Abstract
The aim of this work is to evaluate the effects of purified aromatic-turmerone(ar-turmerione, AR) on murine dendritic cells (DCs). These impacts of AR on DCs from bone marrow derived DCs(BMDCs) were assessed with use of conventional scanning electron microscopy (SEM), fluorescence activated cell sorting (FACS), transmission electron microscopy (TEM), cytochemistry assay, FITC-dextran, bio-assay and enzyme linked immunosorbent assay (ELISA). We found that AR induced phenotypic maturation as evidenced by increased expression of CD86, CD40, CD83, CD80 and major histocompatibility complex II (MHC II). The functional tests showed the activity of acidic phosphatase (ACP) inside the DCs were downregulated after treatment with AR (which occurs when phagocytosis of DCs were decreased). Finally, we proved that AR increased the production of IL-12 and tumor necrosis factor α (TNF-α). These data suggested that AR could promote phenotypic and functional maturation of DCs and this adjuvant-like activity may have potential therapeutic value. It is therefore concluded that AR could exert positive modulation on murine DCs.
Keywords: AR, BMDCs, modulation, maturation
Introduction
Turmeric, Curcuma longa L. (Zingiberaceae family) rhizomes, grows naturally throughout the Indian sub-continent and in tropical countries, particularly in Southeast Asia. It is commonly used as a spice and is well documented for its medicinal properties in Indian and Chinese systems of medicine. It has been widely used for centuries in indigenous medicine for the treatment of several diseases.1 Epidemiological observations, though inconclusive, are suggestive that turmeric consumption may be anti-inflammatory, anti-angiogenic, anti-oxidant, wound healing and other effects. The rhizome of turmeric contains a mixture of three curcuminoids and two turmerones, mainly including curcumin, demethoxycurcumin, bisdemethoxycurcumin, α-turmerone (AL) and aromatic-turmerone (AR).2 Despite the extensively characterized of anti-inflammatory effect of turmeric and its reported effect on T cells and macrophages, no study so far has dealt with the immunomodulatory activities of purified AR on murine BMDCs. Last but not least, the findings revealed the potential use of AR as an immunomodulatory agent.
Dendritic cells (DCs) are a vital lineage of blood cells that controls the immune system. DCs are potent antigen-presenting cells that possess the unique capacity to stimulate naive T cells and induce not only T cell immunity but also T cell tolerance. DCs can also produce cytokines and are susceptible to cytokine-mediated activation. Immature DCs are characterized by high phagocytic capacity and low levels of expression of major histocompatibility complex (MHC) and costimulatory molecules such as CD80, 86 etc. Maturation of DCs is associated with phenotypic changes, including downregulation of phagocytic capacity, upregulation of costimulatory molecules, MHC and secretion of cytokines, transforming them into fully functional antigen-presenting cells (APC) capable of priming naive T cells.3-7
Results
Magnetic activated cell sorting (MACS)
All the cells expressing CD11c in the different wells were isolated respectively using MACS, After the CD11c positive cells were treated with or without turmeric, the purity of the sorted cells were determined by FACS analysis. (Fig. 1)
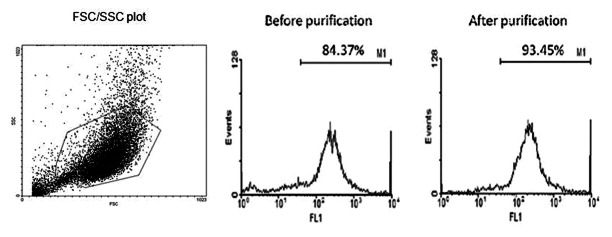
Figure 1. MACS. After cultured with GM-CSF and IL-4 for 6 d, the purity of CD11c+ cells were examined by FACS and the percentage was over 80%. Then purified by MACS, the CD11c+ cells were enriched and the result of FACS approached 94%. FSC/SSC plot was shown in order to get an impression on the purity of the isolated cells.
