al., 1999), and provide information on the circadian patterns of these animals’ general home-cage activity, with increased resolution over previous studies. Not surprisingly, selection for increased wheel-running resulted in higher levels of wheel-running during the dark phase. Interestingly, selection also affected the expression and patterns of other behaviors as discussed below.
We observed no effect of selection on behavior at the 09:00 time period, likely due to the generally low levels of activity during this period. The most prevalent behavior observed during this time was sleep in all animals (data not shown). Selection significantly affected behavior similarly at the 15:00 and 21:00 observation time. During both of these time periods, selected animals exhibited much higher levels of wheel-running behavior, which contributed to generally higher levels of active behavior. This increased activity is independent of wheel-running however, as sedentary selected animals were also more active than sedentary controls. Furthermore, this observed increase in active behavior in selected animals came at the expense of more sedentary behaviors (i.e., inactive and sleeping). This effect is in contrast to previous results collected on these animals at generation 13 (these animals are from generation 35) (Koteja et al., 1999), but is consistent with more recent studies that measured home-cage activity via force plates (Malisch et al., 2009) and infrared detectors (Garland et al., unpublished results). These studies support a positive relationship between wheel-running and general locomotor activity. A direct, positive relationship between these traits is not without controversy. Previous reports suggested that wheelrunning behavior is independent of other stimulated exploratory behaviors, such as open-field activity or emergence (reviewed in Sherwin, 1998). However, more recent studies demonstrate a positive link between home-cage activity and wheel-running behavior (Garland et al., 2011b; Sherwin, 1998), which is more in line with our observations. Importantly, our results (as well as the interpretation of Garland et al. (2011a)) suggest that wheel-running may be a better indicator of general activity levels in mice than measures from paradigms that involve introducing the animal to novel stimuli, such as the open field or other challenge (see also Careau et al., 2012).
When housed without running-wheels, selected animals exhibited lower levels of consummatory behavior than control animals during the 15:00 observation period. This was unexpected given the higher levels of activity (and presumed higher energy expenditure) exhibited by these animals and the established positive association between energy use and wheel-running (Garland et al., 2011b; Novak et al., 2012; Swallow et al., 2001). Further perplexing, access to a running- wheel depressed consummatory behavior in control animals. Thus, two manipulations (selection for increased wheel-running and presenting a running-wheel) that increase activity level resulted in decreased consummatory behavior. Although these findings seem counter-intuitive, it is possible that this observation reflects something other than a decrease in consumption; it could reflect either an increase in feeding efficiency or also changes in circadian feeding patterns when activity levels are increased. In fact, this result directly contradicts a study that more directly measured food consumption in these mice that observed positive effects of both wheel-running and selection on food intake (Swallow et al., 2001). Together, these observations warrant further examination of the effects of these manipulations on consummatory behavior, and also suggest that this selectivebreeding may influence circadian patterns of consumption.
Grooming is a complex behavior that can be highly variable among strains of mice (Kalueff and Tuohimaa, 2005c). Efforts to classify grooming as anxiotypic have presented conflicting results, with increased grooming being associated with increases (Kalueff and Tuohimaa, 2005b) or decreases in anxiety (File et al., 2006). At both dark phase observation periods, selected animals exhibited lower levels of grooming than controls, regardless of housing condition. This may suggest decreased levels of anxiety in our selected animals, as excessive grooming has been associated with increased anxiety (Dunn et al., 1987) (though this association is not universally accepted; File et al., 2006). A wealth of evidence suggests a negative relationship between physical activity and anxiety (Binder et al., 2004; Dishman et al., 2006; Dunn et al., 2001; Fulk et al., 2004; Kalueff and Tuohimaa, 2005a; Salmon, 2001), and in light of the much higher levels of physical activity observed in selected animals, it follows that this decrease in grooming could indicate a decrease in anxiety. Previous studies have investigated the effects of this selection in anxiotypic behavior. While two studies (Bronikowski et al., 2001; Careau et al., 2012) found no effect of selection on anxiotypic behavior using the open-field test, another study (Jonas et al., 2010a) found that one of the selected replicates exhibits more anxiotypic behavior than control animals in the open-field test and the elevated plus maze (two selected replicates were tested in that study, and only one exhibited higher anxiety than controls). Interestingly, the replicate line that exhibited increased anxiotypic behavior in this study (Jonas et al., 2010a) was the same replicate used in our study. Thus, the idea that this decrease in grooming indicates a decrease in anxiety in this selected replicate requires more investigation. In fact, we found no effect of selective-breeding on monoamines in brain areas associated with anxiety (e.g. MeA; Table 1). Alternatively, grooming behavior in this context may reflect restlessness, as opposed to anxiety (Kalueff and Tuohimaa, 2005a). If so, then selective-breeding may influence the way animals deal with restlessness: control animals groom in the face of restlessness, while selected animals express restlessness by increasing other behaviors, possibly rearing and/or wheelrunning. Selected animals exhibit very high levels of rearing when housed without a running-wheel, which could indicate an increase in vigilance (or general restlessness) in selected animals, while control animals express this by an increased in grooming (Zalaquett and Thiessen, 1991).
