Abstract
Endometrial adenocarcinoma is the most frequently diagnosed cancer of the female genital tract in the western world. Studies of complex diseases can be difficult to perform on human tumor samples due to the high genetic heterogeneity in human. The use of rat models is preferable since rat has similarities in pathogenesis and histopathological properties to that of human.
A genomic region including the highly conserved Phf5a gene associated to development of EAC has previously been identified in an association study. PHF5A has been suggested to acts as a transcription factor or cofactor in the up regulation of expression of Gja1 gene in the presence of estrogen. It has earlier been shown that the Phf5a gene is down regulated in rat EAC derived cell lines by means of expression microarrays.
We analyzed the expression of Phf5a and Gja1 by qPCR, and potential relations between the two genes in EAC tumors and non-malignant cell lines derived from the BDII rat model. In addition, the expression pattern of these genes was compared in rat and human EAC tumor samples.
Changes in expression for Phf5a/PHF5A were found in tumors from both rat and human even though the observed pattern was not completely consistent between the two species. By separating rat EAC cell lines according to the genetic background, a significant lower expression of Phf5a in one of the two cross backgrounds was revealed, but not for the other. In contrast to other studies, Phf5a/PHF5A regulation of Gja1/GJA1 was not revealed in this study.
Keywords: BDII, Endometrial cancer, Genetic background, Phf5a, Gja1
Background
Endometrial carcinoma arises from the endometrium, the inner lining of the uterus. Endometrial adenocarcinoma (EAC), the predominant sub type, is the most frequently diagnosed cancer of the female genital tract ranking fourth among the invasive tumors that affect women in the western world. Approximately 85% of the patients with the diagnosis EAC are over 50 years of age [1]. As most cancers, EAC is a complex disease and development of the tumors is influenced by multiple genetic alterations. As the endometrium is a hormone-dependent tissue, tumors developed in this tissue, including EACs, are mainly hormone-dependent [2]. It has been suggested that excess administration of estrogen may act as one of the main factors in predisposition to EAC in women [3].
Studies of complex diseases can be difficult to perform on human tumor samples due to the high genetic heterogeneity in human. Therefore, in studies of complex disease and as a complement to studies in human, model organisms such as inbred rat strains are often used. The use of rat models is preferable since rat has similarities in pathogenesis and histopathological properties to those of human [2,4]. In the EAC susceptible BDII rat strain, more than 90% of the virgin females develop tumors spontaneously during their lifetime. This tumor model has been genetically well characterized, but there is still much important genetic information that remains to be fully understood in this model [5].
Associations between certain marker alleles and tumor incidence in cross progenies, including the susceptible BDII strain and non-susceptible strains, were identified by means of genome-wide screening with microsatellites. It was clear from the data on tumors developed in the inter-strain crosses that the onset of tumors depends not only on the presence of susceptibility alleles from the EAC-prone BDII strain, but is also affected by the contribution from the non-susceptible strains [6,7].
In another study of the same tumor material developed in the inter-strain crosses expression profiling of tumor and pre malignant cell lines was performed. A number of genes were significantly differentially expressed between EAC and pre/non malignant cell lines, and several of these belonged to cancer-associated pathways [8]. One of the genomic regions associated to development of EAC identified in the association studies included Phf5a, and gene expression profiling analysis revealed this gene was down regulated in EAC derived cell lines.
The PHD finger-like domain protein 5a (PHF5A) is ubiquitously expressed and is located in the nucleus. The gene Phf5a and its human counterpart PHF5A (former name Ini) are located on rat chromosome 7 (RNO7q34) and human chromosome 22 (HSA2213q2), respectively. The rat gene consists of four exons, while the human gene have five exons. Both genes encode a highly conserved protein of 110 amino acids that contains a PHD finger domain. The PHD-finger domain is composed by eight amino acids and has been found in two major groups of proteins. One group consists of transcriptional activators, repressors and cofactors, and the second major group consists of proteins involved in chromatin modulating complexes such as acetyltransferase or complexes containing acetyltransferase [9].