MTS assay
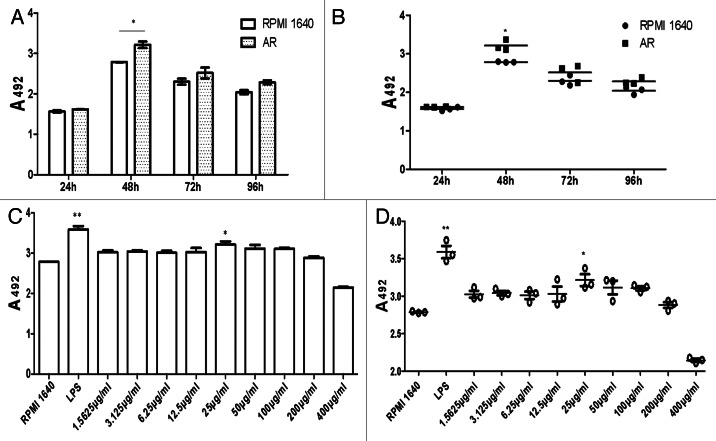
BMDCs were incubated with AR for different period of time (24, 48, 72, 96 h separately). And 24 h after addition of AR, there was no change in cell viability (Fig. 2A and B). To determine optimal concentration of AR, MTS assay was performed at the same time ranged from 1.5625 µg/ml to 400 µg/ml. It was found that the most optimal concentration of AR to boost cell proliferation was 25 µg/ml as shown in Figure 2C and D. All data were represented as means ± S.E.M. (n = 3). *p < 0.05 vs. these in RPMI 1640. **p < 0.01 vs. those in RPMI 1640.
Figure 2. MTS. Proliferation of BMDCs at different period of time points (A and B) and concentration(C and D) of AR. The SEM values were below 10% of the mean value, indicating a good reproducibility of replicates (n = 3).
Inverted phase contrast microscope
The immature BMDCs showed mostly round shape (Fig. 3A), while the mature BMDCs were large cells existing typical irregular protrusions (Fig. 3B and C).
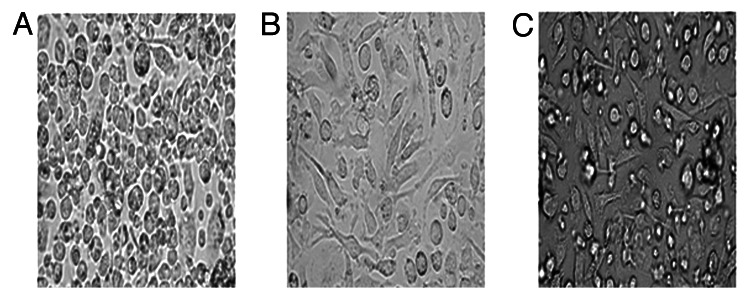
Figure 3. The morphology(×100) under light microscope showed the change of BMDCs. (A) RPMI 1640, (B) LPS, (C) AR.
Transmission electronmicroscopy (TEM)
The monocyte projenitors in bone marrow were cultured for 6 d in GM-CSF and IL-4 in the absence of AR (Fig. 4A) or presence of AR would develope into BMDCs (Fig. 4C). The BMDCs (Fig. 4A) showed a round surface with less cytoplasmic projections and more endocytic vacuoles and lysosomes. However, the BMDCs cultured with LPS or AR showed an irregular morphology with less endocytic vacuoles and lysosomes, more protrusions extending multilevel, clear nuclei, mitochondria rich, well-developed rough endoplasmic reticulum visible in Figure 4B and C.
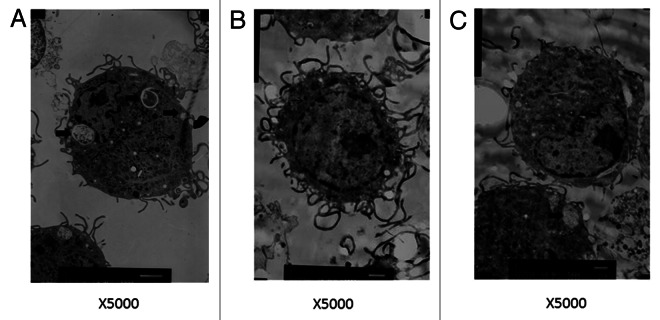
Figure 4. Transmission electron microscopy revealed BMDCs morphology, surface, cytoplasm and organelles. After cultured with AR for 2 d, three characteristic forms of BMDCs were evident. The majority of cells had a more irregular external surface with more cytoplasmic projections, less vacuoles (heavy arrow) and lysosomes(thin arrow). (A) RPMI 1640, (B) LPS, (C) AR.(×5000)
Scanning electronmicroscopy(SEM)
After treatment with AR, the BMDCs showed that there existed many cell surface folds, volume increased further, various kinds of membraneous extensions, some of them long and straight, some fine and branched, others short and bulky (Fig. 5B).
