Abstract
The primary objectives of this study were to: (a) examine the neuroendocrine effects of naltrexone vs. placebo by comparing serum cortisol levels; and (b) test the biobehavioral correlates of naltrexone-induced changes in cortisol. Non-treatment seeking heavy drinkers (n = 37) completed two intravenous alcohol administrations, one after naltrexone (50 mg) and one after placebo. Cortisol levels were measured at baseline and after alcohol intake (BrAC = 0.06 g/dl) on both sessions, as were subjective responses to alcohol. Analyses revealed that naltrexone significantly raised overall cortisol levels compared to placebo. Cortisol levels decreased during alcohol administration and a stronger decrease was observed in the naltrexone condition. Cortisol levels were, in turn, inversely related to some of alcohol’s the reinforcing effects (i.e., alcohol ‘high,’ vigor) and positively associated with some of its unpleasant effects (i.e., sedation and subjective intoxication). These results suggest that naltrexone alters cortisol levels in heavy drinkers and that its effects on subjective responses to alcohol may be related, in part, to naltrexone’s ability to activate the HPA-axis.
Keywords: naltrexone, alcohol, cortisol, HPA-axis, subjective intoxication
Effects of Naltrexone on Cortisol Levels in Heavy Drinkers
Naltrexone (NTX) is an opioid receptor antagonist with empirically supported efficacy, albeit moderate, for the treatment of alcoholism, when used in combination with behavioral treatments (Anton et al., 1999; Anton et al., 2006; Monti et al., 2001; O’Malley et al., 1992; Volpicelli et al., 1992). A few studies, however, have not supported the efficacy of naltrexone (Kranzler et al., 2000; Krystal et al., 2001), and even in studies that favored naltrexone over placebo, not all individuals were found to benefit from this pharmacotherapy (Rohsenow, 2004). Therefore, recent research has focused on understanding mechanisms and moderators of response to naltrexone. To that end, laboratory studies of naltrexone’s biobehavioral mechanisms revealed that this pharmacotherapy dampens alcohol’s positively reinforcing effects (Drobes et al., 2004; McCaul et al., 2001; Ray and Hutchison, 2007; Swift et al., 1994), exacerbates alcohol’s aversive effects (King et al., 1997), and attenuates alcohol craving and self-administration (Anton et al., 2004; Tidey et al., 2008).
The neurobiological mechanisms of action of naltrexone are less well characterized. It is generally posited that naltrexone works by occupying opioid receptors preventing the binding of such receptors by endogenous opioid peptides released upon alcohol intake, which in turn prevents the γ-aminobutric acid (GABA)-mediated release of dopamine in the ventral tegmental area thereby putatively blocking alcohol’s reinforcing effects (Koob and Le Moal, 2008; Kreek, 1996). Opioid peptides may also play a role in alcohol and drug reward through dopamine independent pathways (Koob, 1992). Opioid blockade, mostly tested using naloxone, has been found to increase blood levels of adrenocorticotropic hormone (ACTH), beta-endorphin, and cortisol in humans (Naber et al., 1981; Schluger et al., 1998), although null findings have also been reported (Kemper et al., 1990). These findings suggest a potential role of the hypothalamo-pituitary-adrenocortical (HPA) axis activity in mediating the neurobiological effects of naltrexone. This notion is also consistent with preclinical and clinical data suggesting that HPA-axis stimulation plays an important role in the neurobiology of alcoholism itself (Adinoff et al., 1998; Adinoff et al., 2005) and findings suggesting that blunted HPA-axis response predicts alcohol preference in rodents (Olive et al., 2003) and an increased risk of early relapse in humans (Junghanns et al., 2003; Junghanns et al., 2005).