Finally, selective-breeding affected longitudinal patterns of inactive behavior throughout the study, and these effects were not consistent over the circadian period. During the first observation periods, all animals exhibited equivalently low (near 0) levels of inactive behavior at both the 15:00 and 21:00 observation periods. Near the end of the experiment, control mice increased levels of inactive behavior above selected animals at the 15:00 time period. Conversely, at the 21:00 time period, selected animals increased inactive behavior above those exhibited by control animals at the end of the study. These results may be interpreted as an earlier circadian quiescence in selected mice that develops as these mice age. Both exercise and this selection paradigm influence circadian rhythms (Koteja et al., 2003; Novak et al., 2012, Jonas et al., 2010b), but this is the first time that effects on circadian patterns of home-cage behavior have been reported. Changes in circadian behavioral patterns are implicated in many human disorders, such as anxiety (Shear et al., 1994) and obesity (Carmona-Alcocer et al., 2012), and add relevance to these animals as models of human disease.
3.4. Brain monoamines
We observed selective-breeding effects on monoamines in two brain regions involved in locomotor control, the substantia nigra pars compacta (SNc) and the dorsolateral striatum (caudate putamen; dlCPu). These brain regions are intimately related, as dopamine neurons, which make up the vast majority of cells in the SNc, project to the dlCPu. The dlCPU is a primary central output of the motor cortex (Kandel et al., 2000). In the dlCPu, increased DA release positively modulates voluntary wheel-running and other motivated behaviors (Frank, 2006; Greenwood et al., 2003; McGeorge and Faull, 1989; Rhodes et al., 2005). This ‘nigrostriatal’ system also interacts largely with the raphe serotonin system, with serotonergic inputs from the dorsal raphe positively modulating the nigrostriatal DA output via 5-HT 2A receptors in both the SNc and dlCPu (Kapur and Remington, 1996). Furthermore, DA provides an excitatory effect on dorsal raphe serotonin cells, through D1-like receptors in the raphe (Aman et al., 2007). Compared to controls, selected animals exhibited lower levels of DOPAC in the dlCPu, suggesting decreased nigrostriatal DA activity in selected animals. Fitting with this idea, our data also indicate that communication between the raphe 5-HT and nigrostriatal DA systems is suppressed in selected animals compared to controls. These changes suggest a hypoactive nigrostriatal DA system in selected animals. This idea is in line with a previous pharmacological study that concluded mice from the four replicate selected lines have diminished function in the central DA system (Rhodes et al., 2003). This trait is a hallmark of attention deficit hyperactive disorder, and our results strengthen the case for the use of these selected lines as a model for this disease. The observed reductions in concentrations of monoamine neurotransmitters and their metabolites in these brain areas suggest that this selection protocol results in diminished central monoamine activity in these systems. Importantly, these effects are observed regardless of running-wheel access. Thus, our results highlight the intrinsic effects of this selection protocol on neurophysiology, and support the use of these lines as behavioral and neurophysiological models of human pathologies.
