Abstract
The Drosophila Sex-lethal (Sxl) gene encodes a female-specific RNA binding protein that in somatic cells globally regulates all aspects of female-specific development and behavior. Sxl also has a critical, but less well understood, role in female germ cells. Germ cells without Sxl protein can adopt a stem cell fate when housed in a normal ovary, but fail to successfully execute the self-renewal differentiation fate switch. The failure to differentiate is accompanied by the inappropriate expression of a set of male specific markers, continued proliferation, and formation of a tumor. The findings in Chau et al., (2012) identify the germline stem cell maintenance factor nanos as one of its target genes, and suggest that Sxl enables the switch from germline stem cell to committed daughter cell by posttranscriptional downregulation of nanos expression. These studies provide the basis for a new model in which Sxl directly couples sexual identity with the self-renewal differentiation decision and raises several interesting questions about the genesis of the tumor phenotype.
Keywords: sex determination, germline tumors, stem cell differentiation, nanos, bam, Sxl
Introduction
In Drosophila adults, continuous sperm and egg production depends on a stable population of stem cells that have the capacity to give rise to both self-renewing and differentiating daughter cells.1,2 In both sexes, several germline stem cells (GSCs) reside within a specialized microenvironment located at the anterior end of the gonad. GSCs are prevented from differentiating because they receive strong differentiation-inhibiting signals from their somatic neighbors. The signaling activity, however, is highly restricted. Thus, when the GSC divides, only the daughter cell that remains anchored to the anterior end continues to self-renew. The daughter cell that moves away no longer receives, or responds to, the inhibiting signals and initiates the differentiation program. Defects in this process have drastic consequences. An excess of differentiation leads to stem cell depletion and premature sterility. Failure to enter the differentiation pathway leads to an accumulation of proliferating cells and tumor formation.
Not surprisingly, there are sex-specific differences in the way males and females regulate the self-renewal decision.1,2 Moreover, the sexual identity of the germ cells must match the sex of their somatic neighbors for gametogenesis to occur.3 The mechanism by which somatic cells acquire and maintain their sexual identity is different than the mechanism used by germ cells.3-5 In somatic cells, the choice to be male or female is made early in embryogenesis when X-chromosome number is relayed through regulatory proteins to activate Sex-lethal (Sxl) exclusively in XX animals.6,7 Expression of the Sxl RNA binding protein then serves as an irreversible genetic switch because expression is maintained by a positive feedback splicing mechanism.8,9 In contrast to the early cell autonomous decision made by somatic cells, the sexual identity of embryonic germ cells initially reflects the sex of the surrounding somatic gonadal cells.10-12 Somatic control over germline sexual behavior, however, does not persist after embryogenesis, indicating that sex is then maintained by a cell intrinsic mechanism.13
A number of studies have fingered Sxl as a critical player in maintaining germ cell sexual identity because loss of Sxl function in XX germ cells leads to germ cell tumors that inappropriately express testis-enriched markers.14-18 Here we discuss our recent analysis of Sxl function in the germline,19 which supports a new model linking the self-renewal/differentiation decision with the maintenance of sexual identity and raises some interesting questions about the genesis of germ cell tumors.
