Abstract
Modular pattern generator elements, also known as burst synergies or motor primitives, have become a useful and important way of describing motor behavior, albeit controversial. It is suggested that these synergy elements may comprise part of the pattern shaping layers of a McCrea/Rybak two layer pattern generator, as well as being used in other ways in spinal cord. The data supporting modular synergies ranges across species including man and encompasses motor pattern analyses and neural recordings. Recently, synergy persistence and changes following clinical trauma have been presented. These new data underscore the importance of understanding the modular structure of motor behaviors and the underlying circuitry in order to best provide principled therapies and to understand phenomena reported in the clinic. We discuss the evidence and different viewpoints on modularity, the neural underpinnings identified thus far, and possible critical issues for the future of this area.
Keywords: motor primitives, synergies, modularity, spinal cord, pattern generation, stroke, spinal cord injury
Introduction
We here review ideas and experimental data related to modularity, in particular motor primitives and muscle synergies, and recent work suggesting the direct clinical relevance of these ideas and data. We will highlight gaps to be filled by the research community which are likely, when filled, to produce new therapeutic information and strategies.
Modular systems have components which may be separated and recombined. Modularity thus implies a compositional set of building blocks with different possible arrangements.1,2 Interest in motor modularity has grown greatly recently.3,4 However, the area remains controversial and competing perspectives are presently available on the topic. For example, there are several levels of analysis ––kinematic, muscle, neural, supporting modularity –– and several different sets of ideas about the origins of modularity in each of these. Depending on the perspective, modularity can be seen as a benefit or an impediment to motor control, as a fundamental neural structural feature, as an epiphenomenon of other more fundamental mechanisms, and as offering both clinical opportunities and hindrances. However, it is becoming clear that modularity perspectives at the worst offer a concise shorthand to describe clinical changes and pathology,5–7 and for this reason alone it is important to gain a better insight into modularity. Our understanding of the processes supporting modular motor control and the spinal segmental implementation of modularity remains sketchy,8–12 but filling out this understanding will be the only way to resolve the controversies and leverage modular descriptions to clinical benefit.
Definitions and types of modules
Modularity is apparent at several levels of analysis in motor control. Table 1 summarizes many of the levels and descriptions used, and some examples of biomimetic robotic uses of modularity for comparison. Here we unpack this description.
Table 1.
Modularity terminology and relationships among descriptions and units
| Marr level of description | Increasingly finer resolution elements of action composition and control | Type of data and description | ||||
|---|---|---|---|---|---|---|
|
Task Behavioral task/Kinematic task/Task planning |
Behavior (e.g., Hunt, groom etc.) | Task: (run to capture); akinematic oscillation | Subtask: (swing, stance, foot placement, reach to grasp) | Stroke/kinematic segmentation unit = kinematic stroke or kinematic primitive | Kinematics | |
|
Algorithms Controls/algorithms (kinetics necessary) |
Timing, rhythm & sequence | d Force pattern for subtask, possible co-articulation | Force pattern for individual kinematic stroke, possible co-articulation | Unit building block for force patterns. Force-field primitives = kinetic primitives | Kinetics | |
| bRhythm generator system | cPattern shaping under rhythm generator, descending and sensory control influences or |
gBurst synergies = motor primitives = force-field primitive. Unitary burst (or “pulse”) of a synchronous muscle synergy |
Muscle patterns | |||
| Rhythm generator system | Pattern shaping system | eTime-varying synergies | ||||
|
Implementation Implementations/circuitry |
Neural oscillator or state chaining system | fNeural switching systems or emergent pattern shaping and sequencing processes | Separated neural premotor drives and burst generation | Neural support | ||
| <=> robotic Dynamical movement primitive (oscillator primitives) or finite state machine | <=> robotic Coupling or selection and switching policy, e.g., finite state machine with time outs | <=> robotic Kinematic primitive and PID controller | <=> robotic Kinetic primitive or multi-joint PD control unit | Sample equivalent idea in biomimetic robotics | ||
Note: Missing from the table are the synergy formulations as optimal controlled subspaces, and controlled/uncontrolled manifolds. The missing terms and models of modularity are harder to relate simply and directly to pattern generation and spinal neural circuits in testable hypotheses. In the table, specific relationships or areas need significant work and are important to understand new data on modularity in stroke and spinal cord injury.
The relations of rhythmic and discrete motion at kinematic and production descriptions remain an area of ambiguity.
As might be expected, rhythm/timing generation mechanisms and circuit location remain insufficiently specified.
The structure of pattern shaping remains difficult and controversial. The separation from rhythm generation is controversial. The composition and modularity of pattern is disputed. Modularity can be considered emergent from network dynamics or based on switching and incorporation of explicit modules.
Efficient kinematic and kinetic action may involve co-articulation of modules in sequences and mechanisms should support this contextual adjustment for increasing skill levels in tasks.
Modules in pattern shaping could be fixed sequences of several bursts, also called time varying synergies, that are explicitly related to kinematic modules such as strokes or cycles. Alternatively, pattern shaping modules could be single bursts, that are not uniquely or closely related to kinematic strokes and cycles (e.g., unit bursts/synchronous synergies that instead relate directly to units of biomechanical modularity such as unitary force-fields, and to neural drive (see g below).
The structure of neural switching and pattern sequencing is very poorly understood but is probably crucial to understand processes of co-articulation, merging, splitting, and sequence changes after neural damage and during therapy.
Neural underpinning of unit bursts and drive structure are also part of the key to these clinically meaningful processes.