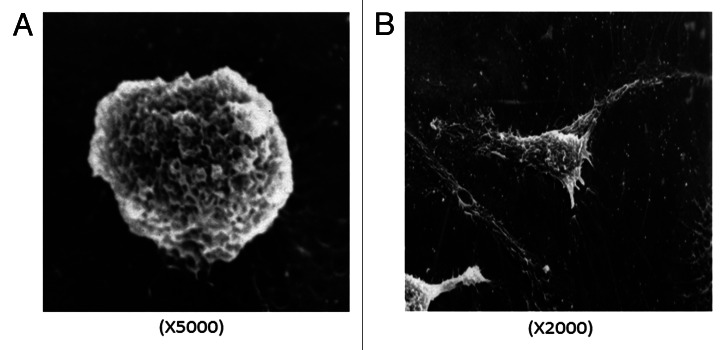
Figure 5. Scanning electron microscopy saw BMDCs morphology. Most of the BMDCs treated with RPMI 1640 were small and round with smooth rim shown in A (×5000). However, BMDCs treated with AR exists many cell surface folds and typical irregular protrusions shown in B (×2000).
FACS analysis
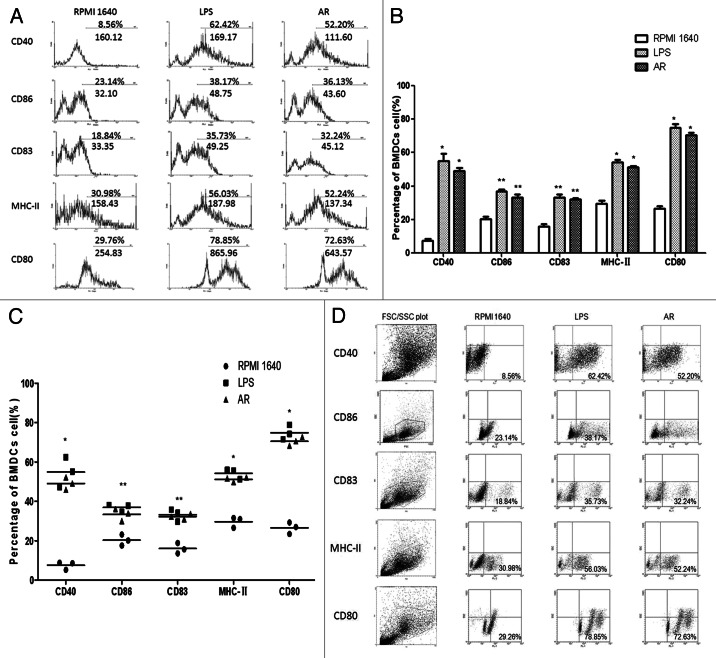
The expression of specific cell surface markers were analyzed using FACS. The BMDCs treated with RPMI 1640 showed the typical expression of cell surface molecules as immature DC. In contrast the BMDC treated with LPS or AR showed a clear change of the expression levels of CD40, CD86, CD83, MHC-II and CD80 (Fig. 6).
Figure 6. (A) AR enhanced the phenotypic maturation of murine DC. The BMDC were phenotypically assessed using FACS analyses to determine the expression levels of specific cell surface molecules, as shown in the percentage of expression. AR clearly increased the expression of CD40, CD86, CD83, MHC-II and CD80 (a representative of three independent experiments). (B and C).AR enhanced the phenotypic maturation of murine DCs. All data were shown as means ± S.E.M.(n = 3). * p < 0.05 vs. these in RPMI 1640. ** p < 0.01 vs. those in RPMI 1640. (D) FSC/SSC plots and scatter plots with all data points were shown in C. The lower left showed the percentage of positive cells, which also proved that AR enhanced the phenotypic maturation of murine DCs.
Cellular immunohistochemistry of phagocytosis by DC
Immunohistochemical staining of BMDC treated or untreated with AR were performed. The number of BMDCs with DAB precipitate in the group with AR or LPS reduced significantly (Fig. 7C and B) as compared with that in the RPMI1640 group (Fig. 7A), which is consistent with the ACP activity assay.