Acute neuroendocrine responses to naltrexone have been examined in a few studies. Naltrexone was found to increase cortisol levels after administration in healthy male volunteers (Volavka et al., 1979) and to raise cortisol and ACTH levels among abstinent alcohol dependent patients (Farren et al., 1999). Both studies, however, had small sample sizes of 10 and 6 participants, respectively. In a recent study, King and colleagues (2002) found that naltrexone increased both ACTH and cortisol levels in healthy participants compared to placebo, and that individuals with a family history positive of alcoholism displayed a heightened ACTH and cortisol response to naltrexone (King et al., 2002). In a study comparing alcohol dependent patients treated with placebo, naltrexone, acamprosate, or both, plasma ACTH and cortisol decreased during early abstinence in the placebo group but not in the active medication groups and increased ACTH and cortisol during treatment was associated with a lower risk of relapse (Kiefer et al., 2006). These findings underscore the need to further elucidate the biobehavioral effects of naltrexone-induced HPA-axis activation.
O’Malley and colleagues (2002) found that non-treatment seeking alcohol dependent individuals treated with naltrexone reported lower levels of alcohol craving upon cue-exposure, higher cortisol levels before drinking, and higher levels of ACTH and cortisol during alcohol intake, as compared to placebo-treated participants. Interestingly, cortisol levels were inversely associated with self-reported alcohol craving (O’Malley et al., 2002). Inasmuch as controlled laboratory studies allow for the examination of the biobehavioral and neurobiological effects of naltrexone, these results suggest that naltrexone-induced reductions in craving may be HPA-axis mediated. In particular, naltrexone could exert its therapeutic effect by restoring the blunted basal activity and reactivity of the HPA system (Adinoff et al., 2005; Kiefer et al., 2006; O’Malley et al., 2002). A more recent study, however, tested saliva cortisol levels in alcohol dependent patients treated with placebo, naltrexone or acamprosate, who participated in two cue-exposure sessions (Ooteman et al., 2007). In this study, there was no significant cue-induced change in cortisol levels and no significant medication effect on cortisol values was observed at baseline or after cue exposure (Ooteman et al., 2007). See Table 1 for a brief review of the literature. Given the conflicting nature of these results, further investigations are needed in order to elucidate the biobehavioral effects of naltrexone-induced HPA-axis activation.
Table 1.
Summary of studies of the effects of naltrexone (NTX) on HPA-axis hormones
| Study | Sample | Naltrexone Dosing | Biological Sample | HPA-Axis Hormones | Findings |
|---|---|---|---|---|---|
| Farren et al., 1999 | Alcohol dependent | Single dose; 0, 25, 50, and 100 mg | Blood | Cortisol ACTH | ↑ cortisol and ACTH levels on NTX compared to placebo; No NTX dose effects on HPA-axis |
| Kiefer et al., 2006 | Alcohol dependent | 50 mg/day, for 12 weeks | Blood | Cortisol ACTH | ↑ cortisol and ACTH levels on NTX and acamprosate groups versus placebo; ↑ cortisol and ACTH during treatment was associated with ↓ risk of relapse |
| King et al., 2002 | Healthy subjects | Single dose; 50 mg | Blood | Cortisol ACTH | ↑ cortisol and ACTH levels on NTX compared to placebo; ↑ cortisol and ACTH response to NTX among FH+ individuals |
| O’Malley et al., 1999 | Alcohol dependent | 50 mg/day, for 6 days | Blood | Cortisol ACTH | ↑ cortisol and ACTH levels on NTX compared to placebo; Negative correlation between cortisol level and alcohol craving |
| Ooteman et al., 2007 | Alcohol dependent(abstinent) | 50 mg/day, for 3 weeks | Saliva | Cortisol | No difference between NTX, acamprosate or placebo on cortisol levels at baseline and after cue-exposure |
| Volavka et al., 1979 | Healthy men | Single dose; 50 mg | Blood | Cortisol ACTH | ↑ cortisol levels on NTX compared to placebo No significant differences in ACTH |
| Present Study | Hazardous drinkers | 50 mg/day, for 3 days | Blood | Cortisol | ↑ cortisol levels on NTX compared to placebo Negative correlation between cortisol and alcohol’s reinforcing effects ↓ cortisol following alcohol infusion compared to sober baseline |
In addition to craving, subjective response to alcohol represents an important biobehavioral mechanism of naltrexone’s effects (King et al., 1997; Swift et al., 1994) and was not tested in any of the preceding investigations. The overarching objective of this study is to further characterize the biobehavioral and neurobiological mechanisms of action of naltrexone as well as their interplay. The primary aim is to extend the literature on the neuroendocrine response to naltrexone and its biobehavioral effects by examining the effects naltrexone, as compared to placebo, on serum cortisol levels and their relation to alterations in the subjective responses to alcohol and craving among hazardous drinkers. The focus on responses to alcohol is particularly relevant as subjective responses to alcohol reward may be influenced by cortisol’s modulation of mesolimbic dopaminergic transmission (Barrot et al., 2000). A secondary aim of this study is to examine the moderating role of family history of alcoholism, which has been associated with neuroendocrine response to opioid blockade (Adinoff et al., 2005; King et al., 2002; Rohsenow et al., 2007; Wand et al., 1998).
