Abstract
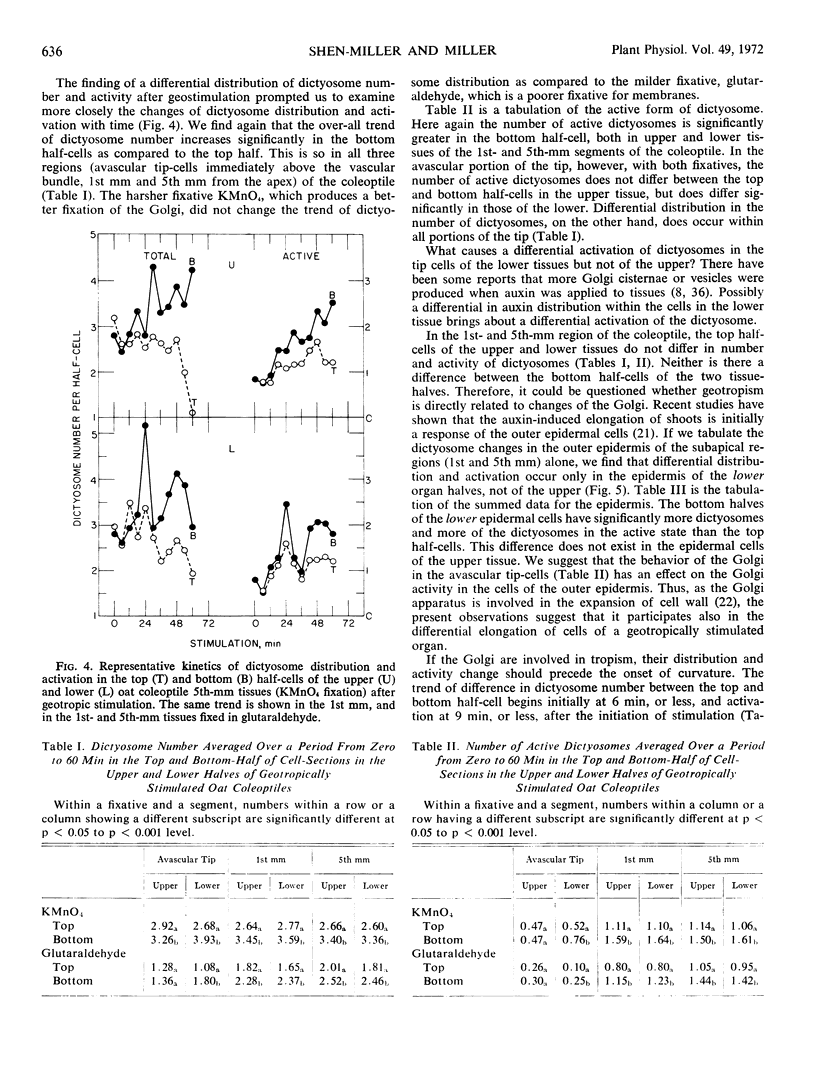
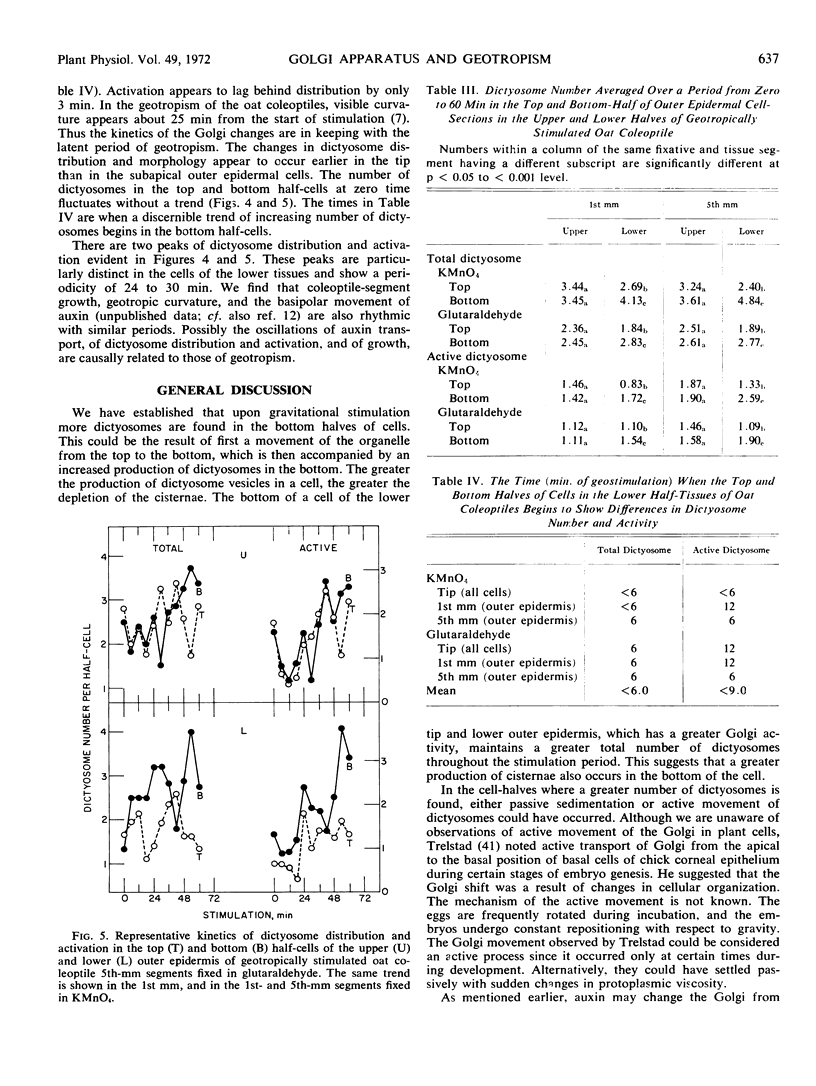
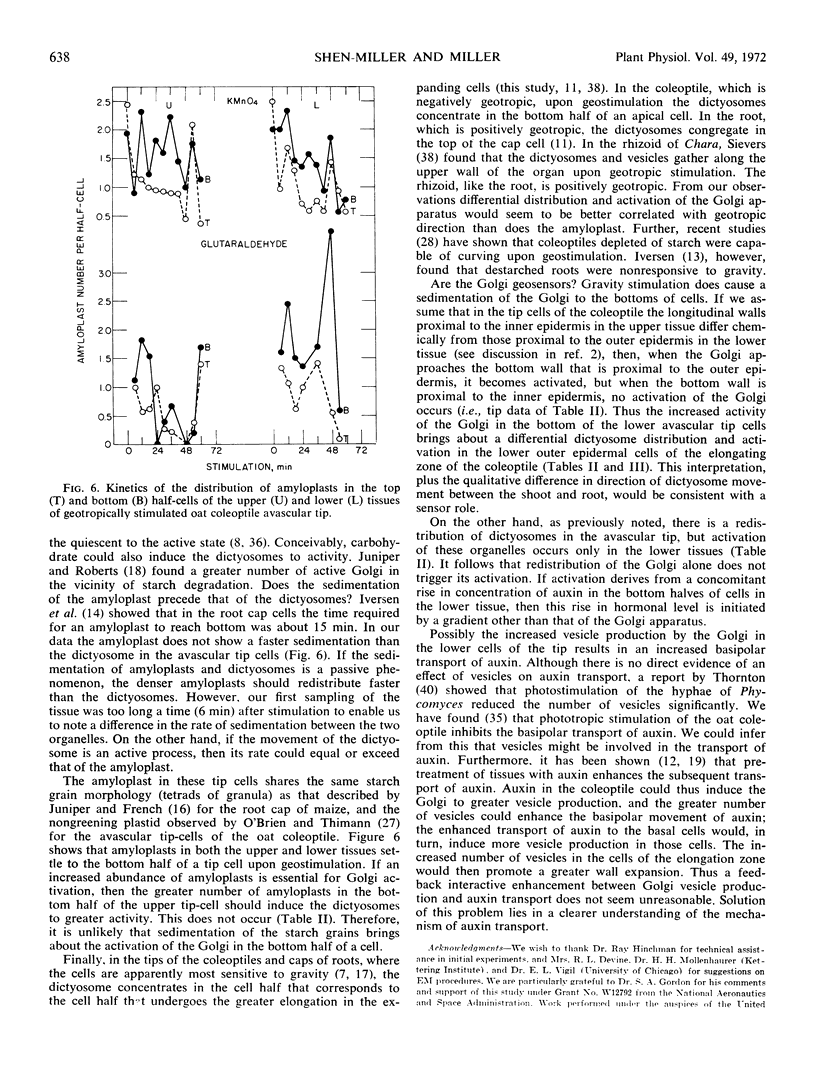
We find a differential distribution of dictyosomes in the avascular tip cells of the oat (Avena sativa) coleoptile upon geostimulation. A differential activation (increased vesicle production) of the dictyosomes with respect to gravity also occurs, but only in the tip cells of the lower tissues. Similar differences in distribution and activation of dictyosomes occur also in cells subjacent to the avascular tip (1st and 5th millimeter from the apex) of both the upper and lower half-tissues. When only the outer epidermal cells below the apex are considered, the differential distribution and activation of dictyosomes occur only in the lower outer epidermis. The changes in distribution of dictyosomes begin at 6 minutes, or sooner, from the start of geostimulation, before the onset of geotropism. The kinetics of amyloplast sedimentation and Golgi movement do not appear to differ in the cells of the avascular tip. We suggest that the Golgi participates in, and possibly initiates, the