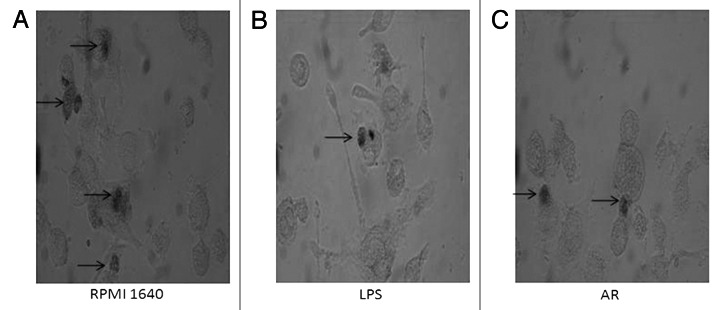
Figure 7. Cellular immunohistochemistry of phagocytosis by DC. The BMDCs in different groups were stained with DAB kit. Most of the immature BMDCs were filled with DAB precipitate. The BMMCs were observed with an inverted phase contrast microscope (CMS GmbH Light Microscopes, Leica Microsystems)(×400).
Phagocytosis study
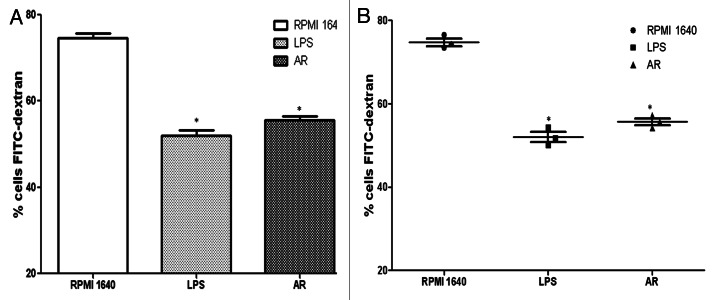
The reduced capacity for antigen uptake is interpreted as a sign of DC maturation. To test the effect on functional maturation of the BMDCs induced with LPS or AR, the endocytotic function was measured by uptake of FITC-dextran. Immature BMDCs showed high levels of endocytoxic activity while the FITC-dextran uptake by BMDC treated with LPS or AR showed low levels of endocytoxic activity. For the analysis of dextran uptake, data for controls at 4°C should be subtracted from the data at 37°C. All data were represented as means ± S.E.M. (n = 3) in Figure 8.
Figure 8. Comparison of FITC-dextran uptaking by immature and mature BMDCs. The BMDCs in different groups were prepared and compared in their ability to uptake FITC-dextran. The cells with lower FITC-dextran uptake were preincubated with LPS or AR. For the analysis of dextran uptaking, the data for controls incubated at 4°C should be subtracted from the 37°C data. All data were represented as means ± S.E.M.(n = 3). * p < 0.05 vs. these in RPMI 1640. ** p < 0.01vs. those in RPMI 1640.
Acid phosphatase (ACP) activity detection
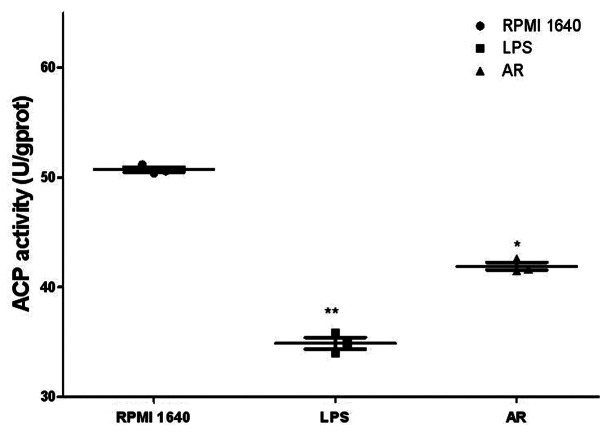
The ACP in the lysosomes is a key enzyme for DCs to digest antigen. So its activity number reflects the degree of maturity of DCs. As shown in Table 1 and Figure 9, AR decreased significantly the activity of ACP in the BMDCs. This is consistent with the cellular immunohistochemistry of phagocytosis by DCs above, which means that the capability for DC to arrest antigen has become weakened, simultaneously with getting stronger in antigen presentation upon maturation. *p < 0.05 vs. these in RPMI 1640. **p < 0.01 vs. those in RPMI 1640.
Table 1. ACP activity of BMDCs after treatment with AR.
| Group | ACP activity (U/gprot) | P |
|---|---|---|
| RPMI 1640 |
50.81 ± 0.07 |
|
| LPS |
34.55 ± 1.51 |
< 0.01 |
| AR | 41.10 ± 0.76 | < 0.05 |
Figure 9. The scatter plots of ACP activity. ACP activity was determined after treatment with AR for 48 h. Results represented the mean ± SEM of three independent experiments.