Kinematic descriptions
Early kinematic analyses undertaken by ethologists supported modular constructions, although these ethological descriptions generally faltered as more detailed analysis of execution was considered.17 Nonetheless, Fentress and Golani,13,14 and more recently Whishaw,15,16 have used such kinematic modular analyses to good effect. Kinematic execution modularity has provided the most insight in work on human reach kinematics and its segmentation. As elements of the segmentation, unitary “kinematic strokes” were noted by Viviani and Terzuolo.18 Explanations for the observed stroke properties were first explored by Hogan and Flash20,21. In general, the motions of limb end-points exhibit straight paths, and have unimodal bell-shaped tangential velocity profiles. These profiles and the resultant stroke segmentations were consistent with kinematic optimization and were well predicted by an endpoint minimization of jerk.20,21 For point to point motions, these features are preserved across loads and in different species and environments.12,22,23 However, likely due to features of biological limb design, the kinematic strokes observed are also consistent with various other kinetic and task optimizations. These other optimizations include minimum torque change and minimized signal dependent noise at the muscle.24 Superposition of collections of kinematic strokes by the central nervous system (CNS) account for various learning, correction, and rehabilitation phenomena.23,25,27 Flash and colleagues continue to develop better descriptions for similar analyses of more complex 3D kinematics.26
Pattern generation and pattern structure descriptions
Rhythmic motions have repeating forms that can be considered modular. Modular and repeatable controls for rhythmic motions have been evaluated at both kinematic and motor pattern levels. Evaluations at the kinematic level include the research of Schaal and colleagues,28,29 and Sternad and Hogan.30,33 Schaal, Ijspeert, and colleagues suggested dynamic primitives in robotics.31,32 Pattern generation was clearly established by seminal work of Wilson, Grillner, and their successors in both vertebrate and invertebrate animals,34 and suggested that central motor pattern was fundamental.35 Examples of modularity at the pattern generator levels would be unit burst generators for hip flexion and hip extension as examined by Stein and colleagues in turtles.36–39 The CNS can organize modular patterns underlying behaviors (usually rhythmic), independent of any feedback or patterned input. Identifying the structure of pattern generator circuitry is an ongoing process. While the neural elements comprising the structure of patterned generators are well identified and modeled in simpler vertebrate circuits,36,40 the topic is significantly less well understood in complex mammalian systems. The biological central pattern generators (CPGs) could represent several types of engineer-style controllers. Neural networks in the CNS could implement dynamic system limit-cycle oscillators. These might be implemented as half center oscillators, as in the original models of Brown and succeeding work. Alternatively, the CPG might represent a kind of finite state machine, one that cycles through its states in the absence of rhythmic inputs. Various pacemaking and burst generating mechanisms in neurophysiology, e.g., in respiration,41 arguably show some features of such a system. Recent data from the paralyzed decerebrate cat supports a hierarchical hybrid CPG in mammalian hindlimb locomotion: a rhythmic or clocking system layer (constructing timing features and perhaps state sequencing) exerts control on a pattern shaping layer.42–48 This hybrid framework is also consistent with component motor primitive/synergy elements (see below) and unit burst elements.36–39
Muscle Synergies
To discover pattern and modularity in unparalyzed animals and humans behaving more broadly, statistical analysis of motor patterns has been used.7,59–511 In animals ranging from the frog to the intact or injured human being, and in both rhythmic and non-rhythmic behaviors, application of statistical decomposition techniques show a remarkably similar breakdown of muscle activation patterns into modular muscle groups (or synergies or drive motor primitives). Other non-statistical physiological methods also support the modules and analysis results.52,53 These muscle groups or synergies are activated as unitary synchronous muscle bursts or pulses, either in sequence or simultaneously, across various test paradigms.51,54–64 They are adjusted and adapted by the CNS in response to the task conditions by selecting among the different synergies, and by changing overall burst amplitudes and onset timings. Dimensionality reduction of the full range of muscle activations that are possible, down to a significantly smaller set of muscle synergies, seems to be the rule. The data are thus consistent with the separate sequencing and control of a small set of premotor drives in each task. The precise structure of the drives has been disputed. Some authors have favored synchronous unit bursts (equivalent to neurophysiological unit burst generators.9,51–53,56,,65), while others have favored time sequences of muscle activations, with the whole sequence acting as an atom or unit of the motor patterns.60,61,63 Pulses of synergistic activity (or unit burst generators) that act as fundamental building blocks of patterns at spinal levels, added or deleted as units, seem to be favored by most data from both fictive, semi-intact, and intact animal behaviors.8,9,36,42,52,53
Linking muscle synergy to biomechanics - unit bursts as force-field primitives
Muscles are intrinsically visco-elastic. The precise mechanical properties vary with activation, history of activation, and shortening or lengthening history. Muscles act in concert via soft tissues, ligament and tendon systems and their moment arms, through the skeletal linkage and its Jacobean properties, to generate forces that can be described as position- and velocity-dependent fields. The simultaneous (synchronous) activation of a collection of muscles produces a well-defined visco-elastic force-field in the limb which modulates with time. This field depends on the initial limb state. In this way force-field descriptions can capture the action of muscle synergies (Fig. 1 and 66–74). Muscles in a synchronous burst synergy have fixed ratios to each other, matching the ratios of cis in Figure 1, e.g., see Figure 2. These force-field descriptions show the property of linear vector superposition, which can be demonstrated when fields are combined through electrical stimulation of spinal cord, or during natural drive co-activations.52,53,65,71 Feedback systems in the spinal cord do not disrupt these visco-elastic force-field structures, but rather seem to support them.53
Figure 1.
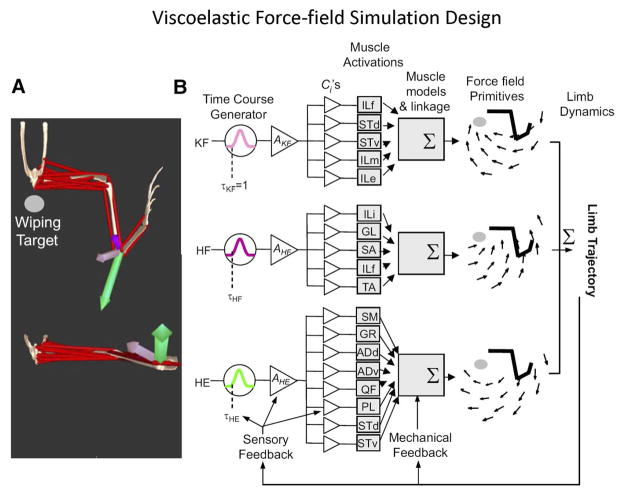
Simulating hindlimb wiping with a detailed model of the spinal primitives. (A) The 13 hindlimb muscles forming the biomechanical model are shown as red lines. Colored arrows mark the force directions of the three force primitives at a fixed limb position during the isometric wiping response: KF (knee flexor primitive), light purple; HE (hip extensor primitive), green; HF (hip flexor primitive), dark purple. (B) The framework used to simulate wiping (left to right): each primitive had a time-course generator, representing the premotor drive burst, which output a normalized waveform (peak = 1.0) at time τ. The variable A scaled this waveform, which was then distributed to each of the muscles within the primitive. Each muscle had a muscle-specific variable C that scaled the excitation wave form. The synergy muscle groups generate contractile forces MF that are transmitted through the limb to produce anisometric endpoint force (at one position) or force field FF (when forces are measured across a range of positions). Normalized force fields produced by each primitive are shown in the far right. When the model limb is freed to move, MFs drive the motion of the model. MF values are in turn regulated by the limb motion (i.e., the force–velocity and force–length properties of muscle and stress–strain properties of in series connective tissue alter MF forces). In this version model, sensory feedback from muscles potentially regulate τ A or C. ILf, iliofibularis; STd, semitendinosus dorsal branch; STv, semitendinosus ventral branch; ILm, iliacus median; Ile, iliacus externus; ILi, iliacus internus; GL, gluteus; SA, sartorius; TA, tibialis anticus; SM, semimembranosus; GR, gracilis; ADd, adductor dorsal; ADv, sdductor ventral; QF, quadratus femoris; PL, peroneus longus. Reproduced from Ref. 83, with permission.
Figure 2.
