Abstract
Objective
To date, little research exists defining bio-behavioral adaptations associated with both marijuana abuse and risk of craving and relapse to other drugs of abuse during early abstinence.
Method
Fifty-nine treatment-seeking individuals dependent on alcohol and cocaine were recruited. Thirty of these individuals were also marijuana (MJ) dependent; 29 were not. Twenty-six socially drinking healthy controls were also recruited. All participants were exposed to three 5-min guided imagery conditions (stress, alcohol/cocaine cue and relaxing), presented randomly, one per day across three consecutive days. Measures of craving, anxiety, heart rate, blood pressure, plasma adrenocorticotrophic hormone and cortisol were collected at baseline and subsequent recovery time points.
Results
The MJ-dependent group showed increased basal anxiety ratings and cardiovascular output alongside enhanced alcohol craving and cocaine craving, and dampened cardiovascular response to stress and cue. They also demonstrated elevated cue-induced anxiety and stress-induced cortisol and adrenocorticotrophic hormone levels, which were not observed in the non-MJ-dependent group or controls. Cue-related alcohol craving and anxiety were both predictive of a shorter number of days to marijuana relapse following discharge from inpatient treatment.
Conclusions
Findings provide some support for drug cross-sensitization in terms of motivational processes associated with stress-related and cue-related craving and relapse.
Keywords: marijuana, stress, drug cue, cocaine craving, alcohol craving, relapse
INTRODUCTION
In 2010, marijuana was shown to represent the illicit drug with the highest rate of past year dependence or abuse in the USA, with the number of recent adult initiates increasing from 49 000 to 247 000 between 2009 and 2010 [Substance Abuse and Mental Health Services Administration (SAMHSA), 2011]. This increase in prevalence may reflect the drugs past public perception of being a comparatively “soft option” in terms of medical and social consequences (Rafael et al., 2005). However, marijuana has also been recognized as a “gateway” drug for more severe substance misuse (Manzanares et al., 2004), which means that individuals who abuse marijuana are much more likely to be at risk for co-abusing other licit and illicit drugs (Agrawal et al., 2004). This, coupled with the fact that the endocannabinoid (eCB) system may represent a neural substrate pivotal to the regulation of core stress system adaptations (Hill and Tasker, 2012; Carvalho and Van Bockstaele, 2012; Häring et al., 2012) and hence the reinforcing effects of other substances (González-Cuevas et al., 2007; Fox and Sinha, 2009), compounds risk of poor outcome during early marijuana abstinence.
Extensive basic science and clinical studies have shown both stimulant and alcohol dependence to reflect a chronic stress state characterized by a tonic up-regulation of autonomic markers as well as extra-hypothalamic corticotropin-releasing factor (CRF) and Norepinephrine (NE) neural circuits (Wand and Dobs, 1991; Ingjaldsson et al., 2003; Thayer et al., 2006; Shively et al., 2007; Fox et al., 2008; Sinha et al., 2009). In response to stressors, including drug cues, blunted cardiovascular and Hypothalamic-Pituitary-Adrenal (HPA) axis responses are documented in alcoholics (Breese et al., 2005; Fox et al., 2007; Sinha et al., 2011), and both dampened and sensitized HPA responses have been recorded in cocaine-dependent men and women (Waldrop et al., 2010; Fox et al., 2006, Fokos and Panagis, 2010). Most importantly, these stress system adaptations are robustly associated with the negative reinforcing aspects of addiction, including sensitized anxiety and negative emotion (Fox and Sinha, 2009) as well as increased craving and relapse in dependent populations (Adinoff et al., 2005; Sinha et al., 2006, 2011; Back et al., 2010; Breese et al., 2011) and risk of dependence in vulnerable populations (Sorocco et al., 2006; Dai et al., 2007).
As widespread support exists for the role of the eCB system in regulating HPA stress system outflow (Berrendero and Maldonado, 2002; Page et al., 2007; D’Souza et al., 2009; Ranganathan et al., 2009), and stress system dysregulation is integral to addiction outcome, the precise nature of chronic marijuana use on stress system adaptations needs to be systematically defined in ecologically relevant polydrug-dependent individuals. In addition, eCB signaling within control regions of the brain including the prefrontal cortex, amygdala and hypothalamus (Hill and Tasker, 2012) suggests that chronic marijuana use may potentially impinge upon a range of regulatory behaviors associated with incentive salience and motivation (Chaperon and Thiébot, 1999; Spano et al., 2004; McGregor et al., 2005; Fattore et al., 2007). Chronic marijuana use may therefore potentially increase craving for other drugs of abuse via eCB-mediated changes within core regulatory stress systems. As such, the extent to which co-dependence on marijuana can potentially induce additional stress system neuroadaptations serving to increase relapse vulnerability for other drugs of abuse also needs to be assessed.
From a general perspective, a broad range of studies from both the clinical and experimental fields have shown marijuana to impinge upon stress system function. For example, research indicates that acute administration of cannabidiol (a major component of cannabis devoid of psychotomimetic effects) can induce anxiolytic and anti-psychotic effects, as well as reduce fear conditioning and attenuate autonomic and behavioral consequences of restraint stress (Resstel et al., 2008; Fusar-Poli et al., 2009; Gomes et al., 2011; Granjeiro et al., 2011). Conversely, high doses of exogenous eCB agonists and antagonists, including Δ9-tetrahydrocannabininol [Delta (9)-THC] and SR141716 (Rimonabant) have been shown to provoke high anxiogenic effects in humans (Zuardi et al., 1982; Gomes et al., 2011). In terms of chronic use in humans, there also exists a wealth of clinical and anecdotal reports indicating that cannabis precipitates episodes of depression, anxiety and psychosis (Rafael et al., 2005), that trauma is associated with high rates of cannabis use (Vlahov et al., 2004; Bonn-Miller et al., 2011) and further that unsuccessful quit attempts are associated with high levels of stress (Rooke et al., 2011)
In the current study, therefore, we assess the response to stress and drug cue, also known to provoke stress system circuitry (Sinha et al., 2003; Fox et al., 2007, 2008) in a group of early abstinent substance abusers who meet current dependence criteria for marijuana, with substance abusers who are not dependent on marijuana and a group of healthy control volunteers. We employ an identical paradigm to our previous studies, where we have shown that exposure to both stressful personalized guided imagery and drug cue-related imagery reliably provokes dissociable stress mechanisms across a wide range of psychobiological domains within both substance abusers and healthy controls (Fox et al., 2007; 2008; Hyman et al., 2007; Chaplin et al., 2008, 2010; Sinha et al., 2009; 2011; Bergquist et al., 2010). As chronic marijuana use may be associated with the dysregulation of core stress systems underlying the negative reinforcing effects of several drugs of abuse, we hypothesize that co-morbid marijuana dependence in poly-substance abusers will increase stress-induced craving for cocaine and alcohol as well as exacerbate subjective, cardiovascular and HPA axis changes that contribute to relapse vulnerability.