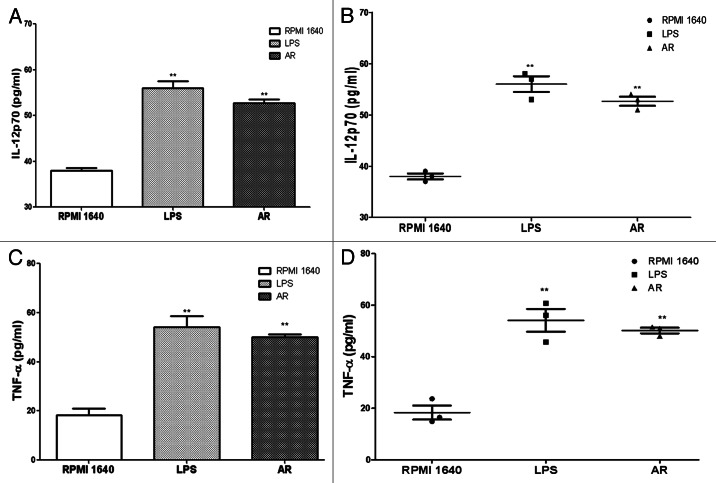
Cytokine secretion by the BMDCs in response to AR
At the end of these culture periods, supernatants were harvested and production of IL-12p70 (Fig. 10A and B) and TNF-α (Fig. 10 and D) were assayed with ELISA. Results were represented as means and SEMs obtained from three independent experiments.
Figure 10. Cytokine assay of IL-12p70 (A and B) and TNF-α (C and D). IL-12p70 and TNF-α secretion was tested by ELISA from culture supernatants of 5 × 104 cells/well immature and mature BMDC. Results were expressed as the mean ± SEM of triplicate samples. AR led to an approximate twice of production in IL-12p70 and TNF-α with * p < 0.05 vs. these in RPMI 1640 and ** p < 0.01 vs. those in RPMI 1640.
Discussion
Turmeric was suggested to have the functions of eliminating blood stasis, promoting the flow of “qi,” stimulating menstrual discharge and relieving pain. Furthermore turmeric widely consumed as food additive and medicine, are believed to possess antimicrobial, antifungal, antiviral, anti-inflammatory activities, wound healing, insecticidal activity, anti-platelet effects, anti-cancer, anti-inflammatory, antioxidant, limit fungal growth and toxin production properties.8-18 Previous studies had shown that AR isolated from turmeric could induce apoptosis in human leukemia cells19 and murine leukemia cells.20 Moreover, AR can be employed as an agent in functional cosmetics to develop safe and effective skin-whitening treatments.21 The AR also possessed anti-oxidant22 and antiplatelet activities.23,24 Now our study provided findings with following major fact:(1) turmeric can remarkably induced the morphological maturation of BMDCs, that was that the matured BMDCs showed a more irregular external surface with more cytoplasmic projection (Figs. 3 and 5),which usually are more receptors such as toll like receptors to fit eating more microbes or antigens, (2) turmeric can remarkably induced phenotypic maturation of BMDCs, that was that AR promoted higher expression of MHC II, CD86, CD83, CD80 and CD40 molecules, which are critical surface molecules as a complex stimulating signals to present an antigen to initiate T cell response (Fig. 6), (3) turmeric can remarkably induced functional maturation of BMDCs, that was that AR deducted phagocytosis ability (Figs. 4, 7 and 8) and ACP activity (Table 1) of BMDCs, which are important parameters indicating the degree of maturation of DCs. Also AR induced DCs to secret higher levels of IL-12 and TNF-α (Fig. 10), which will intensify DC-CD4+T cell pathway, resulting in increased Th1 responses and anti-tumor effect.
DCs are highly specialized immune cells derived from bone marrow stem cell which are unique in their ability to initiate and maintain primary immune responses.25,26 DCs can secrete a diversified panel of chemokines that attract different cell types at different times of the immune response.27 They also express a unique set of costimulatory molecules which permit the activation of naive T cells and thus allow the launching of primary immune response. Their crucial functions comprise antigen presentation, stimulation and regulation of antigen specific T lymphocyte responses. At the same time DCs are at the interface between tolerance and immunity. DCs are also believed to have a major role in the decision whether the immune system will reject or tolerate an antigen (immunity vs. tolerance).28 Though most of the current knowledge relates to the presentation of peptides to T cells in the context of MHC classes I and II molecules, DCs can present glycolipids and glycopeptides to T cells and NKT cells as well as polypeptides to B cells. DCs undergo a complex maturation process from antigen-capturing cells into antigen-presenting cells. Tumors as well as normal tissues under steady-state conditions certainly want to suppress DC function to prevent immune aggression. Numerous ongoing studies aim to evaluate the effectiveness of dendritic cells in preventing tumor relapses and extending patients' survival. Dendritic cells immunogenicity, and from a better understanding of the cell dynamics whereby immune responses are orchestrated.29-34 Here, we discuss these new insights together with an overview of the DC-based clinical studies performed to date. Therefore, in this study, we used AR to promote the maturation process of DCs from antigen-capturing cells into antigen-presenting cells.
