Abstract
Three experiments explored attention to eye gaze, which is incompletely understood in typical development and is hypothesized to be disrupted in autism. Experiment 1 (n=26 typical adults) involved covert orienting to box, arrow, and gaze cues at two probabilities and cue-target times to test whether reorienting for gaze is endogenous, exogenous, or unique; experiment 2 (total n=80: male and female children and adults) studied age and sex effects on gaze cueing. Gaze cueing appears endogenous and may strengthen in typical development. Experiment 3 tested exogenous, endogenous, and/or gaze-based orienting in 25 typical and 27 Autistic Spectrum Disorder (ASD) children. ASD children made more saccades, slowing their reaction times; however, exogenous and endogenous orienting, including gaze cueing, appear intact in ASD.
Keywords: gaze, box, arrow, vision, oculomotor, child
Autistic Spectrum Disorders (ASDs) have an estimated prevalence >1/100 (Kogan, Blumberg, Schieve, Boyle, Perrin, Ghandour, Singh, Strickland, Tevathan, & van Dyck, 2009), making autism a major public health problem. Early recognition is a vital endeavor since early intervention improves outcome (Howlin, Magiati, & Charman, 2009; Reichow & Wolery, 2009). In part, autism is characterized by abnormalities in eye gaze (American Psychiatric Association, 2000; Baron-Cohen, Cox, Baird, Swettenham, Nightingale, Morgan, Drew, & Charman, 1996; Klin, Jones, Schultz, Volkmar, & Cohen, 2002), and failure to orient attention to others' gaze is an attractive hypothesis about autism's causes. There is a well-characterized evolution in the use of gaze over the first five years in typical development (Emery, 2000; Itier & Batty, 2009) that parallels or scaffolds the emergence of other social cognitive abilities, including language and theory of mind. Disruption of this sequence in infancy could trigger a series of further developmental consequences that contribute to the autistic phenotype. For example, a failure to orient to others' gaze could seriously impact the normative development of joint attention and could affect language acquisition (Morales, Mundy, Delgado, Yale, Messinger, Neal, & Schwartz, 2000) by dissociating named objects from a parent's referential intent (because the child would not orient to the object where his/her parent gazed while speaking). Impaired orienting to others' gaze shifts in early childhood could, therefore, contribute to the ontogeny of the social and communicative impairments that are characteristic of autism. A better understanding of attentional orienting, as it is directed by gaze, may then lead to the improvement of screening and diagnosis of autism. A definitive demonstration of abnormal visual attentional orienting to gaze in older autistic children would, therefore, direct more efforts to the study of these processes in infants and toddlers (where such experimentation is significantly more technically difficult (Chawarska, Klin, & Volkmar 2003)); however, clear evidence to the contrary should direct investigators to the study of other processes.
The basic cognitive processes involved in attentional orienting to simple cues have been studied extensively, beginning with and building upon the work of Posner and colleagues (Posner, 1980; Posner & Petersen, 1990; Corbetta, Patel, & Shulman, 2008). Posner's covert orienting of visual attention task allowed investigators to isolate the operation of visual attention from the execution of eye movements (important because attention and oculomotor control systems share neural resources) and to define several key sets of processes: exogenous orienting, endogenous orienting, and inhibition of return (IOR). Exogenous orienting is thought to “automatically” move attention rapidly (within 150 ms) to the location of a visual cue in the periphery. Endogenous orienting “voluntarily” redirects attention to a place where something is expected to occur. IOR (Posner, Rafal, Choate, & Vaughan, 1985) evolves slowly (around 800 ms) and suppresses the return of attention to a previously attended location (all reviewed in: (Vecera & Rizzo, 2003)).
In the late 1990's, researchers realized that these processes might come into play when a shift in one's attention is driven not by a simple cue, but by shifts in another's gaze. These researchers, then, modified the Posner Task (see methods) to study whether attention to gaze is endogenous, exogenous, or some hybrid or unique variant, to characterize the time-course for attentional redirection by gaze, and to decide whether IOR is induced. These studies suggested that gaze cueing occurs rapidly and automatically, but is not typically followed by IOR and was related to central, not peripheral, attention cues (Driver, Davis, Ricciardelli, Kidd, Maxwell, & Baron-Cohen, 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999).
Because of observed differences in autistic gaze patterns, these approaches were then extended to autism research (comprehensive reviews (Frischen, Bayliss, & Tipper, 2007; Itier & Batty, 2009; Nation & Penny, 2008)), asking: If individuals with ASDs use gaze abnormally, is it because attention to gaze is compromised? If so, is there a specific problem with any/all of the key component processes described above when another's gaze is the attention cue (versus a non-social stimulus, such as a box or an arrow)? Foundational work has, unfortunately, generated conflicting findings (Bayliss & Tipper, 2005; Chawarska et al., 2003; Goldberg, Mostow, Vecera, Larson, Mostofsky, Mahone, & Denckla, 2007; Kylliainen & Hietanen, 2004; Ristic, Mottron, Friesen, Iarroci, Burack, & Kingstone, 2005; Senju, Tojo, Dairoku, & Hasegawa, 2004; Swettenham, Condie, Campbell, Milne, & Coleman, 2003; Vlamings, Stauder, van Son, & Mottron, 2005), possibly due to methodological differences across studies (carefully reviewed in (Nation & Penny, 2008): see Table 1 in that paper), as well as an incomplete understanding of the complex, interacting processes involved in attention to gaze.
Table 1.
Demographics for Experiments 1, 2, & 3
| Exp. 1 | Exp. 2 | Exp. 3 Total | Exp. 3 ASD | Exp. 3 Typical | ||
|---|---|---|---|---|---|---|
| Age | 9 – 12 Years | 0 | 40 | 52 | 27 | 25 |
| 18 – 30 Years | 26 | 40 | 0 | 0 | 0 | |
| Gender | Male | 14 | 40 | 42 | 22 | 20 |
| Female | 12 | 40 | 10 | 5 | 5 | |
| Psychotropic Medications | Yes | 0 | 0 | 14 | 14 | 0 |
| No | 26 | 80 | 38 | 13 | 25 | |
| Vision | Normal | 11 | 54 | 41 | 23 | 18 |
| Corrected-Normal | 15 | 26 | 11 | 4 | 7 | |
| Handedness (self-report) | Right | 24 | 67 | 40 | 22 | 18 |
| Left | 2 | 12 | 8 | 3 | 5 | |
| Ambidextrous | 0 | 0 | 1 | 0 | 1 | |
| Not Reported | 0 | 1 | 3 | 2 | 1 |
As the mixed set of findings in the autism gaze cueing literature has been subject to recent scholarly analysis, we refer to the above-cited reviews but highlight several specific examples for purposes of illustration: Swettenham et al. (2003) and Kylliäinen & Hietanen (2004) found no difference between gaze cueing effects across autism and typical development. Goldberg et al. (2007) found impaired orienting to gaze in high-functioning autism. We note that studies using both static (e.g., Senju et al., 2004; Kylliainen & Hietanen, 2004) and dynamic (e.g., Swettenham et al., 2003; Vlamings et al., 2005) gaze cueing stimuli have elicited positive gaze cueing effects in ASD, and Rutherford and Krysko (2008) subsequently demonstrated that terminal eye position, not the direction of stimulus motion, is a key factor. Studies using actual face photos have tended to produce gaze cueing effects in ASD, while those employing schematic stimuli have not (Ristic et al., 2005; Goldberg et al. 2007), a seemingly counter-intuitive pattern, as one might reason that deficiencies in ASD would be more likely to manifest with actual faces. Our findings, below, will show that the stimulus itself (photo versus schematic) does not explain discrepant results in the literature. Ristic et al. (2005) found that high functioning autistic subjects did not orient to non-predictive gaze but did orient to predictive gaze, suggesting automatic but not voluntary orienting to gaze is impaired in autism, and highlighting the, sometimes overlooked, important distinction to be made about the different processes involved in covert orienting under non-predictive (automatic and reflexive processes) versus predictive (endogenous/voluntary processes) cueing conditions. For example, one might be able to compensate for a defective automatic orienting process (which could be crucial for understanding the cognitive contributors to a given phenotype) by virtue of possessing an intact voluntary orienting process based on sensitivity to the statistical regularity of events in the environment. This distinction was our prime motivation for the two cueing probabilities used in our studies.