The alignment between the coding sequence of human PHF5a to mouse Phf5a and rat Phf5a revealed a sequence identity of 91% and 94%, respectively and at the protein level the amino acid sequences are 100% identical between the three species [10].
PHF5A acts as a transcription factor or cofactor in the expression of the gap junction alpha 1 (Gja1) gene, which is normally up regulated in uterus. The PHF5A protein binds to the proximal region of the Gja1 promoter and promotes the up regulation of Gja1 by estrogen [9,11]. The Gja1 gene encodes a Gap junction alpha 1 (former name connexin43) protein that is one of 21 different isoforms that belongs to the connexin family. Connexins are membrane proteins that form channels between adjacent cells and mediate cell-to-cell communication [12-15]. Gja1 is expressed in various tissues like the brain, heart, ovary, uterus and smooth mussels including the myometrium. In the uterus, gap junctions are required for coordination of the contractions at the end of the pregnancy. In the myometrium Gja1 is under control of a steroid hormone as being up regulated by estrogen and down regulated by progesterone [11].
The expression of GJA1 is often down regulated in mammary carcinoma cell lines indicating that in these cases the role of GJA1 in carcinogenesis in maintaining cell differentiation and preventing transformation into cancer cells can not be fulfilled [16,17]. In addition it was found that the expression of GJA1 decreases with increased grade of endometrial adenocarcinoma [18].
The aim of this study was to investigate the expression of Phf5a and its effect on Gja1 expression in tumor and non-malignant cell lines derived from the BDII rat model with different genetic backgrounds. In addition, the expression pattern of these genes was compared to the expression pattern of the corresponding genes in the human tumor samples of FIGO grades I-III.
Results
The gene expression of Phf5a and Gja1 in the rat samples was measured in four groups defined by their cross origin (BDIIxBN)xBDII or (BDIIxSPRD)xBDII and cell type (EAC or NME). Five EACs and 5 NMEs with the BN background and 6 EACs and 6 NMEs with the SPRD background were analysed (Table 1). Gene expression of PHF5A and GJA1 in the human material was measured in 30 human EACs in FIGO grade I-III (10 tumors from each grade), and 26 benign (12 secretory phase and 14 proliferative phase) were analyzed with the students t-test for differences between groups (Table 2).
Table 1.
Overview of the rat tumor material used in this study
| Tumor | Background | Pathology |
|---|---|---|
| NUT43 |
(BDIIxBN)xBDII |
EAC |
| NUT50 |
(BDIIxBN)xBDII |
EAC |
| NUT81 |
(BDIIxBN)xBDII |
EAC |
| NUT97 |
(BDIIxBN)xBDII |
EAC |
| NUT128 |
(BDIIxBN)xBDII |
EAC |
| NUT75 |
(BDIIxBN)xBDII |
NME |
| NUT110 |
(BDIIxBN)xBDII |
NME |
| NUT118 |
(BDIIxBN)xBDII |
NME |
| NUT122 |
(BDIIxBN)xBDII |
NME |
| NUT129 |
(BDIIxBN)xBDII |
NME |
| NUT7 |
(BDIIxSPRD)xBDII |
EAC |
| NUT12 |
(BDIIxSPRD)xBDII |
EAC |
| NUT41 |
(BDIIxSPRD)xBDII |
EAC |
| NUT42 |
(BDIIxSPRD)xBDII |
EAC |
| NUT47 |
(BDIIxSPRD)xBDII |
EAC |
| NUT84 |
(BDIIxSPRD)xBDII |
EAC |
| NUT48 |
(BDIIxSPRD)xBDII |
NME |
| NUT56 |
(BDIIxSPRD)xBDII |
NME |
| NUT58 |
(BDIIxSPRD)xBDII |
NME |
| NUT68 |
(BDIIxSPRD)xBDII |
NME |
| NUT89 |
(BDIIxSPRD)xBDII |
NME |
| NUT91 |
(BDIIxSPRD)xBDII |
NME |
| REF | Rat Embryo Fibroblast |
Table 2.