differential elongation of cells of geotropically stimulated coleoptiles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Jr, Franke W. W., Kleinig H., Falk H., Sitte P. Cellulosic wall component produced by the golgi apparatus of Pleurochrysis scherffelii. Science. 1969 Nov 14;166(3907):894–896. doi: 10.1126/science.166.3907.894. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Jr Observations on the relationship of the golgi apparatus to wall formation in the marine chrysophycean alga Pleurochrysis scherffelii Pringsheim. J Cell Biol. 1969 Apr;41(1):109–123. doi: 10.1083/jcb.41.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., PALADE G. E. PROTEIN SYNTHESIS, STORAGE, AND DISCHARGE IN THE PANCREATIC EXOCRINE CELL. AN AUTORADIOGRAPHIC STUDY. J Cell Biol. 1964 Mar;20:473–495. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Hamilton R. L., Mollenhauer H. H., Mahley R. W., Cunningham W. P., Cheetham R. D., Lequire V. S. Isolation of a Golgi apparatus-rich fraction from rat liver. I. Method and morphology. J Cell Biol. 1970 Mar;44(3):484–491. doi: 10.1083/jcb.44.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard B. G., Thimann K. V. Geotropic response of wheat coleoptiles in absence of amyloplast starch. J Gen Physiol. 1966 May;49(5):1065–1086. doi: 10.1085/jgp.49.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Hernandez W., Leblond C. P. Detection of complex carbohydrates in the Golgi apparatus of rat cells. J Cell Biol. 1969 Feb;40(2):395–414. doi: 10.1083/jcb.40.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Shen-Miller J., Cooper P., Gordon S. A. Phototropism and photoinhibition of basipolar transport of auxin in oat coleoptiles. Plant Physiol. 1969 Apr;44(4):491–496. doi: 10.1104/pp.44.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. The Golgi apparatus in chick corneal epithelium: changes in intracellular position during development. J Cell Biol. 1970 Apr;45(1):34–42. doi: 10.1083/jcb.45.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODING F. B., NORTHCOTE D. H. THE DEVELOPMENT OF THE SECONDARY WALL OF THE XYLEM IN ACER PSEUDOPLATANUS. J Cell Biol. 1964 Nov;23:327–337. doi: 10.1083/jcb.23.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]