METHODS
Participants
All participants were recruited from the community via advertisements posted on the Internet and in local area newspapers. Fifty-nine co-morbid individuals who met DSM-IV criteria for current alcohol dependence and current cocaine dependence were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center for 4–6 weeks of inpatient treatment and study participation. Thirty of these individuals additionally met current dependence criteria for marijuana. Twenty-six socially drinking healthy controls (HCs) were also recruited. All were light social drinkers (25 drinks or less per month) as classified by the Cahalan Quantity Frequency Variability Index (Cahalan et al., 2012). A socially drinking group, rather than drug-naïve comparison group, was used in the current design to allow more thorough examination of the stress-related craving state in both a substance-dependent and non-dependent group. Previous findings using our current imagery paradigm have shown that both stress-related and cue-related imageries induce alcohol craving in light social drinkers (Chaplin et al., 2008; Fox et al., 2008; Sinha et al., 2009). Substance-abusing individuals who met current DSM-IV criteria for dependence on another psychoactive substance other than nicotine were excluded. Healthy controls with current or past diagnoses of any substance dependence were also excluded. All participants were excluded if they were on medications for medical or psychiatric problems. All subjects underwent a thorough medical evaluation to ensure good physical health. Study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine.
Design
A mixed repeated measures design was used. The Between Group factor was Drug Group [marijuana-dependent substance abusers (MJ), non-marijuana-dependent substance abusers (non-MJ) and HCs]. The Within Group factors were Imagery Condition (stress, drug cue and relaxing) and Time point (varying levels for each assessment).
The stress, cue and relaxing imagery conditions were presented on consecutive days with only one stimulus presentation per day. Imagery condition was assigned randomly and counterbalanced across subjects. Staff and subjects were blind to the imagery condition.
Procedures
All substance-abusing patients were admitted to the CNRU of the Connecticut Mental Health Center for study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and a limited and monitored access to visitors. Urine and breathalyzer testing is conducted every three days to ensure drug abstinence. As subjects were treatment seeking, they participated in 4 weeks of group counseling treatment for cocaine and alcohol addiction using the standard drug counseling manual as a guide (Mercer and Woody, 1992). During the first week of inpatient stay, substance-abusing participants were administered structured baseline assessments measuring psychiatric and substance use history. In the second week, scripts for the guided imagery induction were developed as described in previous studies (Sinha et al., 2003; Bergquist et al., 2010). All laboratory sessions were conducted approximately 23 days after admission to allow for normalization of neurobiological changes associated with acute cocaine and alcohol abstinence.
Healthy Control participants were admitted to the Hospital Research Unit of the Yale Clinical Center of Investigation located a block away at Yale/New Haven hospital for a 4-day stay. Within that time, they were required to remain on the hospital unit, within a similar controlled environment to that of the substance-abusing participants. They were given a similar diet, and allowed limited access to visitors and limited staff-accompanied smoke breaks. Baseline demographics, psychiatric and substance use assessments as well as imagery scripts were prepared prior to their admission to the Hospital Research Unit. All social drinking controls were exposed to an alcohol-related script for the drug cue condition.
Imagery script development (for presentation in the laboratory sessions)
In the second week, scripts for the guided imagery induction were written on the basis of methods developed by Lang and colleagues (Lang et al., 1980; Miller et al., 1987) and further adapted in our previous studies (see Sinha et al., 2003, for full details). Briefly, the stress imagery script was based on subjects’ description of a recent personal stressful event that was experienced as “most stressful” (determined by ratings on a 10-point Likert scale where 1 = “not at all stressful” and 10 = “the most stress they felt recently in their life”). Only situations rated as 8 or above were accepted as appropriate for script development. The stress imagery scripts did not include scenarios either relating to or culminating in drug use. The drug cue imagery script was developed by having subjects identify a recent situation that included alcohol-related and cocaine-related stimuli and resulted in subsequent substance use (i.e., being at a bar or watching others smoke crack and drink alcohol). Drug-related scenarios did not include scenarios that involved stressful events such as being arrested. All social drinkers were required to provide alcohol-related scripts. A relaxing imagery script was developed from the subjects’ description of a personal non-drug-related relaxing situation. All scripts were then recorded onto an audiotape to be played in the laboratory sessions. All scripts were recorded by the same female clinician, who was independent to the research study.
Habituation and imagery training session
On a day prior to the laboratory sessions, subjects were brought into the testing room to acclimatize themselves to specific aspects of the study procedures, including the stress of intravenous catheter insertion as well as the subjective rating forms and training in relaxation and imagery procedures. Details on the imagery script development procedures and the imagery and relaxation training procedures have been described previously (Sinha et al., 2003; Sinha, 2008).
Laboratory sessions
Each subject was tested in the same room for the training and three laboratory sessions. On each day of the laboratory session, subjects abstained from breakfast and were allowed a smoke break at 7:30 AM in order to reduce the effects of nicotine withdrawal. Subjects were then taken into the testing room at 8:00 AM. After settling into a sitting position in a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject’s non-preferred arm, in order to periodically obtain blood samples. A blood pressure cuff was placed on the subject’s preferred arm to monitor blood pressure, and a pulse sensor was placed on the subject’s forefinger to obtain a measure of pulse. This was followed by a 45-min adaptation period during which time subjects were asked to practice relaxation. Following the adaptation period, baseline blood was drawn, heart rate and blood pressure were taken and alcohol craving and anxiety rating scales were administered. At 9:00 AM, subjects were provided headphones and given the following instructions for the imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation. Stop imagining when you hear the voice on the tape tell you to stop imaging.” The length of each script was exactly 5 min. Heart rate and blood pressure were continuously monitored during the imagery period. All measures were collected immediately following imagery exposure and again at regular 15-min recovery intervals until 1 h after imagery. If the visual analog scale (VAS) ratings of anxiety remained above baseline levels following the final time point, they were taken through another series of relaxation procedures until their ratings returned to baseline levels. After the last assessment at 10:35 AM, the subject was disconnected from the apparatus and served breakfast.
All subjective, cardiac and blood measures were taken at baseline (−5), immediately following imagery (0 time point) and six recovery time points (+5, +10, +15, +30, +45 and +60 min after imagery).
Laboratory assessments
Subjective measures. Craving: The desire for using alcohol, cocaine and nicotine was assessed using three separate VASs anchored from 1 to 10, where 1 = “not at all” and 10 = “extremely high.”
Anxiety: Participants were required to rate how “tense, nervous or jittery” they felt using a similar 10-point VAS anchored as above.
Physiological measures: A Critikon Dinamap 120 Patient Monitor was used to assess blood pressure. A pulse sensor was attached to the subject’s finger and connected to the Dinamap Monitor to provide a continuous measure of pulse.
Blood samples (HPA markers): Twelve milliliters of blood were collected at each time point in order to assess plasma adrenocorticotrophic hormone (ACTH) and Cortisol. Blood samples were collected in heparinized tubes. All tubes were placed on ice immediately after drawing. Within 30 min of collection, all blood samples were centrifuged at 4 °C, and the plasma was pooled and aliquoted for ACTH and Cortisol assays. Blood samples for HPA axis measures were stored at −70 °C and processed at the Yale Center for Clinical Investigation Core Laboratories using standard radioimmunoassay procedures.