Overview of the human tumor material used in this study
| Sample | Tumor grade | Tissue |
|---|---|---|
| Endometrium |
|
Normal |
| 14 |
Proliferative phase |
Benign |
| 12 |
Secretory phase |
Benign |
| 10 |
Type I |
Malignant |
| 10 |
Type II |
Malignant |
| 10 | Type III | Malignant |
Prior to the statistical analysis of the Ct values, One-Way ANOVA were conducted for analyses of differences among replicates in the rat as well as the human material. There were no significant differences detected among the replicates in either of the data sets. Thus, the average Ct values of the replicates were used in the following calculations of the relative quantitative gene expression. Any undetected Ct values for either of the genes were set to the Ct value of 40 cycles, which corresponds to expression at very low levels.
Pearson´s correlation test on log 2-fold change was performed to explore potential correlation between Phf5a/PHF5A and Gja1/GJA1. No expression correlation between the two genes could be seen, in the rat or human material.
In the rat tumor set, comparison of the gene expression of Phf5a and Gja1 between the EAC and NME samples showed a slight, but not significant, decrease in in expression of Phf5a in the EAC cell lines. The corresponding test for Gja1 revealed no significant differences. In comparisons between all samples of the BN cross origin and the SPRD cross origin, no significant differences were detected for any of the genes investigated (Table 3).
Table 3.
The P-values obtained from the independent sample t-test for differences between groups
| Rat | Phf5a | Gja1 | |
|---|---|---|---|
| EAC |
NME |
0.056 |
0.915 |
| BN |
SPRD |
0.376 |
0.784 |
| BN/EAC |
SPRD/EAC |
0.038* |
0.528 |
| BN/NME |
SPRD/NME |
0.333 |
0.935 |
| BN/EAC |
BN/NME |
0.006** |
0.606 |
| SPRD/EAC |
SPRD/NME |
0.991 |
0.727 |
| Human |
|
PHF5A |
GJA1 |
| Type I-III |
|
0.262 |
0.4461 |
| Prol |
Secr |
0.685 |
0.156 |
| Type I-III |
Prol/Secr |
0.000*** |
0.282 |
| Type I |
Prol/Secr |
0.002** |
0.559 |
| Type II |
Prol/Secr |
0.000*** |
0.517 |
| Type III | Prol/Secr | 0.004*cp | 0.081 |
* P< 0.05.
** P< 0.01.
***P< 0.001.
When we performed the analysis separating groups by tissue type, EAC and NME, and cross background, (BDIIxSPRD)xBDII and (BDIIxBN)xBDII, still no significant differences were detected for Gja1 in any of the comparisons. For Phf5a significant differences were found in comparisons between EACs developed in the BN and those in the SPRD background with a lower expression in the tumor cell lines derived from the progenies of the (BDIIxBN)xBDII crosses (P<0.05). The EAC cell lines developed in the (BDIIxBN)xBDII progeny displayed a significant decrease in Phf5a expression (P<0.01) when compared to the NMEs with the same background (Figure 1A and Table 3).
Figure 1.
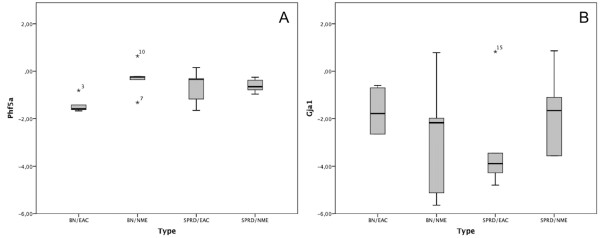
Gene expression of Phf5a (A) and Gja1 (B) in NME and NUT rat cell lines. Gene expression of (A) Phf5a and (B) Gja1 in rat endometrial adenocarcinoma and pre-malignant endometrial cell lines. The bars represent the mean delta delta Ct value in each group. The median in each group is represented by horizontal line.
