Abstract
Activation of caspase-1 leads to pyroptosis, a program of cell death characterized by cell lysis and inflammatory cytokine release. Caspase-1 activation triggered by multiple NLRs (NLRC4, NLRP1b, or NLRP3) leads to loss of lysosomes via their fusion with the cell surface, or lysosome exocytosis. Active caspase-1 increased cellular membrane permeability and intracellular calcium levels, which facilitated lysosome exocytosis and release of host antimicrobial factors and microbial products. Lysosome exocytosis has been proposed to mediate secretion of IL-1β and IL-18; however, blocking lysosome exocytosis did not alter cytokine processing or release. These studies indicate two conserved secretion pathways are initiated by caspase-1, lysosome exocytosis and a parallel pathway resulting in cytokine release, and both enhance the antimicrobial nature of pyroptosis.
INTRODUCTION
Microbial, host-derived, and foreign ‘danger’ signals that gain access to the host cell cytosol are sensed by Nod-like receptors (NLRs) (1). NLR proteins trigger formation of a multiprotein inflammasome complex, which includes the cysteine protease caspase-1 (2). Association of these proteins facilitates the processing and activation of caspase-1 (2), leading to a conserved program of inflammatory cell death termed pyroptosis (3). The features of pyroptosis include cellular DNA damage and rapid formation of plasma membrane pores, resulting in cell lysis and release of inflammatory intracellular contents. Pyroptosis is accompanied by caspase-1-dependent processing and activation of the inflammatory cytokines IL-1β and IL-18 (4).
IL-1β and IL-18 lack classical secretion signals and several methods of cytokine secretion have been proposed. Evidence suggests IL-1β processing in macrophages occurs in the cytosol (5), and membrane pores formed during pyroptosis may allow cytokine release (4). Budding of mature IL-1β-containing microvesicles from the cell surface has also been observed (6–8), which is consistent with cytosolic processing of IL-1β. Other groups have suggested active caspase-1 and cytokines reside in lysosomes, with lysosome exocytosis, or fusion of lysosomes with the cell surface, mediating cytokine release (9–12). Thus, a unifying mechanism for cytokine secretion during pyroptosis has yet to be identified.
In addition to its proposed role in cytokine secretion, lysosome exocytosis is involved in myriad cellular processes ranging from immune function to skin pigmentation (13, 14). In addition to the conventional lysosomal hydrolases that mediate intracellular protein degradation, specialized secretory lysosomes contain a unique set of cell–type specific proteins destined for secretion (14). Examples of secretory lysosomes include lytic granules of cytotoxic T cells, MHC class II compartments of antigen presenting cells, and melanin-containing granules of melanocytes (13, 14). The importance of this exocytic process in host defense is illustrated by the immunodeficiencies that arise in humans with mutations in genes regulating lysosome fusion events (13). Conventional lysosomes have also been demonstrated to fuse with the cell surface after plasma membrane damage (15–18), facilitating membrane repair and rescue cells from lysis (16, 17).
Host activation of caspase-1 controls replication of pathogens in vivo, and contributes to the pathophysiology of several inflammatory disorders (3). Importantly, the protective functions of caspase-1 during infection are not solely due to processing and activation of IL-1β and IL-18 (19, 20), suggesting additional caspase-1-dependent processes are providing protection against infection and contributing to pathological inflammation in vivo. Therefore, defining the mechanistic features of pyroptosis will provide insight into how this form of cell death contributes to inflammatory processes and control of microbial infection. This study identifies lysosome exocytosis as a conserved caspase-1-dependent feature of pyroptosis. We show that caspase-1 activation leads to increased membrane permeability and an influx of calcium, which results in fusion of lysosomes with the cell surface and release of lysosomal contents. Secretion of processed IL-1β and IL-18 in macrophages undergoing pyroptosis occurs independently of lysosome exocytosis. We have demonstrated that multiple stimuli, acting through a diverse set of NLR proteins, lead to two conserved caspase-1-dependent secretion events: the release of processed inflammatory cytokines and lysosome-mediated release of antimicrobial host factors and degraded microbial products.