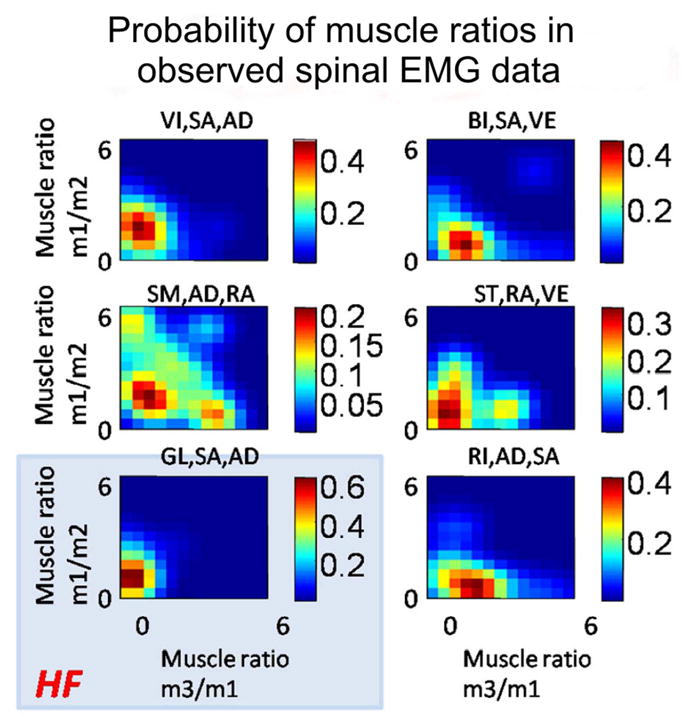
Examining reproducibility of frog primitives. Stability of muscle proportionality ratios in the six main primitives are observed across frogs and across behaviors. The action of the spinal cord across the tested behaviors was to recruit the muscles in fixed ratios and thereby couple muscles so as to generate specific force-field primitives and associated preflex responses. Reflex actions (i.e., feedback effects) modulated these primitives, not individual muscles (see Ref. 53), and thus acted on their component muscles as groups. HF, hip flexor synergy. Muscle Abbrev (nomenclature of Ecker, with equivalent Abbott and Lombard): VI, vastus internus (= iliacus internus); VE, vastus externus (= iliacus externus); BI, biceps (= Iliofibularis); AD, adductor magnus; SA, sartorius; RA, rectus anticus (= quadratus femoris); GL, gluteus. Reproduced from Ref. 83, with permission.
A synergy-driven force-field is a predictable pattern, whether the synergy is activated as a single primitive, as part of a coordinated reflex, or in a voluntary pattern. A ‘force-field motor primitive’ in biomechanics can be associated with a muscle synergy, and corresponds 1:1 to the specific premotor drive bursts (or primitive pulses) occuring in the motor pattern. In effect, the actions of the multiple simultaneously pulsed drives and their associated muscle recruitment effects can be summarized as a sum of the individual muscle biomechanical force effects :
| (1) |
where q is a configuration vector, F is the total limb force-field, Ai is the amplitude of the activation of the ith force-field motor primitive, ai(t) is the normalized activation time course of the ith primitive, and Φi is the normalized visoelastic field associated with the ith premotor drive and its collection of associated muscles. This summation has been demonstrated experimentally.68,69,70,71 Since each field Φi consists of the sum of individual muscle fields, this framework naturally extends from an initially constrained low degree of freedom synergies/motor primitives in reflex and pattern generation through to the fullest voluntary use of spinal cord capabilities of the motor system using individuated muscles that are driven independently from one another.
To generate flexible behaviors and action using the individual premotor drive pulses, the pulses may be repeated and staggered in different combinations:
| (2) |
where τi represents the time shift of the ith drive pulse. The point of this brief mathematical excursion is that a matching between Newton’s laws, a compact control framework, and synergy bursts as motor pattern elements is possible and, taken together, this suggests a simple compositional scheme for movement. This framework ably represents spinal generated behaviors and reflexes53,83 (see Figures 1–3). It also maps cleanly onto the inferred hierarchical control of the pattern shaping systems suggested by McCrea, Rybak and others.
Figure 3.
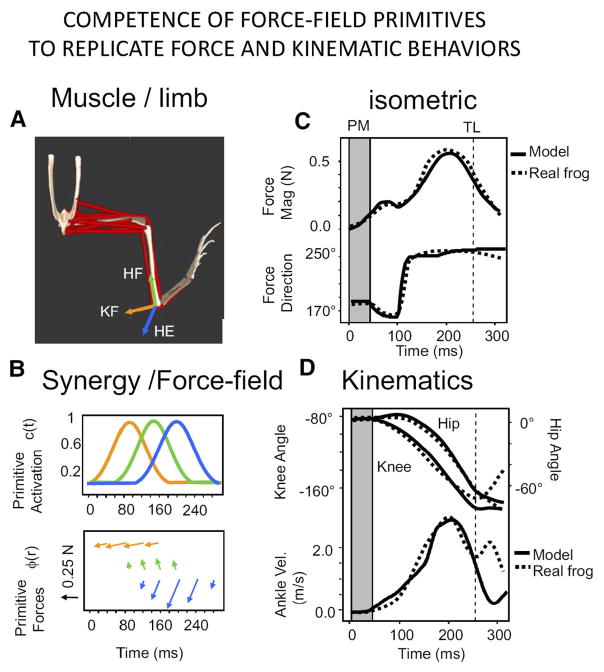
Simulation results using a framework of primitives. Simulating wiping forces and kinematics with the primitive framework and model frog. (A) Model structure. (B) Activation of muscles as synergies. (C) The isometric force pattern produced by the model frog (solid lines) closely matched the force pattern recorded experimentally (dotted lines). (D) After making minor adjustments to the isometric motor pattern (amplitude scaling the ensemble down slightly) the model frog also reproduced the free limb kinematics of the experimental frog. Without downscaling the forces were too strong, indicating potential feedback adjustment in isometric conditions. The top row shows hip and knee angles. The bottom row shows ankle velocity. Dashed line marks the time of target limb contact in the real frog. The gray area (PM) represents the 40-ms premovement period between EMG onset and motion onset that is observed in real frogs.
Support of motor modularity across species
The kinematic stroke modularity seen in human reaching 25 is also exhibited in octopus reaching12,76 in a marine environment using very different effector and neural control circuitry. This suggests fundamental aspects of physics, mechanics and task control may be a basis for the structure of kinematic strokes. Similarly, basic postures and stereotypic motions are exhibited in swimming turns, escape (C-start turns) and other kinematic controls.77,78 Rhythmic kinematics of locomotion in both aquatic, terrestrial and aerial media support common compositional features, this leading Koditschek and Full to the notion of templates and anchors.79 Templates represent the lowest order (lowest dimensional) description of the essence of the physical task and its requirements. Anchors are specific hypotheses regarding the neuromuscular and biomechanical controls used for implementation of the template. In their scheme, motor primitives of any kind (kinematic or kinetic) would be the anchors that allow low dimensional and efficient physical task performance.