Connecting Sexual Identity to the Self-Renewal/Differentiation Decision
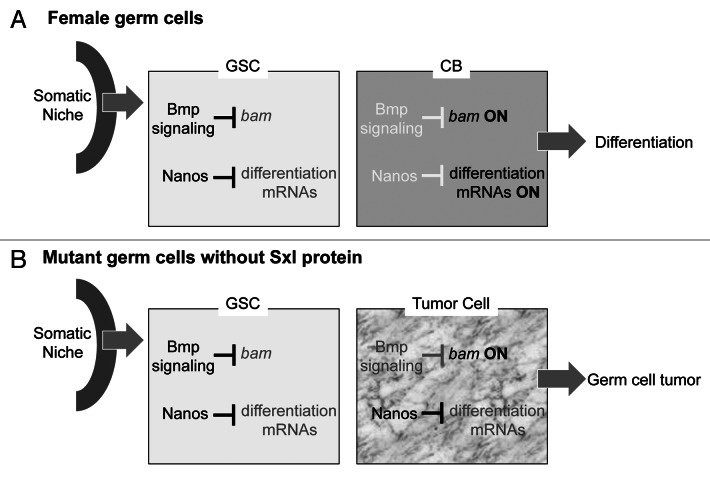
We recently uncovered an unexpected role for Sxl in the lineage progression from stem cell to committed daughter cell through our detailed analysis of the tumorous phenotype.18 In the adult ovary, cell fate switching from a self-renewing GSC to a differentiation-competent daughter cell, called a cystoblast (CB), includes significant accumulation of the differentiation promoting protein Bag-of-marbles (Bam) accompanied by rapid downregulation of a number of self-renewal factors, including Nanos (Fig. 1). In the absence of Sxl protein, mutant germ cells can adopt a GSC fate, but instead of subsequently entering the differentiation pathway, the majority of mutant germ cells are blocked at a stage that is intermediate between a GSC and CB cell—a cell that co-expresses Bam protein and a set of GSC-specific markers, including Nanos protein.18,19
Figure 1. Drawing of the ovarian niche with one GSC cell. The daughter CB lies just outside of the niche. The ovary is composed of about 20 ovarioles each of which contains an linear array of germ cells at progressive stages of development. The somatic niche, the microenvironment that maintains GSC fate by a BMP signaling cascade is located at the tip of each ovariole. (A) In wild type the GSC to CB cell fate switch occurs as one of the daughter cells moves out of this microenvironment permitting the initiation of the differentiation program that includes significant accumulation of the Bam protein and rapid downregulation of a set of GSC specific markers including Nanos protein. Note that Nanos and Bam proteins are expressed in non-overlapping domains. In contrast, Sxl protein (not shown) is expressed in both Nanos- and Bam-expressing cells. (B) Germ cells that lack Sxl protein fail to exit the stem cell stage, continue to proliferate, and form a tumor. GSC markers, including Nanos protein, are co-expressed with Bam in the majority of the tumor cells, except for the presumptive GSCs located at the tip of the ovariole.
In the studies reported in Chau et al., (2012)19 we provide key insight into the cellular mechanism by which Sxl mediates the GSC/CB cell fate switch; namely, we now identify nanos as a Sxl target gene. While previous studies showed that Nanos downregulation in CB cells is regulated at the level of translation,20 the RNA binding proteins controlling the fate of the nanos mRNA had not been identified. We found that this rapid downregulation pattern is limited to female germ cells and is under Sxl control. Moreover, we were able to demonstrate that regulation is direct; nanos mRNA is bound by the female-specific Sxl RNA binding protein in ovarian extracts and nanos silencing is dependent on Sxl binding sites located in the nanos 3′ UTR. These studies therefore point to a post-transcriptional mechanism by which Sxl promotes differentiation through repression of nanos translation.
Incorporating Sexual Identity into the Self-Renewal/Differentiation Regulatory Network
Our studies now add Sxl to the network that controls the GSC to CB cell fate switch. In this integrated model, GSCs are prevented from prematurely differentiating because they receive a strong Bmp signal from their somatic neighbors which inhibits bam transcription.21,22 An additional layer of control is provided by Nanos, and its partner protein Pumilio (Pum), which together repress the translation of differentiation-promoting mRNAs, including brain tumor (brat).23-26 A complete GSC to CB cell fate switch requires several steps. First, when the GSC divides and moves away from the niche the reduced external Bmp signaling leads to bam expression. Second, in Bam-expressing cells Sxl represses the translation of nanos mRNA,19 which allows translation of differentiation-promoting mRNAs, including brat. Third, the newly translated Brat protein partners with Pum to repress translation of self-renewal-promoting mRNAs, including the mRNA encoding the Bmp transducer Mad.26 Finally, negative regulation of Mad by Brat, together with several other highly redundant mechanisms, extinguish the ability of the newborn CB to respond to Bmp signaling.27-32
Sxl is expressed in both GSCs and their progeny, yet its role in silencing nanos must be limited to Bam-expressing cells. Thus an important question regarding Sxl function is how Sxl-mediated regulation of Nanos is restricted to Bam-expressing cells. We propose that Bam itself confers cell type specificity. Previous studies have shown that bam is also required for lowering Nanos protein levels in CB cells.20 However, physical data showing that Bam directly regulates nanos is lacking. Nevertheless, a Sxl/bam partnership is strongly supported by genetic epistasis experiments which show that bam function depends on Sxl activity and, moreover, that Sxl and bam jointly control the entry into the differentiation pathway.18,19 Bam is known to regulate translation in other contexts,33 thus the two proteins could function together to repress nanos translation. Invoking a Sxl/Bam regulatory complex is attractive not only because it explains how Sxl function is limited to differentiating germ cells, but also how bam function substantially differs between males and females.34,35
Our model predicts that by failing to silence nanos, germ cells without Sxl will not express the necessary differentiation-promoting mRNAs, including brat. Furthermore, the failure to express brat is expected to weaken the mutant cells resistance to external sources of Bmp signaling. While this prediction has not been rigorously tested, it is consistent with the observation that in the absence of Sxl protein a minority of mutant germ cells inappropriately respond to Bmp signaling.18 The majority of the mutant germ cells, however, appear to be refractory to Bmp signaling and exhibit robust bam expression. Thus, in the absence of Sxl the other mechanisms responsible for dampening the response to Bmp signaling continue to function.