As a whole, autism gaze cueing studies typically have not included a full battery of control conditions, have not varied cueing probabilities (exception, see (Ristic et al., 2005)), and have lacked rigorous control over eye position. Failure to account for eye position could be a significant contributor to the inconsistent pattern of findings in the literature. If, for example, gaze cueing is intact but weak in ASD, anything that adds noise to the data could obscure it, leading to variant findings. Eye movements would do exactly that, adding time needed to execute the eye movement to the reaction time for a manual response. This paper addresses these issues with three Posner Task experiments, all utilizing rigorous eye position monitoring: Two experiments in typically developing subjects explore important aspects of gaze cueing (in general) that may help us interpret findings in ASD, and a third experiment tests for differences in gaze cueing and attentional orienting to non-social stimuli in ASD and typical children.
First, does gaze cueing utilize brain mechanisms involved in general endogenous (voluntary) orienting, exogenous (automatic, externally-driven) orienting, or some hybrid or unique mechanism? If a unique mechanism were involved, it would be easier to think about how gaze cueing could be disrupted in ASD without impairment to endogenous and exogenous orienting to non-social stimuli. If a general mechanism is employed (only with a different input stimulus: gaze), then exogenous and/or endogenous orienting should be disrupted if gaze cueing is (provided the basic ability to detect and discriminate gaze is intact). Experiment 1 contrasted attentional orienting to gaze with (classical) exogenous and endogenous orienting and IOR by studying typical adults with gaze, box, and arrow attention cues at two cueing probabilities and two cue-target times, in a fully within-subject design.
Second, are there sex and age differences in the strength of orienting to another's gaze shift? Since autism is a disorder with 80% male prevalence (Fombonne, 2005; Kogan et al., 2009), significantly weaker gaze-based orienting in typically developing males (as found in (Bayliss, di Pellegrino, & Tipper, 2005)) could therefore complicate ASD-typical comparisons. Experiment 2 studied the effects of age and sex on attentional orienting for gaze in typically developing subjects. We designed experiment 2 to run a larger number of subjects to provide more rapid answers to contextualize findings from experiments 1 and 3. Beyond the simple question of the magnitude of gaze cueing effects, it is not known how age and sex might interact together with the timecourse of attentional reorienting for gaze. Non-predictive gaze cueing, alone, at three cue-target timing intervals with catch trials, was employed to conduct a more focused analysis of the potential effects of age and sex on the strength and time-course of automatic (also termed “reflexive,” see below) orienting for gaze shifts. Increasingly stronger gaze cueing effects with age might reflect the maturation of specific attentional processes with development and/or the result of early voluntary orienting processes that becomes more “automatic” through one's experience with others' gaze. Explicitly testing general maturation versus over-learning goes beyond the scope of the reported studies; testing other cue conditions and probabilities with the sample size necessary for the particular between-within subjects design used in experiment 2 was, likewise, not pragmatic.
Finally, is there, or is there not a difference in attentional reorienting for a shift in another's gaze in children with ASD? If so, is a gaze cueing deficit in ASD particular to “automatic” orienting and accompanied by deficit(s) in exogenous and/or endogenous orienting to non-social stimuli? Our within-subject manipulation of cue probability, cue type (trial-wise, pseudorandom presentation), and cue-target timing, in a relatively large study sample with strict eye position monitoring, will provide a critical look at these unanswered questions. Experiment 3 compared 9–12 year-old ASD and typical children on the same paradigm used for experiment 1. Box and arrow cues provided within-subject, peripheral and central, non-social controls for this experiment, and the full factorial design allowed for tests of potential disruption to exogenous, endogenous, and gazed-based orienting and IOR in ASD children. Critically, the probability manipulation allowed for analyses of potential differences in automatic versus voluntary gaze-based orienting processes, since as above, one recent study found disrupted orienting to gaze under non-predictive (but not predictive) cueing conditions in ASD (Ristic et al., 2005).
Methods and Materials
General Methods
Subjects
Recruitment and studies were performed according to approved protocols. 271 participants for the three experiments were acquired through a variety of means (see acknowledgements). Pre-screening involved a brief medical (and medication) history, pedigree, and demographics. Subjects were screened out for any history of focal neurological deficit, strabismus, or vision that would not correct to normal acuity with glasses. Typical subjects had no first-degree relatives with an ASD or Attention-Deficit/Hyperactivity Disorder (ADHD) diagnosis.
Assessments
Final participants included 31 typical adults for experiment 1, 40 typical adults and 40 typical children for experiment 2, and 31 typical children and 28 high-functioning ASD children for experiment 3. For experiment 2 subjects were simply screened, as above. Subjects for experiments 1 and 3 were assessed with: vocabulary (VOC) and block design (BD) subscales from the WISC-IV (Wechsler, 2003) or WAIS-III (adults), The Strengths and Weaknesses of ADHD symptoms and Normal behavior scale (SWAN: (Cornish, Manly, Savage, Swanson, Morisano, Butler, Grant, Cross, Bentley, Hollis 2005, )), the Social Responsiveness Scale (SRS: (Constantino, 2002)), Handedness Inventory (Oldfield, 1971), the Child Behavior Checklist (CBCL: (Achenbach, 2001)) or Adult Behavior Checklist (Achenbach, 2003), and a brief neurological exam (performed by the 1st author). Typical subjects were required to have all CBCL/ABCL t-scores <= 60. ASD participants had 1) community MD or PhD clinical diagnoses of Autistic Disorder, Asperger's Disorder, or Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS), 2) research ASD diagnoses as determined in our study with the Autism Diagnostic Observation Schedule (ADOS: (Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles, Rutter, 2000)) and/or Autism Diagnostic Interview Revised (ADI-R: (Lord, Rutter, & Le Couteur, 1994)), and 3) author (child psychiatrists JRP and/or JNC, who reviewed ADOS videos) agreement about diagnosis. Reaction time analyses (below) are based on subsets of these subjects determined by the quality of eye fixation and the need to match the subjects for experiment 3 on VOC and BD. Summaries of the final participants' characteristics and assessments can be seen in Tables 1–4.
Table 4.
Additional Clinical Information for ASD Participants in Experiment 3

|
We were unable to obtain the ADOS on 1 ASD participant, reflected in this table. However, this participant was positive on the ADI-R, has a medical diagnosis of Autistic Disorder, and met DSM-IV Autistic Disorder criteria by expert consensus (authors JRP, JNC, and team).
General Procedures
Our Posner Task variants ran in PsyScope (Carnegie Mellon University, Department of Psychology) on a Macintosh (Apple Computer, Inc.) using the PsyScope Button Box (New Micros, Inc.; Dallas, TX: 1 ms timing resolution). We monitored horizontal eye position (1–2 degrees resolution) with EOG (Powerlab: ADInstruments; Colorado Springs, CO): see Supplementary Figure S1.
Subjects faced a monitor in a dark room and were instructed: “In the following task, please press the green button ONE time as fast as you can but as accurately as you can when you see the small CIRCLE on the left or the right. Keep your eyes on the picture in the center of the screen at all times. These and other pictures may or may not tell where the CIRCLE will appear on each trial. Press the green button to continue.” A modified chin/head rest (Headspot, Tall Option: University of Houston College of Optometry) ensured head position.
We measured reaction time (RT) during this target detection task. Therefore, condition median RT was the dependent measure. A different cue (box, gaze, or arrow) preceded the target on each trial. Cues either indicated the target position (valid) or the other side (invalid) (Figure 1). Experiment-specific designs are described below. The response hand was based on participant hand dominance. Night vision video provided quality assurance during sessions.
Figure 1.
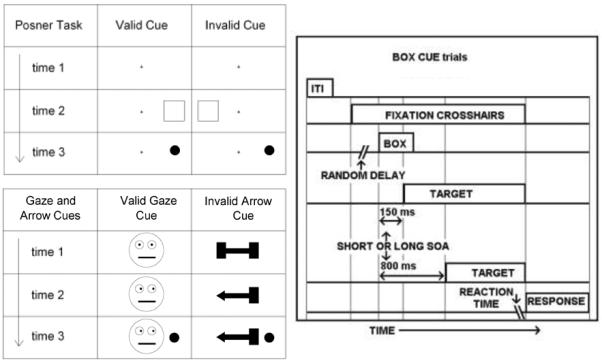
(upper left) In the Posner Task (COVAT) a subject fixates the crosshairs at time 1 and subsequent times. A cue box flashes at time 2. The target appears at time 3 – at the location of the box in the valid condition – or on the other side in the invalid condition. (lower left) This panel shows sequences for valid gaze cueing and invalid arrow cueing trials, respectively (stimuli not to scale). A button press indicates detection of the target, and reaction time is measured. (right) This task schematic (not to scale) illustrates the relative timing of events on box cue trials. ITI = inter-trial interval = 1100 ms. Random Delay = 1000, 1500, or 2000 ms. The box cue lasted 250 ms. Arrow and gaze trials followed an identical sequence, only these cues remained displayed until the button press response. This is because masking or turning off the central cue after its onset would create a luminance transient that would exogenously reorient attention back to the center after it was endogenously and/or reflexively oriented to the periphery by onset of the central cue. Experiment 2 used a gaze cue only, and SOAs were 150, 350, and 550 ms, with 12.5% of all trials containing no targets (catch trials).
