Abstract
Type 1 diabetes (T1D) is an autoimmune disease characterized by the selective destruction of pancreatic β-cells. Although successful islet transplantation provides a promising treatment, high cost, lack of donor organs, immune-mediated destruction of transplanted islets, and side effects from immunosuppressive drugs greatly limit its uses. Therefore, the search for novel and cost-effective agents that can prevent or ameliorate T1D is extremely important to decrease the burden of T1D. In this study, we discovered that epicatechin (EC, 0.5% in drinking water), a flavonol primarily in cocoa, effectively prevented T1D in non-obese diabetic (NOD) mice. At 32 weeks of age, 66.7% control mice had overt diabetes, whereas only 16.6% EC-treated mice became diabetic. Consistently, EC mice had significantly higher plasma insulin levels but lower glycosylated hemoglobin concentrations compared to control mice. EC had no significant effects on food or water intake and body weight gain in NOD mice, suggesting that EC’s effect was not due to alterations in these variables. Treatment with EC elevates circulating anti-inflammatory cytokine interleukin-10 levels, ameliorates pancreatic insulitis, and improved pancreatic islet mass. These findings demonstrate that EC may be a novel, plant-derived compound capable of preventing T1D by modulating immune function and thereby preserving islet mass.
Keywords: epicatechin, flavonol, NOD mice, type 1 diabetes, islets
INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disease characterized by T-cell-mediated destruction of pancreatic β-cells, leading to insulin deficiency 1. T1D is the primary form of diabetes in children, with 95% of childhood diabetes being T1D 2. It is estimated that 70,000 children under 15 years old develop T1D world-wide annually, and the incidence is growing by 3–5% each year 3. Although successful islet transplantation provides a promising approach for T1D treatment, high cost from islet transplantation, lack of sufficient donor organs, ongoing immune-mediated destruction of transplanted islets and side effects from the immunosuppressive drugs greatly limit the widespread use of this procedure 4. Thus, the search for novel and cost-effective agents that can prevent or treat T1D could be an important strategy to decrease the burden from this disease.
A recent epidemiological study found that people living on the San Blas island, who are used to consume a large quantity of flavanol-rich cocoa beverage daily, have a considerably lower incidence of ischemic heart disease, stroke, and diabetes compared to those who live on the mainland of Panama 5. Interestingly, these differences disappeared when San Blas islanders migrated to Panama city where the quantity of cocoa beverage consumption was considerably reduced 6. These data suggest that cocoa may exert health-promoting effects. While the specific cocoa components primarily responsible for these actions are not known, several lines of evidence show that cocoa-derived flavanols, a subtype of flavonoids, may potentially exert various health benefits. Collectively, data from past studies demonstrate that flavanol isolates from cocoa can improve vascular function 7–12, inhibit platelet activation and aggregation 13, suppress the production of various inflammatory molecules 14–20, increase the secretion of anti-inflammatory cytokines IL-4 18, IL-5 19 and transforming growth factor-β 20 from cultured human immune cells, improve the expression of antioxidant enzymes 21–24, reduce blood cholesterol levels 23–25, acutely reduces blood pressure in healthy people 26, but it had no significant effect on hyperglycemia in type 2 diabetic mice 27. In addition, some studies reported that cocoa flavanols may have antioxidant properties and scavenge reactive oxygen species 28–30. The major flavanols present in cocoa are epicatechin (EC), catechin, and procyanidin oligomers 31. After cocoa consumption, EC is found to be the primary (>96%) flavanol in human circulation with the plasma concentration reaching over 6 μM after the intake of cocoa, while other forms of flavanols including procyanidin dimer and catechin are less than 1% and 3%, respectively 32. Consistent with this finding, a recent study provides evidence that EC primarily mediates the beneficial effects of flavanols-rich cocoa in humans 9.
In the development of T1D, inflammation plays a critical role. The infiltration of inflammatory cells into the islets and subsequent insulitis are hallmarks of the pathogenesis of T1D. Activated T-cells and macrophages release several proinflammatory cytokines, such as interleukin-1β (IL-1β), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which are believed to be important mediators leading to β-cell destruction in T1D 33–38. IL-4 was reported to prevent insulitis and T1D by potentiating T helper 2 (Th2) cell function 39, and TGF-β can inhibit the expression of IFN-γ, TNF-α, and IL-1β in T-cells, macrophages, NK cells, and B-cells 40.
In this study, we investigated whether dietary intake of EC can modulate immunity and prevent the onset of T1D using non-obese diabetic (NOD) mice, a most widely used spontaneous T1D model that shares a variety of characteristics of patients with T1D 41. We provided evidence for the first time that dietary supplementation of EC can preserve functional β-cell mass and prevent the onset of T1D in NOD mice.
MATERIALS AND METHODS
Chemicals
EC was purchased from Sigma-Aldrich (purity>90% by HPLC, St. Louis, MO); AIN 93G diet was supplied by Dyet, Inc. (Bethlehem, PA); glucometer and strips were from Kroger Inc. (Cincinnati, Ohio); glycosylated hemoglobin (HbA1c) assay kit was purchased from Henry Schein, Inc. (Melville, NY); insulin ELISA kits were from Mercodia Inc. (Winston-Salem, NC); and cytokine array kits were purchased from Quansys Biosciences (West Logan, UT).