Method
Participants
Participants were 37 (11 females) non-treatment-seeking hazardous drinkers, all of whom met the following inclusion criteria: (1) age between 21 and 35; (2) a score of 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT), indicating a hazardous drinking pattern (Allen et al., 1997); (3) self-reported drinking frequency of 3 or more drinks (2 for women) at least twice per week; (4) no history of adverse reactions to needle puncture; (5) no history of prior treatment for an alcohol use disorder (AUD) and no current interest in treatment for an AUD; (6) no history of medical conditions that would contraindicate study participation; and (7) successfully completing a physical exam to ensure medical eligibility for the trial. Female participants completed a pregnancy test before each alcohol administration session; however, the menstrual cycle in female participants was not recorded. All participants were required to have a breath alcohol concentration (BrAC) of zero before each session. The average age of the sample was 22.2 (SD = 2.3; Range = 21 to 32) and 31 (84%) of the participants were Caucasian, 4 (11%) were Asian, and 2 (5%) were Latino. In this sample, the average number of drinks per drinking episode was 4.9 (SD = 2.3; Range = 2–12), the average drinking frequency was twice per week, and the average AUDIT score was 12.5 (SD = 4.3; Range = 8–21), indicating a hazardous drinking pattern. A total of 13 (35%) participants reported a family history positive for alcoholism. The study was approved by the appropriate human subjects committee and all participants provided written informed consent.
A total of 124 participants (39 women) were screened in the laboratory, 53 completed the physical exam, 7 of whom were ineligible for the study due to a medical reason and 6 of whom decided not to participate in the trial, leaving 40 participants (12 women) who completed the trial. Three participants did not provide a complete set of blood samples, leaving a sample of 37 participants (11 women) who provided complete data for the cortisol analyses reported in this study. This study oversampled for the Asp40 allele of the OPRM1 gene, as described elsewhere (Ray and Hutchison, 2007).
Procedures
Upon arrival at the lab eligible participants read and signed an informed consent form, and completed a series of individual difference measures. Next, participants completed a physical exam at the General Clinical Research Center (GCRC). Each participant completed two alcohol infusion sessions, one after taking naltrexone (50 mg) for 3 days and one after taking a matched placebo for three days. Experimental sessions started between 11:00 am and 1:00 pm and each participant’s start time was identical between the two sessions. Medication was delivered in a counterbalanced and double-blind fashion. The wash-out period between infusions was at least 7 days.
Given the importance of effectively controlling blood alcohol levels in alcohol administration studies (Li et al., 2001; O’Connor et al., 1998; Ramchandani et al., 1999), this study consisted of administering alcohol intravenously, rather than orally. Consistent with the procedures developed in our laboratory (Ray and Hutchison, 2004) the alcohol infusion was performed using a 5% ethanol IV solution and a nomogram was developed taking into account participant’s gender and weight. Participants received IV alcohol at their target infusion rate and BrAC was monitored every 3 to 5 minutes.