Stimuli
The sizes of the stimuli were: box = 4.5 degrees wide × 4.9 degrees high × 0.15 degrees thick; arrow = 1.4 degrees wide; face = 1.8 degrees wide; and target (circle) = 0.2 degrees wide. The target and the center of the box were 6.7 degrees to the left or right of the fixation cross. These stimuli were grey scale on a black background. For all three experiments, facial expression was neutral for the gaze cue, and the target remained on until subject response.
Analyses
A custom program aligned and coded RT and EOG data. We explored relationships between RT, task variables, and subject factors with SAS 9.0 (SAS Institute Inc.; Cary NC) and SPSS 13.0 and 16.0 (SPSS, Inc.).
Key conditions of interest
The three experiments involve multifactorial designs, but certain conditions were also particular targets of analysis. 50% valid box cueing at a short (150 ms) cue-target onset time (stimulus onset asynchrony: SOA) represents the classical condition for isolation of exogenous orienting effects (i.e., stimulus-driven, automatic orienting effects). Similarly, reflexive orienting is a term that has been used for RT speeding at short (150 ms) SOAs for valid, non-predictive (i.e., 50% valid), central (arrow or gaze) cues (Ristic, Friesen, & Kingstone, 2002). Here, there is no physical cue at the subsequent target location, but there is also no expectancy to serve as an endogenous orienting cue. Endogenous orienting is typically seen with arrow cues at 80% validity (late and sometimes early SOAs). A key characteristic of endogenous orienting is the lack of a physical cue at the location of the subsequent target. The expectancy generated by the probabilistic nature (e.g., 80% valid) of the central cue is, itself, the endogenous cue. IOR is best seen with 50% valid box cueing at a late (800 ms) SOA. Other combinations of the independent variables produce RT response patterns that reflect the sum or interaction of the above component processes.
Experiment 1: Typical adults (~50% female); box, arrow, gaze cueing; 2 probabilities and 2 cue-target times
Methods
Subjects performed six alternating (counterbalanced) blocks of 50% and 80% valid cueing. Subjects were not told about the cueing probabilities. SOAs (cue-target onset times) were 150 or 800 ms. There were 96 trials in each 50% valid block and 120 trials in each 80% valid block (648 trials per subject in two 30-minute sessions on two different days with breaks). Other variables (factorial cross of: cue, SOA, validity) were presented pseudo-randomly. Stimulus characteristics and timing variables (see Figure 1) were selected from the literature (especially: (Collie, Maruff, Yucel, Danckert, & Currie, 2000; Maruff, Yucel, Danckert, Stuart, & Currie, 1999)), piloting, and expert consultation (Maruff, personal communication).
Results
Of 31 adults who completed this experiment, 26 fixated (i.e., kept their eyes on the center of the screen for the duration of the trial) on greater than 90% of all trials. The remaining 5 were > 1.5 inter-quartile outliers. The following RT analyses are based on the 26 good fixators: see Table 2.
Table 2.
Clinical Information for Experiment 1
| n=26 | Mean | SD | |
|---|---|---|---|
| Age | 23.9 | 1.9 | |
| WAIS | Block Scaled | 14.4 | 2.4 |
| Vocab Scaled | 15.9 | 2.4 | |
| SRS | Total | 13.8 | 9.9 |
| SWAN * | Total ADHD | 26.9 | 16.6 |
| Inattentive | 13.9 | 8.3 | |
| Hyper-Impulsive | 13.1 | 9.5 | |
| ABCL | Anxious/Depressed | 51.1 | 2.3 |
| Wdrwn/Depressed | 51.0 | 2.2 | |
| Somatic | 50.6 | 1.5 | |
| Thought | 50.8 | 1.7 | |
| Attention | 51.4 | 2.0 | |
| Aggressive | 50.8 | 2.0 | |
| Rule Breaking | 50.4 | 0.9 | |
| Intrusive | 51.1 | 2.3 |
Positive SWAN scores indicate superior attention and negative scores reflect ADHD symptoms.
We computed median RTs for each subject for each condition of the experiment and explored the effects of sex on exogenous orienting (“automatic” orienting for non-predictive, peripheral cues at short SOAs), endogenous orienting (“voluntary” orienting for central, predictive cues), and gaze cueing with a 2 (male, female) × 2 (50%, 80%) × 3 (box, arrow, gaze) × 2 (150 ms, 800 ms) × 2 (valid, invalid) mixed-model, repeated measures ANOVA on these RTs. There was no significant effect of sex [F(1, 24) = .988; p = .330], nor were there any significant interactions between sex and the other independent variables. Therefore, we dropped sex from subsequent analyses. A 2 (50%, 80%) × 3 (box, arrow, gaze) × 2 (150 ms, 800 ms) × 2 (valid, invalid) repeated measures ANOVA showed multiple significant main effects and interactions (we will report statistics that relate in the most salient ways to our central questions; e.g., a main effect of SOA is expected in all experiments and is not fundamentally related to any of the putative processes under study here). We then graphed RTs for valid and invalid cues against SOA at each probability * cue combination (Figure 2) and conducted six 2 (150 ms, 800 ms) × 2 (invalid, valid) repeated measures ANOVAs (one for each probability * cue combination) to test for the significance of differences visualized in these graphs.
Figure 2.
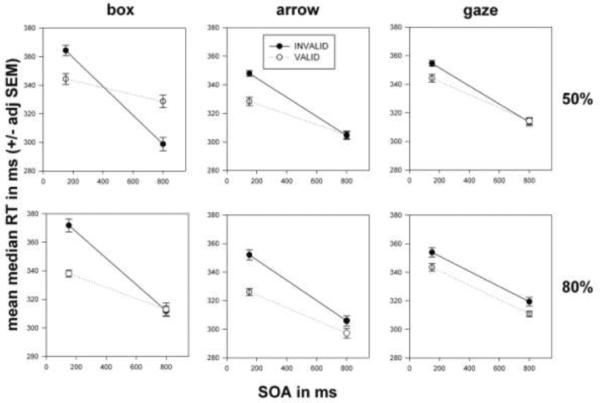
Data from 26 typical adults with good fixation. The plots show group means for condition median RTs (with repeated-measures adjusted SEMs) versus SOA, indexed by cue type (columns: box, arrow, and gaze) and cueing probability (rows: 50% and 80% valid cueing).
Figure 2, box, 50% (upper left panel) shows that RTs were faster for valid box cues at the 150 ms SOA (consistent with exogenous orienting) but slower for valid box cues at 800 ms (consistent with IOR: the slowing of RTs for valid, non-predictive, peripheral cues at late SOAs). A significant SOA * validity interaction1 [F(1, 25) = 45.267; p = .000] characterizes the crossed pattern in this plot. Figure 2, arrow, 50% (upper middle panel) shows faster RTs for valid arrow cues at 150 ms (consistent with “reflexive” orienting to arrows), but there was no RT difference at 800 ms – sideways V-shaped pattern confirmed with a significant SOA * validity interaction [F(1, 25) = 15.920; p = .001]. As with non-predictive arrow cueing, 50% valid gaze cueing (upper right panel) produced faster RTs for valid gaze cues at 150 ms (consistent with “reflexive orienting” – the speeding of RTs for valid, central, predictive cues at short SOAs – for gaze), but no RT difference at 800 ms. A significant SOA * validity interaction [F(1, 25) = 5.532; p = .027] characterized this early facilitation that disappears at 800 ms. The plot for box, 80% (lower left panel) shows RT speeding for valid box cues at 150 ms but no RT difference at 800 ms: the SOA * validity interaction was significant [F(1, 25) = 31.395; p = .000]. Arrow, 80% (lower middle panel) shows faster RTs for valid arrow cues at 150 ms, as well as a smaller difference in RTs at 800 ms. ANOVA confirmed significant effects of validity [F(1, 25) = 18.636; p = .000] and a significant SOA * validity interaction [F(1, 25) = 12.432; p = .002]. In gaze, 80% (lower right panel), RTs are faster for valid gaze cues at both SOAs, which was confirmed with a significant validity effect [F(1, 25) = 12.491; p = .002].
Discussion
Interpretation of a comparison of social and non-social attentional orienting in ASD and typical children would be greatly enhanced by a clearer understanding of the behavior of gaze cues in relationship to endogenous, exogenous, and reflexive orienting, and IOR and the interactions between these processes in typical adults, where such processes are thought to be mature (Brodeur & Enns, 1997; MacPherson, Klein, & Moore, 2003). To our knowledge this is one of only a few within-subject tests of gaze, box, and arrow cueing at multiple cueing probabilities and SOAs. Vecera and Rizzo employed a large subset of this full factorial in control subjects studied along side of patient EVR, but they used words in place of arrows (Vecera & Rizzo, 2004, 2006).