Mice and experimental design
Four-wk-old female NOD/LtJ mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were fed a standard rodent diet at ad libitum and housed in an environmentally-controlled (23 ± 2°C; 12-h light/dark cycle) animal facility. Mice were randomly divided into two groups (n=12) and given either 0% or 0.5% of EC in drinking water. We chose this dose because our recent study showed that 0.5% of EC provided in drinking water is very effective in exerting a number of beneficial effects in obese diabetic mice without causing toxicity 42. Based on allometric scaling 43, this dose of EC is equivalent to daily consumption of 250 g of typical dark chocolate containing 6% EC 44. To ensure the stability of EC, stock compound was stored at −80°C and water bottle was sealed and kept away from light. Fresh EC was made and provided to mice every other day with the same batch of EC through the study. Food intake and body weight were measured biweekly, and water intake was recorded every three days. Non-fasting blood glucose levels were measured in blood from tail vain every 3–5 wk using a glucometer. During the whole period of treatment, the general clinical condition and mortality of the mice was monitored daily. Euthanasia of animals was independently assessed by a veterinarian according to AAALAC guidelines. Mice with body weight less than 25% of their original body weight were euthanized by inhalation of CO2 and censused, and their blood and tissues were collected and included for biochemical analysis. The animal protocol for this study was approved by the Institutional Animal Care and Use Committee at Virginia Tech.
Intraperitoneal glucose tolerance test
For glucose tolerance test, mice at 31 wk of age (n=5/group) were fasted for 12 h and then injected intraperitoneally with a single bolus of glucose (2 g/kg body weight) 45. Blood glucose was measured at time points of 0, 5, 15, 30, 60, and 120 min after glucose administration.
Fasting plasma insulin and HbA1c measurements
At the end of the experiment, mice were fasted overnight and anesthetized for collecting blood samples. Blood HbA1c levels were measured using an assay kit, and plasma insulin concentrations were measured using an ELISA kit.
Pancreatic islet mass and insulitis evaluations
Pancreata were removed after mice were euthanized and immediately fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections at 500–μm apart from each other were deparaffinized, hydrolyzed, and stained with haematoxylin. The relative islet area was determined using point counting stereology as described previously 46, 47. Briefly, a 100-square grid reticle (1 cm2) was used to count points over islet tissue using an Olympus BX51 microscope. The area occupied by islets was divided by total area of pancreatic tissue on the slide to determine relative percentage of islet area. Pancreatic islet mass was calculated by multiplying the relative islet area by the total pancreatic weight. Insulitis was scored as follows according to previously published methods 48, 49 : score 0= no lymphocytic infiltration, score 1= peri-insulitis (less than 20% infiltration), score 2= 20~50% infiltrated islet, score 3= 50~80% infiltrated islet, and score 4= more than 80% infiltration. Five sections were scored for each mouse, and 12 mice from each group were evaluated in this study.
Plasma cytokine measurements
Cytokines from serum were tested using a mouse cytokine array kit (Quansys Biosciences West Logan, UT), including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, monocyte chemoattractant protein-1(MCP-1), IFN-γ, TNF-α, macrophage inflammatory protein-1a (MIP-1a), granulocyte macrophage colony-stimulating factor (GMC-SF), and RANTES.
Statistical analysis
Data were analyzed by one-way or two-way repeated measures ANOVA where appropriate. Significant differences between treatments were analyzed using student’s t-test. The Logrank test was applied to compare the survival distributions of the control and EC-treated groups. Data of immune cell infiltration into islets were subjected to the nonparametric Mann-Whitney U test. Differences were considered significant at p < 0.05.
RESULTS
Dietary supplementation of EC prevents T1D in NOD mice
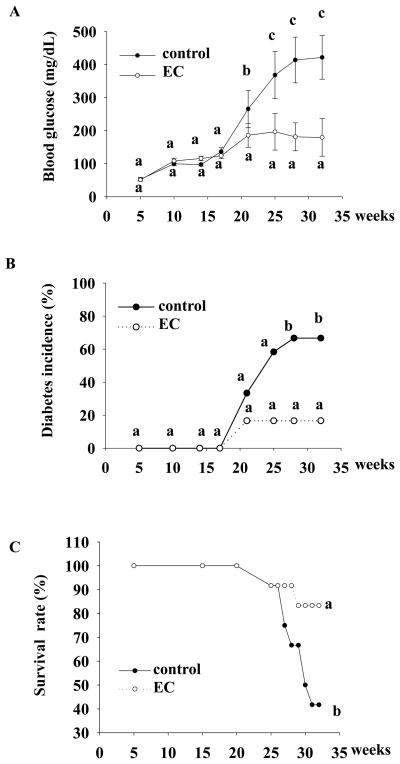
In the present study, we tested if EC has a beneficial effect on T1D by using NOD mice as animal models. We found that EC (0.5% in drinking water) effectively prevented the T1D onset in NOD mice (Figure 1A). At the age of 32 wk, 8 out of 12 mice (66.7%) in control group developed overt diabetes (non-fasting blood glucose over 250 mg/dl), while only 2 out of 12 mice (16.7%) in EC-treated group became diabetic (P=0.04, Figure 1B). Consistent with these data, EC treatment promoted survival of diabetic mice (83.3% in EC group vs. 41.7% in the control) (Figure 1C). All mice sensused for calculating mortality rate, which were healthy before the onset of diabetes, gradually developed severe hyperglycemia and subsequently lost significant amount of body weight, suggesting that these mice died because of diabetes and its complications, which was evaluated and confirmed by a veterinarian. EC treatment did not alter food and water intake as well as body weight of NOD mice before they became diabetic (Table 1), suggesting that the preventive effects of EC on diabetes in NOD mice is not due to alternations of these variables. We didn’t record these variables when mice started developing overt diabetes, because food and water intake and body weight are influenced by diabetes and thus greatly significantly changed after mice become overt diabetic. Therefore, it is impossible to differentiate the effects of EC and diabetes on these parameters after the onset of diabetes. However, we recorded all animal body weight at the time when mice were died from diabetes or euthanized at the end of experiment, which show that EC fed mice had significantly higher body weight than control mice (25.2±3.8 vs. 19.8±4.7), which is largely due to significantly lower incidence of diabetes in EC group than that in the control group, consistent with data showing that EC prevented or delayed the onset of diabetes in NOD mice.