In the human material no significant differences (P>0,05) between the proliferative and the secretory phase of the benign tumors for either PHF5A or GJA1 were seen, and accordingly the two benign classes were merged (Table 3). An ANOVA test on the tumor classes revealed no significant differences among the different tumor grades (P>0,05, but still we analyzed the classes separately. For the gene GJA1 no significant difference in any of the comparisons between malignant and benign material were detected (Table 2, Figure 2B). For PHF5A all tumor classes were up regulated compared to the benign samples, where type II tumors differed most from the benign samples (Table 3, Figure 2A).
Figure 2.

Gene expression of PHF5A (A) and GJA1 (B) in samples of FIGO grade I-III and benign samples. (A) PHF5A and (B) GJA1 gene expression in human endometrial adenocarcinomas of benign samples and FIGO grade I-III tumor samples. The bars represent the mean delta delta Ct value in each group. A horizontal line represents the median in each group.
Discussion
From the results of the association studies in the BDII model of EAC, one small genomic region associated to the development of EAC that included the Phf5a gene was identified [6,7]. In an expression profiling study, the Phf5a gene was shown to be down regulated in certain EAC cell lines from tumors developed in the F2 and N1 progenies [8]. The chromosomal localization of Phf5a is RNO7, band q34, and the PHF5A corresponding human region is located on HSA22 (HSA2213q2), and the gene is highly conserved through evolution.
It has been suggested that the PHF5A protein plays a complex role as a general transcriptional activator for different genes. Trappe et al. (2002) suggested the PHF5A yeast orthologue plays a crucial roll for cell viability and survival [9]. In C. elegans the Phf5a orthologue displays a tissue- and stage-specific pattern of expression during morphogenetic development [19]. In rat myometrium the phf5a protein has been suggested to function as a transcription factor for the gene Gja1 in the presence of estrogen by binding to the proximal promoter region and enhance expression of Gja1[11]. The protein encoded from the gene Gja1 is a component of the gap junctions that form intercellular channels for diffusion of molecules between cells. It has been suggested that GJA1 is down regulated in different human cancer types, presumably through promoter hyper-methylation, and that it displays tumor suppressor activity [17,20]. However, down regulation of GJA1 through its promoter hyper-methylation was shown not to be true for in human colorectal cancer [20,21], and therefore silencing of the GJA1 was suggested be mediated by other mechanisms through estrogen activation. One possible mechanism could be up regulation of Gja1 by Phf5a through the action of estrogen as suggested by Oltra et al. [11].
In this study, we investigated the expression, and a potential correlation of Phf5a and Gja1 in rat cell lines. As the cell lines were derived from tumors developed in crosses between the females of the BDII inbred strain, susceptible to develop EAC and two non-susceptible strains (BN and SPRD), the impact of the genetic background could also be taken into consideration. In human the influence of the genetic background on the development and path of tumourigenesis is difficult to grasp in human clinical materials. Still, there are some cases were specific alleles have a protective or enhancing effect on the expression of mutation alleles in BRCA1/BRCA2[22,23]. In inbred animal models there are good opportunities to design crosses and experiments to enable studies of the impact of the genetic background on the outcome of carcinogenesis. The BDII rat model has been used for studies of EAC onset and development on the DNA, as well as the genomic level. In association studies, it was proved that depending on cross background, (BDIIxSPRD)xBDII and (BDIIxBN)xBDII, different genomic regions were associated to the onset of tumors [7,24]. SKY analysis of genomic aberrations in the cell lines derived from the tumors developed among females in the cross progenies, revealed that some aberrations in the genome were common to both cross backgrounds and others occurred only in one of the cross backgrounds [25].