MATERIALS AND METHODS
Macrophages
Bone marrow-derived macrophages were isolated from the femur exudates of Casp1−/− (a gift from C. Roy, Yale University) and wild-type C57BL/6 (Jackson Laboratory) mice and cultured at 37°C in 5% CO2 in Dulbecco’s minimal essential medium (DMEM, Invitrogen) supplemented with 10% FCS, 5 mM HEPES, 0.2 mg/ml L-glutamine, 0.05 mM β-mercaptoethanol, 50 mg/ml gentamicin sulfate and 10000 U/ml penicillin and streptomycin with 30% L-cell-conditioned medium (21). After 6 to 7 days of incubation, macrophages were collected by washing with ice-cold PBS containing 1 mM EDTA, resuspended in supplemented antibiotic free DMEM containing 5% FCS and allowed to adhere for 18 to 24 h before infection. Macrophages were primed with 100ng/ml LPS approximately 18 hours before Salmonella infection when assaying IL-1β secretion. Bone marrow-derived macrophages from lethal toxin (LT)-susceptible Balb/c mice (Jackson Laboratory) were used to examine LT-induced pyroptosis.
Infections were done in supplemented antibiotic-free DMEM containing 5% FCS and 5mM glycine unless otherwise indicated. Ac-YVAD-CMK was added to 200μM 1 hour before infection. Calcium-free DMEM lacking CaCl2 and containing 1mM EDTA was added to cells 1 hour before infection. All infections were done in the presence of 5mM glycine, which prevented cell lysis in response to all stimuli used to activate caspase-1 ((4, 22) and Fig. S1A). Several experiments including calcium flux and TMR dextran loss were done in parallel in the presence or absence of glycine and showed identical results.
Induction of macrophage cell death
Macrophages were pretreated with 50ng/ml ultra pure LPS from Salmonella minnesota (List Biologicals) prior to treatment with 20μM nigericin or 5mM ATP (Sigma) in the presence of 50ng/ml LPS. For lethal toxin treatment, both PA and LF were at 1 μg/ml (List Biologicals). Salmonella strain SL1344 and a sipB::aphT derivative (23)(T3SS-null) were used for all experiments except calcium flux and zymosan release assays, which were done using Salmonella strain LT2. Salmonella expressing GFP contained plasmid pDW5 (24). Bacteria were grown for infection experiments as described previously (22). Briefly, overnight cultures back-diluted 1:15 into L-broth containing 0.3 M sodium chloride were grown at 37°C with shaking for 3 hours, washed and resuspended in sterile PBS, and used to infect macrophages at an MOI of 10:1.
Fluorescence staining
Macrophages grown on glass coverslips were incubated with 0.5mg/ml TMR dextran for 1 hour followed by a 3 hour chase with fresh media. Macrophages were then infected with Salmonella in the presence of 5μM carboxyfluorescein-YVAD-fluoromethyl ketone (FAM-YVAD; Immunochemistry Technologies). Macrophages were washed thoroughly to remove unbound FAM-YVAD, fixed, and DNA was stained with To-Pro3 (Molecular Probes). To identify surface LAMP1 exposure, intact macrophages were incubated with anti-LAMP1 antibodies (BD Pharmingen), then fixed and stained with PE-conjugated secondary antibodies and the nuclear stain, To-Pro3. To confirm that binding of anti-LAMP1 antibodies is restricted to the cell surface, FAM-YVAD-treated macrophages were infected with Salmonella for 20 minutes and then stained with antibodies specific for the cytoplasmic protein, ASC, a component of the multiprotein inflammasome complex. ASC- and LAMP1-specific staining was compared in the presence and absence of permeabilization with Cytofix/Cytoperm (BD Biosciences). Coverslips were mounted using ProLong antifade reagent (Molecular Probes) and analyzed using a Leica SL confocal microscope in the W.M. Keck Center for Advanced Studies in Neural Signaling.
Immunobloting
Macrophages were infected in serum-free DMEM, and at the indicated timepoints the supernatant was removed, sterilized using a 0.22μm filter, and concentrated using a 10,000MWCO Centricon Plus-20 centrifugal filter device (Millipore). Supernatants were separated by 15% SDS-PAGE, transferred to nitrocellulose membranes, and protein processing and release was analyzed by western blot using anti-IL-18 M19 (Santa Cruz Biotechnology), anti-IL1β AF-401-NA (R & D Systems), or anti-cathepsin D C-20 (Santa Cruz Biotechnology) and peroxidase-conjugated secondary antibodies. Immunoblots were developed with an enhanced chemiluminescence system (Amersham Biosciences).
