Abstract
When obtaining samples for population genetic studies, it is essential that the sampling is random. For Drosophila, one of the crucial steps in sampling experimental flies is the collection of eggs. Here an egg collection method is presented, which randomizes the eggs in a water column and diminishes environmental variance. This method was compared with a traditional egg collection method where eggs are collected directly from the medium. Within each method the observed and expected standard deviations of egg-to-adult viability were compared, whereby the difference in the randomness of the samples between the two methods was assessed. The method presented here was superior to the traditional method. Only 14% of the samples had a standard deviation higher than expected, as compared with 58% in the traditional method. To reduce bias in the estimation of the variance and the mean of a trait and to obtain a representative collection of genotypes, the method presented here is strongly recommended when collecting eggs from Drosophila.
Keywords: Egg-to-adult viability, density control, random sampling, reliability, sampling error
Introduction
To obtain a sample of fruit flies, e.g., Drosophila melanogaster, or other insect species for experimental work one needs to control the density of larvae during development to avoid crowding effects on phenotypes such as size.1 Various methods are used in different laboratories, but many studies control the number of eggs within each vial by directly transferring eggs from an egg laying medium to medium in a vial. This commonly used method will be the focus of this study and referred to as the traditional method. However, this method introduces the possibility of non-random sampling, which in turn results in biased estimates of the traits of interest. In the traditional egg collection method, a line of D. melanogaster is divided into a number of small containers (bottles or vials) with medium, and eggs are picked from these containers, whereby each container only represents a fraction of the genotypes present in the line. Furthermore, the females do not oviposit their eggs in a random pattern across the medium and the pattern may vary within lines.2,3 Thus eggs picked from one container will not be a random sample of the line as a whole, and will not be a random sample of the small ‘container-line’ if eggs are picked from a small area within the container. Here, I describe another procedure of egg picking, based on Moth and Barker,6 which ensures the collection of random samples of eggs from the line at hand. This method will be referred to as the suspension method, and will be compared with the traditional method by looking at the standard deviation of the egg-to-adult viability in a high number of lines. As egg-to-adult viability follows a binomial distribution (1) where the probability of x successes (P(x)) can be calculated from the number of trials (n) and the probability of success, in this case the mean hatching success (p), the standard deviation will only depend on the mean and the number of replicates.
A higher standard deviation than expected in egg-to-adult viability can be caused by three events: (1) sampling error, (2) larval density effects on mortality level1,4 and (3) non-random samples. Sampling error occurs when the egg picker miscounts the number of eggs or damages some of the eggs during transfer from the egg-picking site to the new medium. This source of variance will always be present, and can only be mitigated by practice and care. However, differences in the handling of eggs between the two egg collection methods may affect the mean and the standard deviation of egg-to-adult viability. Larval density differences among vials within a line will result in a difference in the chance of survival.1,4,5 As an increased standard deviation would increase the effect of larval density on mortality levels, it can bias the results by strengthening any differences between the two methods. Non-random samples result in a higher standard deviation of egg-to-adult viability than expected by differentiating the chance of survival in the different vials from the given line. The risk of obtaining non-random samples will be the focus of this study, and the degree to which the two egg collection methods differ with respect to random sampling will be assessed. This will be done for each method by comparing the observed and the expected standard deviations. A similar method as the one described here can be used for many other insect species but in this paper I discuss the specifics related to work on D. melanogaster.
Results and Discussion
To visualize the possible departure of the observed standard deviation from the expected, each of the observed means was used to simulate five binomial distributions, producing the expected correlation between the means and the standard deviations of the two methods. In Figure 1 the correlation of the mean and standard deviation of the observed and expected data are plotted for the traditional (A) and suspension (B) methods. Figure 1 clearly illustrates that the data of the suspension method follows the expected correlation better than the data of the traditional method. Using the traditional method 58.0% of the samples has a significantly higher standard deviation than expected, while only 14.4% of the samples differ significantly from expected with the suspension method. As the sampling in the suspension method is completely randomized and the effect of different larval densities will be very small when the maximum number of larvae in a vial is 20,1 all the significant samples can be ascribed to sampling error and type I errors (5% expected). The discrepancy caused by sampling error is assumed to be the same for the two methods. This is supported by similar egg-to-adult viability estimates for the two methods (difference in survival: 0.3% ± 2.5, n = 7). However, this still leaves a large fraction of significant samples in the traditional method unaccounted for, strongly suggesting a failure to obtain random samples. These results confirm that the traditional method fails to produce a representative set of genotypes from a line, and that the mean and standard deviation estimates obtained with this method can be misleading. Thus wrong conclusions may be drawn if this method is used with a low number of replicates. In an experimental setup where five containers with medium are set up with 50 flies in each and 40 eggs are picked from each container, one might for example end up with 40 eggs laid by two females, giving eggs from only eight females in total, instead of 125 females. The mean and standard deviation estimates from such a setup can be highly biased. Consequently the suspension method provides a better estimate of the mean, from a representative collection of genotypes and has the advantage of being as fast or faster than the traditional method. Furthermore the suspension method only exposes the parent flies to a few environments, whereas the traditional method typically consists of a high number of small containers, each constituting a unique environment, thus increasing the environmental variance.