Statistical analysis
Linear Mixed Effect Models (Laird and Ware, 1982) were implemented to analyze the baseline and response data, using SPSS (version 19; SPSS Inc., Chicago, IL, USA). Between-subjects factor of Drug Group (MJ, non-MJ and HC) and within-subjects factors of Condition (stress, cue and relaxing) and Time point (varying levels) were the fixed effects, and Subjects was the random effect. In order to account for baseline variability across each testing day, change from baseline was used for all measures in order to assess response to the imagery exposure. Bonferroni tests were used as adjustments for all multiple comparisons. Pearson’s product moment correlational analysis and standard regression models were used for extended analysis in the MJ group, in order to ascertain relationships between marijuana use, anxiety, craving and relapse. Area under the curve response data were used for these analyses.
RESULTS
Participants
In the current sample of participants, the healthy controls were younger and spent a greater number of years in education compared with both of the substance abuse groups. Both the MJ and non-MJ substance-abusing groups were well matched in terms of drug use and demographics, with the exception of race and age. As expected, the MJ group used a significantly greater amount of marijuana in the 3 months prior to treatment entry; they were also older and comprised a higher number of African Americans compared with the non-MJ group. As such, age and race were treated as covariates for all analyses (Table 1).
Table 1.
Participant demographic and clinical characteristics (means and standard deviations are shown)
| N = 85 | Substance abusers n = 29 | Substance abusers with marijuana dependence n = 30 | Healthy controls N = 26 | p* |
|---|---|---|---|---|
| Gender no. male | 17 (58.6%) | 18 (60.0%) | 10 (40%) | NS |
| Race | >0.02 | |||
| No. Caucasian | 18 (62.1%) | 9 (30%) | 14 (56%) | |
| No. African American | 8 (27.6%) | 20 (66.7%) | 7 (28%) | |
| Other | 3 (10.3%) | 1 (3.3%) | 4 (16%) | |
| Age | 37.1 ± 6.4 | 33.7 ± 6.9 | 28.1 ± 1.4 | <0.0001 |
| Years in education | 12.4 ±0.3 | 12.3 ± 0.4 | 15.1 ± 0.4 | <0.0001 |
| Smoking status no. regular smokers (%) | 25 (86.2%) | 26 (86.7%) | 6 (24%) | <0.0001 |
| Years of cocaine use | 8.5 ± 5.4 | 8.2 ± 5.3 | 0 | <0.0001 |
| Years of alcohol use | 15.7 ±8.9 | 11.9 ±7.3 | 4.8 ± 1.2 | <0.0001 |
| Years of marijuana use | 5.3 ±13.2 | 13.2 ± 5.5 | 1.0 ± 0.6 | <0.0001 |
| No. of days used in the last month | ||||
| Cocaine | 12.4 ±12.3 | 10.1 ±11.5 | 0 | <0.0001 |
| Alcohol | 14.5 ±12.4 | 9.4 ± 11.7 | 3.8 ± 3.6 | <0.0001 |
| Marijuana | 0.6 ± 2.2 | 9.1 ± 12.7 | 0 | <0.0001 |
| Amount used in the last month | ||||
| Cocaine (grams) | 25.2 ± 23.7 | 36.3 ± 52.5 | 0 | <0.0001 |
| Alcohol (drinks) | 203.7 ±138.4 | 159.8 ± 119.4 | 16.4 ±13.5 | <0.0001 |
| Marijuana (joints) | 5.3 ± 10.5 | 96.6 ± 185.7 | 0 | <0.0001 |
| No. lifetime depression | 8 (27.6%) | 8 (26.7%) | 1 (4%) | <0.0001 |
| No. lifetime anxiety (including PTSD**) | 10 (34.5%) | 12 (40%) | 1 (4%) | <0.0001 |
| No. lifetime anxiety (without PTSD**) | 2 (6.9%) | 2 (6.7%) | 0 | <0.0001 |
Overall statistical difference between all three groups. Shaded area represents significant differences (p < 0.05) between the two substance-abusing groups only.
PTSD: Post Traumatic Stress Disorder.
Baseline findings
Subjective
Significant basal variations in Drug Group were observed with regard to Nicotine Craving [F(2, 59) = 4.9, p = 0.01, without covariates; F(2, 54) = 3.9, p <0.03, with covariates], where both the MJ group and non-MJ group reported higher ratings of nicotine craving compared with the healthy controls. Following the inclusion of covariates, only the MJ group demonstrated significantly higher basal ratings of Nicotine Craving compared with the controls (MJ dep >HC, p = 0.04; non-MJ >HC, p <0.03, without covariates; MJ dep >HC, p = 0.04; non-MJ >HC, p = ns, with covariates).
A significant main effect of Drug Group for Anxiety [F(2, 82) = 3.7, p = 0.03, without covariates; F(2, 76) = 3.5, p <0.04, with covariates] also indicated that the MJ group reported higher ratings of baseline Anxiety compared with controls (MJ dep >HC, p = 0.03, with and without covariates).
Cardiovascular
At baseline, the MJ group showed enhanced heart rate and blood pressure compared with the healthy controls. This remained a trend following the inclusion of covariates. A main effect of Drug Group was observed for basal Heart rate [F(2, 82) = 4.6, p = 0.01, without covariates; F(2, 77) = 3.1, p = 0.05, with covariates], indicating that the MJ group demonstrated higher heart rate compared with controls (p = 0.01 without covariates; p = 0.07 with covariates).
A main effect of Drug Group was also observed for basal Systolic Blood Pressure (SBP) [F(2, 82) = 3.0, p = 0.05, without covariates; F(2, 77) = 2.6, p = 0.07, with covariates] again showing a trend for higher levels of SBP in the MJ group compared with the healthy controls (p = 0.07 without covariates; p = 0.08 with covariates). A main effect of Drug Group for Diastolic Blood Pressure (DBP) [F(2, 82) = 4.4, p <0.02, without covariates; F(2, 77) = 3.1, p = 0.05, with covariates] again showed that the MJ group had increased basal DBP compared with controls (p = 0.01 without covariates; p = 0.10 with covariates).
Response to imagery
Subjective rating scales
Alcohol craving
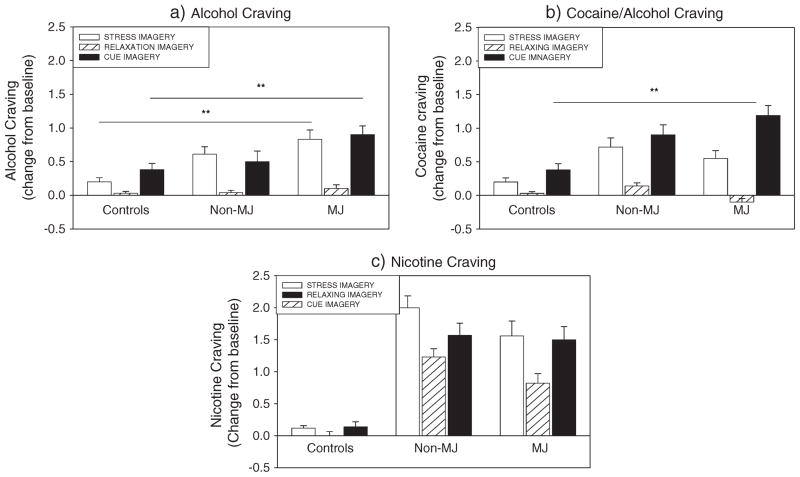
A significant Drug Group × Imagery Condition interaction was observed for Alcohol Craving [F(4, 1649) = 3.2, p = 0.01, without covariates; F(4, 1609) = 3.3, p = 0.01, with covariates], where the MJ group reported significantly higher ratings of Alcohol Craving following exposure to stress (p = 0.01 without covariates; p = 0.02 with covariates) and cue (p = 0.05 without covariates; p <0.08 with covariates) compared with the control group (Figure 1a).