To the best of our knowledge, this is possibly the first report on the immunomodulatory activity exerted by AR on murine BMMCs. These may contributes to a better understanding of its modulating effects on immune system, and complicated mechanisms at molecular level via which AR regulates immune system. Nowadays it is known that the consumption of AR is associated with many beneficial effects on human health. In contrast to this point, its use as an herbal medicinal product is more than seldom. The research in-vitro and in-vivo has shown various activities, such as anti-inflammatory, antiviral, antifungal, cytokines release, antioxidant, immunomodulatory, enhancing of the apoptotic process and antiangiogenic properties. Indeed numerous studies are now evaluating the efficacy of turmeric as the adjuvant setting for different types of cancers. So we hope the role of AR on DCs could be benefit for anticancer immunotherapy.35,36 But such studies require large numbers of patients and long follow up periods to determine the impact of AR on disease free and overall survival, thus, more time is required to see the results. However a research of this investigation is now beginning in our group.
Materials and methods
Reagents and antibodies
Standard ar-turmerone (AR) was purchased from ChromaDex™, (Catalog number: Asb-00020596–010). The mAbs used in this study were purchased from eBioscience and BD PharMingen respectively. IL-4 (Catalog number: 315–03) and GM-CSF (Catalog number:214–14) were obtained from PeproTech Inc., The ELISA assay kits for IL-12p70 and TNF-α analysis were purchased from eBioscience. LPS (Catalog number: L-2880. Ten ng/ml was used) as a positive control was a product of Sigma-Aldrich. MACS (Miltenyi Biotec). Other chemicals frequently used in our laboratory were all products from Sigma-Aldrich or BD PharMingen.
Mice
The female C57BL/6 mice with 4–6weeks old involved in this study were from pathogen free animal house, China Medical University.
Bone marrow-derived dendritic cells (BMDCs) culture
After removing all muscle tissues with gauze from the femurs and tibias, the bones were placed in a 60 mm dish with 70% alcohol for 1 min, washed twice with PBS and transferred into a fresh dish with RPMI 1640. Both ends of the bones were cut with scissors in the dish, and then the marrow was flushed out using 1 ml of RPMI 1640 with a 1ml syringe. The tissue was suspended, passed through nylon mesh to remove small pieces of bone and debris, and red cells were lysed with ammonium chloride. Then 106 /ml cells were placed in 24-well plates in 1 ml of RPMI 1640 supplemented with 10% fetal bovine serum, recombinant murine granulocyte macrophage colony stimulating factor (GM-CSF) (10 ng/ml), interleukin (IL)-4 (10 ng/ml), 2 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin. After incubation for 4 h, the medium containing non-adherent cells was removed and replaced with fresh medium as described above. Supplemented medium was replaced every three days and on day 6 of culture, all the cells expressing CD11c in the different wells were isolated respectively using MACS according to the manufacturer’s instruction and were seeded into new wells with fresh medium. Finally, the CD11c-positive cells were treated with or without turmeric at a dose of 25 µg/ml for another 48 h, and the purity of the sorted cells were determined by fluorescence activated cell sorting (FACS) analysis (Fig. 1). and the separated BMDCs were used for the phenotypic studies.
MTS assay
With 100 µl BMDCs suspension at 1 × 105 cell/ml were seeded in 96 well microtiter plate (Corning incorporated Costar). Added 100 µl AR in concentration range from 1.5625 µg/m to 400 µg/ml to the wells respectively, triplicate/conc. The effect of different AR concentrations on the proliferation of DCs was determined by MTS/PMS analysis as the kinetics of the responses and the shape of BMMCs were observed with an inverted phase contrast microscope (CMS GmbH Light Microscopes, Leica Microsystems). Optical density was read by iMark Microplate Reader (BIO-BAD) at 492 nm.