Without considering the genetic background of rat female progenies that developed tumors, the expression of Phf5a in EAC cell lines was shown to be slightly lower than the NME samples, but not significantly lower. By separating the cell lines according to cross background, a significant lower expression of Phf5a in the EAC derived cell lines with the BN background compared to the EACs from the SPRD background was revealed. Phf5a was not differentially expressed in comparison with the non-malignant cell lines in the SPRD background, but in the BN background (Figure 1A). Accordingly, cross set-ups such as in the BDII rat model, permit findings that otherwise is difficult to uncover.
The normal function of GJA1/Gja1 is in the myometrium is coordination of the contractions at the end of the pregnancy, and is under control of a steroid hormone Expression of GJA1 has been shown to be down regulated in mammary tumors, lung cancer and endometrial adenocarcinoma [18,26-29], as GJA1/Gja1 normally maintains cell differentiation and prevents transformation into cancer cells [11,16,17]. In addition it has been found that the expression of GJA1 decreases with increased grade of endometrial adenocarcinoma [18].
This earlier results could not be validated in this study as the Gja1 gene was not down regulated in the rat EAC cell lines, and no significant differences between the different cross backgrounds or between EACs and NMEs were detected. It is known that the PHF5A protein binds to the proximal region of the Gja1 promoter and promotes the up regulation of Gja1 by estrogens as described in transfections experiments [9,11]. In these experiments, it was stated that PHF5A was localized in the nucleus, where it binds to the GJA1 promoter and up regulates the expression of GJA1 in the presence of estrogens in a tissue specific and dose dependent way. In contrast to the studies describes above [11], it could not be corroborated that Phf5a regulate the expression of Gja1 in experimental EACs as no correlation between the expression of Gja1 and Phf5a could be seen (Table 3, Figure 1B).
The result of the expression study of Phf5a in rat could not be verified in the human samples. Although PHF5A was also down regulated in the human tumor samples, but unexpectedly, it was more down regulated in the human benign samples.
Conclusions
To conclude, changes in expression in tumors for one of the genes, PHF5A/Phf5a were found both in human and rat, even if the pattern of changes is not completely consistent between the two species. The reason for this discrepancy has to be further investigated. We could not confirm suggestions made in earlier studies, where it was shown that PHF5A regulate the expression of GJA1. The impact of estrogen in this regulation has been investigated in the same study [11]. We plan to investigate and validate this in the material used in an extended study, where we will also investigate the function of PHF5A as a promoter for other genes.
The utmost important finding in this study was that, by using an experimental model, we could successfully show that certain aberrations in the tumors that lead to changes in gene expression, and subsequent changes in protein expression is dependent on the genetic background. Thus, the importance of using animal models as a complement to clinical studies is obvious.
Material and methods
Rat tumor material
In order to study genetic aspects of EAC development, intercross (F1, F2) and backcross (N1) populations were set up by breeding BDII females to males from two different strains with low EAC incidence (BN and SPRDCu3). Females were examined weekly, suspected tumors were were surgically removed and pathologically examined, and cell cultures were established when possible [24]. The tumors were pathologically classified as EAC, or other uterine tumors. In some cases no cancer cells were detected when pathologically examined. These tissues were referred to non-malignant endometrium (NME) (Table 1) [8,30]. In this study cell lines from tumors and pre/nonmalignant tissues in the backcross female progeny were used (NUT).
In vitro cell culture conditions
Primary cell cultures from endometrial material (EAC and NME) and rat embryo fibroblast (REF) cell line were cultured in Dulbecco’s modified Eagle medium (DMEM, Invitrogen) supplemented with 100 IU/100 μg/ml penicillin/streptomycin, L-glutamine, MEM amino acids, MEM Non-Essential Amino acids, MEM Vitamins solution and 10% heat-inactivated fetal bovine serum, for 3–5 passages in order to obtain the required amount of cells. The cells were grown at 37°C with a 5% CO2 and humidity at 95% and harvested by trypzination at a confluence of 80-90%. In this study, a total of 22 primary cell cultures (11 EAC and 11 NME) were used (Table 1).