Calcium flux
Macrophages were incubated with PBS containing 2.5μM fluo-4 and 0.01% Pluronic F127 (Molecular Probes) at room temperature for 20 minutes. Cells were washed once and resuspended in serum-free media containing 2.5mM probenecid and 5mM glycine (Sigma) and incubated for 30 minutes at 37°C in 5% CO2 before infection or treatment. Images were taken of multiple fields prior to treatment (time=0) and every 15s for 20–25 minutes following treatment using a DeltaVision Live Cell microscope (Applied Precision) in the W. M. Keck Center for Advanced Studies in Neural Signaling. Temperature was maintained at 37°C without CO2 throughout the experiment. Time-lapse videos were generated using DeltaVision SoftWorx.
Release of yeast particles from lysosomes
Macrophages were incubated with 0.5mg/ml Alexa-488 dextran for 1 hour followed by a 3 hour chase with fresh media containing Alexa-594 zymosan. Extracellular zymosan was washed away and replaced with media containing 5mM glycine, which protects cells from caspase-1-dependent cell lysis (25). Macrophages were infected with Salmonella and images were taken of multiple fields every 20s for 20–25 minutes following treatment using a DeltaVision Live Cell microscope (Applied Precision). During this time period, caspase-1-dependent cell lysis is negligible in the presence of 5mM glycine (Fig. S1 A and (25)). Temperature was maintained at 37°C without CO2 throughout the experiment. Prior to taking the final image, the membrane-impermeant dye trypan blue (0.2% trypan blue in 0.2M sodium citrate buffer, pH 4.4/0.15M NaCl) was added to quench extracellular fluorescence.
Isolation of supernatant proteins and antimicrobial activity assay
Wild-type macrophages were incubated in phenol red-free, serum-free media containing 5mM glycine with or without 1.8mM CaCl2 and infected with Salmonella for 30 minutes. Wild-type and Casp1−/− macrophages were stimulated with 50ng/ml LPS for 3 hours followed by incubation in phenol red-free, serum-free media containing 5mM glycine and 5mM ATP with or without 1.8mM CaCl2. Supernatants were harvested and filter sterilized, CaCl2 was added back to those lacking calcium, and then supernatants were acidified to pH 2–3 using trifluoroacetic acid (TFA). An Oasis HLB Plus cartridge (Waters Corporation) was wetted with acetonitrile and washed once in 0.1% TFA before application of acidified supernatant. The cartridge was washed thoroughly with 0.1% TFA and proteins were eluted in 60% acetonitrile/0.1% TFA. The samples were lyophilized and stored at −80°C. Lyophilized samples were reconstituted in 0.1% TFA to 500x the starting concentration. Approximately 2×106 Salmonella/ml were incubated in LB containing 40x concentrated supernatant and incubated at 37°C for 1 hour and then diluted and plate on LB to determine the number of colony forming units.
RESULTS
Pyroptosis is accompanied by lysosome exocytosis
Lysosome exocytosis has been proposed to mediate release of caspase-1-processed cytokines in response to ATP (9, 10); therefore, the fate of lysosomal compartments was examined in Salmonella-infected macrophages undergoing pyroptosis. Macrophages were incubated with TMR-dextran to identify lysosomes (Fig. 1 A) and then infected with Salmonella in the presence of the fluorescent probe (FAM-YVAD), which specifically binds to active caspase-1 (26). Wild-type Salmonella infection triggered activation of caspase-1, and many caspase-1 positive macrophages were devoid of lysosomes (Fig. 1 A, Fig. S1 B). Loss of lysosomes required injection of proteins into the host cell cytosol via the bacterial type III secretion system (T3SS), as T3SS-null Salmonella failed to stimulate caspase-1 activation and remained lysosome positive (Fig. 1 A). The reduced number of lysosomes during pyroptosis could be explained by their fusion with the cell surface as a result of lysosome exocytosis, and this was quantified by examining surface exposure of the lysosomal transmembrane protein LAMP1. Salmonella infection resulted in surface localization of LAMP1 on ~35% of macrophages, compared with <5% of uninfected or T3SS-null (SipB-) Salmonella-infected macrophages (Fig. 1 B, C). These data indicate activation of caspase-1 is accompanied by loss of lysosomal compartments and surface exposure of LAMP1 via lysosome exocytosis.
Figure 1. Caspase-1 activation and lysosome exocytosis during pyroptosis.