Motor patterns and muscle/pattern modularity across species
Features of the motor patterns, stereotypic bursting, and synergies supporting both stereotypic and more flexible behaviors are common from invertebrates to man. Examples of common features have been reviewed and remarked on extensively.80 Recent work from Lacquaniti, Ivanenko, Dominici and colleagues has shown the similarity of rhythmic pattern construction in guinea fowl, rat, cat and human, and across human development, linking rhythm generation and primitives across species.64
Interpreting modular structures: task, algorithm, and implementation
Modularity as exhibited across species in kinematic, kinetic, and motor pattern features could be considered in several ways. The neuroscientist David Marr 81 suggested considering nested levels of task, algorithm and implementation in analyzing CNS function in behavior. This scheme is useful in modularity because it highlights the points of convergence and divergence in different accounts of modularity. At the task level, modularity may simply represent task features that must be managed as constraints or task affordances in the real world: most locomotion and physical engines employ cyclic features, etc. and often have, in the parlance of Full and Koditschek, similar templates. At the algorithmic level, if task solutions are modular, how is this accomplished computationally? What are the computational anchors? How flexible and general are the algorithms employed in different biological systems, in different tasks, and at different levels of the neuraxis? Control theory as a field has developed numerous principled and powerful ways of constructing and managing controls that often converge on modular solutions, and the field continues to advance rapidly. Complex issues such as problems of degrees of freedom are proving more tractable than originally supposed, though still by no means paper tiger problems that can be ignored.84 Optimization methods in control lead to modular solutions.85–89 It is natural to wonder how much of the biological circuitry represents the implementation of a set of powerful, flexible, and principled control algorithms.90–97 Alternatively, how much of biological circuitry represents hacks, heuristics, and the historical baggage from evolution? Engineers all recognize that real-world systems are a compromise between what is ideal and what is possible, given the manufacturing, design, material and other costs available. We do not know how best to balance this cost as we think about biology and motor evolution, ontogeny, and learned interactions. Data and simulation results are often consistent with both perspectives.98,99 It seems that algorithmic flexibility, generality, and learning time must be weighed against the evolutionary pressure to function rapidly (e.g., the wildebeest calf, hatchling sea turtle). The pack-and-go cost of added neural hardware for flexibility in weight and energy consumption must be balanced against other options for using the mass and energy. Some of these kinds of trade-offs are observed in modern consumer technologies such as mobile phones, which also must survive in the marketplace in competition with other choices. Not every phone type is equally smart, or similarly controlled, and each has its niche. While some researchers view modules as built into the neural structure of the CNS by evolution,8 others see them as arising from an optimal control implementation operating on the anatomical affordances in the musculoskeletal system. In this last view, modularity arises as an optimal strategy and division of labor by the optimal controller, which parcels task dimensions into a controlled and an uncontrolled manifold, (i.e., different subspaces) with the controlled task operating in low dimensions.94–96
Some behavioral needs that can always be anticipated are partly embedded in the mechanical design of limbs. De novo neural motor solutions in each generation may waste time and lose access to scarce resources, and be selected against. Further, in biological systems, all requirements for function are not available a priori in early development. Future control needs must be anticipated or learning must be channeled to support the critical functions needed later, beyond the current locally optimal learning. Developmental research supports much pre-structuring of circuits, prior to feedback use.103–106 Nonetheless, subsequent adaptation and extensibility of behaviors are also needed, and automaton-like behavior is clearly insufficient.100–102 Optimization and adaptation in adults is essential. Modularity, if well crafted by evolution, should support both early function and later adjustment to conditions and new affordances.
How might the circuitry and algorithms suggested by these different views arise and be supported in the spinal cord? Are they built-in by evolution, learned in child development, or developed denovo in task solutions? In Marr’s scheme,81 the implementation details of neural systems, where available, may help resolve these conundrums. Current data suggest that different perspectives can be correct, depending on species, age, task, and the specific CNS circuit regions that are examined.
Implementation of spinal primitives and modules –spinal structures
Several lines of investigation support spinal circuits explicitly geared toward modularity. The competence of modular systems is largely established. From the perspective of synergy-based modularity, work in frog, cat, and monkey bears strongly on these issues. In our lab, Corey Hart has demonstrated with single unit extracellular recordings in the frog spinal cord that interneurons with mono- or di-synaptic projections to motor pools replicate the patterns and weightings of synergy drives.107 Other interneurons showed a range of features that further support the idea that the frog reflex circuitry is organized to drive and control onset timing, amplitude, and combinations of synergy and primitives in spinal reflexes (107 and unpublished data). Recordings by Berkowitz and colleagues in turtles support similar shared and specialized neural structures that might match with synergy/primitives analyses of motor pattern.108–110 Behavioral state-dependent control and altered output patterns from a spinal circuit in this case are expected to arise from rearranging the onset timing and combinations of synergy primitives, not from radical alteration of these drive circuits. Within such a framework, duplication of similar projecting drive circuit neurons for specialized use in various contexts could occur. Specialized drive systems that contribute to reaching were characterized in the work of Alstermark and colleagues on the C3–C4 interneuron systems (reviewed by Alstermark and Isa, 111). The precise premotor drive and motoneuron projection patterns of these neurons are not sufficiently characterized to show whether there are truly discrete sets of modular drive projections, but the roles and even genetic developmental processes of the C3–C4 systems in mice, cats, and monkeys are now beginning to yield to modern methods.112 The work of Seki and colleagues, recording in monkey spinal cord, also supports possible modular groupings in upper limb controls.113 Taken together, these studies all represent modern extensions of the modular ideas initiated by Hall, Brown and Sherrington.121 In mouse CPG, the extensive data from the combination of physiological and genetic dissection of the interneuron systems may in future be related to ideas of modular spinal organization107 and to hierarchical pattern generator organization.42,43 The explicit linkages of developmental mechanisms, genetics, and resulting interneuron form and projection details remain to be determined but will surely yield to this powerful strategy.
Learning and subsuming spinal modular structure through development
A scaffold of basic infrastructure for modular control and pattern generation is likely to be common across tetrapods. Through development this may be put into use very rapidly, or more gradually shaped by learning. Dominici and colleagues have recently performed an analysis and comparison of human and cross-species development of locomotion.64 Their data support the idea that the basic modular pattern generation of tetrapods is, in man, gradually specialized for bipedalism through development. The quadrupedal infrastructure is refined: some is preserved and some is modified to satisfy the novel biomechanical constraints of bipedalism. Their account is very much in keeping with a hybrid perspective of evolutionarily embedded bootstrap circuitry, onto which novel extensions and optimizations are layered. 8,114 In some instances the older circuits are subsumed to form elements of the new control, but in others instances these may be reshaped or replaced to satisfy the novel needs.