Figure 1.
Bar graphs showing alcohol craving and cocaine craving between the marijuana (MJ)-dependent group, non-MJ-dependent group and controls
In addition, increased Alcohol Craving was reported following exposure to stress in both the MJ and non-MJ groups, compared with relaxing imagery (p <0.0001 in all cases with and without covariates). This stress-induced increase was not observed in the control group. All three groups reported significant cue-related craving compared with the relaxing condition (C >N: p <0.0001 in MJ and non-MJ; p = 0.01 in healthy controls with and without covariates).
Cocaine craving
A significant Drug Group Imagery Condition interaction was observed for Cocaine Craving [F(4, 1430) = 10.0, p <0.0001, without covariates; F(4, 1389) = 9.9, p <0.0001, with covariates], where the MJ group reported significantly higher ratings of cocaine craving following exposure to cue compared with the healthy controls (p <0.0001 with and without covariates).
In addition, increased Cocaine Craving was reported following exposure to stress in both the MJ and non-MJ groups, compared with relaxing imagery (p <0.0001 in all cases with and without covariates). This stress-induced increase was not observed in the control group. All three groups reported significant cue-related craving compared with the relaxing condition (C >N: p <0.0001 in MJ and non-MJ; in healthy controls: p = 0.004 without covariates; p = 0.003 with covariates) (Figure 1b)
Nicotine craving
A main effect of Drug Group [F(2, 60) = 9.9, p <0.0001, without covariates; F(2, 56) = 5.5, p = 0.006, with covariates] indicated that both the MJ group and non-MJ group reported significantly higher Nicotine Craving compared with the healthy controls (MJ >HC, p = 0.007; non-MJ >HC, p <0.0001, without covariates. MJ >HC, p <0.03; non-MJ >HC, p <0.02, with covariates).
In addition, a significant Drug Group × Imagery Condition interaction was observed [F(4, 1195) = 4.8, p = 0.001, without covariates; F(4, 1155) = 5.2, p 0.0001, with covariates], where the significantly higher Nicotine Craving was reported in the stress compared with the cue condition in the non-MJ group (p = 0.007 with and without covariates). This cue-related difference was not observed in either the MJ group or the healthy controls.
Both the MJ group and the non-MJ group reported significantly higher Nicotine Craving following exposure to stress compared with the healthy controls (MJ >HC, p = 0.002; non-MJ >HC, p <0.0001, without covariates. MJ >HC, p = 0.005; non-MJ >HC, p = 0.002, with covariates) and also following exposure to cue compared with the healthy controls (MJ >HC, p = 0.003; non-MJ >HC, p = 0.001, without covariates. MJ >HC, p = 0.01; non-MJ >HC, p = 0.03, with covariates).
The MJ group also demonstrated a stress-related and cue-related increase in Nicotine Craving compared with their intra-individual relaxing condition (p <0.0001, in all cases with and without covariates). This stress-related and cue-related increase in Nicotine Craving was also observed in the non-MJ group (p <0.0001, without covariates; p = 0.05, with covariates) but was not observed in the healthy control group.
Anxiety
A significant Drug Group × Imagery Condition × Time point interaction was observed for Anxiety [F(24, 1649) = 1.7, p = 0.02, without covariates; F(24, 1609) = 1.6, p = 0.03, with covariates]. This interaction reflects the fact that following exposure to cue the MJ group reported significantly higher ratings of Anxiety compared with the healthy control group (p = 0.001, without covariates; p = 0.002, with covariates) and the non-MJ group (p = 0.02, without covariates; p <0.03, with covariates) at the +82 recovery time point. This was also observed at the +90 time point (MJ >HC: p <0.0001; MJ >non-MJ, p = 0.002, without covariates; MJ >HC: p <0.0001; MJ >non-MJ, p = 0.004, with covariates) as well as the +105 time point (MJ >HC: p = 0.01; MJ >non-MJ, p = 0.02, without covariates; MJ >HC: p = 0.01; MJ non-MJ, p <0.04, with covariates).
Additionally, the MJ group reported significantly higher ratings of Anxiety following exposure to the cue imagery condition compared with their ratings following the intra-individual relaxing condition at the +82 time point (C >N, p <0.0001 with and without covariates). This was also the case at the +90 recovery time point (C >N, p = 0.002, without covariates; p = 0.001, with covariates) and the +105 recovery time point (C >N, p <0.04, with and without covariates). These cue-induced increases in anxiety during recovery were not observed in either the healthy controls or the non-MJ group.
Following exposure to stress imagery, the MJ group also reported significantly higher Anxiety ratings compared with the healthy control group at the +82 recovery time point (p = 0.001, without covariates; p <0.02, with covariates) (Figure 2a–c).
Figure 2.
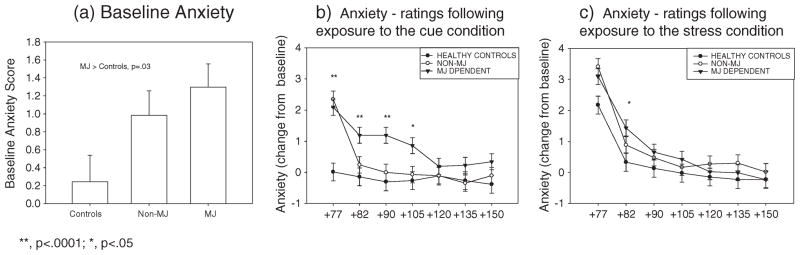
Line graphs showing anxiety ratings between the marijuana (MJ)-dependent group, non-MJ-dependent group and controls at (a) baseline, (b) following cue imagery exposure and (c) following stress imagery exposure
Cardiovascular measures
Heart rate
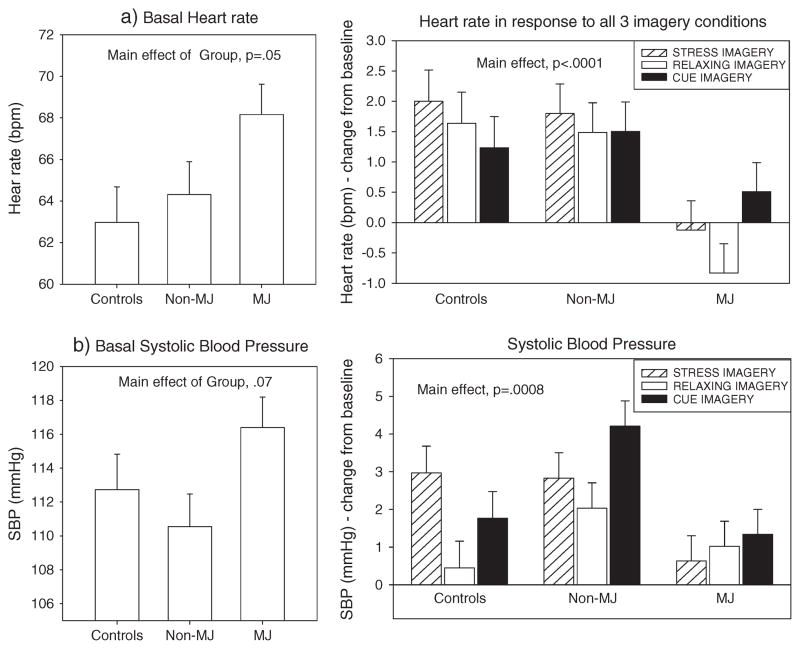
A main effect of Drug Group was observed [F(2, 82) = 5.7, p = 0.005, without covariates; F(2, 3334) = 12.0, p <0.0001, with covariates], indicating that the MJ group demonstrated a significantly decreased heart rate across all three imagery conditions compared with both the healthy controls (p <0.02 without covariates; p <0.0001 with covariates) and the non-MJ group (p = 0.01 without covariates; p <0.0001 with covariates) (Figure 3a).