(A) Bone marrow derived macrophages were incubated with TMR-dextran to label lysosomes (red) and infected for 20 minutes with wild type (w.t.) or T3SS-null (SipB-) Salmonella (ST), or left uninfected (UI), in the presence of FAM-YVAD-FMK to label active caspase-1 (green). DNA was stained using TO-PRO3 (blue). (B) Macrophages were infected with GFP-expressing Salmonella (green) for 20 minutes, or left uninfected, and surface exposure of LAMP-1 (red) was determined by immunofluorescence staining of intact cells. DNA was stained using TO-PRO3 (blue). Scale bars are 10μm. (C) The percentage of cells with surface LAMP-1; data are means and standard deviations calculated from multiple fields from 3 experiments. *P<0.0001.
Active caspase-1 mediates membrane pore formation, calcium influx and lysosome exocytosis
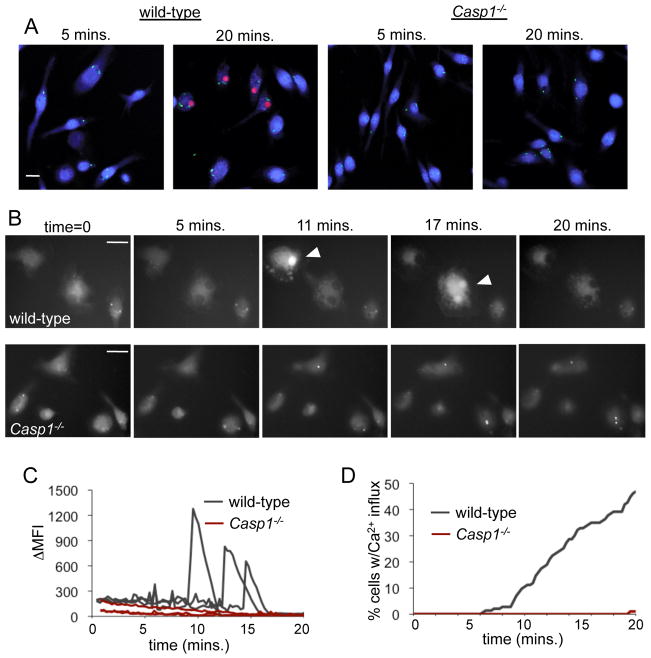
Fusion of both conventional and secretory lysosomes with the cell surface requires an influx of calcium ions from the extracellular media (16). Caspase-1 activation leads to formation of plasma membrane pores between 1.1 and 2.4 nm in diameter; large enough to allow the influx of extracellular calcium ions required for lysosome exocytosis (4). We examined the kinetics of pore formation during pyroptosis by monitoring uptake of ethidium bromide (EtBr) during infection with GFP-expressing Salmonella. At 5 minutes post infection the majority of wild-type macrophages were infected, but had not yet taken up EtBr, and by 20 minutes approximately 50% of macrophages were EtBr positive (Fig. 2 A). Casp1−/− macrophages failed to take up EtBr at any timepoint in response to Salmonella infection (Fig. 2 A), indicating pore formation during pyroptosis is a caspase-1-dependent process.
Figure 2. Active caspase-1 mediates pore formation and calcium influx.
(A) Wild-type and Casp1−/− macrophages were infected with GFP-expressing Salmonella (green) in the presence of glycine for the indicated period of time and stained with ethidium bromide (red) to identify macrophages with membrane pores and SYTO62 (blue) to label all macrophages. (B–D) Wild-type and Casp1−/− macrophages were loaded with the calcium indicator fluo-4, infected with Salmonella, and images were taken every 15 seconds for 20 minutes. Representative images are shown (B), with arrowheads indicating macrophages with increased intracellular calcium. Scale bars are 20μm. (C) The change in mean fluorescence intensity of individual wild-type and Casp1−/− macrophages during Salmonella infection. (D) The percentage of cells with increased intracellular calcium calculated from multiple fields containing greater than 100 cells total. Representative of 3 experiments.
Despite formation of caspase-1-dependent membrane pores, plasma membranes remain intact and retain cytoplasmic LDH early in pyroptosis (Fig. S1 A and (4)). Membrane integrity during LAMP1 staining was confirmed using antibodies specific for the cytoplasmic protein ASC, a component of the multiprotein inflammasome complex. Antibody-mediated detection of ASC foci was only possible when the macrophages were first permeabilized with Cytofix/Cytoperm (Fig S1 C), demonstrating that Salmonella-induced LAMP1 staining (Fig 1 B) is specific for molecules exposed on the cell surface during lysosome exocytosis of otherwise intact cells.