During the experimental sessions, participants were seated in a recliner chair and the IV was placed in their non-dominant arm. Last use of alcohol and alcohol withdrawal symptoms were not recorded in this study, although all participants were required to have a BrAC of zero at the beginning of each alcohol administration session. Moreover, withdrawal symptoms were unlikely given the self-reported drinking quantity and frequency in this sample. Participants were told that they would receive IV doses of alcohol but remained blind to their BrAC throughout the trial. Participants completed a baseline assessment packet before receiving the intravenous (IV) doses of alcohol (i.e., when BrAC = 0.00 g/dl) and provided identical assessment measures at three points in the ascending arm of the blood alcohol curve (BrAC: 0.02, 0.04, and 0.06 g/dl). After the third assessment point at the ascending limb, the infusion procedure was stopped and participants were given a meal. All participants stayed in the lab until their BrAC was below 0.02 g/dl. Blood samples for cortisol analyses were collected on both medication conditions (i.e., naltrexone and placebo) at baseline (BrAC = 0.00 g/dl) and at the final target BrAC level (i.e., 0.06 g/dl). Therefore, the only behavioral assessment points of interest to this study are those that match the assessment of cortisol (i.e., at baseline and at BrAC = 0.06 g/dl).
Medication Procedures
Participants were required to take the first medication (naltrexone 50 mg or placebo) once a day for two days prior to the first experimental session and on the morning of their appointment. After the first session participants were given the second medication, which they took in the same fashion. Active medication and placebo were counterbalanced and double-blinded. Compliance was examined by packing the medication and placebo into capsules with 50 mg of riboflavin. Urine samples were collected prior to each infusion session and were analyzed for riboflavin content under an ultraviolet light, which makes the riboflavin detectable (Del Boca et al., 1996). All samples tested positive for riboflavin content verifying that the last dose was taken. Participant report and pill minder data were employed to verify compliance with the first two medication doses.
Measures
Participants completed a battery of individual difference measures that included demographics, drinking behavior, and family history of alcohol problems using the Family History Assessment Module (FHAM) for alcohol use disorders and limited to affected first degree relatives. During each alcohol infusion session, measures of subjective responses to alcohol and alcohol craving were administered at baseline and at the ascending and descending limbs of intoxication. The following measures were used: (1) Alcohol Urge Questionnaire (AUQ). The AUQ consists of eight items related to urge to drink alcohol; each item is rated on a 7-point Likert scale and anchored by “Strongly Disagree” and “Strongly Agree.” The AUQ has demonstrated high internal consistency in previous laboratory studies (Bohn et al., 1995; MacKillop, 2006). (2) Subjective High Assessment Scale (SHAS). The SHAS was used to assess subjective feelings of alcohol intoxication. This measure has been adapted by Schuckit (1984) and has since been widely used in alcohol challenge studies. The SHAS consists of 13-items such as: drunk, high, nauseated, dizzy, and drug effect (Schuckit, 1984). (3) Biphasic Alcohol Effects Scale (BAES). The BAES assesses feelings of alcohol-induced stimulation and sedation, each subscale consisting of 7 items (Martin et al., 1993). (4) Profile of Mood States (POMS). The short version of the POMS (McNair, 1971) was used in this study to assess mood changes following alcohol administration. The following subscales of the POMS, each composed of 10 items, were used in this study: vigor, tension, positive and negative mood. (5) Alcohol Rating Scale (ARS). The ARS measures participants’ responses to the hedonic properties of alcohol, including alcohol-induced feelings of “high.”
Cortisol Assay
Blood samples for cortisol were collected by the nursing staff and placed into heparinized tubes (0.15 μl heparin) and placed on ice immediately after blood drawing. Within 15 minutes of collection, blood was centrifuged at 4°C and the serum transferred to a microtube stored at −20°C. The cortisol assay was performed at the Neurogenetics Core Laboratory at the Mind Research Network using a cortisol enzyme immunoassay (EIA) provided by Assay Designs (Catalog number 900–071) and protocol instructions were followed exactly as directed. The optical density of the EIA plate was read at 405 nm using a Multiskan Ascent plate reader from Thermo Scientific and results were extrapolated using standard curves and a 4 parameter logistic curve fitting algorithm. The sensitivity of the cortisol assay is 0.22 μg/dl, the inter assay coefficient of variation is 2.9%, and the inter assay coefficient of variation is 6.0%.