Transmission Electronmicroscopy(TEM)
Collected BMDCs, which were treatment with LPS (10 ng/ml) and AR (25 µg/ml) for 48 h were fixed for 0.5 h at 4°C in 2.5% glutaraldehyde in 0.1 M sodium cacodylate containing 1% sucrose and 2 mM calcium chloride at pH 7.4 to preserve surface morphology. Cell preparation steps included glutaraldehyde fixation, cleaning, glutaraldehyde fixation, slicing and finally, the prepared samples were analyzed by TEM (JEOL JEM-1200EX).
Scanning Electronmicroscopy(SEM)
After seeding BMDCs onto coverslips, we treated BMMCs with LPS or AR for 48 h. Following fixation in 2% glutaraldehyde (GA), preparations were kept at 4°C until postfixation with 1% osmium tetroxide and then treated with 1% tannic acid, dehydrated in ethanol and critical point dried from carbon dioxide. Specimens were sputter-coated with gold and analyzed in a SEM (JEOL JSM-T300).
FACS analysis
For surface labeling 5 × 105 or more cells of cultured BMDCs served as testing group (after treatment with LPS or AR for 48 h) and control group. The cells were first washed three times in phosphate-buffer saline(PBS) with 2% Fetal Bovine Serum (FBS), and incubated with 10 µl anti-CD40, anti-CD86, anti-CD83, anti-CD80 and anti-MHC II antibodies for 30 min at 4°C for optimal staining, and then washed twice in PBS containing 2% FBS and the cell pellet was re-suspended in the residual volume (~100 µl). The cells were analyzed immediately. The data, obtained from the analysis of the cells by FACS Calibur (Becton Dickinson), were then analyzed using WinMDI 2.9 (Joseph Trotter, BD Biosciences).
Cellular immunohistochemistry of phagocytosis by DC
The BMDCs (1 × 105/ml), treated with LPS or AR for 48 h were cultured with 0.08 mg/ml horseradish peroxidase and then fixed with methanol for 4 h, followed by staining with DAB kit. Finally, the samples were observed under light microscope.
Phagocytosis study
The cells were suspended in RPMI 1640, 10% FCS and 25 mM HEPES, pH 7.4 and incubated with 1 mg/ml of FITC–dextran (Mr = 40,000; Sigma) at 4°C for 2 h, subsequently at 37°C for 1 h and finally washed 3 times with ice-cold PBS, 2% FBS and 0.01% NaN3. The samples were checked by FACS Calibur (Becton Dickinson).
Acid phosphatase (ACP) activity detection
The concentration of BMDCs was adjusted to 1 × 106/ml. The ACP activity of the BMDCs cells after treatment with LPS or AR for 48h was measured at OD520 nm by the phenol-4-AAP (amino antipyrine) method in conjunction with ACP testing kit (Jiancheng Bio-engineering Institute of the South).
Cytokine assay of IL-12p70 and TNF-α
BMDCs were plated in 96-well flat-bottom plates at a concentration of 5 × 104 cells/well either with medium alone, AR or with LPS. Supernatants were collected after 48 h and tested for their IL-12p70 and TNF-α contents by ELISA kits. The absorbance at 450 nm (A450) was determined using a iMark Microplate Reader (BIO-RAD).
Statistical analysis
Statistical analysis was performed using the statistical program SPSS (Statistical Package for Social Sciences, Version 16.0) for Windows. All variables were presented as mean ± SEM. Differences were evaluated by ANOVA for multiple groups and by the student t-test two groups using the Prism (Graph Pad Software). T test used for post hoc analysis indicated significance when p < 0.05 by ANOVA.
Acknowledgments
This work was supported financially by China Liaoning provincial foundation for international collaboration, No.2006305007 (to Feng Ping Shan).