The influx of calcium into Salmonella-infected macrophages was addressed using the calcium indicator fluo-4, which has increased fluorescence intensity in response to calcium binding. Increased intracellular calcium levels were observed in individual macrophages approximately 8 minutes after Salmonella infection (Fig. 2 B, Video S1). In contrast, infection of Casp1−/− macrophages did not result in increased intracellular calcium (Fig. 2 B, Video S2). The change in mean fluorescence intensity of individual wild-type and Casp1−/− macrophages was quantified, and representative traces are shown (Fig. 2 C). Fifty percent of wild-type Salmonella-infected macrophages underwent a calcium influx during 20 minutes of infection, compared with <2% of Salmonella-infected Casp1−/− macrophages during the same period (Fig. 2 D). Removing calcium from the extracellular media prevented increased intracellular calcium, confirming the influx of the calcium was from the extracellular milieu and not intracellular calcium stores (unpublished data). These data are consistent with caspase-1-induced membrane pores allowing a calcium influx from the extracellular media.
To directly examine the role of caspase-1 activation and extracellular calcium in lysosome exocytosis, macrophages were infected with Salmonella in the presence of the caspase-1 inhibitor YVAD-CMK or in calcium-free media, and surface LAMP1 positive cells were quantified. Surface LAMP1 was detected on 44% of Salmonella-infected macrophages, and this was reduced to 7% in the presence of YVAD-CMK (Fig. 3 A, B). In the absence of extracellular calcium, only 4% of Salmonella-infected macrophages became surface LAMP1 positive (Fig. 3 A, B). Calcium-free media had no effect on the uptake of Salmonella into macrophages (Fig. S1 D), nor does the absence of extracellular calcium alter Salmonella-induced pyroptosis (25). Lysosome exocytosis also results in secretion of lysosomal proteins (14), and we confirmed release of lysosomal cathepsin D into the supernatant during Salmonella infection (Fig. 3 C). Consistent with the pattern of surface LAMP1 exposure, cathepsin D secretion during infection was inhibited in the presence of YVAD-CMK or calcium-free media (Fig. 3 C). These data suggest that during Salmonella infection caspase-1 activation leads to an increase in membrane permeability and an influx of calcium from the extracellular media. Elevated intracellular calcium facilitates lysosome exocytosis, resulting in surface LAMP1 exposure and lysosomal protein secretion.
Figure 3. Lysosome exocytosis requires caspase-1 activity and extracellular calcium.

(A–C) Macrophages were infected with Salmonella (ST) for 20 minutes in the presence of 200μM Ac-YVAD-CMK (+YVAD) or the absence of extracellular calcium (-Ca2+). (A) Surface exposure of LAMP-1 (red) determined by immunofluorescence staining of intact cells. DNA was stained using TO-PRO3 (blue). Scale bars are 20 μm. (B) Quantification of cells with surface LAMP-1; data are means and standard deviations calculated from multiple fields from 3 experiments. (C) Secretion of lysosomal cathepsin D into the supernatant was analyzed by western blot. Representative of 3 experiments. *P<0.0001.
Caspase-1-dependent lysosome exocytosis is a conserved host response
Previous research has indicated caspase-1-dependent membrane pore formation occurs in response to multiple stimuli (25), suggesting events downstream of pore formation, like lysosome exocytosis, may also be conserved. Salmonella activates caspase-1 via NLRC4. Additional stimuli that signal through distinct NLRs were assayed for their ability to trigger caspase-1-dependent lysosome exocytosis. Treatment of wild-type macrophages with Bacillus anthracis lethal toxin or the H+/K+ antiporter nigericin (which activate caspase-1 via NLRP1b and NLRP3, respectively (27, 28)) resulted in the surface exposure of LAMP1 (Fig. 4 A, B). This process required active caspase-1, as Casp1−/− or YVAD-treated macrophages did not become surface LAMP1 positive under the same conditions (Fig. 4 A, B). These data confirmed caspase-1-dependent lysosome exocytosis is a conserved response to a diverse group of stimuli.
Figure 4. Activation of caspase-1 by the NLRs Nalp1b and Nalp3 also leads to the conserved process of lysosome exocytosis.