In experiment 1, we reproduced classically described exogenous (facilitation of RTs for valid non-predictive box cues at 150 ms SOAs) and endogenous orienting effects (facilitation of RTs for predictive arrow cues) and IOR (RT slowing for valid non-predictive box cues at 800 ms SOAs). Non-predictive gaze cueing produced a pattern of results that looks very similar to non-predictive arrow cueing (reflexive orienting2) but different from non-predictive box cueing: there was no IOR for gaze at the long SOA, consistent with reported findings (Driver et al., 1999). This observation fits with the notion that IOR occurs at the physical location of transient stimuli, as Friesen and Kingstone (2003) demonstrated that gaze cueing does not create IOR at the gazed at location, but IOR does develop at the location of an abrupt-onset gaze cue, itself. As with the non-predictive conditions, predictive gaze cueing produced a pattern of results that looks very similar to predictive arrow cueing but different from predictive box cueing. With predictive gaze and arrow cueing, endogenous orienting (stronger at the 800 ms SOA) appears to sum with reflexive orienting (seen in non-predictive cueing at the 150 ms SOA); i.e., the early reflexive effects, seen in non-predictive cueing, are still present for both predictive arrow and gaze cueing, but now, because of additional late endogenous effects resulting from the predictive nature of the cues, valid RTs are faster than invalid at the 800 ms SOA, leading to the main effects of validity for both arrow and gaze cues in the 80% condition. The pattern seen with predictive box cueing emerges in a similar way because early endogenous effects combine with exogenous effects (at the 150 ms SOA), and late endogenous effects (from the expectancy generated at 80% valid cueing) presumably cancel out IOR at the 800 ms SOA. These results will help contextualize findings from experiment 2, a more focused examination of non-predictive gaze cueing utilizing a finer sampling of SOAs, and from experiment 3, which employs the identical design as experiment 1 in ASD and typical children.
Experiment 2: Children & adults (50% female): 50% valid gaze cueing at 3 SOAs with catch trials
Methods
Basic procedures were similar to experiment 1, but only gaze cues at 50% valid cueing were used. A photo3 of a female face (displacement of the pupils at cue onset created the appearance of a gaze shift) replaced the cartoon animation used in experiments 1 and 3. SOAs were 150 ms, 350 ms, and 550 ms, and 12.5% of trials were catch (no target) trials. A single block of 96 trials was completed in one session. There was no response-time window (nor was there in experiments 1 or 3); excessively delayed responses were handled as stated, here and in experiments 1 and 3, by using median (versus mean or filtered mean) RTs, a common method for RT experiments.
Results
We explored the effects of age and sex on gaze cueing with a 2 (male, female) × 2 (adult, child) × 3 (150 ms, 350 ms, 550 ms SOA) × 2 (valid, invalid) mixed-model, repeated measures ANOVA. Figure 3, shows that adults were faster than children [F(1, 76) = 30.036; p =.000]. Adults also fixated better (94.3 +/− 2.1% of trials) than children (82.6 +/− 3.4% of trials) [t(57.4284) = 2.932; p = .005]. There was neither a significant main effect of sex [F(1, 76) = 1.094; p =.299] nor a significant sex * validity interaction [F(1, 76) = 3.597; p =.062]. There were no other significant interactions between sex and the other independent variables. Consequently, we dropped sex from subsequent analyses. A 2 (adult, child) × 3 (150 ms, 350 ms, 550 ms) × 2 (valid, invalid) repeated measures ANOVA on raw RTs showed a main effect of validity [F(1, 78) = 32.148; p=.000]. ANOVA using Z-score transformed RTs (plotted in Figure 3) showed a similar main effect [F(1, 78) = 70.072; p=.000] but revealed a significant validity * age group interaction [F(1, 78) = 6.465; p =.013], confirming the visual impression (at least for the transformed RTs) that the gaze cueing effect is larger for adults than for children.
Figure 3.
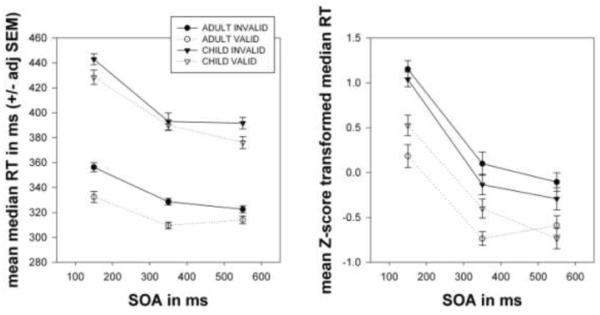
Data from 80 typical subjects (half children, half female). (left) The plot shows group means for condition median RTs (with repeated-measures adjusted SEMs) versus SOA. (right) This plot shows Z-score transformed condition median RTs. Note how the lines for adults (circles) bracket those for children (triangles).
Discussion
In this study, there were no significant effects of sex. Adults fixated better than children. We are convinced the children understood the instructions, but it was somehow more difficult for them to keep their eyes still. The children's saccades may have contributed to their slower RTs. Excluding bad fixators would have eliminated too many subjects. There was too much colinearity for ANCOVA, and this experiment was not engineered for trial-wise elimination of saccades (see experiment 3, below, which was). If raw RTs are considered, age did not affect the strength of the gaze cueing effect. We, however, believe it is proper to normalize (e.g., Z-score transform) RTs in the presence of significant between-group differences because such differences artificially exaggerate between-within interactions in mixed-model RT studies (Faust, Balota, Ferraro, & Spieler, 1999). The point is not that transforming the data eliminates all between-within interactions. Rather, it decreases spurious interactive effects, allowing true main effects and interactions to shine through. Z-score transformed RTs showed that adults had a greater gaze-cueing effect than the children. This result is consistent with the interpretation that gaze cueing uses endogenous orienting mechanisms that become more reflexive over the course of development (see: (Brignani, Guzzon, Marzi, & Miniussi, 2009)). We are mindful of previous work showing reflexive gaze cueing in preschoolers (Ristic, Friesen, & Kingstone, 2002) and toddlers (Chawarska et al., 2003). Our experiment, however, sought to directly compare children to adults. We are well aware that many developmental reaction time studies do not employ normalization for group differences in mean RT. We think this is incomplete, and so we have presented our data both ways so that the reader can draw his/her own conclusions about our data.
Experiment 3: ASD and typical 9–12 year-olds (~20% female): box, arrow, gaze cueing; 2 probabilities and 2 SOAs
Methods
The design was identical to experiment 1, except for the between-group comparison, and the children could complete the covert orienting task in 3 days instead of 2 if necessary.
Results
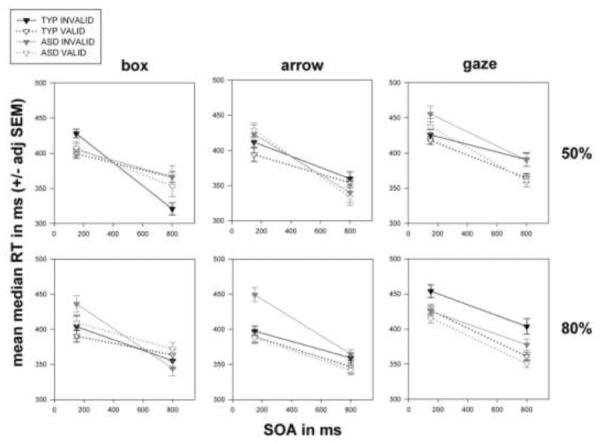
We could match 52 (27 ASD, 25 typical) task completers on both WISC-IV vocabulary and block design scores (VOC and BD: see Table 3 and legend). Matching was achieved at the group level. We explored the effects of diagnosis (typical, ASD) on exogenous orienting, endogenous orienting, and gaze cueing with a 2 (typical, ASD) × 2 (50%, 80%) × 3 (box, arrow, gaze) × 2 (150 ms, 800 ms) × 2 (valid, invalid) mixed-model, repeated measures ANOVA (similar to our analysis for experiment 1, only with the addition of the between-subjects factor). Figure 4 shows that ASD children were slower, but they were not significantly so in this VOC-and BD-matched dataset (so we opted, below, for presentation of ANOVA results on raw median RTs versus Z-score transformed RTs, except where transformation proved useful for checking the robustness of between-within interactions). We also explored the effects of saccades on RT (see below). There were multiple significant omnibus effects.
Table 3.