Figure 1.
EC prevents the onset of diabetes in NOD mice. Five-wk old NOD-LtJ female mice were given 0.5% EC in drinking water. Aged-matched control mice were given regular water. Non-fasting blood glucose (A), the incidence of diabetes (B), and survival rate (C) were recorded. Data are expressed as mean ± SE (A) or as percentage (B, C). Means without a common letter differ (n=12 mice/group), P<0.05 .
Table 1.
EC has no effect on food and water intake and body weight in non-diabetic NOD mice.
| Age (wk) | Water intake (ml/d/mouse) | Food intake (g/d/mouse) | Body weight (g) | |||
|---|---|---|---|---|---|---|
| Control | EC | Control | EC | Control | EC | |
| 5 | 2.92(0.09)* | 3.02 (0.00) | 2.36 (0.01) | 2.47 (0.06) | 14.7 (0.2) | 15.1 (0.2) |
| 7 | 3.90 (0.07) | 3.46 (0.02) | 2.44 (0.01) | 2.45 (0.02) | 20.6 (0.2) | 20.5 (0.2) |
| 9 | 3.38 (0.10) | 3.23 (0.23) | 2.37 (0.01) | 2.31 (0.04) | 21.1 (0.2) | 20.6 (0.2) |
| 11 | 2.92 (0.09) | 3.31 (0.28) | 2.73 (0.04) | 2.84 (0.05) | 22.2 (0.2) | 21.4 (0.2) |
| 13 | 3.42 (0.12) | 3.58 (0.33) | 2.87 (0.05) | 2.88 (0.05) | 22.8 (0.2) | 22.0 (0.2) |
| 15 | 2.96 (0.18) | 3.11 (0.41) | 2.78 (0.03) | 2.76 (0.06) | 22.5 (0.2) | 22.5 (0.2) |
| 17 | 3.92 (0.33) | 3.47 (0.24) | 2.89 (0.02) | 2.98 (0.09) | 23.4 (0.2) | 23.5 (0.3) |
Data are mean (± SE).
EC improves glucose tolerance and HbA1c levels
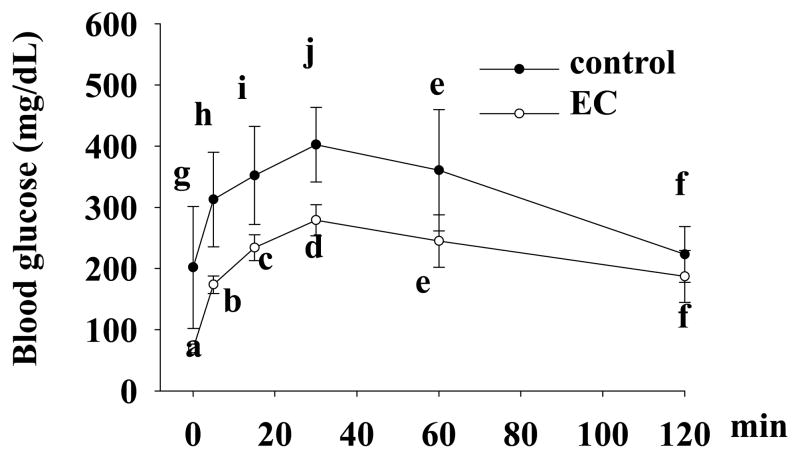
We performed glucose tolerance tests on mice that were still alive at 31 wk of age. Treatment with EC lowered blood glucose by about 50% (ANOVA, p<0.05) during the first 60 min of glucose tolerance test (Figure 2), which however, could be largely due to the improved fasting blood glucose by EC treatment. To our surprise, blood glucose levels after 1 h of glucose administration were not significantly different between two groups. To further confirm the anti-diabetic effect of EC in NOD mice, we measured blood levels of HbA1c, which reflect an average of blood glucose over a period of two to three months 50. Consistently, HbA1c concentrations were significantly lower in EC-treated mice as compared to those in the control mice (5.2± 0.36 vs. 7.4±0.76, p=0.02) (Table 2).