(A) Wild-type and Casp1−/− C57BL/6 macrophages were treated with LPS for 3 hours followed by addition of nigericin for 45 minutes to activate caspase-1 via NLRP3. (B) Caspase-1 was activated via NLRP1b by treating Balb/c macrophages with B. anthracis lethal toxin for 60 minutes. Macrophages were treated with 200μM Ac-YVAD-CMK (+YVAD) where indicated. (A–B) Surface exposure of LAMP1 (red) was determined by immunofluorescence staining of intact cells. DNA was stained using TO-PRO3 (blue). (C) LPS pretreated wild-type and Casp1−/− macrophages were loaded with the calcium indicator fluo-4, treated with ATP, and images were taken every 15s for 20 minutes. (t=0 and 30s shown) (D) Wild-type and Casp1−/− C57BL/6 macrophages were treated with LPS for 3 hours followed by addition of ATP for 45 minutes. Wild-type macrophages were treated in the absence of extracellular calcium (-Ca2+) where indicated. Surface exposure of LAMP-1 (red) was determined by immunofluorescence staining of intact cells. DNA was stained using TO-PRO3 (blue). All scale bars are 20μm. All images are representative of 3 experiments.
In macrophages and monocytes treated with the P2X7 receptor ligand ATP, caspase-1 is activated but lysosome exocytosis is caspase-1-independent (10–12). One possible explanation for this distinct mechanism of lysosome exocytosis is the ability of ATP treatment to initiate a rapid calcium influx prior to detectable caspase-1 activation. Increased intracellular calcium was detected within 30 seconds of ATP stimulation of both wild-type and Casp1−/− macrophages (Fig. 4 C), in contrast to the delayed calcium influx observed during Salmonella infection (Fig. 2 D). Surface LAMP1 was apparent in both wild-type and Casp1−/− macrophages after ATP treatment and was inhibited by removing calcium (Fig. 4 D), unlike Salmonella infection where surface LAMP1 exposure was both caspase-1- and calcium-dependent (Fig. 3). Thus, stimuli capable of mediating calcium influxes independently of caspase-1 activation (10, 17) can initiate lysosome exocytosis without requiring caspase-1 activation; however, caspase-1 activation via the NLRs NLRP1b, NLRP3, and NLRC4 leads to the conserved processes of pyroptosis and lysosome exocytosis.
Caspase-1-dependent cytokine secretion occurs independently of lysosome exocytosis
During pyroptosis, the inflammatory cytokines IL-1β and IL-18 are cleaved and released from the cell. Membrane pore-mediated secretion (4), lysosome exocytosis (9, 10), and multivesicular body release (11, 12) have all been proposed as mechanisms of secretion. During Salmonella infection, mature IL-18 and IL1β were released from macrophages, and secretion was inhibited by YVAD-CMK (Fig. 5 A). Inhibition of lysosome exocytosis by removal of calcium from the extracellular media (Fig. 3, 4 D) did not affect cytokine processing and release (Fig. 5 A), indicating lysosome exocytosis is not required for cytokine release during Salmonella infection. The same pattern of mature cytokine secretion was observed after ATP treatment, where IL-1β cleavage and secretion required caspase-1 activity but was not altered by inhibition of lysosome exocytosis (Fig. 5 B). Therefore, mature cytokine secretion during pyroptosis occurs by a caspase-1-dependent process distinct from lysosome exocytosis.
Figure 5. Secretion of the caspase-1 substrates IL-18 and IL-1β occurs independently of lysosome exocytosis.

Western blot detection of cleaved IL-18 and IL-1β released into the supernatant by macrophages (A) 20 minutes after Salmonella infection in the presence of 200μM Ac-YVAD-CMK (+YVAD) or the absence of extracellular calcium (-Ca2+) or (B) 45 minutes of ATP treatment in the absence of caspase-1 (Casp1−/−) or extracellular calcium (-Ca2+). Representative of 2 experiments.
Lysosome exocytosis mediates release of antimicrobial factors and microbial products during pyroptosis
Phagocytic uptake of microbes often leads to their transport into acidic phagolysosomal compartments for destruction. Our data suggests that intact or degraded microbes residing in lysosomes may be released by lysosome exocytosis during pyroptosis. We examined the ability of macrophages undergoing pyroptosis to release phagocytosed yeast particles via lysosome exocytosis. Macrophages were loaded with Alexa 488-dextran (to identify lysosomes) and Alexa 594-labelled yeast particles, and the majority of yeast particles were located within lysosomes in both wild-type and Casp1−/− macrophages (Fig. 6 A–C). Macrophages were infected with Salmonella and monitored by live cell microscopy to observe whether lysosomal compartments (containing both Alexa 488-dextran and Alexa 594-yeast) underwent exocytosis and fused with the cell surface. Release of Alexa 594-yeast into the extracellular medium was confirmed using the membrane-impermeant dye trypan blue, which quenches extracellular fluorescence (Fig. 6 A). Thirty-six percent of wild-type macrophages released one or more yeast particles during the course of Salmonella infection (Fig. 6 B, D). In contrast, <10% of Casp1−/−macrophages released yeast particles during Salmonella infection (Fig. 6 C, D), indicating caspase-1 can mediate release of microbial products within lysosomes. These studies were performed in the presence of glycine and at timepoints prior to cell lysis (Fig S1 A). Therefore, release of lysosomal contents during the early stages of pyroptosis is mediated by exocytosis.