Clinical Information for 52 (27 ASD and 25 typical) 9–12 year-old children in Experiment 3
| ASD Mean | ASD SD | Typ. Mean | Typ. SD | df | t | p | ||
|---|---|---|---|---|---|---|---|---|
| Age | 11.1 | 1.2 | 11.0 | 1.2 | 50 | −0.37 | 0.713 | |
| WISC | Block Scaled | 12.3 | 2.8 | 11.8 | 2.5 | 50 | −0.575 | 0.568 |
| Vocab Scaled | 10.3 | 2.6 | 11.2 | 2.1 | 50 | 1.521 | 0.134 | |
| SRS | Total | 101.7 | 22.1 | 18.9 | 13.9 | 50 | −16.015 | 0.000 |
| SWAN * | Total ADHD | −22.4 | 14.0 | 10.3 | 17.0 | 50 | 7.615 | 0.000 |
| Inattentive | −11.6 | 8.5 | 5.1 | 9.1 | 50 | 6.857 | 0.000 | |
| Hyper-Impsv | −11.0 | 6.9 | 5.2 | 9.1 | 50 | 7.295 | 0.000 | |
| CBCL ** | Anx/Dep | 64.9 | 11.5 | 51.8 | 3.4 | 30.9 | −5.662 | 0.000 |
| Wdrwn/Dep | 64.0 | 9.7 | 50.5 | 1.8 | 27.9 | −7.176 | 0.000 | |
| Somatic | 61.2 | 8.4 | 51.6 | 2.5 | 30.8 | −5.707 | 0.000 | |
| Social | 64.3 | 10.0 | 50.6 | 1.7 | 27.6 | −6.949 | 0.000 | |
| Thought | 69.3 | 7.9 | 51.0 | 2.0 | 29.4 | −11.657 | 0.000 | |
| Attention | 68.2 | 8.3 | 50.9 | 1.8 | 28.5 | −10.570 | 0.000 | |
| Rule Breaking | 56.6 | 6.8 | 50.9 | 1.6 | 29.2 | −4.261 | 0.000 | |
| Aggressive | 62.2 | 10.0 | 50.7 | 1.9 | 28.0 | −5.844 | 0.000 |
59 (31 typical and 28 ASD) children (BD and VOC > 6 or known average/above average academics but did not complete BD or VOC: 1 typical child became upset, and 1 ASD child did not meaningfully engage in VOC) completed the task. In 57 task completers with VOC scores, typical children had higher scores (12.17 +/− .517) than ASD children (10.26 +/− .491) [t(54.999) = 2.677; p = .010]. In this group mean RT had a significant negative correlation with VOC (r = −.372, p = .004). Because this is an RT study, we then matched the groups on VOC by eliminating 5 typical (and 0 ASD) subjects with VOC >= 16, resulting in the 52 children described in this table.
Positive SWAN scores indicate superior attention and negative scores reflect ADHD symptoms.
T-Test dfs for CBCL scores reflect corrections for unequal variance.
Figure 4.
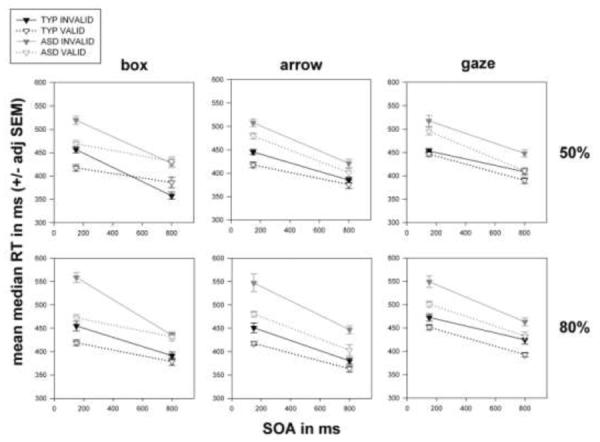
Data from 25 typical and 27 ASD children. The format is similar to Figure 2. The global pattern of effects is strikingly similar between the ASD and typical children. Note, especially the plot for gaze, 50%, where if anything, the validity effect for the ASD children at the 150 ms SOA appears larger than that for the typical children. See also gaze, 50% in figure 5.
We then conducted six 2 (typical, ASD) × 2 (150 ms, 800 ms) × 2 (invalid, valid) repeated measures ANOVAs. Figure 4, box, 50% (upper left panel) shows that both groups of children have intact exogenous orienting, since RTs were faster within both diagnostic groups for valid box cues at 150 ms. RTs appear slower for valid box cues at 800 ms for typical children (consistent with IOR) with no apparent RT difference for ASD children. ANOVA showed a significant effect of validity [F(1, 50) = 4.376; p = .042] and a significant SOA * validity interaction [F(1, 50) = 24.342; p = .000]. However, despite the suggestive group difference in IOR the diagnosis * SOA * validity interaction failed to reach significance [F(1, 50) = .284; p = .596].
The plot for arrow, 50% (upper middle panel) shows that both groups of children have intact “reflexive” orienting to non-social symbols: i.e., RTs were faster within both diagnostic groups for valid arrow cues at 150 ms, with less RT difference at the late SOA in either group. Validity was significant [F(1, 50) = 9.154; p = .004], but the SOA * validity interaction was not [F(1, 50) = 1.958; p = .168].
Gaze, 50% (upper right panel) shows little apparent RT difference for valid and invalid gaze cues at 150 ms for typical children, but RTs were visibly faster for valid gaze cues among ASD children (pattern opposite the central hypothesis). Children within both groups appear faster for valid gaze cues at 800 ms. Here, the validity effect was significant [F(1, 50) = 13.032; p = .001], but despite the visual suggestion, neither the diagnosis * validity [F(1, 50) = 2.156; p = .148] nor the diagnosis * SOA * validity [F(1, 50) = .026; p = .872] interactions reached significance.
The plot for box, 80% (lower left panel) shows RTs were faster for valid box cues at 150 ms – and more so among the ASD children. There was little apparent RT difference at 800 ms in both groups. Validity [F(1, 50) = 25.747; p = .000], SOA * validity [F(1, 50) = 14.211; p = .000], and the SOA * validity * diagnosis interaction [F(1, 50) = 4.456; p = .040] reached significance. Note, however, that in ANOVA on Z-score transformed RTs, the SOA * validity * diagnosis interaction was not significant [F(1, 50) = 2.199; p = .144]. The lower middle panel (arrow, 80%) shows RT speeding for valid arrow cues at both SOAs, with the validity effect appearing possibly smaller for typical children – this was not, however, confirmed in ANOVA on raw RTs: validity [F(1, 50) = 28.216; p = .000] but validity * diagnosis [F(1, 50) = 3.898; p = .054]. Gaze, 80% (lower right panel) produced faster RTs for valid cues at both SOAs in both groups. Validity was significant [F(1, 50) =24.827; p = .000], but there were no significant interactions with diagnosis.
Typical children fixated better (66.6 +/− 4.9% of trials) than ASD children (41.1 +/− 6.7% of trials) [t(46.571) = 3.059; p = .004]. We were able to compare across groups the degree of concordance between the direction of eye movements and the direction of the cues for a limited number of children for whom we felt percent concordance values would be reliable. We manually coded the direction of eye movements and analyzed 800 ms SOA trials, only, so there would be no confusion about saccades for cues versus targets, for the n=5 ASD and n=5 typical worst fixators (to ensure at least 8 fixation breaks per child per cue at this SOA to base a percent directional concordance). ANOVA showed a main effect of cue [F(2, 16) = 15.958; p = .000] but no main effect of diagnosis [F(1, 8) = .644; p = .445] and no cue * diagnosis interaction [F(2, 16) = .545; p = .590]. Percent concordance values (arrow, box, gaze) were: 72.7 +/− 6.4%, 90.9 +/− 1.9%, 76.5 +/− 4.2% for ASD and 80.0 +/− 5.2%, 92.1 +/− 3.0%, 79.9 +/− 3.2% for typical children.
In light of a recent report of abnormal oculomotor control in attention-deficit/hyperactivity disorder (ADHD) (Loe, Feldman, Yasui, & Luna, 2009) and the tight correlation between ASD and ADHD symptoms in our sample (for SRS versus SWAN: r = −.731, p = .000), we analyzed % trials fixated for ASD children as a function of ADHD symptoms (SWAN scores) but found no correlation (r = −.195, p = .329). We did find significant correlations between % fixated and VOC (r = .382, p = .049), CBCL social problems (r = .402, p = .038), and CBCL thought problems (r = .539, p = .004) (but not SRS, ADOS, or ADI-R scores) in the ASD group (note: the typical subjects were selected for CBCL T-scores <= 60, so there were no meaningful such analyses in that group).