Figure 3.
Bar graphs showing basal and response heart rate and blood pressure between the marijuana (MJ)-dependent group, non-MJ-dependent group and controls
Systolic blood pressure
A main effect of Drug Group was also observed for SBP [F(2, 82) = 3.3, p = 0.04, without covariates; F(2, 354) = 5.1, p = 0.006, with covariates], indicating that the MJ group demonstrated significantly lower SBP compared with the non-MJ group across all three imagery conditions (p <0.04 without covariates; p = 0.008 with covariates).
A significant Drug Group × Imagery Condition interaction was also observed [F(4, 1863) = 3.8, p = 0.004, without covariates; F(4, 474) = 1.4, p = ns, with covariates]. This indicated that the MJ group demonstrated lower SBP compared with the healthy control group following exposure to stress (p = 0.05 without covariates; p = 0.2 with covariates) and lower SBP compared with the non-MJ group, following exposure to cue (p = 0.008 without covariates; p = 0.01 with covariates) (Figure 3b).
HPA axis markers
Plasma cortisol
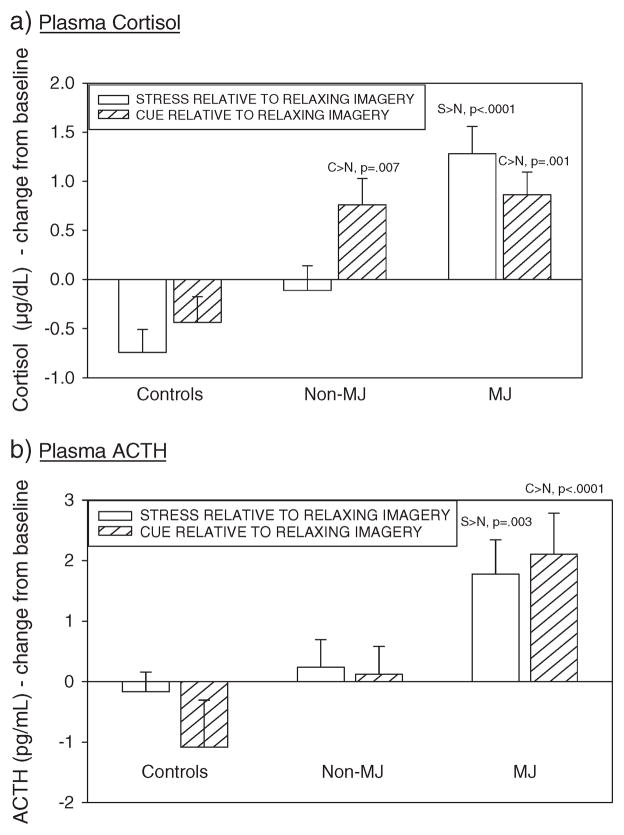
A significant Drug Group × Imagery Condition interaction was observed [F(4, 1270) = 10.0, p <0.0001, with and without covariates], indicating that the MJ group demonstrated significantly higher levels of Cortisol following exposure to the stress imagery condition compared with the intra-individual-relaxing control condition (S >N, p 0.0001, with and without covariates). This stress-induced increase was not observed in either the healthy controls or the non-MJ group. Both the MJ group and the non-MJ group demonstrated increases in cue-related Cortisol (MJ group; C >N: p = 0.001 with and without covariates; non-MJ group; C >N: p = 0.006 without covariates; p = 0.007 with covariates), which was not demonstrated in the healthy control group (Figure 4a).
Figure 4.
Bar graphs showing differences in adrenocorticotrophic hormone (ACTH) and cortisol response to stress and cue relative to the relaxing imagery condition between the marijuana (MJ)-dependent group, non-MJ-dependent group and controls
Plasma ACTH
Similarly, a significant Drug Group Imagery Condition interaction was observed [F(4, 1272) = 3.9, p = 0.003, without covariates; F(4, 1272) = 4.0, p = 0.004, with covariates], showing that the MJ group demonstrated significantly higher levels of ACTH following exposure to the stress imagery condition compared with the intra-individual relaxing control condition (S >N, p = 0.003, with and without covariates). The MJ group also demonstrated significantly higher levels of ACTH following exposure to the cue imagery condition compared with the intra-individual-relaxing control condition (C>N, p <0.0001, with and without covariates). These stress-related and cue-related increases in ACTH were not observed in either the healthy control group or the non-MJ group (Figure 4b).
Extended analysis in the MJ group. Relationship between craving, anxiety and relapse
Mean number of days to marijuana relapse in the MJ group was 40.5 ± 36.9. Extended analysis using Pearson’s product moment coefficient showed that cue-induced alcohol craving and cue-induced anxiety were both associated with the number of days to marijuana relapse following discharge from inpatient treatment (Alcohol Craving: r = −0.14, p = 0.04; Anxiety: r = −0.21, p = 0.002). The association between cue-related Cocaine Craving and days to marijuana relapse approached significance (r = −0.13, p = 0.07). Stress-induced Anxiety was also associated with the number of days to marijuana relapse (r = −0.11, p = 0.03).
Three standard regression models were subsequently conducted to assess the extent to which (i) cue-related Alcohol Craving, (ii) cue-related Anxiety and (iii) stress-related Anxiety predicted the number of days to marijuana relapse following discharge from inpatient treatment. Age and Race were included as covariates in all three models. Findings indicated that increased reports of both cue-induced Alcohol Craving and cue-induced Anxiety were predictive of a shorter number of days to marijuana relapse, accounting for 19% and 17% of the variance over the 90-day follow-up period, respectively (Alcohol craving: β = −0.19, R2 = 0.19, t = −4.1, p <0.0001; Anxiety: β = −0.15, R2 = 0.17, t = −3.2, p = 0.002). Stress-related increases in Anxiety did not predict the number of days to marijuana relapse.