Statistical Analysis
To test the primary aims of this study, a 2 × 2 repeated measures analyses of variance (ANOVAs) was conducted to examine the effects of Medication (i.e., naltrexone vs. placebo) and Alcohol (i.e., pre vs. post) on cortisol serum levels. Both Medication and Alcohol were within-subjects factors. Additionally, Pearson correlations were used to examine associations between serum cortisol levels and subjective responses to alcohol at matched assessment points (i.e., baseline and when BrAC reached 0.06 g/dl). To test the secondary aim of the study, a mixed 2 × 2 × 2 ANOVA was conducted by adding Family History (positive vs. negative) to the model described above. Corrections for Type I error were considered but ultimately rejected on the basis of the argument that Type I error needs to be considered for each hypothesis separately, not for the number of variables in the whole set of analyses reported (Dar et al., 1994). In the present analyses multiple measures are assessing the single hypothesis regarding differential subjective response to alcohol as a function of cortisol levels, thereby suggesting that corrections for Type I error may not be warranted.
Results
Baseline Comparisons
There were no significant differences in BrACs in the Naltrexone vs. Placebo conditions, F (1,36) = 1.58, p =.22. There was no effect of gender on cortisol levels at any of the four assessment points (p >.05). Therefore, it is highly unlikely that gender or other variables measured at baseline confounded the statistical analyses presented herein.
Naltrexone and Alcohol Effects
The 2 × 2 repeated measures ANOVA, described above, revealed a significant main effect of medication, such that cortisol levels were higher on naltrexone, as compared to placebo [F (1, 36) = 17.2, p <.001]. There was also a significant main effect of alcohol, such that cortisol levels decreased after alcohol infusion (BAC = 0.06 g/dl) [F (1, 36) = 19.3, p <.0001]. A significant Alcohol × Medication interaction indicated that naltrexone was associated with greater decreases in cortisol level after alcohol infusion [F (1, 36) = 6.67, p <.05], see Figure 1.
Figure 1.
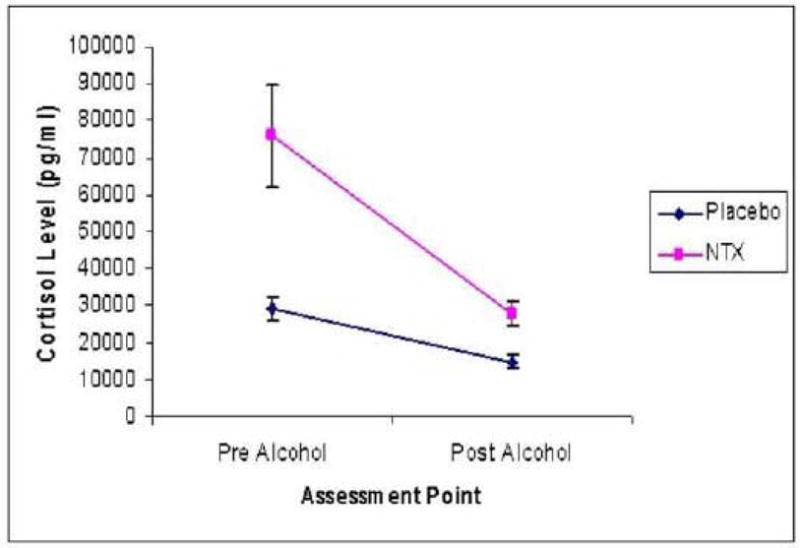
Means and standard errors for cortisol level on the naltrexone and placebo conditions both pre and post alcohol infusion.
Cortisol Levels and Subjective Responses to Alcohol
Baseline cortisol level, in the placebo condition, was inversely related to Vigor at baseline [r = −.47, p <.01]. In the naltrexone condition, post alcohol cortisol level was associated with higher self-reported Sedation [r = 41, p <.05] and Subjective Intoxication [SHAS; r =.50, p <.05] at matched assessment points (BAC = 0.06 g/dl). Additionally, when examining relationships across pre-post alcohol assessment, baseline cortisol level, in the placebo condition, was negatively associated with alcohol “high” [r = −.36, p <.05], Stimulation [r = −.34, p <.05], and Vigor [r = −.39, p <.05] at the target BAC (i.e., 0.06 g/dl), suggesting that baseline cortisol level is negatively associated with the reinforcing subjective effects of alcohol. This is relevant in light of the clinical literature suggesting that higher basal levels of cortisol may contribute to the relapse-preventing effects of naltrexone (Adinoff et al., 2005; Kiefer et al., 2006). See Table 2 for a complete list of correlations between cortisol levels and subjective responses to alcohol.