This is consistent with the points emphasized by Valero-Cuevas and colleagues, namely that neural modularity constraints will both support and limit biomechanical and behavioral control options, not unlike limb design and the tendon anatomy in the hand.94–96 The fullest flexibility of behavior is achieved by transcending these constraints where it is feasible to do so. The degree of departure from the early infrastructure in an adult individual will determine the relative importance and therapeutic value of the evolutionarily older circuitry. Various groups are now examining how modularity and spinal modularity impact on the motor learning of able bodied individuals.61 Older tetrapod circuitry could potentially either aid or hinder learning and rehabilitation after some kinds of injury. Depending on the final adult movement compositionality in able bodied individuals, it is conceivable that the older circuitry could re-emerge as pathology.8 However, despite this risk, when thoracic and lumbar circuits are isolated from descending controls in complete spinal cord injury, there may be few options in the clinic but to engage the older and now isolated spinal circuits for therapeutic purposes. It is becoming clear that recruiting these isolated spinal circuits in fact often offers great promise.115–117 Nonetheless, it is important to understand the end state of spinal circuitry after development and motor learning in man in order to best utilize this promise. Taken together, this all suggests an important role for spinal modularity and pattern generator elaboration in the understanding of disease processes and trauma, and in the design of effective clinical rehabilitaton. Newer studies of human clinical outcomes using modularity analyses support this perspective.
Clinical ramifications of modularity
Recently, the modularity perspectives developed in animal models have begun to be tested in clinical settings. These studies have involved both the upper and lower limb. More is known about animal pattern generation In the lower limb, and work from various groups supports modularity and pattern generation contributions in man.7,54,55 Clark, Neptune, Ting, Zajac and Kautz have shown that, following stroke, the modularity reported by themselves and others in able-bodied individuals can collapse to still lower dimensions.7 This collapse looks like a merging of the healthy modules or synergies. The degree of collapse maybe related to the degree of deficit. With rehabilitation, the merged synergies may again separate themselves and the degree of this re-differentiation may relate to the degree of rehabilitation improvement. These data provide a compelling account of the pathology and recovery processes with clear links to the hierarchical pattern generation and synergy literature. Bizzi and colleagues have examined stroke in the upper limb.5,6 The C3–C4 systems of Alstermark and the overall modularity described by Drew, Krouchev and colleagues in cats50 may be relevant. By comparing the unaffected and affected arms, and the muscle pattern structures recorded in reaching movement tasks in each, they have shown that the same modularity or premotor drive structures often exist in both limb. However, drive use is disordered in pattern in the affected limb. Beyond this, they also observed merging, as was seen in the lower limb by Clark and colleagues. This merging process could be directly correlated with the degree of deficit on clinical scales. Such merging is seen in animal models in deafferentation or other manipulations.82 The data from both upper and lower limb human studies suggest that the basic synergy circuits are embedded in the spinal cord and brainstem. Taken together, the Cheung/Bizzi and Clark/Neptune/Ting data suggest that loss of cortical controls causes initial failures in the temporally differentiated control of spinal interneuron systems for synergies in man, but that temporally differentiated controls can be re-established. The Cheung/Bizzi work also showed a further process at work in late chronic stroke. This involved the splitting of some synergies in the affected arm from those that were observed in the unaffected arm (i.e., splitting in the affected limb of the modules observed in the healthy and unaffected limb). The splitting process they observed was interpreted as a possible compensatory reorganization of brainstem and spinal controls that was only possible on a timescale of several or more years. The finer subdivision of modules, as opposed to module merging and separation, seems to take very long periods of concerted effort by patients, suggesting a robustness of modules and a need for prolonged practice or difficult identification of latent circuits to create these subdivisions. Stroke recovery also involves changes in kinematic modules or elements, though these take a different form,23 with kinematic merging indicating increasing function and skill. What spinal mechanisms support such processes? Transient addition, deletion, merging, and switching processes in modular pattern elements are part of the normal operation of spinal cord reflex and pattern generating systems as underscored by work in turtle by Stein,118,119 Berkowitz,120 and the data in frog discussed above.52,53,82,107 It is likely the pathological merging observed in trauma relates closely to these low level mechanisms and their neural support. Understanding splitting, merging, and control of component synergy drives and bursts may thus form a key to more principled rehabilitation. Animal models of injury and rehabilitation that exhibit similar merging and splitting phenomena through injury and recovery are likely to provide such understanding.
Conclusions : future issues and known unknowns
Current data support a well defined spinal infrastructure in tetrapods that is layered onto earlier trunk locomotor swimming systems, to augment or supplant them.114 This infrastructure seems to have both rhythm generation and separated modular pattern shaping layers. The modular shaping may comprise specific premotor drive systems, and these are likely to participate, albeit in modified fashion, in adult human movements. Activating the isolated lumbar circuits to restore some level of stepping in severe spinal cord injury recruits all of these systems. Our knowledge of the modular drive systems is still very sketchy. Understanding the organization and operations in the pattern shaping part of the CPG hierarchy is likely to have strong clinical significance. In injury, modular mergings or timing collapses of synergies occur. Later, on timescales of several years, splitting of normal synergies may be possible. We do not know in detail how merging, splitting, and rhythm to pattern shaping interactions operate at the circuit level, what neural systems they employ, or how best to support these circuits in a rehabilitative or other intervention. These latter data are likely to arise more quickly in basic animal models, using neural recordings in reduced preparations and fictive and genetic methods. The resulting knowledge of how the building blocks of pattern interact and support one another in the context of descending and other controls is essential to develop principled integrative therapies and interventions for the clinic.