Glossary
Abbreviations:
- AR
aromatic-turmerone, ar-turmerone
- ACP
acidic phosphatase
- DCs
dendritic cells
- BMDCs
bone-marrow derived dendritic cells
- LPS
lipopolysaccharide
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- DAB
3, 3′-diaminobenzidine
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy- methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- PMS
phenazine methosulphate
- MACS
magnetic activated cell sorting
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21526
References
- 1.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 2.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 3.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–56. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 5.Muth S, Schütze K, Schild H, Probst HC. Release of dendritic cells from cognate CD4+ T-cell recognition results in impaired peripheral tolerance and fatal cytotoxic T-cell mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:9059–64. doi: 10.1073/pnas.1110620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. 2011;10:336–45. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donald A, Fitch FW. The road to the discovery of dendritic cells, a tribute to Ralph Steinman. Cell Immunol. 2012;2012273:95–8. doi: 10.1016/j.cellimm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Jayaprakasha GK, Negi PS, Anandharamakrishnan C, Sakariah KK. Chemical composition of turmeric oil--a byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Z Naturforsch C. 2001;56:40–4. doi: 10.1515/znc-2001-1-207. [DOI] [PubMed] [Google Scholar]
- 9.Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;2005:720–4. doi: 10.1016/j.foodchem.2005.06.037. [DOI] [Google Scholar]
- 10.Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.) Food Chem Toxicol. 2010;48:1026–31. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Balaji S, Chempakam B. Toxicity prediction of compounds from turmeric (Curcuma longa L) Food Chem Toxicol. 2010;48:2951–9. doi: 10.1016/j.fct.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Sindhu S, Chempakam B, Leela NK, Suseela Bhai R. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem Toxicol. 2011;49:1188–92. doi: 10.1016/j.fct.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Martin RC, Aiyer HS, Malik D, Li Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. Food Chem Toxicol. 2012;50:227–31. doi: 10.1016/j.fct.2011.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling J, Wei B, Lv G, Ji H, Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012;130:229–35. doi: 10.1016/j.foodchem.2011.07.039. [DOI] [Google Scholar]
- 15.Miquel J, Bernd A, Sempere JM, Díaz-Alperi J, Ramírez A. The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geriatr. 2002;34:37–46. doi: 10.1016/S0167-4943(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 16.Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agric Food Chem. 1999;47:4297–300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 17.Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Chemistry and biological activities of C. longa. Trends Food Sci Technol. 2005;16:533–48. doi: 10.1016/j.tifs.2005.08.006. [DOI] [Google Scholar]
- 18.Prakash P, Misra A, Surin WR, Jain M, Bhatta RS, Pal R, et al. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb Res. 2011;127:111–8. doi: 10.1016/j.thromres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Aratanechemuge Y, Komiya T, Moteki H, Katsuzaki H, Imai K, Hibasami H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L) in two human leukemia cell lines, but not in human stomach cancer cell line. Int J Mol Med. 2002;9:481–4. [PubMed] [Google Scholar]
- 20.Ji M, Choi J, Lee J, Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14:253–6. [PubMed] [Google Scholar]
- 21.Park SY, Jin ML, Kim YH. Aromatic-turmerone inhibits a-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch Dermatol Res. 2011;303:737–44. doi: 10.1007/s00403-011-1155-7. [DOI] [PubMed] [Google Scholar]
- 22.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C. 2002;57:828–35. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 23.Lee HS. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol. 2006;97:1372–6. doi: 10.1016/j.biortech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Yue GGL, Chan BCL, Hon P-M, Lee MYH, Fung K-P, Leung P-C, et al. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem Toxicol. 2010;48:2011–20. doi: 10.1016/j.fct.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 27.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–8. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuler G. Dendritic cells: indispensable? Cancer J. 2011;17:337–42. doi: 10.1097/PPO.0b013e3182350077. [DOI] [PubMed] [Google Scholar]
- 29.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101–13. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 30.Blanco PA, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood. 2012;119:106–14. doi: 10.1182/blood-2011-06-360768. [DOI] [PubMed] [Google Scholar]
- 32.Nagato K, Motohashi S, Ishibashi F, Okita K, Yamasaki K, Moriya Y, et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment after α-Galactosylceramide-Pulsed antigen presenting cells. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9697-9. [DOI] [PubMed] [Google Scholar]
- 33.Park MH, Lee JS, Yoon JH. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol. 2012 doi: 10.1002/jso.23095. [DOI] [PubMed] [Google Scholar]
- 34.Reichardt VL, Brossart P, Kanz L. Dendritic cells in vaccination therapies of human malignant disease. Blood Rev. 2004;18:235–43. doi: 10.1016/j.blre.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Ullricha E, Ménarda C, Flamenta C, Termea M, Mignota G, Bonmorta M, et al. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev. 2008;1:79–92. doi: 10.1016/j.cytogfr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Ballestreroa A, Boya D, Morana E, Cirmenaa G, Brossartb P, Nencionia A. Immunotherapy with dendriticcells for cancer. Adv Drug Deliv Rev. 2008;2:173–83. doi: 10.1016/j.addr.2007.08.026. [DOI] [PubMed] [Google Scholar]