In this experiment, we could eliminate individual trials with eye movements. Figure 5 shows RT plots for 36 (23 typical, 13 ASD) children who fixated on >= 25% of trials. Care should be taken in trying to interpret the finer pattern of results, as ~50% of the data have been eliminated. Two main points can be made, however: 1) RT ranges for the ASD and typical children now overlap (all six panels), indicating that eye movements accounted, at least in part, for the general slowing seen for ASD children when the data were based on all trials; and 2) the upper right panel (gaze, 50%) shows faster RTs for valid versus invalid trials for both typical and ASD children (validity was significant [F(1, 34) = 11.533; p = .002], but validity * diagnosis [F(1, 34) = .303; p = .586] and SOA* validity * diagnosis [F(1, 34) = .041; p = .840] were not), indicating that the strictest possible comparison still shows no group difference.
Figure 5.
Fixation-only data from 23 typical and 13 ASD children. Format similar to Figure 2. Here, we eliminated trials with eye movements prior to computing each subject's condition median RTs. Subjects who fixated on >= 25% of trials could generate a median RT for each condition. See text for limits of interpretation, but note that now grey and black lines overlap (c.f., figure 4) and see, especially, gaze, 50%, where there is still a significant cueing effect without an interaction with diagnosis.
Since a recent study (Chawarska, Volkmar, and Klin, 2010) found shorter saccadic RTs away from central social (but not non-social) attentional cues in ASD versus typically developing or developmentally delayed toddlers, we conducted a 2 (typical, ASD) × 2 (arrow, gaze) × 2 (150 ms, 800 ms) × 2 (invalid, valid) repeated measures ANOVA, which showed that RTs were slower for gaze versus arrow cues [F(1, 34) = 38.736; p = .000], but there was no cue * diagnosis interaction [F(1, 34) = 1.485; p = .231]. We saw a similar pattern with repeated measures ANOVA using fixated plus non-fixated trials in the full 52 subject dataset: a main effect of cue [F(1, 50) = 20.006; p = .000] but no cue * diagnosis interaction [F(1, 50) = .187; p = .667].
Discussion
ASD children were slower overall, but not significantly so after matching on VOC (which correlated negatively and significantly with RT) in addition to BD. The overall patterns of results were, otherwise, strikingly similar between the ASD and typical children. Importantly, ASD children oriented equally strongly to gaze compared to typical children. One might consider follow-up with a double-cue procedure (MacPherson et al., 2003) to explore further the visually suggestive but non-significant group differences in IOR (figures 4 and 5, box, 50%). The only significant interaction between validity and diagnosis occurred at the 150 ms SOA for probabilistic box cueing. The direction of this interaction was in favor of a larger effect in ASD; this interaction did not remain significant after Z-score transformation; and there were no diagnosis * validity interactions for probabilistic arrow or gaze cueing. ASD children made more eye movements, but we are sure they understood the task. Anecdotally, a number of ASD children commented that they were trying to keep their eyes still but could not. Observed correlations between fixation and CBCL social and thought problems ran opposite expected directions, such that more social and thought problems went with better fixation in the presence of ASD. We can only speculate this might reflect over-focus translating into better task performance (also anecdotally reported by our team). We did not design this experiment as an oculomotor control study and originally intended simply to account for fixation. With that disclaimer, the limited analysis we were able to conduct on the data acquired showed that there were no group differences in the degree of concordance between the direction of eye movements and the direction of the arrow, box and gaze cues. Even though ASD children broke fixation more, for both ASD and typical children, when fixation was broken, eyes moved in the cued direction. We also found no evidence in support of ASD children responding more rapidly than typical children for social versus non-social central attentional cues, as has recently been reported in toddlers with ASD (Chawarska et al., 2010).
Importantly, RT analyses using fixated-only trials showed 1) eye movements account for the ASD children's slower RTs, 2) covert orienting is intact in ASD (ASD children don't have to move their eyes to orient attention), 3) the condition that shows this best is the gaze cueing condition, and 4) therefore, in a rigorous test of our motivating hypothesis, gaze cueing is not disrupted in ASD children.
General Discussion
Three experiments in typical and ASD subjects explored the nature of visual attentional redirection in response to another's shift in eye gaze. The experiments in typically developing children and adults explored gaps in our present knowledge about gaze cueing that have particular relevance to autism research: the nature of reorienting for gaze in relationship to processes known to be involved in reorienting for non-social stimuli, potential changes in the strength of gaze cueing with development, and potential effects of sex. Then, a mixed design study compared ASD to typically developing children in a fully within-subject manipulation of cue type (gaze, box, and arrow), cue-target onset time (SOA), and cueing probability with stringent eye-position monitoring. Our hope was that the full-factorial design with consideration of the potential impact of eye movements on manual reaction times for these within-subject manipulations should help to resolve inconsistencies in the autism gaze-cueing literature (Chawarska, et al., 2003; Goldberg et al., 2007; Kylliainen & Hietanen, 2004; Ristic et al., 2005; Senju et al., 2004; Swettenham et al., 2003; Vlamings et al., 2005). The full factorial design additionally allowed us to address the integrity of basic visual attentional operations for non-social stimuli in ASD children.
Experiment 1 showed that gaze cueing looks more similar to arrow cueing than to box cueing in typical adults: we found reflexive behavior (early facilitation at short SOAs during non-probabilistic cueing) for both gaze and arrows. This finding (see also the strikingly similar patterns for gaze and arrow cueing in experiment 3) suggests gaze cueing uses general endogenous orienting mechanisms (see: (Brignani et al., 2009)), as opposed to specialized brain systems dedicated for attentional reorienting to gaze (Kingstone, Friesen, & Gazzaniga, 2000; Ristic et al., 2002; Vuilleumier 2002; Kingstone, Tipper, Ristic, & Ngan, 2004; Akiyama, Kato, Muramatsu, Saito, Umeda, & Kashima, 2006a; Akiyama, Kato, Muramatsu, Saito, Nakachi, & Kashima, 2006b; Nation & Penny, 2008). Significantly slower mean RT for gaze versus arrow cues, as we found for both ASD and typical children in experiment 3, could reflect basic perceptual processing rather than the engagement of different attentional systems, and our argument about the pattern of results in experiment 1 favors one system for gaze and arrows that receives different inputs. Little prior imaging work involves studies of gaze cueing, as opposed to other aspects of gaze (Mosconi, Mack, McCarthy, & Pelphrey, 2005; Pelphrey, Morris, & McCarthy 2005). Extant fMRI gaze-cueing reports (Kingstone et al., 2004; Hietanen, Nummenmaa, Nyman, Parkkola, & Hamalainen, 2006; Tipper, Handy, Giesbrecht, & Kingstone, 2008; Greene, Mooshagian, Kaplan, Zaidel, & Iacoboni, 2009) have not resolved the issue of shared versus distinct neural systems supporting gaze-based and non-social orienting. One gaze cueing fMRI study demonstrated selective activation of the superior temporal sulcus (STS) when gaze cueing was compared against cueing with a (an ambiguous, perceptually identical) non-social stimulus (Kingstone et al., 2004). Interestingly, a different group reported a patient with a right STS lesion, who demonstrated impaired gaze cueing but intact arrow cueing (Akiyama et al., 2006a; Akiyama et al., 2006b). Any number of lesions, however, that affect aspects of gaze perception could impair gaze cueing by eliminating input to general-purpose attentional systems (Posner & Petersen, 1990). The cited STS imaging and lesion reports may be more about gaze perception than attention. If this interpretation is correct, the preponderance of the evidence (see (Tipper et al., 2008): also using perceptually identical, ambiguous gaze / non-social cues; and: (Greene et al., 2009)) may weigh in favor of same systems with different inputs. More research is needed. We note the case of patient EVR who has a bilateral frontal lobe lesion that eliminated orienting to both gaze and instruction but not to peripheral boxes (Vecera & Rizzo 2004; Vecera & Rizzo 2006). Recent event related potential work also suggests that gaze cueing operates through machinery that sub-serves basic endogenous orienting (Brignani et al., 2009).
Experiment 2 suggests that reflexive orienting to gaze strengthens through experience. A subsequent study would be needed to test the mechanism responsible for this (e.g., by comparing the effect of age/maturation alone to the effect of over-practice on gaze-cueing versus orienting to a purely endogenous cue, such as a random number that is arbitrarily associated with different target sides). There were no significant effects of sex in experiments 1 or 2, contrary to recent findings from another group (Bayliss et al., 2005) that, in part, motivated us to look at this – therefore, we do not believe weaker gaze cueing in males explains some prior findings of disrupted gaze cueing in autism. It is not clear why Bayliss et al. found an effect of sex (for both gaze and arrows) and we did not. Our results, however, were consistent across different experiments involving both children and adults and for schematic (experiment 1) and real face photo (experiment 2) gaze cueing stimuli.