Acknowledgments
Supported by NIH NS40412, NS54894 and NSF CRCNS IIS-0827684
References
- 1.Callebaut W, Rasskin-Gutman . Modularity: Understanding the Development and Evolution of Natural Complex Systems. MIT Press; 2005. [Google Scholar]
- 2.Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8(12):921–31. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- 3.Schouenborg J. Modular organization and spinal somatosensory imprinting. Brain Res Rev. 2002;40:80–91. doi: 10.1016/s0165-0173(02)00191-1. [DOI] [PubMed] [Google Scholar]
- 4.Slotine JJ, Lohmiller W. Modularity, evolution, and the binding problem: a view from stability theory. Neural Netw 2001. 2001 Mar;14(2):137–45. doi: 10.1016/s0893-6080(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 5.Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci U S A 2009. 2009b Oct 30; doi: 10.1073/pnas.0910114106. [epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung VC, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A 2012. 2012 Sep 4;109(36):14652–6. doi: 10.1073/pnas.1212056109. Epub 2012 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010 Feb;103(2):844–57. doi: 10.1152/jn.00825.2009. Epub 2009 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giszter SF, Hart CB, Silfies SP. Spinal cord modularity: evolution, development, and optimization and the possible relevance to low back pain in man. Exp Brain Res. 2010 Jan;200(3–4):283–306. doi: 10.1007/s00221-009-2016-x. Epub 2009 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giszter S, Patil V, Hart C. Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective. Prog Brain Res. 2007;165:323–46. doi: 10.1016/S0079-6123(06)65020-6. Review. [DOI] [PubMed] [Google Scholar]
- 10.Giszter SF. Motor Primitives. In: Squire Larry R., editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2008. [Google Scholar]
- 11.Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 2009. 2009 Oct 12;19(6):601–7. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flash T, Hochner B. Motor primitives in vertebrates and invertebrates. Current Opinion in Neurobiology. 2005;15(6):660–6. doi: 10.1016/j.conb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Fentress JC. Development of grooming in mice with amputated forelimbs. Science. 1973;179(74):704–5. doi: 10.1126/science.179.4074.704. [DOI] [PubMed] [Google Scholar]
- 14.Golani I, Fentress JC. Early ontogeny of face grooming in mice. Dev Psychobiol 1985. 1985 Nov;18(6):529–44. doi: 10.1002/dev.420180609. [DOI] [PubMed] [Google Scholar]
- 15.Sacrey LA, Karl JM, Whishaw IQ. Development of rotational movements, hand shaping, and accuracy in advance and withdrawal for the reach-to-eat movement in human infants aged 6–12 months. Infant Behav Dev 2012. 2012 Jun;35(3):543–60. doi: 10.1016/j.infbeh.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Whishaw IQ, Travis SG, Koppe SW, Sacrey LA, Gholamrezaei G, Gorny B. Hand shaping in the rat: conserved release and collection vs. flexible manipulation in overground walking, ladder rung walking, cylinder exploration, and skilled reaching. Behav Brain Res 2010. 2010 Jan 5;206(1):21–31. doi: 10.1016/j.bbr.2009.08.030. Epub 2009 Aug 28. [DOI] [PubMed] [Google Scholar]
- 17.McFarland DJ, Houston A. Quantitative Ethology: The state space approach. Pitman; 1981. [Google Scholar]
- 18.Viviani P, Terzuolo C. Trajectory determines movement dynamics. Neuroscience 1982. 1982 Feb;7(2):431–7. doi: 10.1016/0306-4522(82)90277-9. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein N. The co-ordination and regulation of movements. Pergamon Press; Oxford: 1967. [Google Scholar]
- 20.Hogan N. An organizing principle for a class of voluntary movements. J Neurosci. 1984;4(11):2745–54. doi: 10.1523/JNEUROSCI.04-11-02745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. 1985;5(7):1688–703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdet E, Milner TE. Quantization of human motions and learning of accurate movements. Biological Cybernetics. 1998;78:307–318. doi: 10.1007/s004220050435. [DOI] [PubMed] [Google Scholar]
- 23.Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, Stein J, Hogan N. Smoothness during stroke recovery. J Neurosci. 2002;22(18):8297–8304. 8299. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci 2001. 2001 Nov 1;5(11):487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- 25.Sosnik, Hauptmann, Karni, Flash When practice leads to co-articulation: the evolution of geometrically defined movement primitives. Exp Brain Res (2004) 2004;156:422–438. doi: 10.1007/s00221-003-1799-4. [DOI] [PubMed] [Google Scholar]
- 26.Polyakov F, Stark E, Drori R, Abeles M, Flash T. Parabolic movement primitives and cortical states: merging optimality with geometric invariance. Biol Cybern 2009. 2009 Feb;100(2):159–84. doi: 10.1007/s00422-008-0287-0. [DOI] [PubMed] [Google Scholar]
- 27.Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. J Neurosci. 1998;18(23):10105–15. doi: 10.1523/JNEUROSCI.18-23-10105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaal S, Sternad D, Osu R, Kawato M. Rhythmic arm movement is not discrete. Nat Neurosci 2004. 2004 Oct;7(10):1136–43. doi: 10.1038/nn1322. Epub 2004 Sep 26. Erratum in: Nat Neurosci. 2004 Nov;7(11):1279. [DOI] [PubMed] [Google Scholar]
- 29.Schaal S, Ijspeert A, Billard A. Computational approaches to motor learning by imitation, Philosophical Transaction of the Royal Society of London: Series B. Biological Sciences. 2003;358(1431):537–547. doi: 10.1098/rstb.2002.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan N, Sternad D. On rhythmic and discrete movements: reflections, definitions and implications for motor control. Exp Brain Res 2007. 2007 Jul;181(1):13–30. doi: 10.1007/s00221-007-0899-y. Epub 2007 May 26. [DOI] [PubMed] [Google Scholar]
- 31.Ijspeert A, Nakanishi J, Schaal S. Learning attractor landscapes for learning motor primitives. In: Becker S, Thrun S, Obermayer K, editors. Advances in Neural Information Processing Systems. Vol. 15. Cambridge, MA: MIT Press; 2003. pp. 1547–1554. [Google Scholar]
- 32.Ijspeert A, Nakanishi J, Pastor P, Hoffmann H, Schaal S. Dynamical Movement Primitives: Learning Attractor Models for Motor Behaviors. Neural Computation. 2013;25:328–373. doi: 10.1162/NECO_a_00393. [DOI] [PubMed] [Google Scholar]
- 33.Hogan N, Sternad D. Dynamic primitives of motor behavior. Biol Cybern 2012. 2012 Dec;106(11–12):727–39. doi: 10.1007/s00422-012-0527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grillner S. The motor infrastructure : from ion channels to neuronal networks. Nat Neurosci Rev. 2003;4:673–686. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 35.Kiehn O, Hounsgard J, Sillar KT. Basic building blocks of vertebrate CPGs. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks and Motor Behavior. MIT press; Cambridge MA: 1997. pp. 47–60. [Google Scholar]
- 36.Stein PS. Neuronal control of turtle hindlimb motor rhythms. Journal of Comparative Physiology A Neuroethology, Sensory and Neural Behavior Physiology. 2005;191(3):213–29. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- 37.Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev 2008. 2008 Jan;57(1):118–24. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci 2002. 2002 Aug 1;22(15):6800–9. doi: 10.1523/JNEUROSCI.22-15-06800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein PS, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol 2004. 20024 May;91(5):2380–4. doi: 10.1152/jn.01184.2003. [DOI] [PubMed] [Google Scholar]