Figure 2.
EC improves glucose tolerance in NOD mice. Overnight-fasted mice were injected intraperitoneally with a bolus of glucose (2g/kg body weight), followed by measurements of blood glucose at 0, 5, 15, 30, 60, and 120 min after glucose injection. Data are expressed as mean ± SE (n=5 mice/group). Means without a common letter differ, P<0.05.
Table 2.
EC lowers HbA1c and increases plasma insulin levels.
Data are mean (± SE).
P<0.05, (n=9 and 12 mice in control and EC groups, respectively).
EC treatment increases plasma insulin levels and pancreatic islet mass
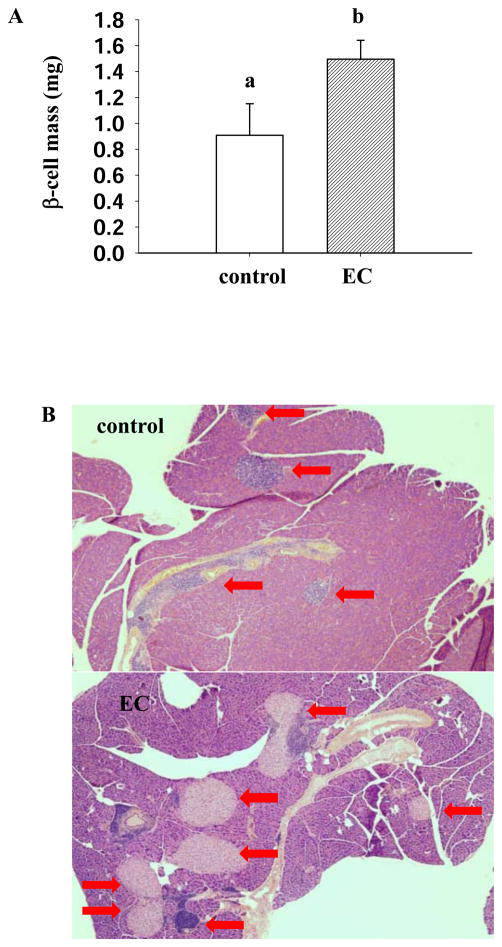
To determine if the improved blood glucose results from increased insulin secretion, we measured and compared plasma insulin levels in control mice and EC-treated mice. Mice treated with EC had significantly higher plasma insulin levels (0.392±0.06 μg/L vs. 0.129±0.03 μg/L, p=0.01) as compared with those in untreated mice (Table 2). Consistent with this result, EC treatment significantly improved pancreatic islet mass (1.5±0.15 mg vs.0.9±0.24 mg, p=0.025) (Figure 3).
Figure 3.
EC supplementation improves pancreatic islet mass in NOD mice. Islet mass was calculated by multiplying pancreas weight by relative β-cell area on tissue slides (n=12). Images shown are representative pancreas section from control and EC treated mice (A). Pancreatic islets are identified by arrows (B). Data are expressed as mean±SE. Means without a common letter differ, P<0.05.
EC improves insulitis and increases plasma IL-10 and IL-12 levels
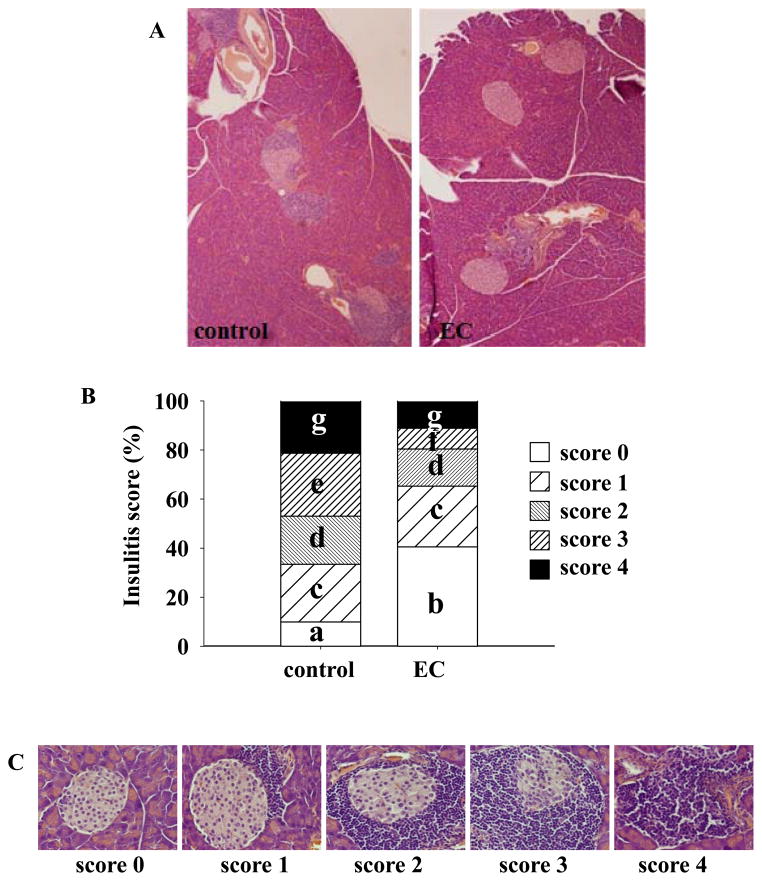
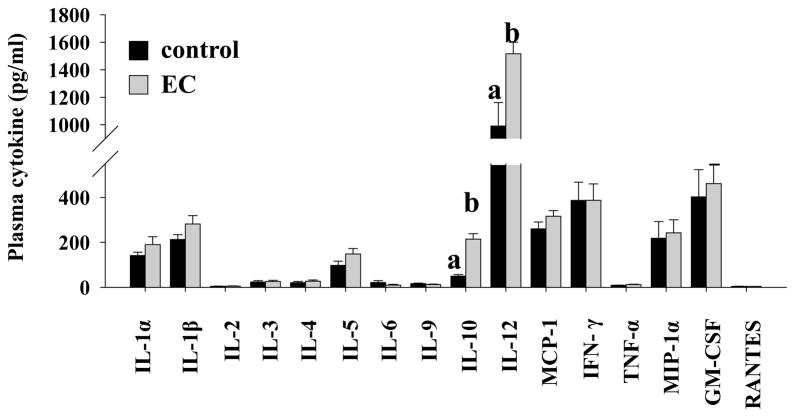
In both T1D patients and NOD mice, invasion of pancreatic islets by immune cells and subsequent islet inflammation (insulitis) primarily causes β-cell destruction. Therefore, we evaluated whether the anti-diabetic action of EC is associated with decreased insulitis. We observed that the degree of lymphatic infiltration into islets from EC-treated mice was lower than that in control mice. EC-fed mice had significantly higher proportion of immune cell-free islets (p=0.02) but fewer islets with clear infiltration (Figure 4), which is in agreement with the decreased occurrence of diabetes in EC-treated mice. We then measured an array of immune regulatory cytokines and chemokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, MCP-1, IFN-γ, TNF-α, MIP-1α, GMCSF, and RANTES) in the circulation. EC-treated mice increased plasma IL-10 (P=0.000002) and IL-12 (P=0.005) levels, while other cytokines and chemokines were not affected (Figure 5).
Figure 4.
EC treatment ameliorates pancreatic islet insulitis. Pancreatic sections were stained with hematoxylin and assessed for insulitis as described in the “Materials and Methods” section. Representative histological sections of pancreas from control and EC-treated mice (A), insulitis scores (B), and representative images for different grades of insulitis (C) are shown. Twelve mice in each group and 5 sections per mouse were scored. Means without a common letter differ, P<0.05.