4. Conclusion
Selective breeding an increased propensity to exercise on wheels results in dynamic changes in behavior, resulting in generally increased levels of activity, and possibly, differences in circadian rhythm. We also observed changes in brain monoamine systems that could explain these behavioral differences, and support the idea that these selected lines of mice can be used as animal models of human pathology.
5. Experimental procedures
5.1. Animals
House mice (Mus domesticus; originally from the outbred Hsd:ICR strain) from one of four replicate lines selectively bred for increased voluntary wheel-running and one non-selected control line were used in this study (Swallow et al., 1998a). Specifically, 26 selected males (from lab designation Line 8) and 28 control males (from lab designation Line 2) were obtained from generation 35 stock. Because we used only two of the eight total lines, further studies will be required to verify the generality of our results (Garland et al., 2011a; Jonas et al., 2010a, 2010b). Following shipment by air from the University of California, Riverside to the University of South Dakota, mice were housed in groups of 6–8 individuals for approximately two months in acrylic cages with wire lids (Technoplast, Allentown, PA; 55 × 35 × 20 cm3) to allow animals to acclimate to our facility. For the duration of the study, mice were maintained at 23 °C on a 12L:12D reverse light cycle (light from 22:00–10:00 h). Bedding (Harlan Teklad Sani-Chips) was changed weekly at the end of the light period, with food (Harlan Teklad Rodent Diet (W) 8604) and water available ad libitum. All procedures were carried out with approval by the University of South Dakota Animal Care and Use Committee and were conducted in accordance with the “Guiding Principles on the Care and Use of Animals” as approved by the Council of the American Physiological Society.
5.2. Voluntary wheel-running
Following the approximately 2 month acclimation period, we reassigned mice (age=96.5±1.4 days; mean±S.E.M.) to individual Nalgene cages (47 × 25 × 20 cm3) equipped with wire lids with (n=24; 12 selected, 12 control) or without (n=30; 14 selected, 16 control) an activity wheel (Nalgene F-size wheels, 1.084m circumference) for 8 weeks. Wheels were located within the cages and were freely available at all times. This environment differs from the environment in which they were phenotyped for selective-breeding, where a separate, but connected chamber houses the activity wheel (Swallow et al., 1998a). Throughout the study, we weighed mice during weekly bedding changes (09:00–10:00 h). We monitored voluntary wheel-running every 60 s for the duration of the 8-week experiment via computer with VitalView software (Mini Mitter Company, Inc., Sunriver, OR), allowing the assessment of running distance, running duration, and average running speed (Swallow et al., 1998a). Wheel-running data are expressed as daily averages, unless otherwise noted.
5.3. Behavioral assessment
We observed the behavior all mice during the final 5 weeks of the study (weeks 4–8 of wheel access) via instantaneous scan sampling (Martin and Bateson, 1993) by a single observer at three specific times during a 24-h period, starting at 09:00, 15:00, and 21:00 h. These times correspond to the last hour of the light phase (09:00), and the mid-point (15:00) and final hour (21:00) of the dark phase. We chose these times to achieve a comprehensive behavioral assessment of the mice throughout the active (dark) phase of the circadian period. During each behavioral observation period, a single observer made and recorded instantaneous observations of all mice, and repeated this for ten rotations; this process took approximately 45min. The observer entered the room, and remained stationary in a dark corner of the room, out of the animals’ sight for 5–10 min until all animals were accustomed to his presence. Behavioral scoring then commenced from a central point in the room, approximately 2 m from the home-cages. Animals did not exhibit any apparent disturbance by the observer’s presence. We scored behaviors into the following mutually exclusive categories: grooming (licking at fur and rubbing paws), sleeping (motionless with eyes closed or curled in sleeping position), inactive (motionless with eyes open), consumption (eating or drinking), and active (moving in cage, running, rearing, etc.). We included wheel-running in the active category in analyses for mice with wheel access, and also analyzed it separately for comparison with computer-generated wheel-running data. We express behavioral data as frequencies for each category. The week (4–8) during which we collected behavioral data served as a repeated factor in our analysis to detect longitudinal changes in behavior throughout the study.