Experiment 3 tested the hypothesis that disrupted orienting to gaze contributes to autism. Potential abnormalities in basic attentional orienting processes in autism have posed challenges for making sense of the mixed findings in the autism gaze cueing literature. Investigators have reported impaired early orienting to peripheral flashed boxes and central arrows in autism (Townsend, Harris, & Courchesne, 1996; Wainwright-Sharp & Bryson, 1993) (exception, see (Iarocci & Burack, 2004)) and impaired disengagement of attention from attended stimuli (Allen & Courchesne, 2001; Casey, Gordon, Mannheim, & Rumsey, 1993; Landry & Bryson, 2004). It may be hard to say whether there is a specific problem with gaze cueing in autism if there is uncertainty about the integrity of exogenous and endogenous orienting.
We controlled for eye movements and included box and arrow comparison conditions. We found that ASD children showed an almost identical pattern of results (across all conditions) when compared to typical children (in groups matched for age, VOC, and BD). The minor differences (seen in probabilistic box cueing) were in favor of the ASD children showing more robust cueing. This could reflect a greater cost for invalid predictive box cues in ASD, but this interaction with diagnosis was not robust (see experiment 3 discussion). Both the presence of robust cueing effects with central cues that remained on during the entire trial and the increased number of saccades away from the central cues or fixation cross argue against a primary deficit in attentional disengagement in autism. Chawarska et al.'s (2010) recent study similarly argues against a general deficit in attentional disengagement in autism but reports speeded saccades away from central face (but not non-social) cues to peripheral targets in toddlers with ASD. We found no significant diagnosis * cue interaction in experiment 3. Possible explanations for our different result include the ages of the subjects and the stimuli, design, and trial structure. Chawarska et al. (2010) used real faces, and the children made eye movements; our arrow and face stimuli were simple line drawings, and children made manual responses. Chawarska et al.'s (2010) recent study involved a blocked design, where non-social cueing trials always preceded social cueing trials, and the non-social trial sequence involved the constant display of a scrambled face, while the social trials involved sequences of closed and open eyes and direct and/or averted gaze. Our arrow and gaze cueing trials were presented in trial-wise pseudorandom sequence and were identical in every respect save the cue, itself (arrow or gaze). A follow-up study might test 9–12 year-olds with their design and toddlers with ours.
One major finding in experiment 3 was an increase in the number of eye movements in ASD versus typical children. Though excess eye movement in a covert orienting study might be considered a study limitation, our central hypothesis was rejected through analysis of fixated trials only. We wish to stress that we are sure the children understood the task. Fixation in the ASD children did not correlate with ADHD symptoms, ADOS, ADI-R, or SRS scores. Fixation was better in ASD children with worse CBCL social and thought problem scores. Problems with oculomotor control in autism have previously been reported (Goldberg, Landa, Lasker, Cooper, & Zee, 2000; Goldberg, Lasker, Zee, Garth, Tien, & Landa, 2002; Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Minshew, Luna, & Sweeney, 1999) and may warrant further study. We note that our finding is completely consistent with those of Mahone et al. (Mahone, Powell, Loftis, Goldberg, Denckla, & Mostofsky, 2006), who demonstrated impaired motor persistence in ASD on a lateral gaze fixation task (Kertesz, Nicholson, Cancelliere, Kassa, & Black, 1985). The number of eye movements and the demonstrated effect of saccades on manual RTs (likewise, the way RT normalization can change the interpretation of some results) are cautionary messages for future developmental RT studies requiring eye fixation (consider also fixation effects on fMRI data; see e.g.: (Dalton, Nacewicz, Johnstone, Schaefer, Gernsbacher, Goldsmith, Alexander, & Davidson, 2005; Hadjikhani, Joseph, Snyder, Chabris, Clark, Steele, McGrath, Vangel, Aharon, Feczko, Harris, & Tager-Flusberg, 2004)).
Our major finding from the 50% valid gaze cueing condition in experiment 3 was no effect of diagnosis on orienting to gaze, regardless whether trials with eye movements were included or excluded. We have noted that studies which used actual face photos tended to produce positive gaze cueing effects in ASD, while those employing schematic stimuli have not (Ristic et al., 2005; Goldberg et al., 2007) – which seems the opposite that one might expect if one thought deficiencies in ASD would be more likely to manifest with actual faces. Our findings with impoverished cartoon stimuli show that the stimulus itself (photo versus schematic) does not explain extant discrepant results in the literature, as we now report a positive gaze cueing effect with schematic stimuli. This finding stands in contrast to the finding from Ristic et al. (2005), who also compared predictive to non-predictive cueing in older ASD and typical subjects. Their finding of intact probabilistic (related to voluntary attentive processes) but disrupted non-predictive (related to reflexive attentive processes) gaze cueing in ASD was an extremely important consideration in the initial stages of our work. Ristic et al. (2005) monitored fixation informally, but we used EOG and accounted for the effects of eye movement on manual reaction time. While their study involved 47 subjects, their probability manipulation was a between-subject manipulation: 23 subjects were randomized to the predictive condition and 24 to the non-predictive. In our study, all 52 children in the age and IQ-matched set of task completers performed both probabilities due to our within-subject comparison. Such a demonstration with a fully within-subject, factorial manipulation of cueing probability, cue type, and cue-to-target onset times and strict account for eye position makes a strong case for this important ability being intact in ASD. Importantly, our result in older children adds weight to similar findings from Chawarska et al.'s (2003) overt orienting study in toddlers, which argues against disrupted reflexive orienting to gaze in infancy as a primary contributor to ASD.
Use of gaze in the first years of life in typical development progresses from an early sensitivity to direct versus averted gaze, to joint and shared attention – a sequence that unfolds alongside, and may in part support, the early development of language and theory of mind (see recent scholarly reviews: (Emery, 2000; Frischen et al., 2007; Itier & Batty, 2009). Reflexive attentional orienting for shifts in others' gaze in typical development (Driver et al., 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999) may provide a signature for the important relationship that gaze perception and attention to gaze hold for a host of sophisticated social-cognitive abilities. Our findings in ASD would imply that intact gaze cueing is not sufficient (it may be necessary but not sufficient, but these experiments can't establish that) for the suite of social behaviors that develop typically but are disrupted in autism; i.e., one can orient to others' gaze yet show impaired social responsiveness. While our reported studies involved tests of visuospatial attentional redirection for gaze, convergent research on attentional capture for social stimuli may provide additional answers about the perceptual and attentional contributors to social abnormalities in ASD.
In conclusion, exogenous orienting, endogenous orienting, and gaze cueing appear intact in high-functioning ASD children. Therefore, disrupted attentional redirection for shifts in others' gaze does not appear to be the explanation for gaze abnormalities and problems of social relatedness in autism.
Supplementary Material
Acknowledgments
We thank all of the families who generously participated in this study. We thank Maggie M. Gross for study coordination and clinical assessments, Ansley Stanfill for technical support, Fran Miezin for computer engineering, and Patricia LaVesser for clinical assessments. Subjects were recruited with the assistance of the Interactive Autism Network (IAN) Research Database at the Kennedy Krieger Institute and Johns Hopkins Medicine - Baltimore, sponsored by the Autism Speaks Foundation. We thank the Washington University School of Medicine Volunteers for Health (VFH) program; Autism Speaks; Missouri Families for Effective Autism Treatment (MO-FEAT); the Illinois Center for Autism; and other local research laboratories, clinics, schools, and community doctors' offices for their help in advertising the studies. Research funding included: R21 MH079958, K12 EY16336, T32 DA07261 (John Pruett); The Blanch F. Ittleson Endowment Fund (Richard Todd); McDonnell Center for Higher Brain Function grant “Cueing visual-spatial attention with biologically-relevant versus non-biological stimuli in children and adults with and without autism” (Steve Petersen).
Footnotes
Drs. Pruett and Petersen report no biomedical financial interest or potential conflicts of interest. Dr. Todd is deceased but had no biomedical financial interest or potential conflicts of interest. Dr. Constantino receives royalties on the Social Responsiveness Scale, which is published and distributed by Western Psychological Services. Ms. LaMacchia, Ms. Hoertel, Ms. Squire, and Ms. McVey report no biomedical financial interest or potential conflicts of interest.
Aspects of this paper were presented as posters at the 2009 Society for Neuroscience and American Academy of Child and Adolescent Psychiatry annual meetings.
In an interaction the dependent variable changes different amounts for a given change of one independent variable, depending on the levels of another independent variable. E.g., here, the effect of validity on RT is different at the two different SOAs.