Connecting the Loss of Sexual Identity to Tumorigenesis
Interestingly, a number of studies, including our own, have observed that the failure to silence nanos in germ cells is not sufficient to cause the tumorous phenotype characteristic of Sxl loss of function.19,20,26 Even though forced expression of nanos can delay differentiation, resulting in an accumulation of extra stem-like germ cells, it does not interfere with gametogenesis.19,26 The conclusion that nanos dysregulation is not what drives tumor formation is supported by our double mutant studies which show that while nanos is necessary for accelerating tumor growth, the majority of surviving double mutant germ cells continue to resemble a tumor cell.19 Thus other genes and pathways under Sxl control must be necessary to elicit malignant transformation.
What other genes and pathways are under Sxl control? A comprehensive list of Sxl target genes is not yet available. We do known, however, that germ cell tumors resulting from the lack of Sxl inappropriately express a number of testis-enriched markers.18 Control of this male gene expression network, however, does not require nanos, as double mutant germ cells continue to express these male markers (unpublished). Remarkably, bam ovarian tumors express the same set of testis-enriched markers.18 We therefore propose that Sxl and bam co-regulate at least two independent pathways, one of which leads to downregulation of nanos translation and the other that silences this male-specific gene expression network (Fig. 2).
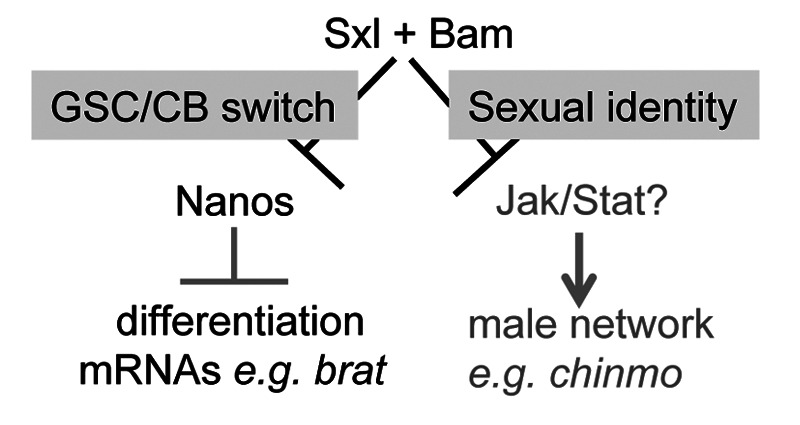
Figure 2. Model for integration of female sexual identity with the self-renewal/differentiation decision by a Sxl/Bam partnership. Recent studies suggest that Nanos maintains GSC cell fate by repressing RNAs, such as brat, required for differentiation. In CBs, Bam/Sxl inhibits nanos translation, thereby promoting differentiation. In addition, Sxl/Bam maintains female sexual identity by repressing a male-specific network of genes that includes chinmo, most likely through attenuation of Jak/Stat signaling.