Relationship between craving, anxiety and previous marijuana use
As marijuana use is often associated with a high prevalence of anxiety-related disorders (Bonn-Miller et al., 2011; Bujarski et al., 2012), a complex reciprocal relationship may exist between both in terms of their effects on craving and motivation for drug use. Although momentary assessment studies have shown that anxiety increases the negative reinforcing effects of marijuana use (Buckner et al., 2011, 2012), preclinical and clinical research has indicated that chronic marijuana use over time will serve to sensitize neural stress systems (Koob and Le Moal, 2008). We therefore conducted standard regression analyses, with marijuana use 3 months prior to treatment and baseline anxiety as predictor variables, and stress-induced and cue-induced alcohol and cocaine craving as dependent variables. Findings indicated that previous marijuana use was a significant predictor of cue-induced alcohol craving (β = 0.29, R2 = 0.17, t = 2.12, p <0.04) and cue-induced cocaine craving (β = 0.43, R2 = 0.22, t = 2.89, p = 0.007) after controlling for the main effect of baseline anxiety. Neither marijuana use nor anxiety predicted stress-related craving.
DISCUSSION
Current findings indicate that MJ-dependent substance abusers who are also co-dependent for alcohol and cocaine abuse demonstrate selective tonic and phasic stress system adaptations specific to their MJ dependence. In comparison to both well-matched substance abusers who were not dependent on cannabis and healthy socially drinking controls, MJ-dependent individuals showed higher generalized anxiety as well as an up-regulated cardiovascular basal drive and enhanced alcohol craving and cocaine craving following exposure to stress and cue. Consistent with prior research, increased stress-induced and cue-induced craving in the MJ group was also accompanied by reduced cardiovascular output, increased anxiety and enhanced HPA axis function compared with the other groups. Extended analysis also indicated that enhanced cue-related alcohol craving and anxiety were predictive of time to marijuana relapse. As such, initial findings suggest that co-morbid MJ dependence may exacerbate stress-induced and cue-induced craving for other drugs of abuse by potentially altering selective mechanisms of stress system function specific to marijuana use.
Most notably, overall findings support the existence of behavioral cross-sensitization in terms of some of the motivational processes associated with craving and relapse. For example, substance abusers who were co-morbidly dependent on marijuana additionally reported significantly higher ratings of alcohol craving following exposure to stress-related and cue-related imagery compared with both well-matched substance abusers not dependent on marijuana as well as healthy controls. They also reported significantly higher ratings of cocaine craving following exposure to cue, although no group differences were observed in terms of nicotine craving. These findings provide broad support for the possible link between the eCB receptor system and motivation to consume alcohol and cocaine (Wiskerke et al., 2008).
For example, CB-sub1 receptor stimulation by Δ8-THC and WIN 55,212-2 has been shown to dose-dependently enhance the effects of conditioned cue on re-instatement to psychostimulants (Anggadiredja et al., 2004; González-Cuevas et al., 2007). Similarly, with regard to ethanol seeking, an absence of withdrawal symptoms and reductions in cue-conditioned and stress-induced drinking following alcohol cessation have both been documented in CB1 knock-out mice (Racz et al., 2003; Soria et al., 2005). When considered together, preclinical studies such as these broadly support current findings by highlighting the association between exogenous cannabinoid changes to the eCB system and stress-induced and cue-induced motivational aspects of cocaine and alcohol seeking.
The fact that the current MJ group did not demonstrate similar increases in nicotine craving may be related to variation in consumption expectancies compared with cocaine and alcohol. Although substance-abusing participants were kept on a locked inpatient facility with no access to cocaine or alcohol, they were allowed four regular smoke breaks per day including prior to and following the laboratory study, in order to curb nicotine withdrawal-related symptoms. As consumption expectancies are known to influence cue-related craving (Marlatt et al., 1973; Berg et al., 1981; Kaplan et al., 1984), knowledge of a subsequent smoke break may have served to curb nicotine craving in the substance-abusing groups.
In this study, the potential for motivational cross-sensitization in terms of drug seeking is further highlighted by the fact that elevations in cue-related alcohol craving are also predictive of a shorter number of days to marijuana relapse. A trend was also observed for cue-related cocaine craving. This may be related to the fact that the negative reinforcing properties of cocaine and alcohol (Sinha et al., 2003, 2011; Koob and Le Moal, 2005; Fox et al., 2007; 2008) may be ameliorated by using cannabis (González-Cuevas et al., 2007). In view of this, previous human studies have shown that acute cannabis administration potentiates the positive subjective effects of both alcohol and cocaine, by altering the bioavailability of both (Perez-Reyes et al., 1988; Chait and Perry, 1994; Lukas et al., 1994). This also corroborates preclinical studies that have highlighted the anxiolytic effects of CB1 agonists following cocaine, alcohol and stress exposure (Hayase et al., 2005; Fokos and Panagis, 2010) and may provide an underlying mood-related mechanism for predicting cannabis relapse in cocaine-dependent and alcohol-dependent individuals.
In the current study, the chronic effects of marijuana following 3 weeks of abstinence were associated with significantly higher basal and phasic ratings of anxiety, which were accompanied by enhanced alcohol and cocaine craving. In particular, the sensitized anxiety response in the MJ group was compounded following exposure to cue and remained persistently elevated up until approximately 30 min post-imagery. This is concordant with previous research linking stress-related and cue-related craving to dissociable aspects of emotional distress. Although cue-induced alcohol and cocaine craving has been associated with increases in appetitive “vigilance”-related emotions, including anxiety, fear and arousal, response to stress has been more associated with negative affect such as sadness and anger (Fox et al., 2007). Furthermore, as a complex reciprocal relationship potentially exists between chronic marijuana use and anxiety disorders (Bonn-Miller et al., 2011), it may be reasonable to predict persistently elevated levels of anxiety following exposure to cue in the MJ group.
Persistently elevated cue-induced anxiety was also a significant predictor of marijuana relapse. It may be the case therefore that increased anxiety symptomatology in MJ-dependent polydrug abusers enhances the negative reinforcing effects of chronic drug use (Sinha, 2001; Fox and Sinha, 2009) and promotes a greater adaptive allostatic “shift” towards a sensitized stress system (Koob, 2004; Kalivas and Volkow, 2005; Koob and Le Moal, 2008; Sinha, 2008). This in turn may increase generalized drug and alcohol craving and relapse risk (Fox et al., 2007; Sinha, 2008; Sinha et al., 2009; 2011). Conversely, however, it is also interesting to note that baseline marijuana use was predictive of cue-related alcohol and cocaine craving even when baseline increases in anxiety were held constant. As such, understanding more fully the relative contribution of both marijuana consumption and anxiety with regard to marijuana seeking may represent an important avenue for future treatment development research.
The current MJ group also demonstrated variations in both tonic and phasic cardiovascular output compared with the other experimental groups. At baseline, however, only a trend for higher heart rate and systolic blood pressure was recorded in the MJ group following the inclusion of covariates, suggesting that some of this variance may have been accounted for by age and race. This is consistent with research indicating that both age and African American heritage are risk factors for hypertension (Kaplan, 1994). Although baseline findings are consistent with the acute effects of marijuana (Hollister, 1988; Vandrey et al., 2011), only a few studies to date have assessed the cardiovascular effects of withdrawal in cannabis-dependent individuals. These studies suggest that a general up-regulation of basal blood pressure and heart rate may be observed following 48 h of abrupt cannabis cessation as a possible rebound effect following tolerance to the repeated acute effects (Jones et al., 1981; Jones, 2002; Vandrey et al., 2008, 2011). As such, our findings hold some support for this; however, it is important to note that the basal up-regulation observed in the current MJ group may not be of clinical significance as blood pressure was not within the standard hypertensive range (>140 mm/Hg for SBP and >90 mm/Hg for DBP).