Human tumor material
A total of 30 EACs in FIGO grade I-III (10 tumors from each grade) embedded in archival formalin fixed paraffin (FFPE) were used in the study. As control, 26 benign FFPE endometrial tissues (12 of secretory phase and 14 of proliferative phase) were used. As the reference sample, one sample from normal endometrium was used in the normalization process (Table 2) [31] for further details).
Exctraction av RNA and RT-PCR
Total RNA was isolated from the EAC/NME cell lines (Table 1) using the Qiagen® AllPrep RNA Mini Kit according to the manufacturer’s protocol. Total RNA was extracted from human endometrial paraffin imbedded tissue samples (Table 2) using Qiagen® AllPrep RNA FFPE Kit. RT-PCR was performed on 500 ng of total RNA, using the High Capacity RNA-to-cDNA Kit according to the manufacturer’s protocol (Applied Bio-systems, USA).
Quantitative PCR (qPCR) of Phf5a and Gja1 in rat tumors
A total of 11 EAC and 11 NME cell lines were included in the qPCR analysis. The house keeping gene, Gapdh was used as an endogenous control and the Rat embryo fibroblast cell line (REF) was used as an exogenous control. Template cDNA was added to TaqMan Universal Master Mix (Applied Biosystems, USA) in a 25 μl reaction with specific pre-designed probes for Phf5,a Gja1 and Gapdh (Applied Biosystems). Reactions were performed in triplicates and the averages threshold cycle number was used for further analysis. Relative gene expression quantification was calculated according to the comparative Ct method using Gapdh as an endogenous control and REF as calibrator. Final results were determined as follows: 2–(ΔCt sample–ΔCt calibrator), where ΔCt values of the calibrator and sample were determined by subtracting the Ct value of the target gene from the value of the Gapdh gene.
Quantitative PCR (qPCR) of PHF5A and GJA1 in human tumors
Total RNA from paraffin imbedded tissue samples was used for qPCR. The house keeping gene, GAPDH was used as an endogenous control and RNA from the endometrial tissue was used as an exogenous control. The qPCR reaction setup followed the same procedure as for the rat samples.
Statistical analysis
For statistical evaluations of gene expression data, Ct values for differences among replicates was analyzed by ANOVA. For comparisons of expression differences between normal and malignant tissues, independent sample t-test was applied on the log 2-fold change (PASW Statistics 20, SPSS Inc, Chicago, USA). In both tests the null hypotheses were assuming no differences between replicates, and no differences between tissue types, respectively. The Pearson correlation test was performed to check for expression correlation between Phf5a/PHF5A and Gja1/ GJA1. The significance levels were set to P<0.05 in all statistical tests. Furthermore, the tumor material from the rat samples was analyzed for differences in gene expression between the two backgrounds (BN and SPRD).
Abbreviations
EAC: Endometrial adenocarcinoma; NME: Normal/pre-malignant endometrium; NUT: Backcross, rat uterine tumor; FIGO: International Federation of Gynecology and Obstetrics.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EF contributed with ides, designed the study, performed all the experiment, data analysis and drafted the manuscript. KKL supervised the project, contributed with ideas and took part in the preparation of the manuscript. Both authors have read and approved the final version of the manuscript.
Contributor Information
Eva Falck, Email: eva.falck@his.se.
Karin Klinga-Levan, Email: karin.klinga.levan@his.se.
Acknowledgement
This work was supported by, the Erik Philip-Sörensen Foundation, the Nilsson-Ehle Foundation and Wilhelm and Martina Lundgren Foundation. We are grateful to Department of Pathology, Örebro University Hospital, Sweden for providing us with human tumor material for this study.