It will be interesting, therefore, to determine whether the sexually inappropriate gene expression network unleashed by the loss of sexual identity is what drives tumorigenesis. In this regard, we find it intriguing that chronologically inappropriate morphogenesis (chinmo) is ectopically expressed in ovarian tumors (unpublished). In the adult, chinmo expression is normally limited to the testis, with expression in both the germline and somatic cells.36-38 Moreover, chinmo is positively and cell-autonomously regulated at the transcription level by the Janus kinase-Signal transducer and activator of transcription (Jak/Stat) signaling pathway.38 Although Jak/Stat signaling is used reiteratively in the somatic cells of both the ovary and the testis, activation in the germline is strictly male-specific.38-43 Female GSCs do not activate the Jak/Stat signaling pathway. Thus, our finding that tumor cells express chinmo suggests that the normally male-specific Jak/Stat pathway is inappropriately activated. How might the absence of Sxl protein lead to the erroneous activation of the pathway? One possibility is that mutant germ cells respond as if they were male germ cells to the activating cytokine Unpaired secreted from the somatic gonadal cells. Although entirely speculative at this time, a role for sex-inappropriate activation of the Jak/Stat pathway in ovarian tumor formation is consistent with numerous studies that have connected hyperactive Jak/Stat signaling to other Drosophila tumor models,44-50 and human cancers.51
Beyond Drosophila
Our studies focused on how Sxl jointly controls the exit from the stem cell state and the maintenance of germline sexual identity offers new insight into the female-specific exit strategy used by germ cells to enter into the differentiation pathway. The challenge in coming years will be to understand the functional connections between the failure to make this cell fate transition, sexually inappropriate gene expression, and tumorigenesis.
Although the gene regulatory networks that control sex determination vary between species, the link between germ cell differentiation, sexual identity, and germ cell cancer may extend beyond Drosophila. In humans, germ cell tumors occur frequently in individuals with intersex disorders.52,53 There is also increasing evidence that testicular germ cell tumors arise from disruptions in sex-specific processes that control differentiation.54-57 Altogether these studies suggest that the information obtained in Drosophila may provide a valuable foundation for investigating the mechanisms underlying mammalian germ cell tumors and may, in the future, lead to the design of effective strategies to restrict tumor growth.
Acknowledgments
I thank L. Kulnane, J. McDonald, and R. Sousa-Neves for helpful comments. The National Institutes of Health (NIH) grant R01-GM61039 provided support for my work on Sxl.
Glossary
Abbreviations:
- Sxl
Sex-lethal
- GSC
germline stem cell
- CB
Cystoblast
- bam
bag of marbles
- brat
brain tumor
- chinmo
chronologically inappropriate morphogenesis
- Jak/Stat
Janus kinase-Signal transducer and activator of transcription
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/22687
References
- 1.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–71. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann R. Germline stem cells: origin and destiny. Cell Stem Cell. 2012;10:729–39. doi: 10.1016/j.stem.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray SM, Yang SY, Van Doren M. Germ cell sex determination: a collaboration between soma and germline. Curr Opin Cell Biol. 2010;22:722–9. doi: 10.1016/j.ceb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salz HK, Erickson JW. Sex determination in Drosophila: The view from the top. Fly (Austin) 2010;4:60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salz HK. Sex determination in insects: a binary decision based on alternative splicing. Curr Opin Genet Dev. 2011;21:395–400. doi: 10.1016/j.gde.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson JW, Quintero JJ. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol. 2007;5:e332. doi: 10.1371/journal.pbio.0050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salz HK. Male or female? The answer depends on when you ask. PLoS Biol. 2007;5:e335. doi: 10.1371/journal.pbio.0050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cline TW. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics. 1984;107:231–77. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991;65:229–39. doi: 10.1016/0092-8674(91)90157-T. [DOI] [PubMed] [Google Scholar]
- 10.Nöthiger R, Jonglez M, Leuthold M, Meier-Gerschwiler P, Weber T. Sex determination in the germ line of Drosophila depends on genetic signals and inductive somatic factors. Development. 1989;107:505–18. doi: 10.1242/dev.107.3.505. [DOI] [PubMed] [Google Scholar]
- 11.Wawersik M, Milutinovich A, Casper AL, Matunis E, Williams B, Van Doren M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature. 2005;436:563–7. doi: 10.1038/nature03849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casper A, Van Doren M. The control of sexual identity in the Drosophila germline. Development. 2006;133:2783–91. doi: 10.1242/dev.02415. [DOI] [PubMed] [Google Scholar]
- 13.Casper AL, Van Doren M. The establishment of sexual identity in the Drosophila germline. Development. 2009;136:3821–30. doi: 10.1242/dev.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schüpbach T. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of sex-lethal in Drosophila melanogaster. Genetics. 1985;109:529–48. doi: 10.1093/genetics/109.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinmann-Zwicky M, Schmid H, Nöthiger R. Cell-autonomous and inductive signals can determine the sex of the germ line of drosophila by regulating the gene Sxl. Cell. 1989;57:157–66. doi: 10.1016/0092-8674(89)90181-5. [DOI] [PubMed] [Google Scholar]