Figure 5.
Supplementation of EC increases plasma IL-10 and IL-12 levels. Blood was drawn from fasted mice and plasma samples were used for measurements of various cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, MCP-1, IFN-γ, TNF-α, MIP-1α, GM-CSF, and RANTES (n=9 mice in control group, n=12 in EC group). Means without a common letter differ, P<0.05.
DISCUSSION
T1D is an insulin-deficient disease caused by T-cell–mediated autoimmune destruction of pancreatic β-cells. In the present study, we found that EC supplemented in drinking water 0.5% (w/v) can effectively prevent the onset of diabetes and subsequently promoted survival of NOD mice. Consistently, EC treatment significantly improved glucose tolerance and lowered HbA1c levels which were associated with increased circulating insulin levels, improved insulitis, and preserved pancreatic islet mass. These findings provide evidence for the first time that EC may be a natural agent that can protect pancreatic islets from autoimmune-mediated destruction, and thereby prevent the development of T1D.
In the present study, long-term treatment with EC had no effect on food and water intake and body weight gain of non-diabetic NOD mice, suggesting that this compound likely has no significant toxic effect and that an anti-diabetic effect of EC is not a secondary action whereby it modulates these variables in mice. Indeed, our recent study demonstrated that long-term dietary treatment with EC at this dose has an array of beneficial effects without causing toxicity in obese diabetic mice 42. Consistently, it was reported that dietary intake of 1.5 g/kg/day of EC did not induce any toxicological relevant changes in rats (48).
Blood glucose levels are tightly regulated through the coordinated actions of several organs. The intestine is the first key organ involved in glucose homeostasis where carbohydrate in the meal is digested and released glucose is transported to the circulation 51. While catechins were reported to suppress intestinal absorption of glucose in rodents 52, which could contribute to postprandial glycemic control and body weight gain, the anti-diabetic action of EC in NOD mice should be primarily ascribed to its ultimate protection on functional pancreatic islets, as demonstrated by significantly larger β-cell mass in EC-treated mice. Consistently, mice given EC had significantly higher circulating insulin levels compared to those in the controls. Furthermore, EC treatment improved fasting blood glucose levels and intraperitoneal glucose tolerance, which largely reflects a direct response of β-cells to circulating glucose 53–55.