References
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Vollmer G. Endometrial cancer: experimental models useful for studies on molecular aspects of endometrial cancer and carcinogenesis. Endocr Relat Cancer. 2003;10(1):23–42. doi: 10.1677/erc.0.0100023. [DOI] [PubMed] [Google Scholar]
- Cavanagh D, Fiorica JV, Hoffman MS, Durfee J, Nicosia SV. Adenocarcinoma of the endometrium: an institutional review. Cancer Control. 1999;6(4):354–360. doi: 10.1177/107327489900600405. [DOI] [PubMed] [Google Scholar]
- Samuelson E, Hedberg C, Nilsson S, Behboudi A. Molecular classification of spontaneous endometrial adenocarcinomas in BDII rats. Endocr Relat Cancer. 2009;16(1):99–111. doi: 10.1677/ERC-08-0185. [DOI] [PubMed] [Google Scholar]
- Deerberg F, Kaspareit J. Endometrial carcinoma in BD II/Han rats: model of a spontaneous hormone-dependent tumor. J Natl Cancer Inst. 1987;78(6):1245–1251. [PubMed] [Google Scholar]
- Roshani L, Beckman B, Szpirer S, Hedrich HJ, Klinga-Levan K. The rat as a model for identification of susceptibilty genes involved in the development of endometrial carcinoma. Rat Genome. 2000;6:76. doi: 10.1002/ijc.1553. [DOI] [PubMed] [Google Scholar]
- Roshani L, Mallon P, Sjostrand E, Wedekind D, Szpirer J, Szpirer C, Hedrich HJ, Klinga-Levan K. Genetic analysis of susceptibility to endometrial adenocarcinoma in the BDII rat model. Cancer Genet Cytogenet. 2005;158(2):137–141. doi: 10.1016/j.cancergencyto.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Olsson B, Klinga-Levan K. Gene expression profiling predicts a three-gene expression signature of endometrial adenocarcinoma in a rat model. Cancer Cell Int. 2009;9:12. doi: 10.1186/1475-2867-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe R, Ahmed M, Glaser B, Vogel C, Tascou S, Burfeind P, Engel W. Identification and characterization of a novel murine multigene family containing a PHD-finger-like motif. Biochem Biophys Res Commun. 2002;293(2):816–826. doi: 10.1016/S0006-291X(02)00277-2. [DOI] [PubMed] [Google Scholar]
- Oltra E, Fulvia V, Werner R, D’Urso G. A novel RING-finger-like protein Ini1 is essential for cell cycle progression in fission yeast. J Cell Sci. 2003;117:967–974. doi: 10.1242/jcs.00946. [DOI] [PubMed] [Google Scholar]
- Oltra E, Pfeifer I, Werner R. Ini, a small nuclear protein that enhances the response of the connexin43 gene to estrogen. Endocrinology. 2003;144(7):3148–3158. doi: 10.1210/en.2002-0176. [DOI] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83(4):1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10(4–6):173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131(3):570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal. 2006;18(5):592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12(3–4):225–256. doi: 10.1615/critrevoncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66(20):9886–9894. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- Schlemmer SR, Novotny DB, Kaufman DG. Changes in connexin 43 protein expression in human endometrial carcinoma. Exp Mol Pathol. 1999;67(3):150–163. doi: 10.1006/exmp.1999.2286. [DOI] [PubMed] [Google Scholar]
- Trappe R, Schulze E, Rzymski T, Frode S, Engel W. The Caenorhabditis elegans ortholog of human PHF5a shows a muscle-specific expression domain and is essential for C. elegans morphogenetic development. Biochem Biophys Res Commun. 2002;297(4):1049–1057. doi: 10.1016/S0006-291X(02)02276-3. [DOI] [PubMed] [Google Scholar]
- Piechocki MP, Burk RD, Ruch RJ. Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis. 1999;20(3):401–406. doi: 10.1093/carcin/20.3.401. [DOI] [PubMed] [Google Scholar]