- 40.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev 2008. 2008 Jan;57(1):94–102. doi: 10.1016/j.brainresrev.2007.07.018. Epub 2007 Aug 22. Review. [DOI] [PubMed] [Google Scholar]
- 41.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 2007. 2007;165:201–20. doi: 10.1016/S0079-6123(06)65013-9. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog Brain Res 2007. 2007;165:235–53. doi: 10.1016/S0079-6123(06)65015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 2008. 2008 Jan;57(1):134–46. doi: 10.1016/j.brainresrev.2007.08.006. Epub 2007 Sep 5. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. Journal of Neurophysiology. 2005;94(2):1120–32. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- 45.Quevedo J, Stecina K, Gosgnach S, McCrea DA. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol. 2005;94(3):2045–52. doi: 10.1152/jn.00175.2005. [DOI] [PubMed] [Google Scholar]
- 46.Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 2006. 2006a Dec 1;577(Pt 2):617–39. doi: 10.1113/jphysiol.2006.118703. Epub 2006 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol 2006. 2006b Dec 1;577(Pt 2):641–58. doi: 10.1113/jphysiol.2006.118711. Epub 2006 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas J, Chizeck IEEE Transactions of Biomedical Engineering. 1995;42(11):117–1127. doi: 10.1109/10.469379. [DOI] [PubMed] [Google Scholar]
- 49.Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006;5(4):2199–212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- 50.Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol. 2006;96(4):1991–2010. doi: 10.1152/jn.00241.2006. [DOI] [PubMed] [Google Scholar]
- 51.Hart CB, Giszter SF. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci 2004. 2004 Jun 2;24(22):5269–82. doi: 10.1523/JNEUROSCI.5626-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kargo WJ, Giszter SF. Rapid corrections of aimed movements by combination of force-field primitives. Journal of Neuroscience. 2000a;20:409–426. doi: 10.1523/JNEUROSCI.20-01-00409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kargo WJ, Giszter SF. Individual premotor drive pulses, not time-varying synergies, are the units of adjustment for limb trajectories constructed in spinal-cord. J Neuroscience. 2008;28(10):2409–25. doi: 10.1523/JNEUROSCI.3229-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olree KS, Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern 1995. 1995 Oct;73(5):409–14. doi: 10.1007/BF00201475. [DOI] [PubMed] [Google Scholar]
- 55.Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006;95(6):3426–37. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 56.Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93(1):609–13. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- 58.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. 2006;96(3):1530–46. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 59.Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. Journal of neurophysiology. 2007;98(4):2144–56. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 60.d’Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. PNAS. 2005;102(8):3076–3081. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.d’Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching by muscle synergy combinations. J Neurosci. 2006;26(30):7791–7810. doi: 10.1523/JNEUROSCI.0830-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- 63.Muceli S, Boye AT, d’Avella A, Farina D. Identifying representative synergy matrices for describing muscular activation patterns during multidirectional reaching in the horizontal plane. J Neurophysiol. 2010;103(3):1532–42. doi: 10.1152/jn.00559.2009. [DOI] [PubMed] [Google Scholar]
- 64.Dominici N, Ivanenko YP, Cappellini G, d’Avella A, Mondì V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 2011. 2011 Nov 18;334(6058):997–9. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- 65.Giszter SF, Kargo WJ. Conserved temporal dynamics and vector superposition of primitives in frog wiping reflexes during spontaneous extensor deletions. Neurocomputing. 2000;32–33:775–783. [Google Scholar]
- 66.Bizzi E, Mussa-Ivaldi FA, Giszter S. Computations underlying the execution of movement: a biological perspective. Science. 1991;253(5017):287–291. doi: 10.1126/science.1857964. [DOI] [PubMed] [Google Scholar]
- 67.Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog spinal cord. Journal of Neuroscience. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mussa-Ivaldi FA. From basis functions to basis fields: Using vector primitives to capture vector patterns. Biol Cybern. 1992;67:479–489. doi: 10.1007/BF00198755. [DOI] [PubMed] [Google Scholar]
- 69.Mussa-Ivaldi FA, Bizzi E. Motor learning through the combination of primitives. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2000;355(1404):1755–69. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mussa-Ivaldi FA, Giszter SF. Vector field approximation: a computational paradigm for motor control and learning. Biol Cybern. 1992;67:491–500. doi: 10.1007/BF00198756. [DOI] [PubMed] [Google Scholar]
- 71.Mussa-Ivaldi FA, Giszter SF, Bizzi E. Linear combination of primitives in vertebrate motor control. Proceedings and National Academy of Sciences. 1994;91:7534–7538. doi: 10.1073/pnas.91.16.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemay MA, Grill WM. Modularity of motor output evoked by intraspinal microstimulation in cats. Journal of Neurophysiol. 2004;91(1):502–14. doi: 10.1152/jn.00235.2003. [DOI] [PubMed] [Google Scholar]
- 73.Boyce VS, Lemay MA. Modularity of endpoint force patterns evoked using intraspinal microstimulation in treadmill trained and/or neurotrophin-treated chronic spinal cats. J Neurophysiol. 2009;101(3):1309–20. doi: 10.1152/jn.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Experimental Brain Research. 1999;129(3):401–16. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- 75.Tresch M, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nature Neuroscience. 1999;2:162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- 76.Sumbre G, Fiorito G, Flash T, Hochner B. Octopuses use a human-like strategy to control precise point-to-point arm movements. Curr Biol. 2006;16(8):767–72. doi: 10.1016/j.cub.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 77.Fetcho JR, McLean DL. Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications. Ann N Y Acad Sci 2010. 2010 Jun;1198:94–104. doi: 10.1111/j.1749-6632.2010.05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryczko D, Dubuc R, Cabelguen JM. Rhythmogenesis in axial locomotor networks: an interspecies comparison. Prog Brain Res 2010. 2010;187:189–211. doi: 10.1016/B978-0-444-53613-6.00013-7. [DOI] [PubMed] [Google Scholar]
- 79.Full RJ, Koditschek DE. Templates and anchors: neuromechanical hypotheses of legged locomotion on land. J Exp Biol 1999. 1999 Dec;202(Pt 23):3325–32. doi: 10.1242/jeb.202.23.3325. [DOI] [PubMed] [Google Scholar]
- 80.Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 2009. 2009 Dec;19(6):572–86. doi: 10.1016/j.conb.2009.10.011. Epub 2009 Nov 10. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vision Marr D. A Computational Investigation into the Human Representation and Processing of Visual Information. WH Freeman; 1983. Vision. [Google Scholar]