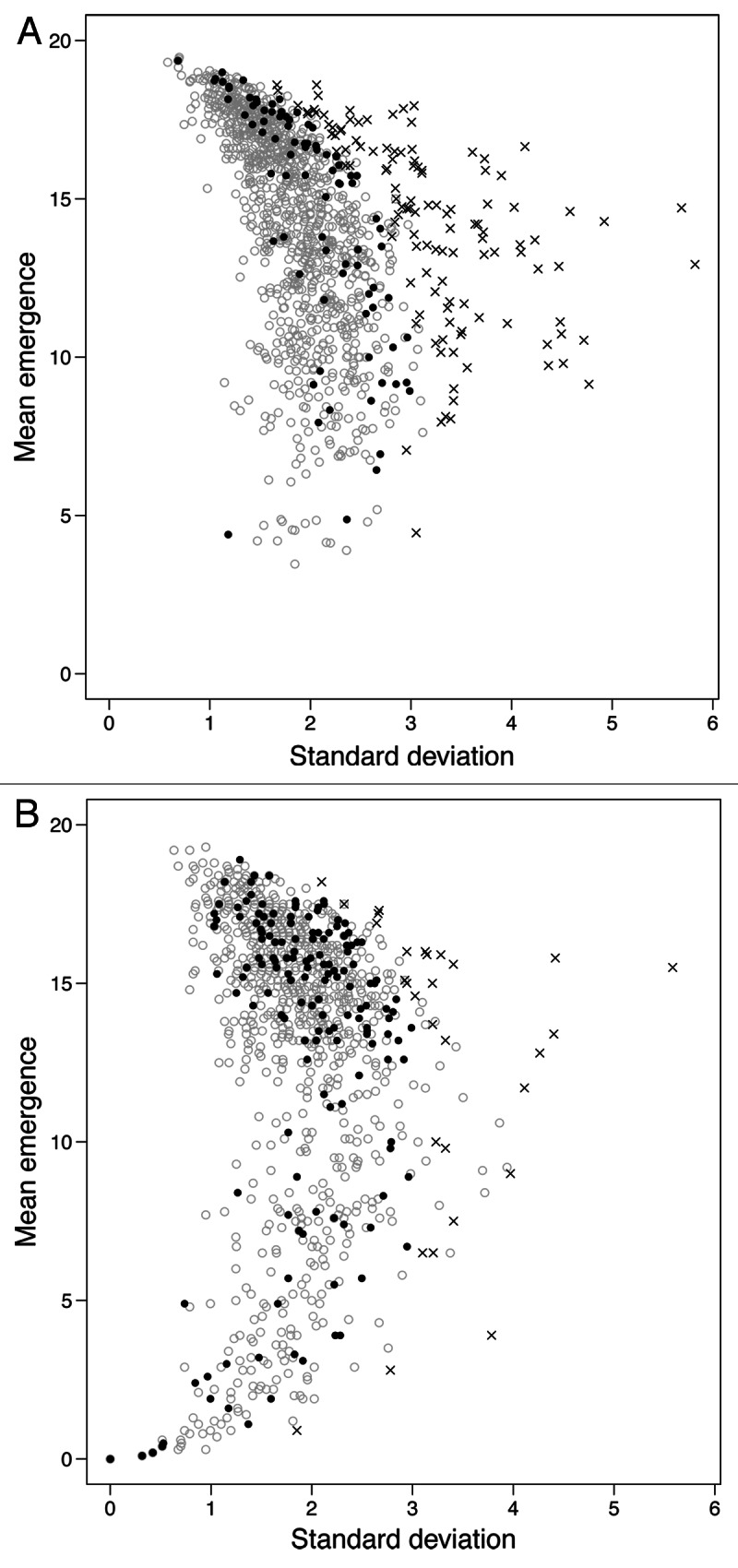
Figure 1. The correlation between the standard deviation and the mean number of emerging flies from 20 eggs for the observed and expected data of the traditional (A) and the suspension (B) method. The p-values indicate whether a sample’s standard deviation is significantly different from its expected value. ○ = Expected, ● = Observed estimates with a non-significant standard deviation (p ≥ 0.05), x = Observed estimates with a significant standard deviation (p < 0.05).