- Sirnes S, Honne H, Ahmed D, Danielsen SA, Rognum TO, Meling GI, Leithe E, Rivedal E, Lothe RA, Lind GE. DNA methylation analyses of the connexin gene family reveal silencing of GJC1 (Connexin45) by promoter hypermethylation in colorectal cancer. Epigenetics. 2011;6(5):602–609. doi: 10.4161/epi.6.5.15237. [DOI] [PubMed] [Google Scholar]
- Kirchhoff T, Gaudet MM, Antoniou AC, McGuffog L, Humphreys MK, Dunning AM, Bojesen SE, Nordestgaard BG, Flyger H, Kang D, Yoo KY, Noh DY, Ahn SH, Dork T, Schurmann P, Karstens JH, Hillemanns P, Couch FJ, Olson J, Vachon C, Wang X, Cox A, Brock I, Elliott G, Reed MW, Burwinkel B, Meindl A, Brauch H, Hamann U, Ko YD. Breast cancer risk and 6q22.33: combined results from Breast Cancer Association Consortium and Consortium of Investigators on Modifiers of BRCA1/2. PLoS One. 2012;7(6):e35706. doi: 10.1371/journal.pone.0035706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pankratz VS, Fredericksen Z, Tarrell R, Karaus M, McGuffog L, Pharaoh PD, Ponder BA, Dunning AM, Peock S, Cook M, Oliver C, Frost D, Sinilnikova OM, Stoppa-Lyonnet D, Mazoyer S, Houdayer C, Hogervorst FB, Hooning MJ, Ligtenberg MJ, Spurdle A, Chenevix-Trench G, Schmutzler RK, Wappenschmidt B, Engel C, Meindl A, Domchek SM, Nathanson KL, Rebbeck TR, Singer CF. Common variants associated with breast cancer in genome-wide association studies are modifiers of breast cancer risk in BRCA1 and BRCA2 mutation carriers. Hum Mol Genet. 2010;19(14):2886–2897. doi: 10.1093/hmg/ddq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshani L, Wedekind D, Szpirer J, Taib Z, Szpirer C, Beckman B, Rivière M, Hedrich HJ, Klinga-Levan K. Genetic identification of multiple susceptibility genes involved in the development of endometrial carcinoma in a rat model. Int J Cancer. 2001;94(6):795–799. doi: 10.1002/ijc.1553. [DOI] [PubMed] [Google Scholar]
- Falck E, Behboudi A, Klinga-Levan K. The impact of the genetic background on the genome make-up of tumor cells. Genes Chromosomes Cancer. 2012;51(5):438–446. doi: 10.1002/gcc.21929. [DOI] [PubMed] [Google Scholar]
- Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 1999;59(16):4104–4110. [PubMed] [Google Scholar]
- Chen JT, Cheng YW, Chou MC, Sen-Lin T, Lai WW, Ho WL, Lee H. The correlation between aberrant connexin 43 mRNA expression induced by promoter methylation and nodal micrometastasis in non-small cell lung cancer. Clin Cancer Res. 2003;9(11):4200–4204. [PubMed] [Google Scholar]
- Saito T, Nishimura M, Kudo R, Yamasaki H. Suppressed gap junctional intercellular communication in carcinogenesis of endometrium. Int J Cancer. 2001;93(3):317–323. doi: 10.1002/ijc.1350. [DOI] [PubMed] [Google Scholar]
- Lesniewicz T, Kanczuga-Koda L, Baltaziak M, Jarzabek K, Rutkowski R, Koda M, Wincewicz A, Sulkowska M, Sulkowski S. Comparative evaluation of estrogen and progesterone receptor expression with connexins 26 and 43 in endometrial cancer. Int J Gynecol Cancer. 2009;19(7):1253–1257. doi: 10.1111/IGC.0b013e3181a40618. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Klinga-Levan K. Expression analysis of human endometrial adenocarcinoma in an inbred rat model. Adv Exp Med Biol. 2008;617:503–509. doi: 10.1007/978-0-387-69080-3_50. [DOI] [PubMed] [Google Scholar]
- Falck E, Karlsson S, Carlsson J, Helenius G, Karlsson M, Klinga-Levan K. Loss of glutathione peroxidase 3 expression is correlated with epigenetic mechanisms in endometrial adenocarcinoma. Cancer Cell Int. 2010;10:46. doi: 10.1186/1475-2867-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]