5.4. Brain collection
Following the 8-week experimental period, we killed all mice via cervical dislocation followed by rapid decapitation, between 10:00 and 12:00 h. Brains were rapidly dissected from the skull (within 1 min), frozen on dry ice to a microscope slide, and then stored at −80 °C until sectioning.
5.5. Microdissection and monoamine analysis
We cut frozen brains into 300 μm serial sections using a Leica® CM 1800-3 cryostat (Leica Instruments GmbH, Nussloch, Germany) at −11 °C. We thaw mounted sections onto glass slides and refroze them at −80 °C for microdissection. We identified brain regions using a rat brain microdissection guide (Palkovits and Brownstein, 1988) modified for the size of the mouse brains, and microdissected them using 100–300 μm inside diameter punch. We analyzed the hippocampus (CA1 and CA3), central nucleus of the amygdala (CeA), medial nucleus of the amygdala (MeA), nucleus accumbens shell (NaccSh) and core (NAccC), dorsal striatum (dorsal caudate putamen; dCPu), paraventricular nucleus of the hypothalamus (PVN), substantia nigra pars compacta (SNc), ventral tegmental area (VTA), dorsal raphe (dRN), and locus ceruleus (LC). We chose these brain nuclei because they are monoaminergic cell body or terminal regions associated with physical activity, motivation, and/or stress (Chaouloff et al., 1987; Dishman et al., 1997; Rhodes et al., 2003, 2005; Sutoo and Akiyama, 2003; Wightman and Robinson, 2002).
We analyzed monoamines in these regions using high performance liquid chromatography (HPLC) with electrochemical detection, determining norepinephrine (NE), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC, a metabolite of DA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA, a metabolite of 5-HT) concentrations (Renner and Luine, 1984). We expelled tissue into 60 μL of sodium acetate buffer (pH 5) containing 2,3-dihydroxybenzoic acid (DHBA) as an internal standard, adding ascorbic acid oxidase (AAO) solution (Sigma Chemical Co., St. Louis, MO, 1 mg AAO/10 mL H2O; 2 μL) to each sample prior to centrifugation. We analyzed the supernatant (45 μl) for monoamines using HPLC (Waters Associates, Milford, MA) and an LC-4B potentiostat (Bioanalytical Systems, West Lafayette, IN; +0.6 V Ag/AgCl reference). We determined total protein using the precipitate dissolved in 110 μL of 0.4 M NaOH and via Bradford assay (Bradford, 1976). We express neurotransmitter concentrations as pg amine/μg protein. We estimate dopaminergic and serotonergic activities by dividing the metabolite concentration by the concentration of the respective neurotransmitter.
5.6. Statistical analyses
We use two-way, repeated measures analysis of variance (RMANOVA) to analyze wheel-running data (selected line-=between-subjects factor, day of study=within-subjects factor). We compared the effects of selection and wheel access on body mass using a three-way RMANOVA (selected line and wheel access=between-subjects factors; week=within-subjects factor). Behavioral scan sampling generated frequency data; therefore, we used an arcsine transformation to these data before running three-way repeated measures MANOVA (RM-MANOVA; selected line and wheel access – between-subjects factor, week – within-subjects factor) (Zar, 1996); each behavioral category served as a dependent variable. We used the Greenhouse-Geiser statistic to test the assumptions of sphericity. We used post-hoc univariate ANOVA to determine individual behaviors that contribute to effects detected with the MANOVA model. We used SPSS 18.0 (SPSS Inc., Chicago, IL) to perform these analyses, and we set α at P<0.05. To compensate for the 31 simultaneous multiple comparisons in the post hoc behavioral analyses, we used the FDR procedure (‘Qvalue’ library run in the R statistical package. The R Foundation for Statistical Computing). Based on these analyses, we reset the significance level at P<0.03 to control the table-wise α level at P<0.05 for statistical tests involving post hoc behavioral analyses.