Figure 6. During pyroptosis, lysosome exocytosis mediates release of microbial products and antimicrobial factors.
Wild-type (A,B) and Casp1−/− (C) macrophages were loaded with Alexa-488 dextran to label lysosomes (green) and Alexa-594 yeast particles (red) and infected with Salmonella. Images were taken every 15–20s for 20 minutes and representative images show yeast particles within dextran-containing lysosomes (green+red) and subsequent lysosome exocytosis and release of yeast particles (red). (A) Prior to taking the final image, the membrane-impermeant dye trypan blue was added to quench extracellular fluorescence. Lower panels show the Alexa-594 channel alone. Dextran-positive compartments containing (a) and lacking (b) zymosan particles were released over the 20 min period of pyroptosis induction (kinetics shown in Fig. S1 E). Arrows designate (a) particles with reduced fluorescence upon quenching, thus demonstrating that they have been released from the macrophage during lysosome exocytosis. Not all yeast particles were released during this 20 min period (c). Several Alexa-594 yeast particles, labeled (d), did not colocalize with Alexa-488 dextran at t=0 and their fluorescence was quenched by trypan blue, suggesting that they remained extracellular during induction of pyroptosis. B–C) Representative images showing the kinetics of zymosan release from lysosomes of wild-type macrophages (B); release is caspase-1-dependent and therefore absent in Casp1−/− macrophages (C). Scale bars are 10μm. (D) The percentage of macrophages releasing one or more particles during infection; data are means and standard deviations from duplicate samples from multiple experiments. *P<0.05. (E) Supernatants from Salmonella-infected wild-type macrophages undergoing lysosome exocytosis (in the presence of calcium, S+LE) or in the absence of lysosome exocytosis (in the absence of calcium, S−LE) were concentrated and incubated with Salmonella for 1 hour and plated for CFU. All are representative of 3 experiments. *P=0.0004
Recent studies have indicated host lysosomal proteins retain their antimicrobial activity when released into the extracellular space (17, 29), suggesting lysosome exocytosis could also function to control replication of extracellular bacteria in the vicinity of cells undergoing pyroptosis. To examine this possibility, wild-type macrophages were infected with Salmonella and secreted factors were collected under conditions where lysosome exocytosis occurred (S+LE) or where lysosome exocytosis was inhibited (S−LE). Exocytosed factors were concentrated, incubated with exponentially growing Salmonella, and the surviving bacteria were quantified. Only 1.4% of the initial number of Salmonella were recovered after incubation with S+LE; however, when incubated with S−LE, bacterial survival increased to >22% (Fig. 6 E). The increased antimicrobial activity of supernatants containing lysosomal components was also observed using macrophages treated with ATP (unpublished data). These data indicate lysosome exocytosis enhances the antimicrobial nature of pyroptosis by releasing molecules that have direct antimicrobial activity against extracellular bacteria.
DISCUSSION
We demonstrate that caspase-1-dependent lysosome exocytosis is a conserved feature of pyroptosis. Activation of caspase-1 stimulates membrane perturbations and an influx of calcium from the extracejhllular milieu. Increased intracellular calcium levels promote fusion of lysosomes with the cell surface and secretion of lysosomal contents. Lysosome exocytosis facilitates release of antimicrobial factors and microbial products from cells undergoing pyroptosis, but does not mediate cytokine secretion. These data indicate caspase-1 activation stimulates multiple secretory events (Fig. S2), and the factors released during pyroptosis have diverse functions that contribute to effective host responses to microbial infection.