While the pathogenic mechanisms and T-cell mediated autoimmune process that destroy pancreatic β-cells in T1D are complex and are still not fully defined 56–59, it is clear from past studies that the infiltration of immune cells into the islets and subsequent insulitis are hallmarks of the pathogenesis of T1D. Activated T-cells and macrophages produce pro-inflammatory cytokines, such as IL-1β, IFN-γ, and TNF-α, which are believed to be important mediators leading to β-cell destruction in T1D 33–38. In the present study, we found that EC intake significantly reduced immune cell infiltration in the islets. This finding demonstrates that the preservation of functional β-cell mass by dietary intake of EC is likely mediated through preventing immune cell infiltration and thereby β-cell destruction. To further examine whether EC targets the immune systems to modulate immunity in NOD mice, we measured the circulating levels of inflammation-related cytokines, which are indicators of immune cell activity. EC had no effect on most of cytokines tested in this study. Paradoxically, we observed that EC increased plasma levels of pro-inflammatory cytokine IL-12, which was reported to enhance T1D development 60. However, this effect of EC appears to be moderate. On the contrary, plasma IL-10 levels in EC-treated mice were 6.8-fold higher compared to those in untreated mice. IL-10 is an anti-inflammatory cytokine that is primarily secreted by Th2 cells and regulatory T-cells 61, 62. Previous studies demonstrated that administration of IL-10 or IL-10 gene transfer prevents insulitis and diabetes in NOD mice 63–66, suggesting that IL-10 may have an important role in the control of T1D development. Based on these observations, it is reasonable to speculate that the effect of EC on insulitis and thereby T1D onset may be mediated by stimulating IL-10 production, which warrants further investigation.
While data from this study suggest that the anti-diabetic effect of EC might be due to modulation of immunity, thereby protecting islets from immune cell-mediated destruction of pancreatic β-cells, the underling mechanism for this action by EC is still unclear. It is well recognized that oxidative stress may play a potential role in the initiation of chronic inflammation and various degenerative diseases including diabetes, which is always associated with a decline in antioxidant levels in a number of tissues. EC is considered a potent free radical scavenger at pharmacological doses 67, and its biological effects are frequently attributed to a presumably antioxidant activity. At physiologically relevant doses however, EC displayed very low ability of scavenging free radicals 68. Our recent study showed that dietary intake of epigallocatechin gallate (EGCG), a similar antioxidant primarily present in green tea, also prevented T1D in NOD mice 69. Interestingly, unlike EC, which prevented insulitis and therefore protected islets from immune cell-mediated destruction, EGCG did not prevent immune cell infiltration into pancreatic islets but may directly promoted islet survival 69. These findings suggest that the preventive effect of EC in the pathogenesis of T1D could be primarily mediated via antioxidant-independent mechanisms.
In conclusion, we provided evidence for the first time that dietary supplementation of EC can prevent the onset of T1D in NOD mice. This protective effect is likely due to EC prevention of islets from immune cell-infiltration-mediated destruction of pancreatic islets, thereby preserving functional β-cell mass. However, further studies are still needed to define the underling mechanism for this action by EC, which will provide the basis for further pre-clinical and clinical trials to evaluate its preventive and therapeutic potential for T1D.
Acknowledgments
This study was supported by grants from the American Diabetes Association Awards (1-08-JF-30, 7-11-BS-84 to DL), the National Center for Complementary and Alternative Medicine of National Institute of Health (1R21AT004694 to DL), and the Biodesign and Bioprocessing Research Center, College of Agriculture and Life Sciences, Virginia Tech.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
References
- 1.Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67:376–387. [PubMed] [Google Scholar]
- 2.Soltesz G, Patterson CC, Dahlquist G. Worldwide childhood type 1 diabetes incidence--what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. doi: 10.1111/j.1399-5448.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 3.Danne T, Lange K, Kordonouri O. New developments in the treatment of type 1 diabetes in children. Arch Dis Child. 2007;92:1015–1019. doi: 10.1136/adc.2006.094904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009;4:e4226. doi: 10.1371/journal.pone.0004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayard V, Chamorro F, Motta J, Hollenberg Nk. Does flavanol intake influence mortality from nitrix oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama. International journal of medical sciences. 2007;4:53–58. doi: 10.7150/ijms.4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenberg NK, Naomi F. Is it the dark in dark chocolate? Circulation. 2007;116:2360–2362. doi: 10.1161/CIRCULATIONAHA.107.738070. [DOI] [PubMed] [Google Scholar]