- 16.Wei G, Oliver B, Pauli D, Mahowald AP. Evidence for sex transformation of germline cells in ovarian tumor mutants of Drosophila. Dev Biol. 1994;161:318–20. doi: 10.1006/dbio.1994.1032. [DOI] [PubMed] [Google Scholar]
- 17.Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science. 2011;333:885–8. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
- 18.Chau J, Kulnane LS, Salz HK. Sex-lethal facilitates the transition from germline stem cell to committed daughter cell in the Drosophila ovary. Genetics. 2009;182:121–32. doi: 10.1534/genetics.109.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau J, Kulnane LS, Salz HK. Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis. Proc Natl Acad Sci USA. 2012;109:9465–70. doi: 10.1073/pnas.1120473109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc Natl Acad Sci USA. 2009;106:9304–9. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–91. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–64. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 23.Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–90. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- 24.Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 2004;14:981–6. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–9. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- 26.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011;20:72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–90. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- 28.Iovino N, Pane A, Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009;17:123–33. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–90. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Xia L, Zheng X, Zheng W, Zhang G, Wang H, Tao Y, et al. The niche-dependent feedback loop generates a BMP activity gradient to determine the germline stem cell fate. Curr Biol. 2012;22:515–21. doi: 10.1016/j.cub.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 31.Lu W, Casanueva MO, Mahowald AP, Kato M, Lauterbach D, Ferguson EL. Niche-associated activation of rac promotes the asymmetric division of Drosophila female germline stem cells. PLoS Biol. 2012;10:e1001357. doi: 10.1371/journal.pbio.1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- 33.Shen R, Weng C, Yu J, Xie T. eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc Natl Acad Sci USA. 2009;106:11623–8. doi: 10.1073/pnas.0903325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gönczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–71. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- 35.Insco ML, Leon A, Tam CH, McKearin DM, Fuller MT. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc Natl Acad Sci USA. 2009;106:22311–6. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–9. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–20. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–68. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–8. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.López-Onieva L, Fernández-Miñán A, González-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–40. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- 43.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–10. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Feng S, Thomas S, Wang J. Diverse tumor pathology due to distinctive patterns of JAK/STAT pathway activation caused by different Drosophila polyhomeotic alleles. Genetics. 2012;190:279–82. doi: 10.1534/genetics.111.135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herranz H, Hong X, Hung NT, Voorhoeve PM, Cohen SM. Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes Dev. 2012;26:1602–11. doi: 10.1101/gad.192021.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng S, Huang J, Wang J. Loss of the Polycomb group gene polyhomeotic induces non-autonomous cell overproliferation. EMBO Rep. 2011;12:157–63. doi: 10.1038/embor.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–8. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YH, Huang ML. Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye. Dev Dyn. 2010;239:2522–33. doi: 10.1002/dvdy.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nat Genet. 2009;41:1150–5. doi: 10.1038/ng.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González I, Simón R, Busturia A. The Polyhomeotic protein induces hyperplastic tissue overgrowth through the activation of the JAK/STAT pathway. Cell Cycle. 2009;8:4103–11. doi: 10.4161/cc.8.24.10212. [DOI] [PubMed] [Google Scholar]
- 51.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pleskacova J, Hersmus R, Oosterhuis JW, Setyawati BA, Faradz SM, Cools M, et al. Tumor risk in disorders of sex development. Sex Dev. 2010;4:259–69. doi: 10.1159/000314536. [DOI] [PubMed] [Google Scholar]
- 53.Hersmus R, Stoop H, White SJ, Drop SL, Oosterhuis JW, Incrocci L, et al. Delayed Recognition of Disorders of Sex Development (DSD): A Missed Opportunity for Early Diagnosis of Malignant Germ Cell Tumors. Int J Endocrinol. 2012;2012:671209. doi: 10.1155/2012/671209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–4. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanetsky PA, Mitra N, Vardhanabhuti S, Vaughn DJ, Li M, Ciosek SL, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum Mol Genet. 2011;20:3109–17. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kratz CP, Han SS, Rosenberg PS, Berndt SI, Burdett L, Yeager M, et al. Variants in or near KITLG, BAK1, DMRT1, and TERT-CLPTM1L predispose to familial testicular germ cell tumour. J Med Genet. 2011;48:473–6. doi: 10.1136/jmedgenet-2011-100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turnbull C, Rapley EA, Seal S, Pernet D, Renwick A, Hughes D, et al. UK Testicular Cancer Collaboration Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42:604–7. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]