Several pathogens use specialized secretion systems to inject proteins into the host cell cytosol, and this requires formation of a membrane-spanning translocation pore in the host cell membrane. It has been suggested that the pore formed by the T3SS stimulates a calcium influx and fusion of lysosomes with the cell surface (30); however, we have demonstrated that pores formed in the macrophage membrane during Salmonella and Yersinia infection are dependent on host caspase-1 (4, 31) (Fig. 2 A). Casp1−/− macrophages, or macrophages treated with caspase-1 inhibitor, fail to undergo a calcium influx or lysosome exocytosis in response to Salmonella infection (Fig. 2 B and 3 A), and infection of macrophages with Yersinia also stimulates lysosome exocytosis in a caspase-1-dependent manner (unpublished data). This indicates that during infection host caspase-1, and not the bacterial secretion system per se, is critical for allowing the calcium influx required for lysosome exocytosis. We have shown caspase-1 activation mediates lysosome exocytosis and others have demonstrated caspase-1 plays a role in limiting intracellular bacterial survival via phagosome maturation (32–34). An overlapping set of host cell processes regulate lysosome exocytosis and phagosome maturation (30, 35), and our studies and others implicate caspase-1 as a regulator of both lysosome exocytosis and phagosome maturation during bacterial infection (32–34). Macrophages that have active caspase-1 restrict bacterial replication by enhancing transit to lysosomes, and cells destined to undergo pyroptosis continue to exert antimicrobial activity by releasing host antimicrobial factors.
Fusion of conventional lysosomes with the cell surface is able to mediate membrane repair in multiple cell types (16). However, the stimuli used in our studies led to robust caspase-1 activation and ultimately resulted in cell lysis, indicating lysosome exocytosis was not sufficient to completely rescue cells from lysis under these conditions. Caspase-1 activation is clearly not an all or nothing phenomenon, as macrophages and other cell types can activate caspase-1 without immediately undergoing cell death (32, 36–39). It is possible that intermediate levels of caspase-1 activation result in membrane alterations and cytokine secretion, with subsequent lysosome exocytosis repairing membrane defects, rescuing cells from lysis, and simultaneously releasing anti-microbial factors. Once threshold levels of active caspase-1 are reached, membrane defects outstrip membrane repair and cell death occurs. Further experiments will be required to address the ability of lysosome exocytosis to rescue cells from pyroptosis.
Lysosomes contain factors with known antimicrobial activity in the context of the acidified lysosome; however, some lysosomal contents retain antimicrobial activity in the extracellular milieu ((29, 40) and Fig. 6 E). Several proteins with adjuvant activity have also been localized to lysosomes (14, 41, 42). For intracellular pathogens like Salmonella, the lysosomes of infected macrophages may also contain degraded bacteria and their fusion with the cell surface could mediate the release of antigens for presentation by neighboring cells, and consistent with this, bacterial lipids are released from M. tuberculosis-infected macrophages (43). We have demonstrated caspase-1-dependent release of microbial products during pyroptosis. Together these findings suggest inflammatory host cell suicide by pyroptosis is not only an innate defense mechanism, but also drives concurrent release of inflammatory cytokines, molecules with direct antimicrobial activity, and microbial products and other inflammatory contents that could function to amplify the inflammatory response by sensitizing nearby host cells to pyroptosis (31). Additionally, release of antigens in the presence of caspase-1-dependent inflammation would facilitate robust adaptive immune responses.
Supplementary Material
Acknowledgments
We would like to thank the members of the Cookson Lab for helpful discussions and Matt Johnson for providing technical assistance.
This work was supported by National Institutes of Health Grants U54 AI57141 and P50 HG02360, National Institute of General Medical Sciences Public Health Service National Research Service Award Grant T32 G07270 (to T.B.), the Helen Riaboff Whiteley Fellowship (to T.B.), and Poncin and Achievement Rewards for College Scientist Fellowships (to S.L.F.).
References
- 1.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 3.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 5.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 7.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 8.Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 9.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci U S A. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 12.Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305:55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- 14.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 17.Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci U S A. 2005;102:5192–5197. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol. 2007;9:2543–2551. doi: 10.1111/j.1462-5822.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 20.Miao EA, I, Leaf A, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaniga K, Tucker S, Trollinger D, Galan JE. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol. 2006;61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- 25.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 27.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 29.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 31.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 34.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174:997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 37.Miggin SM, Palsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, Rothwell N, Golenbock D, Fitzgerald KA, O’Neill LA. NF-kappaB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci U S A. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Koo IC, Ohol YM, Wu P, Morisaki JH, Cox JS, Brown EJ. Role for lysosomal enzyme beta-hexosaminidase in the control of mycobacteria infection. Proc Natl Acad Sci U S A. 2008;105:710–715. doi: 10.1073/pnas.0708110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 42.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80:31–40. doi: 10.1078/0171-9335-00131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.