We originally questioned reports of reflexive arrow cueing (Tipples, 2002); we purposely implemented a large target eccentricity to central stimulus size ratio to bias against finding this result; but our own data have convinced us about the robust nature of reflexive arrow cueing.
We used a photo in experiment 2 because early piloting for experiment 3 raised concerns that typical children might not show a gaze cueing effect for cartoon cues. After collection of more data, we decided to keep the cartoon for experiment 3. It is our belief that the nature of the cue (photo or cartoon) matters little for the issues at hand. A small number of typical children were tested with both the photo and cartoon, and they showed robust gaze cueing effects for both stimuli. A larger study would be needed to assess for any quantitative differences across these two types of gaze cues.
Explanation of dfs: acceptable EOG quality on 74/80 subjects, followed by DF-correction for unequal variances across groups.
References
- Achenbach T. CBLC/6–18 profile for boys - syndrome scales. ASEBA. 2001 [Google Scholar]
- Achenbach T. Adult behavior checklist for ages 18-59. ASEBA. 2003 [Google Scholar]
- Akiyama T, Kato M, et al. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia. 2006a;44(2):161–70. doi: 10.1016/j.neuropsychologia.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, et al. Gaze but not arrows: a dissociative impairment after right superior temporal gyrus damage. Neuropsychologia. 2006b;44(10):1804–10. doi: 10.1016/j.neuropsychologia.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baron-Cohen S, Cox A, Baird G, Swettenham J, Nightingale N, Morgan K, et al. Psychological markers in the detection of autism in infancy in a large population. British Journal of Psychiatry. 1996;168(2):158–163. doi: 10.1192/bjp.168.2.158. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, di Pellegrino G, Tipper SP. Sex differences in eye gaze and symbolic cueing of attention. Quarterly Journal of Experimental Psychology A. 2005;58(4):631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP. Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. British Journal of Psychology. 2005;96(Pt 1):95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Brignani D, Guzzon D, Marzi CA, Miniussi C. Attentional orienting induced by arrows and eye-gaze compared with an endogenous cue. Neuropsychologia. 2009;47(2):370–381. doi: 10.1016/j.neuropsychologia.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Brodeur DA, Enns JT. Covert visual orienting across the lifespan. Canadian Journal of Experimental Psychology. 1997;51(1):20–35. doi: 10.1037/1196-1961.51.1.20. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Gordon CT, Mannheim GB, Rumsey JM. Dysfunctional attention in autistic savants. Journal of Clinical Experimental Neuropsychology. 1993;15(6):933–946. doi: 10.1080/01688639308402609. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Development. 2003;74(4):1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F, et al. Limited attentional bias for faces in toddlers with autism spectrum disorders. Arch Gen Psychiatry. 2010;67(2):178–85. doi: 10.1001/archgenpsychiatry.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie A, Maruff P, Yucel M, Danckert J, Currie J. Spatiotemporal distribution of facilitation and inhibition of return arising from the reflexive orienting of covert attention. Journal of Experimental Psychology: Human Perception and Performance. 2000;26(6):1733–1745. doi: 10.1037//0096-1523.26.6.1733. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The social responsiveness scale. Western Psychological Services; Los Angeles: 2002. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular Psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6(5):509–540. [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24(6):581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Ferraro FR, Spieler DH. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. Journal of Clinical Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5(3):490–495. [Google Scholar]
- Friesen CK, Kingstone A. Abrupt onsets and gaze direction cues trigger independent reflexive attentional effects. Cognition. 2003;87(1):B1–10. doi: 10.1016/s0010-0277(02)00181-6. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Mooshagian E, et al. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychol Res. 2009;73(4):499–511. doi: 10.1007/s00426-009-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Landa R, Lasker A, Cooper L, Zee DS. Evidence of normal cerebellar control of the vestibulo-ocular reflex (VOR) in children with high-functioning autism. Journal of Autism and Developmental Disorders. 2000;30(6):519–524. doi: 10.1023/a:1005631225367. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40(12):2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostow AJ, et al. Evidence for impairments in using static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism. J Autism Dev Disord. 2007;38(8):1405–13. doi: 10.1007/s10803-007-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, et al. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33(1):406–13. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. American Journal of Intellectual and Developmental Disabilities. 2009;114(1):23–41. doi: 10.1352/2009.114:23;nd41. [DOI] [PubMed] [Google Scholar]
- Iarocci G, Burack JA. Intact covert orienting to peripheral cues among children with autism. Journal of Autism and Developmental Disorders. 2004;34(3):257–264. doi: 10.1023/b:jadd.0000029548.84041.69. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neuroscience and Biobehavioral Reviews. 2009;33(6):843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Nicholson I, et al. Motor impersistence: a right-hemisphere syndrome. Neurology. 1985;35(5):662–6. doi: 10.1212/wnl.35.5.662. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Friesen CK, Gazzaniga MS. Reflexive joint attention depends on lateralized cortical connections. Psychological Science. 2000;11(2):159–166. doi: 10.1111/1467-9280.00232. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain and Cognition. 2004;55(2):269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of Parent-Reported Diagnosis of Autism Spectrum Disorder Among Children in the US, 2007. Pediatrics. 2009;124(4):1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Attention orienting by another's gaze direction in children with autism. Journal of Child Psychology and Psychiatry. 2004;45(3):435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6(5):541–567. [Google Scholar]
- Loe IM, Feldman HM, Yasui E, Luna B. Oculomotor performance identifies underlying cognitive deficits in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(4):431–440. doi: 10.1097/CHI.0b013e31819996da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- MacPherson AC, Klein RM, Moore C. Inhibition of return in children and adolescents. Journal of Experimental Child Psychology. 2003;85(4):337–351. doi: 10.1016/s0022-0965(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Powell SK, et al. Motor persistence and inhibition in autism and ADHD. J Int Neuropsychol Soc. 2006;12(5):622–31. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- Maruff P, Yucel M, Danckert J, Stuart G, Currie J. Facilitation and inhibition arising from the exogenous orienting of covert attention depends on the temporal properties of spatial cues and targets. Neuropsychologia. 1999;37(6):731–744. doi: 10.1016/s0028-3932(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52(5):917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Mundy P, Delgado CEF, Yale M, Messinger D, Neal R, et al. Responding to joint attention across the 6- through 24-month age period and early language acquisition. Journal of Applied Developmental Psychology. 2000;21(4):283–298. [Google Scholar]
- Mosconi MW, Mack PB, et al. Taking an “intentional stance” on eye-gaze shifts: a functional neuroimaging study of social perception in children. Neuroimage. 2005;27(1):247–52. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: is it normal? Is it automatic? Is it social? Developmental Psychopathology. 2008;20(1):79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, et al. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(Pt 5):1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: neural basis and function. Cognitive Neuropsychology. 1985;2(3):211–228. [Google Scholar]
- Reichow B, Wolery M. Comprehensive synthesis of early intensive behavioral interventions for young children with autism based on the UCLA young autism project model. Journal of Autism and Developmental Disorders. 2009;39(1):23–41. doi: 10.1007/s10803-008-0596-0. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin Review. 2002;9(3):507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: The case of autism. Brain Research: Cognitive Brain Research. 2005;24(3):715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Krysko KM. Eye direction, not movement direction, predicts attention shifts in those with autism spectrum disorders. J Autism Dev Disord. 2008;38(10):1958–65. doi: 10.1007/s10803-008-0592-4. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and and arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45(3):445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Condie S, Campbell R, Milne E, Coleman M. Does the perception of moving eyes trigger reflexive visual orienting in autism? Philosophical Transactions of the Royal Society Lond B: Biological Sciences. 2003;358(1430):325–334. doi: 10.1098/rstb.2002.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Handy TC, et al. Brain responses to biological relevance. J Cogn Neurosci. 2008;20(5):879–91. doi: 10.1162/jocn.2008.20510. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychonomic Bulletin Review. 2002;9(2):314–318. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society. 1996;2(6):541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. Spatial attention: normal processes and their breakdown. Neurologic Clinics. 2003;21(3):575–607. doi: 10.1016/s0733-8619(02)00103-2. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. What are you looking at? Impaired 'social attention' following frontal-lobe damage. Neuropsychologia. 2004;42(12):1657–1665. doi: 10.1016/j.neuropsychologia.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. Eye gaze does not produce reflexive shifts of attention: evidence from frontal-lobe damage. Neuropsychologia. 2006;44(1):150–159. doi: 10.1016/j.neuropsychologia.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. Journal of Autism and Developmental Disorders. 2005;35(3):267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Perceived gaze direction in faces and spatial attention: a study in patients with parietal damage and unilateral neglect. Neuropsychologia. 2002;40(7):1013–26. doi: 10.1016/s0028-3932(01)00153-1. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23(1):1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth ed. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