Table 2.
Correlations between cortisol levels (pre and post alcohol) and measures of subjective response to alcohol in the placebo and naltrexone conditions at matched assessment points
| Assessment | Pre Alcohol Cortisol PLAC | Post Alcohol Cortisol PLAC | Pre Alcohol Cortisol NTX | Post Alcohol Cortisol NTX |
|---|---|---|---|---|
| Urge to Drink (AUQ) | −0.02 | 0.06 | −0.06 | −0.14 |
| Intoxication (SHAS) | 0.10 | −0.07 | 0.01 | 0.50** |
| Stimulation (BAES) | −0.20 | −0.17 | −0.17 | 0.10 |
| Sedation (BAES) | 0.19 | −0.02 | 0.17 | 0.41* |
| Vigor (POMS) | −0.47** | −0.17 | −0.19 | −0.19 |
| Tension (POMS) | −0.04 | −0.08 | 0.01 | 0.03 |
| Positive Mood (POMS) | −0.23 | −0.19 | −0.23 | −0.14 |
| Negative Mood (POMS) | 0.10 | 0.01 | 0.19 | 0.08 |
| Alcohol “high” (ARS)a | −0.10 | −0.08 |
Note: p <.05;
p <.01;
Alcohol “high” was only assessed post alcohol intake.
Family History as a Moderator
Analyses did not support a moderating role of family history of alcoholism (FH) on the relationship between naltrexone and cortisol levels, such that there was no main effect of FH on cortisol levels [F (1, 35) = 1.46, p =.24], or FH × Medication [F (1, 35) = 2.67, p =.11] and FH × Medication × Alcohol [F (1, 35) = 2.12, p =.15] interactions.
Discussion
Results of this study revealed that naltrexone significantly raised serum cortisol levels, as compared to placebo, among hazardous drinkers. These findings advance previous work suggesting that naltrexone’s neurobiological mechanisms involve HPA-axis activation (Kiefer et al., 2006; King et al., 2002; O’Malley et al., 2002). From the perspective of the biobehavioral mechanisms of action of naltrexone, results indicated that basal cortisol levels are inversely related to some of the reinforcing effects of alcohol (i.e., high, vigor) and positively associated with some of alcohol’s unpleasant effects (i.e., sedation and subjective intoxication). This is relevant in light of the clinical literature suggesting that higher basal levels of cortisol may contribute to the relapse-preventing effects of naltrexone (Adinoff et al., 2005; Kiefer et al., 2006). Thus, it is plausible that the relapse-preventing effects of naltrexone may be due to differential responsivity to alcohol’s reinforcing effects at higher basal levels of cortisol.
However, there were no significant associations between cortisol levels and self-reported craving for alcohol, contrary to previous findings (O’Malley et al., 2002). There are multiple methodological considerations that may account for the null findings regarding alcohol craving. For instance, O’Malley et al. (2002) studied alcohol dependent participants whereas the current study focused on hazardous drinkers, most of whom are unlikely to meet criteria for alcohol dependence. Additionally, the intravenous alcohol administration lacks the exposure to alcohol cues and a previous study suggested that the oral alcohol administration is more effective at eliciting alcohol craving (Ray et al., 2007).
The present results however, are complementary to those of O’Malley and colleagues (2002) as that study did not examine subjective responses to alcohol but focused exclusively on alcohol craving. Together, these findings suggest that the inverse relationship between cortisol levels and craving for alcohol (O’Malley et al., 2002) may be due to the fact that cortisol levels are inversely related to the reinforcing effects of alcohol and positively associated to its unpleasant effects, such that individuals experiencing lower levels of alcohol-induced reward, at higher cortisol levels, report lower alcohol craving. Future studies examining naltrexone-induced HPA-axis activation and its effects on alcohol craving and subjective responses to alcohol among alcohol dependent patients are warranted to more fully integrate these findings.