In conclusion the suspension method can improve the randomness of egg samples and thereby the quality of the results obtained in experiments involving large-scale egg collection in Drosophila and possibly other insect genera.
Materials and Methods
Egg collection method—the suspension method
The suspension method is based on that of Moth and Barker6 and has similarities to Ralchev.7 Approximately 500 reproductively active flies are put into two 500 mL plastic bottles with 25 mL of a 2.5% agar medium and a pile of 1:1 yeast/water paste in the middle, and left there for approximately 15 h at 25°C. Hereafter all the eggs laid are on the surface of the agar medium or the sides of the bottles. The flies and excess yeast are removed, and 20 mL of a 29 g/100 mL sucrose/water solution is added to the bottles. The eggs are then gently washed into the sucrose solution with a small brush and poured into a 100 mL measuring cylinder. The bottles are washed again, and the volume in the measuring cylinder is made up to a 100 mL sucrose suspension. After a few minutes all the eggs will float to the surface, and any remaining yeast paste will be dissolved in the water column or settled at the bottom. The eggs are collected from the surface with a pipette and transferred to a filter paper (15 μm) placed in a funnel, where 30 mL sucrose solution is used to wash away any remaining yeast. Then the eggs can be picked from the filter paper, which should be kept moist with the sucrose solution. The strategy described above is designed to avoid yeast on the eggs, and a few steps can be done quicker if that is not of high priority. Likewise a standard oatmeal-sugar-yeast Drosophila medium with 3% agar can be used instead of the agar medium. Egg production may be higher on this type of medium.
Comparing the traditional and suspension methods
In both methods the egg-to-adult viability of a line was assessed by transferring 20 eggs from density controlled parents into a number of vials containing 7 mL medium. Egg-to-adult viability was assessed as the mean number of emerging flies across replicate vials with the standard deviation obtained from the variation between replicate vials. Egg-to-adult viability of 42 D. melanogaster lines with different inbreeding levels and at different temperatures was measured using the traditional method (giving a total of n = 205 mean and standard deviation estimates). Each estimate was produced by transferring approximately 30 flies into each of 15 vials containing spoons with 1.5 mL of oatmeal-sugar-yeast-agar Drosophila medium. After 15 h eggs were picked from the spoons into the vials. For the suspension method, egg-to-adult viability was measured for 19 lines of D. melanogaster exposed to different degrees of nutritional stress, and different inbreeding levels (n = 209). By assuming that the survival of eggs follows a binomial distribution, one can simulate expected samples from an observed sample mean and number of replicates. To test whether the observed standard deviation of egg-to-adult viability differed significantly from the expected, 10,000 binomial distributions were simulated from each of the estimated means, whereby the null distribution for the observed standard deviation was produced. A two-tailed test was then performed to compare the null distribution and the standard deviation of the observed sample. All statistical analyses and simulations were done in R.5
Acknowledgments
I am grateful to J. Stuart F. Barker, Janneke Wit, Torsten Nygaard Kristensen and Volker Loeschcke for stimulating discussions on the method described here and useful comments on the manuscript, and to Doth Andersen for her help in the laboratory. The Graduate School of Science and Technology at Aarhus University in Denmark supported my research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/22758
References
- 1.Sang JH. The ecological determinants of population growth in a Drosophila culture; larval and pupal survival. Physiol Zool. 1949;22:183–202. doi: 10.1086/physzool.22.3.30152044. [DOI] [PubMed] [Google Scholar]
- 2.del Solar E, Ruiz G. Behavioral analysis of the choice of oviposition site by single females of Drosophila melanogaster (Diptera: Drosophilidae) J Insect Behav. 1992;5:571–81. doi: 10.1007/BF01048005. [DOI] [Google Scholar]
- 3.del Solar E, Palomino H. Choice of oviposition in Drosophila melanogaster. Am Nat. 1966;100:127–33. doi: 10.1086/282406. [DOI] [Google Scholar]
- 4.Lewontin RC. The effects of population density and composition on viability in Drosophila melanogaster. Evolution. 1955;9:27–41. doi: 10.2307/2405355. [DOI] [Google Scholar]
- 5.Development Core Team. R: A language and environment for statistical computing. 2012.
- 6.Moth JJ, Barker JSF. Interspecific competition between Drosophila melanogaster and Drosophila simulans: Effects of adult density, species frequency, light, and dietary phosphorus-32 on fecundity. Physiol Zool. 1981;54:28–43. [Google Scholar]
- 7.Ralchev KH, Harisanova NT. A convenient method for mass production and harvesting of synchronous Drosophila embryos. Drosoph Inf Serv. 1985;61:196–8. [Google Scholar]