We used two-way ANOVAs to compare monoamine levels of control and selected mice housed with or without a running-wheel (factors: Selection and Wheel; SPSS 18.0, Chicago, IL). To compensate for the large number of simultaneous multiple comparisons in the monoamine analyses, we used the pFDR procedure (Storey and Tibshirani, 2003a, 2003b). We generated the overall proportion of true null hypotheses and the corresponding q-values with the ‘Qvalue’ library run in the R statistical package (The R Foundation for Statistical Computing) using the ‘Bootstrap’ option. Based on these analyses, we reset the significance level at P<0.0004 to control the table-wise α level at P<0.05 for statistical tests involving monoamine values (Table 1).
Acknowledgments
We owe thanks to Stacia M. Desantis for her help with the behavioral statistical analysis. This project was supported by NIH grant P20 RR-015567, which is designated as a Center of Biomedical Research Excellence (COBRE) to J.G.S. and K.J.R., by the USD J.F. and M.P. Nelson Endowment to J.G.S., and by grants from NSF (IOB-0448060 and EPSCoR in South Dakota 0091948 to J.G.S, IOS-1121273 to T.G., and IOS-0921874 to K.J.R).
References
- Aman T, Shen R, Haj-Dahmane S. D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther. 2007;320:376. doi: 10.1124/jpet.106.111690. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul J. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Perusse L, Stephens T. Heredity, activity level, fitness and health. In: Stephens T, editor. Physical Activity, Fitness and Health: International Proceeding and Concensus Statement. Vol. 1. Human Kinetics; Chicago, IL: 1994a. pp. 106–118. [Google Scholar]
- Bouchard C, Shepard RJ, Stephens T. Physical activity fitness and health. In: Stephens T, editor. Physical Activity, Fitness and Health: International Proceeding and Concensus Statement. Vol. 1. Human Kinetics; Chicago, IL: 1994b. pp. 77–88. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Carter PA, Swallow JG, Girard IA, Rhodes JS, Garland T., Jr Open-field behavior of house mice selectively bred for high voluntary wheel-running. Behav Genet. 2001;31:309–316. doi: 10.1023/a:1012283426530. [DOI] [PubMed] [Google Scholar]
- Careau V, Bininda-Emonds OR, Ordonez G, Garland T., Jr Are voluntary wheel running and open-field behavior correlated in mice? Different answers from comparative and artificial selection approaches. Behav Genet. 2012;42:830–844. doi: 10.1007/s10519-012-9543-0. [DOI] [PubMed] [Google Scholar]
- Carmona-Alcocer V, Fuentes-Granados C, Carmona-Castro A, Aguilar-Gonzalez I, Cardenas-Vazquez R, Miranda-Anaya M. Obesity alters circadian behavior and metabolism in sex dependent manner in the volcano mouse Neotomodon alstoni. Physiol Behav. 2012;105:727–733. doi: 10.1016/j.physbeh.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Carter PA, Swallow JG, Davis SJ, Garland T., Jr Nesting behavior of house mice (Mus domesticus) selected for increased wheel-running activity. Behav Genet. 2000;30:85–94. doi: 10.1023/a:1001967019229. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Laude D, Merino D, Serrurrier B, Guezennec Y, Elghozi JL. Amphetamine and alpha-methyl-p-tyrosine affect the exercise-induced imbalance between the availability of tryptophan and synthesis of serotonin in the brain of the rat. Neuropharmacology. 1987;26:1099–1106. doi: 10.1016/0028-3908(87)90254-1. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Dishman RK. The new emergence of exercise neurobiology. Scand J Med Sci Sports. 2006;16:379–380. doi: 10.1111/j.1600-0838.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dunn A, Berridge C, Lai Y, Yachabach T. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exercise. 2001;33:587–597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials. 2002;23:584–603. doi: 10.1016/s0197-2456(02)00226-x. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prevent Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Feder ME, Garland T, Jr, Marden JH, Zera AJ. Locomotion in response to shifting climate zones: not so fast. Annu Rev Physiol. 2010;72:167–190. doi: 10.1146/annurev-physiol-021909-135804. [DOI] [PubMed] [Google Scholar]