- 82.Kargo WJ, Giszter SF. Afferent roles in hindlimb wiping reflex: free limb kinematics and motor patterns. J Neurophysiology. 2000b;83(3):1480–1501. doi: 10.1152/jn.2000.83.3.1480. [DOI] [PubMed] [Google Scholar]
- 83.Kargo WJ, Ramakrishnan A, Hart CB, Rome L, Giszter SF. A simple experimentally-based model using proprioceptive regulation of motor primitives captures adjusted trajectory formation in spinal frogs. J Neurophysiology. 2010;103(1):573–90. doi: 10.1152/jn.01054.2007. Epub 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loeb GE. Overcomplete musculature or under specified tasks? Motor Control. 4: 81–83. doi: 10.1123/mcj.4.1.81. discussion 97–116, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Loeb GE, He J, Levine WS. Spinal cord circuits: are they mirrors of musculoskeletal mechanics? J Mot Behav. 1989 Dec;21(4):473–91. doi: 10.1080/00222895.1989.10735495. [DOI] [PubMed] [Google Scholar]
- 86.Loeb GE, Levine WS, He J. Understanding sensorimotor feedback through optimal control. Cold Spring Harb Symp Quant Biol 1990. 1990;55:791–803. doi: 10.1101/sqb.1990.055.01.074. [DOI] [PubMed] [Google Scholar]
- 87.Sanger TD. Human arm movements described by a low-dimensional superposition of principal components. J Neurosci. 2000;20(3):1066–72. doi: 10.1523/JNEUROSCI.20-03-01066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanger TD. Optimal unsupervised motor learning for dimensionality reduction of nonlinear control systems. IEEE Trans Neural Netw 1994. 1994;5(6):965–73. doi: 10.1109/72.329694. [DOI] [PubMed] [Google Scholar]
- 89.Chabra M, Jacobs RA. Properties of Synergies Arising from a Theory of Optimal Motor Behavior. Neural Computation. 2006;18:2320–2342. doi: 10.1162/neco.2006.18.10.2320. [DOI] [PubMed] [Google Scholar]
- 90.Todorov E. Optimality principles in sensorimotor control. Nat Neurosci. 2004;7(9):907–15. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todorov E, Ghahramani Z. Unsupervised Learning of Sensory-Motor Primitives Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE; Cancun Mexico. 2003. [Google Scholar]
- 92.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5(11):1226–35. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 93.Todorov E, Li W, Pan X. From task parameters to motor synergies: A hierarchical framework for approximately-optimal control of redundant manipulators. J Robot Syst. 2005;22(11):691–710. doi: 10.1002/rob.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valero-Cuevas FJ. A mathematical approach to the mechanical capabilities of limbs and fingers. Adv Exp Med Biol. 2009b;629:619–33. doi: 10.1007/978-0-387-77064-2_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol, 2009. 2009;102(1):59–68. doi: 10.1152/jn.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valero-Cuevas FJ, Yi JW, Brown D, McNamara RV, 3rd, Paul C, Lipson H. The tendon network of the fingers performs anatomical computation at a macroscopic scale. IEEE Trans Biomed Eng. 2007;54(6 Pt 2):1161–6. doi: 10.1109/TBME.2006.889200. [DOI] [PubMed] [Google Scholar]
- 97.Liu D, Todorov E. Evidence for the flexible sensorimotor strategies predicted by optimal feedback control. J Neurosci. 2007;27(35):9354–68. doi: 10.1523/JNEUROSCI.1110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nat Neurosci 2007. 2007 Oct;10(10):1329–36. doi: 10.1038/nn1986. Epub 2007 Sep 16. [DOI] [PubMed] [Google Scholar]
- 99.Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci U S A 2009. 2009 May 5;106(18):7601–6. doi: 10.1073/pnas.0901512106. Epub 2009 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5(4):191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- 101.Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007. 2007 Feb;189(2):155–69. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 102.Pang MY, Lam T, Yang JF. Infants adapt their stepping to repeated trip-inducing stimuli. J Neurophysiol. 2003;90(4):2731–40. doi: 10.1152/jn.00407.2003. [DOI] [PubMed] [Google Scholar]
- 103.Clarac F, Brocard F, Related Vinay L. The maturation of locomotor networks. Prog Brain Res. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. [DOI] [PubMed] [Google Scholar]
- 104.Frank E, Mendelson B. Specification of synaptic connections mediating the simple stretch reflex. J Exp Biol. 1990 Oct;153:71–84. doi: 10.1242/jeb.153.1.71. Review. [DOI] [PubMed] [Google Scholar]
- 105.Mendelson B, Frank E. Specific monosynaptic sensory-motor connections form in the absence of patterned neural activity and motoneuronal cell death. J Neurosci 1991. 1991 May;11(5):1390–403. doi: 10.1523/JNEUROSCI.11-05-01390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haverkamp LJ. Anatomical and physiological development of the Xenopus embryonic motor system in the absence of neural activity. J Neurosci 1986. 1986 May;6(5):1338–48. doi: 10.1523/JNEUROSCI.06-05-01338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hart CB, Giszter SF. Neural Underpinnings of Motor Primitives. J Neurosci. 2010;30(4):1322–36. doi: 10.1523/JNEUROSCI.5894-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berkowitz A. Physiology and morphology of shared and specialized spinal interneurons for locomotion and scratching. J Neurophysiol. 2008;99(6):2887–901. doi: 10.1152/jn.90235.2008. Epub 2008 Apr 2. [DOI] [PubMed] [Google Scholar]
- 109.Berkowitz A, Hao ZZ. Partly Shared Spinal Cord Networks for Locomotion and Scratching. Integr Comp Biol. 2011 Jun 22; doi: 10.1093/icb/icr041. [DOI] [PubMed] [Google Scholar]
- 110.Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Frontiers in behavioral neuroscience. 2010;4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci 2012. 2012;35:559–78. doi: 10.1146/annurev-neuro-062111-150527. Epub 2012 Apr 9. [DOI] [PubMed] [Google Scholar]
- 112.Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 2012. 2012 Jul 12;487(7406):235–8. doi: 10.1038/nature11206. [DOI] [PubMed] [Google Scholar]
- 113.Takei T, Seki K. Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J Neurosci 2010. 2010 Dec 15;30(50):17041–50. doi: 10.1523/JNEUROSCI.4297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauscent A, Le Ray D, Cabirol-Pol MJ, Sillar KT, Simmers J, Combes D. Development and neuromodulation of spinal locomotor networks in the metamorphosing frog. J Physiol Paris 2006. 2006 Nov-Dec;100(5–6):317–27. doi: 10.1016/j.jphysparis.2007.05.009. Epub 2007 Jun 8. Review. [DOI] [PubMed] [Google Scholar]
- 115.Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011. 2011 Jun 4;377(9781):1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother 2011. 2011 Oct;11(10):1351–3. doi: 10.1586/ern.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol 2001. 2001 May 15;533(Pt 1):15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Earhart GM, Stein PS. Scratch-swim hybrids in the spinal turtle: blending of rostral scratch and forward swim. J Neurophysiol 2000. 2000 Jan;83(1):156–65d. doi: 10.1152/jn.2000.83.1.156. [DOI] [PubMed] [Google Scholar]
- 119.Earhart GM, Stein PS. Step, swim and scratch motor patterns in the turtle. J Neurophysiol 2000. 2000 Nov;84(5):2181–90. doi: 10.1152/jn.2000.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 120.Hao ZZ, Spardy LE, Nguyen EB, Rubin JE, Berkowitz A. Strong interactions between spinal cord networks for locomotion and scratching. J Neurophysiol. 2011 Oct;106(4):1766–81. doi: 10.1152/jn.00460.2011. Epub 2011 Jul 6. [DOI] [PubMed] [Google Scholar]
- 121.Sherrington CS. The integrative action of the nervous system. New Haven, CT: Yale University Press; 1961. [Google Scholar]