Conversely, the present results are consistent with several studies that did not find support for an association between alcohol craving and cortisol levels in alcohol dependent individuals (Leggio et al., in press; Ooteman et al., 2007). In sum, the results of this study further support previous findings (Kiefer et al., 2006; King et al., 2002; O’Malley et al., 2002) suggesting that naltrexone is associated with HPA-axis activation. However, these results and others (Leggio et al., in press; Ooteman et al., 2007), suggest that cortisol may not be a direct biological marker of alcohol craving and that perhaps other HPA-axis related hormones (e.g., aldosterone) could reflect alcohol craving in a more direct way, at least among alcohol dependent patients (Leggio et al., 2008). Differences in sample characteristics (see Table 1), such as gender and age may help account for inconsistencies in the literature. For example, participants in this study were generally younger than treatment samples and previous research has shown age and sex differences in cortisol levels in healthy individuals (Nakamura and Yakata, 1984).
Results also suggested a main effect of alcohol and an alcohol by naltrexone interaction such that alcohol intake was found to lower cortisol levels and cortisol decreases were stronger in the naltrexone condition, as compared to placebo. The decrease in cortisol levels following intravenous alcohol administration is somewhat surprising, yet previous studies have shown decreases, albeit small, on cortisol levels during alcohol administration (Gianoulakis et al., 1996). Interestingly, a prior study of a low dose of intravenous alcohol across the menstrual cycle in women with premenstrual syndrome and controls found that cortisol levels decreased during both the placebo and alcohol infusion (Nyberg et al., 2005). One methodological difference that may help explain these findings is that the intravenous alcohol administration, as compared to the oral administration, prevents the fist pass metabolism of alcohol by the liver. A review of the literature has suggested that acetaldehyde, rather than ethanol per se, may be responsible for direct adrenal stimulation by alcohol (Cobb and Van Thiel, 1982). If that is the case, the lack of first pass by the liver in the IV alcohol administration may serve to reduce adrenal stimulation which may account for the decrease in cortisol levels following alcohol infusion. Although intriguing, the effects of alcohol on cortisol should be interpreted with caution, particularly due to the lack of a placebo-alcohol condition. Importantly, the start time of the testing session was kept the same within subjects, yet there was a two hour window of time in which sessions began thereby causing between-subjects variability. This is relevant to the test of alcohol’s effects given the diurnal pattern of cortisol variation (Smyth et al., 1997). Additional studies with a placebo alcohol condition are needed to further clarify these initial findings.
The secondary study objective was to examine family history of alcoholism as a putative moderator of naltrexone-induced HPA-axis activation. Although family history has received prior empirical support as a moderator of naltrexone response (King et al., 2002; King et al., 1997; Rohsenow et al., 2007), it did not moderate naltrexone-induced changes in cortisol level in this study. Interestingly, a recent study failed to find an association between cortisol responsivity and family history of alcoholism in abstinent alcohol-dependent men (Hardin and Adinoff, 2008). Further investigation of family history as a moderator of naltrexone’s effects in larger samples seems warranted.
These results should be interpreted in light of the study’s strengths and limitations. Study strengths include the double-blind within-subjects counterbalanced design and an IV alcohol administration that produces high levels of control over BrACs and allows us to focus more directly on the pharmacology of alcohol. Limitations include the lack of a placebo alcohol condition (e.g., using a saline solution), a sample that was relatively small and composed of young non treatment-seeking hazardous drinkers that may not generalize to clinical samples, the lack of ACTH determination, and the use of an alcohol infusion administration which differs from oral alcohol consumption (Ray et al., 2007). This study did not control for menstrual cycle in female participants, which in turn may impact cortisol levels in response to alcohol (Nyberg et al., 2005). Although future studies are necessary to further clarify these relationships, on balance, these results suggest that naltrexone alters cortisol levels in hazardous drinkers and that its effects on subjective responses to alcohol and drinking per se may be related, in part, related to naltrexone’s ability to activate the HPA-axis.