- 7.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 8.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiss C, Schroeter H, Balzer J, Kleinbongard P, Matern S, Sies H, Kelm M. Endothelial function, nitric oxide, and cocoa flavanols. J Cardiovas c Pharmacol. 2006;47(Suppl 2):S128–135. doi: 10.1097/00005344-200606001-00007. discussion S172–126. [DOI] [PubMed] [Google Scholar]
- 11.Rees DD, Palmer RM, Hodson HF, Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. British J of Pharm. 1989;96:418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaha AM, Vallanceb P, Harrisonc D. NO in the cardiovascular system. Cardiovasc Res. 1999;43:507–508. doi: 10.1016/s0008-6363(99)00181-9. [DOI] [PubMed] [Google Scholar]
- 13.Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 14.Noreen Y, Serrano G, Perera P, Bohlin L. Flavan-3-ols isolated from some medicinal plants inhibiting COX-1 and COX-2 catalysed prostaglandin biosynthesis. Planta Med. 1998;64:520–524. doi: 10.1055/s-2006-957506. [DOI] [PubMed] [Google Scholar]
- 15.Schewe T, Sadik C, Klotz LO, Yoshimoto T, Kuhn H, Sies H. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol Chem. 2001;382:1687–1696. doi: 10.1515/BC.2001.204. [DOI] [PubMed] [Google Scholar]
- 16.Sanbongi C, Suzuki N, Sakane T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humans in vitro. Cell Immunol. 1997;177:129–136. doi: 10.1006/cimm.1997.1109. [DOI] [PubMed] [Google Scholar]
- 17.Mao T, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa procyanidins and human cytokine transcription and secretion. J Nutr. 2000;130:2093S–2099S. doi: 10.1093/jn/130.8.2093S. [DOI] [PubMed] [Google Scholar]
- 18.Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev Immunol. 2002;9:135–141. doi: 10.1080/1044667031000137601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TKM, JVdW, CLK, HHS, MEG Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J Med Food. 2002;5:17–22. doi: 10.1089/109662002753723188. [DOI] [PubMed] [Google Scholar]
- 20.Mao TK, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa flavonols and procyanidins promote transforming growth factor-beta1 homeostasis in peripheral blood mononuclear cells. Exp Biol Med (Maywood) 2003;228:93–99. doi: 10.1177/153537020322800113. [DOI] [PubMed] [Google Scholar]
- 21.Ramiro-Puig E, Urpi-Sarda M, Perez-Cano FJ, Franch A, Castellote C, Andres-Lacueva C, Izquierdo-Pulido M, Castell M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J Agric Food Chem. 2007;55:6431–6438. doi: 10.1021/jf070487w. [DOI] [PubMed] [Google Scholar]
- 22.Yeh CT, Yen GC. Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. J Nutr. 2006;136:11–15. doi: 10.1093/jn/136.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Osakabe N, Baba S, Yasuda A, Iwamoto T, Kamiyama M, Takizawa T, Itakura H, Kondo K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- 24.Mathur S, Devaraj S, Grundy SM, Jialal I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- 25.Fraga CG, Actis-Goretta L, Ottaviani JI, Carrasquedo F, Lotito SB, Lazarus S, Schmitz HH, Keen CL. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin Dev Immunol. 2005;12:11–17. doi: 10.1080/10446670410001722159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 27.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–355. doi: 10.1016/j.nut.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Modak B, Contreras ML, Gonzalez-Nilo F, Torres R. Structure-antioxidant activity relationships of flavonoids isolated from the resinous exudate of Heliotropium sinuatum. Bioorg Med Chem Lett. 2005;15:309–312. doi: 10.1016/j.bmcl.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 29.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 30.Silva MM, Santos MR, Caroco G, Rocha R, Justino G, Mira L. Structure-antioxidant activity relationships of flavonoids: a re-examination. Free Radic Res. 2002;36:1219–1227. doi: 10.1080/198-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 31.Zhu QY, Holt RR, Lazarus SA, Orozco TJ, Keen CL. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysis. Exp Biol Med (Maywood) 2002;227:321–329. doi: 10.1177/153537020222700504. [DOI] [PubMed] [Google Scholar]
- 32.Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 33.Mandrup-Poulsen T, Helqvist S, Molvig J, Wogensen LD, Nerup J. Cytokines as immune effector molecules in autoimmune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity. 1989;4:191–218. doi: 10.3109/08916938909003049. discussion 219–134. [DOI] [PubMed] [Google Scholar]
- 34.Pankewycz OG, Guan JX, Benedict JF. Cytokines as mediators of autoimmune diabetes and diabetic complications. Endocrine Reviews. 1995;16:164–176. doi: 10.1210/edrv-16-2-164. [DOI] [PubMed] [Google Scholar]
- 35.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 36.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46:255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 37.Li L, El-Kholy W, Rhodes CJ, Brubaker PL. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48:1339–1349. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 38.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51:311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 39.Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitch TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–4692. [PubMed] [Google Scholar]
- 40.Piccirillo CA, Chang Y, Prud’homme GJ. TGF-beta1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J Immunol. 1998;161:3950–3956. [PubMed] [Google Scholar]