Although basal up-regulation of heart rate and blood pressure in the MJ group may not signify clinical hypertension following 4 weeks of abstinence, it may still be a salient factor contributing to the dampened phasic response observed following exposure to stress-related and cue-related stimuli (Sinha et al., 2009), potentially reflecting a ceiling level of physiological response undermining the ability to respond effectively to stress or cue. For example, the MJ group was unable to mount an elevated SBP response appropriate for exposure to stress, exposure to cue and sensitized levels of anxiety. They also demonstrated a generalized down-regulation of heart rate and blood pressure across all three imagery conditions compared with the non-MJ-abusing polydrug group and the healthy controls. Most importantly, the inability to demonstrate an appropriate bio-physiological engagement to stress has been associated with relapse factors in alcoholics (Adinoff et al., 2005; Junghanns et al., 2005; Fox et al., 2007; Sinha et al., 2009, 2011) and co-morbid alcohol-dependent and cocaine-dependent men (Fox et al., 2009) as well as risk of dependence in vulnerable populations (Zimmerman et al., 2004; Sorocco et al., 2006). As this suppressed response to stress and cue was not observed in the non-MJ-dependent group, it may reflect an additional risk factor for craving, associated selectively with the co-morbid actions of marijuana on vascular function.
Current findings also showed that the MJ group demonstrated significant elevations in ACTH after stress and cue exposure, relative to their own baseline levels, as well as stress-induced elevations of cortisol that were not observed in either the non-MJ-dependent substance abusers or the healthy controls. Very broadly, this is in keeping with extensive preclinical research that has examined the role of eCB signaling in stress system regulation where the administration of exogenous cannabinoids including SR141716 (Rimonabant) and THC dose-dependently activate the HPA axis by stimulating CRH, ACTH and corticosterone secretion (Manzanares et al., 1999; Brown and Dobs, 2002; Patel et al., 2004; Pagotto et al., 2006; Wade et al., 2006; Steiner et al., 2008).
Although these studies represent acute paradigms, some research in both animals and humans have also shown that tolerance develops quickly, culminating in a blunting of cortisol in response to subsequent intravenous THC exposure (Murphy et al., 1998; Pagotto et al., 2006; D’Souza et al., 2008; Ranganathan et al., 2009). As such, the elevations in stress-related and cue-related ACTH and cortisol observed in the MJ group may reflect a “rebound” up-regulation mechanism following marijuana cessation. Of the few studies that have examined HPA axis changes following cannabis cessation, stress paradigms have not been incorporated. Although one study documented a 2.5-fold increase in CRH and a 1/3 increase in corticosterone in rodents following antagonist-elicited cannabis withdrawal (de Fonseca et al., 1997), this was not replicated in a similar recent human study (Goodwin et al., 2012). Similarly, an early human study also found no significant change in cortisol concentrations among 30 healthy male cannabis smokers after 6 days of abstinence (Cohen, 1976). These human studies may hold some support for the unchanged basal findings in the present study, however; again, future research is warranted in order to fully elucidate cannabis-related adaptations to HPA axis function in polydrug-dependent individuals.
Interpretation of current findings is restricted by the fact that subjective craving for marijuana was not assessed in the current sample of MJ-dependent individuals. As such, it is difficult to ascertain completely the true extent of cross-sensitization with regard to motivation for drug seeking, particularly in terms of assessing the role of marijuana craving on compulsive alcohol and cocaine seeking. Additionally, although relapse-related subjective and bio-physiological stress system adaptations were observed to a greater extent in the MJ-dependent group, compared with the other experimental groups, the lack of MJ-craving ratings makes it challenging to attribute these adaptations directly to motivation for marijuana use. Despite this, findings do indicate that a higher frequency of marijuana use in the 3 months prior to inpatient treatment is predictive of greater cue-induced anxiety, alcohol craving and cocaine craving in the MJ-dependent group. Although this may potentially be attributable to greater overall substance use in the MJ group, it is important to consider that both substance-abusing groups were statistically matched on alcohol and cocaine use. Interpretation of findings are also limited to a certain extent by the fact that the healthy group did not reflect a population of regular smokers and, as such, may not have provided an optimal control group for measuring nicotine craving. However, this does not detract from the fact that there were no significant variations in both stress-induced and cue-induced nicotine craving between the two dependent groups.
Additionally, this is one of the first studies to show that MJ-dependent polydrug users may demonstrate selective tonic and phasic subjective, cardiovascular and HPA stress system adaptations during early abstinence that may be specific to their marijuana use and associated with risk in a range of drug-abusing populations. Most notably, in the current study, these adaptations included a higher level of cue-induced anxiety and craving for alcohol, both of which were also significantly predictive of time to marijuana relapse. Increases in cue-related cocaine craving approached statistical significance. As such, findings support the need to examine motivational cross-sensitization much more thoroughly as a contributing factor to overall relapse vulnerability in polydrug-dependent individuals
Acknowledgments
We would like to thank the staff at the Clinical Neuroscience Research Unit, the Substance Abuse Center at the Connecticut Mental Health Center and the Yale Center for Clinical Research for their assistance in completing this study.