- File S, Mabbutt P, Walker J. Comparison of adaptive responses in familiar and novel environments: modulatory factors. Ann N Y Acad Sci. 2006;525:69–79. doi: 10.1111/j.1749-6632.1988.tb38596.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R, Jones S, Caron M. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Carter PA. Evolutionary physiology. Ann Rev Physiol. 1994;56:579–621. doi: 10.1146/annurev.ph.56.030194.003051. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evol: Int J Organic Evol. 2002;56:1267–1275. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc Roy Soc Biol Sci. 2011a;278:574–581. doi: 10.1098/rspb.2010.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011b;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard I, McAleer MW, Rhodes JS, Garland T., Jr Selection for high voluntary wheel-running increases speed and intermittancy in house mice (Mus domesticus) J Exp Biol. 2001;204:4311–4320. doi: 10.1242/jeb.204.24.4311. [DOI] [PubMed] [Google Scholar]
- Girard I, Garland T., Jr Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J Appl Physiol. 2002;92:1553–1561. doi: 10.1152/japplphysiol.00465.2001. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HEW, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha1beta-adrenergic receptor mRNA in the rat raphe nuclei. Soc Biol Psychiatry. 2005;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Guderley H, Houle-Leroy P, Diffee GM, Camp DM, Garland T., Jr Morphometry, ultrastructure, myosin isoforms, and metavolic capacities of the “mini muscles” favoured by selection for high activity in house mice. Comp Biochem Physiol Part B: Biochem Mol Biol. 2006;144:271–282. doi: 10.1016/j.cbpb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Henn FA. Dopamine: a marker of psychosis and final common driver of schizophrenia psychosis. Am J Psychiatry. 2011;168:1239–1240. doi: 10.1176/appi.ajp.2011.11091346. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy P, Garland T, Jr, Swallow JG, Guderley H. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol. 2000;89:1708–1717. doi: 10.1152/jappl.2000.89.4.1608. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Genetic models in applied physiology selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol. 2003;94:1682–1688. doi: 10.1152/japplphysiol.00556.2002. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, French D, Carroll FI, Kunko PM. Continuous infusion of selective dopamine uptake inhibitors or cocaine produces time-dependent changes in rat locomotor activity. Behav Brain Res. 1999;99:201–208. doi: 10.1016/s0166-4328(98)00104-1. [DOI] [PubMed] [Google Scholar]
- Jonas I, Schubert KA, Reijne AC, Scholte J, Garland T, Jr, Gerkema MP, Scheurink AJ, Nyakas C, van Dijk G. Behavioral traits are affected by selective breeding for increased wheel-running behavior in mice. Behav Genet. 2010a;40:542–550. doi: 10.1007/s10519-010-9359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas I, Vaanholt LM, Doornbos M, Garland T, Jr, Scheurink AJ, Nyakas C, van Dijk G. Effects of selective breeding for increased wheel-running behavior on circadian timing of substrate oxidation and ingestive behavior. Physiol Behav. 2010b;99:549–554. doi: 10.1016/j.physbeh.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005a;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur J Pharmacol. 2005b;508:147–153. doi: 10.1016/j.ejphar.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI) Behav Brain Res. 2005c;160:1–10. doi: 10.1016/j.bbr.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw Hill; New York, NY: 2000. [Google Scholar]
- Kapur S, Remington G. Serotonin–dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T., Jr Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheelrunning behavior. Pharmacol Biochem Behav. 2012;101:528–537. doi: 10.1016/j.pbb.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Koteja P, Swallow JG, Carter PA, Garland T., Jr Different effects of intensity and duration of locomotor activity on circadian period. J Biol Rhythms. 2003;18:491–501. doi: 10.1177/0748730403256998. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Salzman W, Gomes FR, Rezende EL, Jeske DR, Garland T., Jr Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol Biochem Zool. 2007;80:146–156. doi: 10.1086/508828. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T., Jr Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- Martin PR, Bateson PPG. Measuring Behaviour: An Introductory Guide. Cambridge University Press; Cambridge [England]; New York, NY, USA: 1993. [Google Scholar]
- Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res. 2010;210:155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Meek TH, Eisenmann JC, Garland T., Jr Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obesity (Lond) 2009;34:960–969. doi: 10.1038/ijo.2010.25. [DOI] [PubMed] [Google Scholar]
- Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kir HM, Akar F, Erden F. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91:1252–1262. doi: 10.1016/j.lfs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Nehrenberg DL, Hua K, Estrada-Smith D, Garland T, Jr, Pomp D. Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity (Silver Spring) 2009;17:1402–1409. doi: 10.1038/oby.2009.51. [DOI] [PubMed] [Google Scholar]
- Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against highfat diet-induced insulin resistance. Am J Physiol—Endocrinol Metab. 2007;293:E31–E41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. Elsevier; Amsterdam, Netherlands: 1988. [Google Scholar]
- Porter DM, Bell CC. The use of clonidine in posttraumatic stress disorder. J Natl Med Assoc. 1999;91:475–477. [PMC free article] [PubMed] [Google Scholar]
- Renner KJ, Luine VN. Determination of monoamines in brain nuclei by high performance liquid chromatography with electrochemical detection: Young vs. middle-aged rats. Life Sci. 1984;34:2193–2199. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Hosack HR, Girard I, Kelley AE, Mitchell GS, Garland T., Jr Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology. 2001;158:120–131. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T., Jr Differential sensitivity to acute administration of Ritalin, apormophine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheelrunning behavior. Psychopharmacology. 2003;167:242–250. doi: 10.1007/s00213-003-1399-9. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117:1243–1257. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Riley AL. The effects of haloperidol on cocaine-induced conditioned taste aversions. Physiol Behav. 2012;105:1161–1167. doi: 10.1016/j.physbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Shear MK, Randall J, Monk TH, Ritenour A, Tu X, Frank E, Reynolds C, Kupfer DJ. Social rhythm in anxiety disorder patients. Anxiety. 1994;1:90–95. doi: 10.1002/anxi.3070010208. [DOI] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003a;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci. 2003b;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13:1–14. doi: 10.1016/s0969-9961(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998a;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Garland T, Jr, Carter PA, Zhan W, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus) J Appl Physiol. 1998b;84:69–76. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T., Jr Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J Exp Biol. 1999;202:2513–2520. doi: 10.1242/jeb.202.18.2513. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T., Jr Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol B—Biochem Syst Environ Physiol. 2001;171:651–659. doi: 10.1007/s003600100216. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Schutt M, Palm F, Vogel C, Meier M, Schmitt A, Lesch KP, Mossner R, Sommer C. Lack of the serotonin transporter in mice reduces locomotor activity and leads to gender-dependent late onset obesity. Int J Obesity. 2010;34:701–711. doi: 10.1038/ijo.2009.289. [DOI] [PubMed] [Google Scholar]
- Walsh B, Hooks B, Hornyak JE, Koch LG, Britton SL, Hogan MC. Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol. 2006;100:1765–1769. doi: 10.1152/japplphysiol.01533.2005. [DOI] [PubMed] [Google Scholar]
- Waters R, Renner K, Pringle R, Summers C, Britton S, Koch L, Swallow J. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008a;93:1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RP. Behavioral, endocrine and neurochemical consequences of bidirectional selection for endurance capacity in female NIH rats, In Biology, Doctor of Philosophy. University of South Dakota; Vermillion, SD: 2007. p. 179. [Google Scholar]
- Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, Swallow JG. Selection for aerobic capacity affects corticosterone, monoamines and wheelrunning activity. Physiol Behav. 2008b;93:1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Welkley JE, Warren GL, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. Treadmill exercise training and estradiol increase plasma ACTH and prolactin after novel footshock. J Appl Physiol. 1996;80:931–939. doi: 10.1152/jappl.1996.80.3.931. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Zalaquett C, Thiessen D. The effects of odors from stressed mice on conspecific behavior. Physiol Behav. 1991;50:221–227. doi: 10.1016/0031-9384(91)90524-r. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice Hall; Upper Saddle River, NJ: 1996. [Google Scholar]