- 41.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 42.Si H, Fu Z, Babu PV, Zhen W, Leroith T, Meaney MP, Voelker KA, Jia Z, Grange RW, Liu D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J Nutr. 2011;141:1095–1100. doi: 10.3945/jn.110.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langer S, Marshall LJ, Day AJ, Morgan MR. Flavanols and methylxanthines in commercially available dark chocolate: a study of the correlation with nonfat cocoa solids. J Agric Food Chem. 2011;59:8435–8441. doi: 10.1021/jf201398t. [DOI] [PubMed] [Google Scholar]
- 45.Ruohonen ST, Pesonen U, Moritz N, Kaipio K, Roytta M, Koulu M, Savontaus E. Transgenic mice overexpressing neuropeptide Y in noradrenergic neurons: a novel model of increased adiposity and impaired glucose tolerance. Diabetes. 2008;57:1517–1525. doi: 10.2337/db07-0722. [DOI] [PubMed] [Google Scholar]
- 46.Weibel ER. The value of stereology in analysing structure and function of cells and organs. J Microsc. 1972;95:3–13. doi: 10.1111/j.1365-2818.1972.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 47.Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Todorov I, Lin CL, Atkinson M, Kandeel F, Forman S, Zeng D. Elimination of insulitis and augmentation of islet beta cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci U S A. 2007;104:2337–2342. doi: 10.1073/pnas.0611101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Signore A, Annovazzi A, Giacalone P, Beales PE, Valorani MG, Vestri AR, Ruberti G, Manfrini S, Pozzilli P, Bulfone-Paus S. Reduced cumulative incidence of diabetes but not insulitis following administration of chimeric human IL-15-murine IgG2b in NOD mice. Diabetes Metab Res Rev. 2003;19:464–468. doi: 10.1002/dmrr.400. [DOI] [PubMed] [Google Scholar]
- 50.Aldasouqi SA, Solomon DJ, Bokhari SA, Khan PM, Muneera S, Gossain VV. Glycohemoglobin A1c: A promising screening tool in gestational diabetes mellitus. Int J Diabetes Dev Ctries. 2008;28:121–124. doi: 10.4103/0973-3930.45271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knauf C, Cani PD, Kim DH, Iglesias MA, Chabo C, Waget A, Colom A, Rastrelli S, Delzenne NM, Drucker DJ, Seeley RJ, Burcelin R. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57:2603–2612. doi: 10.2337/db07-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skopec MM, Green AK, Karasov WH. Flavonoids have differential effects on glucose absorption in rats (Rattus norvegicus) and American robins (Turdis migratorius) J Chem Ecol. 2010;36:236–243. doi: 10.1007/s10886-010-9747-9. [DOI] [PubMed] [Google Scholar]
- 53.Reimer RA, Russell JC. Glucose tolerance, lipids, and GLP-1 secretion in JCR:LA-cp rats fed a high protein fiber diet. Obesity (Silver Spring) 2008;16:40–46. doi: 10.1038/oby.2007.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vialettes B, Vague P, Lassmann V, Simon MC. Islet transplantation in diabetic rats. Long-term follow-up of glucose tolerance. Acta Diabetol Lat. 1979;16:1–8. doi: 10.1007/BF02590757. [DOI] [PubMed] [Google Scholar]
- 55.Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparre T, Larsen MR, Heding PE, Karlsen AE, Jensen ON, Pociot F. Unraveling the pathogenesis of type 1 diabetes with proteomics: present and future directions. Mol and Cel Proteomics. 2005;4:441–457. doi: 10.1074/mcp.R500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 57.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–994. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 58.Tisch R, Wang B. Dysrulation of T cell peripheral tolerance in type 1 diabetes. Adv Immunol. 2008;100:125–149. doi: 10.1016/S0065-2776(08)00805-5. [DOI] [PubMed] [Google Scholar]
- 59.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 60.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmun Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Balasa B, Davies JD, Lee J, Good A, Yeung BT, Sarvetnick N. IL-10 impacts autoimmune diabetes via a CD8+ T cell pathway circumventing the requirement for CD4+ T and B lymphocytes. J Immunol. 1998;161:4420–4427. [PubMed] [Google Scholar]
- 62.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nitta Y, Tashiro F, Tokui M, Shimada A, Takei I, Tabayashi K, Miyazaki J. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum Gene Ther. 1998;9:1701–1707. doi: 10.1089/hum.1998.9.12-1701. [DOI] [PubMed] [Google Scholar]
- 64.Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 2000;7:2099–2104. doi: 10.1038/sj.gt.3301334. [DOI] [PubMed] [Google Scholar]
- 65.Goudy K, Song S, Wasserfall C, Zhang YC, Kapturczak M, Muir A, Powers M, Scott-Jorgensen M, Campbell-Thompson M, Crawford JM, Ellis TM, Flotte TR, Atkinson MA. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proc Natl Acad Sci U S A. 2001;98:13913–13918. doi: 10.1073/pnas.251532298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z, Chen M, Wu R, Fialkow LB, Bromberg JS, McDuffie M, Naji A, Nadler JL. Suppression of autoimmune diabetes by viral IL-10 gene transfer. J Immunol. 2002;168:6479–6485. doi: 10.4049/jimmunol.168.12.6479. [DOI] [PubMed] [Google Scholar]
- 67.Sheehan EW, Zemaitis MA, Slatkin DJ, Schiff PL., Jr A constituent of Pterocarpus marsupium (−)-epicatechin as a potential antidiabetic agent. J Nat Prod. 1983;46:232–234. doi: 10.1021/np50026a018. [DOI] [PubMed] [Google Scholar]
- 68.Galleano M, Verstraeten SV, Oteiza PI, Fraga CG. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch Biochem Biophys. 2010;501:23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Fu Z, Zhen W, Yuskavage J, Liu D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br J Nutr. 2011;105:1218–1225. doi: 10.1017/S0007114510004824. [DOI] [PMC free article] [PubMed] [Google Scholar]