This study was supported in part by grants K01-DA029040 (Fox), R01-DA11077 (Sinha), P50-DA16556 (Sinha), R0I-AA13892 (Sinha), K02-DA17232 (Sinha) and P50-DA16556 (Sinha) from the National Institutes of Health, Bethesda, MD, USA.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34(7):1227–37. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29 (8):1470–8. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106(1):21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JC, Laberg A, Skutle A, Ohman A. Instructed versus pharmacological effects of alcohol in alcoholics and social drinkers. Behav Res Ther. 1981;19:55–66. doi: 10.1016/0005-7967(81)90112-1. [DOI] [PubMed] [Google Scholar]
- Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue related experiences in cocaine and alcohol dependent individuals. Exp Clin Psychopharmacol. 2010;18(3):229–37. doi: 10.1037/a0019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163(1):111–7. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25(3):485–91. doi: 10.1037/a0021945. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29(2):185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129(2):149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Dobs AS. Endocrine effects of marijuana. J Clin Pharmacol. 2002;42 (11 Suppl):90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Smits JA, et al. Anxiety sensitivity and marijuana use: an analysis from ecological momentary assessment. Depress Anxiety. 2011;28(5):420–426. doi: 10.1002/da.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Crosby RD, Wonderlich SA, Schmidt NB. Social anxiety and cannabis use: an analysis from ecological momentary assessment. J Anxiety Disord. 2012;26(2):297–304. doi: 10.1016/j.janxdis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski SJ, Norberg MM, Copeland J. The association between distress tolerance and cannabis use-related problems: the mediating and moderating roles of coping motives and gender. Addict Behav. 2012;37(10):1181–1184. doi: 10.1016/j.addbeh.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. American drinking practices. Rutgers Center for Alcohol Studies; New Brunswick, NJ: 1969. Monograph No. 6. [Google Scholar]
- Carvalho AF, Van Bockstaele EJ. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(1):59–67. doi: 10.1016/j.pnpbp.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Perry JL. Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994;115(3):340–9. doi: 10.1007/BF02245075. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiébot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13(3):243–81. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–50. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Fox HC, Siedlarz KM, Bergquist K, Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum Psychopharmacol. 2010;25(5):368–76. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. The 94-day cannabis study. Ann N Y Acad Sci. 1976;282:211–220. doi: 10.1111/j.1749-6632.1976.tb49900.x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32(3):293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology (Berl) 2009;202(4):569–78. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Endocannabinoid regulation of relapse mechanisms. Pharmacol Res. 2007;56(5):418–27. doi: 10.1016/j.phrs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Fokos S, Panagis G. Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol. 2010;24(5):767–77. doi: 10.1177/0269881109104904. [DOI] [PubMed] [Google Scholar]
- de Fonseca FR, Carrera MRA, Navarro M, Koob GF, Weiss F. Activation of corticotropin releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17(2):103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 2006;185(3):348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong K-I, Siedlarz KM, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33(4):796–80. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, et al. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcsm. 2009;44(6):575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 2011;213(2–3):465–73. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- González-Cuevas G, Aujla H, Martin-Fardon R, López-Moreno JA, Navarro M, Weiss F. Subchronic cannabinoid agonist (WIN 55,212-2) treatment during cocaine abstinence alters subsequent cocaine seeking behavior. Neuropsychopharmacology. 2007;32(11):2260–6. doi: 10.1038/sj.npp.1301365. [DOI] [PubMed] [Google Scholar]
- Goodwin RS, Baumann MH, Gorelick DA, et al. CB1 — cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. Am J Drug Alcohol Abuse. 2012;38(1):114–119. doi: 10.3109/00952990.2011.600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granjeiro EM, Gomes FV, Guimarães FS, Corrêa FM, Resstel LB. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol Biochem Behav. 2011;99(4):743–8. doi: 10.1016/j.pbb.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Häring M, Guggenhuber S, Lutz B. Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience. 2012;204:145–158. doi: 10.1016/j.neuroscience.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behav Pharmacol. 2005;16 (5–6):395–404. doi: 10.1097/00008877-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister LE. Cannabis—1988. Acta Psychiatr Scand Suppl. 1988;345:108–18. doi: 10.1111/j.1600-0447.1988.tb08576.x. Review. [DOI] [PubMed] [Google Scholar]
- Hyman S, Fox HC, Sinha R. Stress and drug-cue induced craving in opioid dependent individuals on naltrexone. Exp Clin Psychopharmacol. 2007;15 (2):134–43. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54(12):1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42 (11 Suppl):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–5. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. Review. [DOI] [PubMed] [Google Scholar]
- Kaplan NM. Ethnic aspects of hypertension. Lancet. 1994;344:450–452. doi: 10.1016/s0140-6736(94)91774-4. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Meyer RE, Virgilio LM. Physiological reactivity to alcohol cues and the awareness of an alcohol effect in a double-blind placebo design. Br J Addict. 1984;79:439–442. doi: 10.1111/j.1360-0443.1984.tb03893.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Allostatic view of motivation: implications for psychopathology. Nebr Symp Motiv. 2004;50:1–18. Review. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Kouri E, Fukuzako H, Mendelson JH. Marihuana smoking increases plasma cocaine levels and subjective reports of euphoria in male volunteers. Pharmacol Biochem Behav. 1994;48(3):715–21. doi: 10.1016/0091-3057(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Fuentes JA. Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta(9)-tetrahydrocannabinol in rats. Brain Res. 1999;839(1):173–179. doi: 10.1016/s0006-8993(99)01756-4. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Urigüen L, Rubio G, Palomo T. Role of endocannabinoid system in mental diseases. Neurotox Res. 2004;6(3):213–24. doi: 10.1007/BF03033223. Review. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: an experimental analogue. J Abnorm Psychol. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Dam KD, Mallet PE, Gallate JE. Delta9-THC reinstates beer- and sucrose-seeking behaviour in abstinent rats: comparison with mid-azolam, food deprivation and predator odour. Alcohol Alcohol. 2005;40(1):35–45. doi: 10.1093/alcalc/agh113. [DOI] [PubMed] [Google Scholar]
- Mercer D, Woody G. Addiction counseling. Unpublished manuscript. University of Pennsylvania /VAMC Center for Studies of Addiction; 1992. [Google Scholar]
- Miller G, Levin DN, Kozak MJ, Cook EW, McLean A, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cogn Emotion. 1987;1:367–390. [Google Scholar]
- Murphy LL, Muñoz RM, Adrian BA, Villanúa MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5(6 Pt B):432–46. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86(1):162–8. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. Review. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145(12):5431–8. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Hicks RE, Bumberry J, Jeffcoat AR, Cook CE. Interaction between marihuana and ethanol: effects on psychomotor performance. Alcohol Clin Exp Res. 1988;12(2):268–76. doi: 10.1111/j.1530-0277.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23(6):2453–8. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Braley G, Pittman B, et al. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203(4):737–44. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael B, Wooding S, Stevens G, Connor J. Comorbidity: cannabis and complexity. J Psychiatr Pract. 2005;11(3):161–76. doi: 10.1097/00131746-200505000-00004. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Corrêa FM, Guimarães FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154(3):869–76. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Norberg MM, Copeland J. Successful and unsuccessful cannabis quitters: comparing group characteristics and quitting strategies. Subst Abuse Treat Prev Policy. 2011;6:30. doi: 10.1186/1747-597X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Mietus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates (Macaca fascicularis) Psychopharmacology(Berl) 2007;192(2):183–191. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwagar Z, Siedlatz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Siedlarz KA, Bergquist KT, Kreek MJ. Stress and cue-induced alcohol craving, anxiety and adrenal sensitivity are predictive of alcohol relapse outcomes. Arch Gen Psychiatry. 2011;68(9):942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizábal V, Touriño C, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30 (9):1670–80. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59(3):210–7. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol. 2004;143(3):343–50. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33 (8):1165–70. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-41, HHS Publication No (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, 3rd, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J of Psychophysiol. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Umbricht A, Strain EC. Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med. 2011;5(1):16–20. doi: 10.1097/ADM.0b013e3181d2b309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Ahern J, Resnick H, Kilpatrick D. Sustained increased consumption of cigarettes, alcohol, and marijuana among Manhattan residents after September 11, 2001. Am J Public Health. 2004;94(2):253–4. doi: 10.2105/ajph.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MR, Degroot A, Nomikos GG. Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharmacol. 2006;551(1–3):162–7. doi: 10.1016/j.ejphar.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, et al. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010;35(6):798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitaryadrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13(2):225–38. doi: 10.1111/j.1369-1600.2008.00109.x. Review. [DOI] [PubMed] [Google Scholar]
- Zimmerman U, Spring K, Koller G, Holsboer F, Soyka M. Hypothalamic-pituitary-adrenal system regulation in recently detoxified alcoholics is not altered by one week of treatment with acamprosate. Pharmacopsychiatry. 2004;37:98–102. doi: 10.1055/s-2004-818986. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76(3):245–50. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]