Acknowledgments
This research was supported by grants from the National Institute on Alcoholism and Alcohol Abuse (AA14847 and AA012238) to LAR and KEH, a grant from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health (M01-RR00051), and a grant from the Alcoholic Beverage Research Foundation to JM. The authors wish to thank Amy Audette, Stephanie DiCristophoro, Keira Odell, and the staff at the General Clinical Research Center at the University of Colorado, at Boulder, for their contribution to data collection in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Cobb CF, Van Thiel DH. Mechanism of ethanol-induced adrenal stimulation. Alcohol Clin Exp Res. 1982;6:202–206. doi: 10.1111/j.1530-0277.1982.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Dar R, Serlin RC, Omer H. Misuse of statistical test in three decades of psychotherapy research. J Consult Clin Psychol. 1994;62:75–82. doi: 10.1037//0022-006x.62.1.75. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Farren CK, O’Malley S, Grebski G, Maniar S, Porter M, Kreek MJ. Variable dose naltrexone-induced hypothalamic-pituitary-adrenal stimulation in abstinent alcoholics: a preliminary study. Alcohol Clin Exp Res. 1999;23:502–508. [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Hardin E, Adinoff B. Family history of alcoholism does not influence adrenocortical hyporesponsiveness in abstinent alcohol-dependent men. Am J Drug Alcohol Abuse. 2008;34:151–160. doi: 10.1080/00952990701877011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kemper A, Koalick F, Thiele H, Retzow A, Rathsack R, Nickel B. Cortisol and beta-endorphin response in alcoholics and alcohol abusers following a high naloxone dosage. Drug Alcohol Depend. 1990;25:319–326. doi: 10.1016/0376-8716(90)90158-b. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology. 2000;22:493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Opiates, opioids and addiction. Mol Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, Vonghia L, Miceli A, Capristo E, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients. A longitudinal study. doi: 10.1111/j.1530-0277.2008.00792.x. Alcohol Clin Exp Res in press. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Miceli A, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Renin and aldosterone but not the natriuretic peptide correlate with obsessive craving in medium-term abstinent alcohol-dependent patients: a longitudinal study. Alcohol. 2008;42:375–381. doi: 10.1016/j.alcohol.2008.03.128. [DOI] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O’Connor S, Ramchandani VA. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res. 2001;25:136–144. [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic- pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational & Industrial Testing Service; 1971. [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–1647. [PubMed] [Google Scholar]
- Naber D, Pickar D, Davis GC, Cohen RM, Jimerson DC, Elchisak MA, Defraites EG, Kalin NH, Risch SC, Buchsbaum MS. Naloxone effects on beta-endorphin, cortisol, prolactin, growth hormone, HVA and MHPG in plasma of normal volunteers. Psychopharmacology (Berl) 1981;74:125–128. doi: 10.1007/BF00432677. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Yakata M. Age- and sex-related differences in urinary cortisol level. Clin Chim Acta. 1984;137:77–80. doi: 10.1016/0009-8981(84)90314-0. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology. 2005;30:892–901. doi: 10.1016/j.psyneuen.2005.04.016. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Koenig HN, Camarini R, Kim JA, Nannini MA, Ou CJ, Hodge CW. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacology (Berl) 2003;165:181–187. doi: 10.1007/s00213-002-1248-2. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur Neuropsychopharmacol. 2007;17:558–566. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE. The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. J Stud Alcohol Drugs. 2007;68:379–384. doi: 10.15288/jsad.2007.68.379. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ. What place does naltrexone have in the treatment of alcoholism? CNS Drugs. 2004;18:547–560. doi: 10.2165/00023210-200418090-00001. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Miranda R, Jr, McGeary JE, Monti PM. Family history and antisocial traits moderate naltrexone’s effects on heavy drinking in alcoholics. Exp Clin Psychopharmacol. 2007;15:272–281. doi: 10.1037/1064-1297.15.3.272. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Mallya A, Bauman J, Pevnick J, Cho D, Reker D, James B, Dornbush R. Hormonal and other effects of naltrexone in normal men. Adv Exp Med Biol. 1979;116:291–305. doi: 10.1007/978-1-4684-3503-0_17. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]


