Abstract
Bipolar disorder is associated with persistent declarative memory disturbances, but the neural basis of these deficits is not well understood. We used fMRI to investigate brain activity during performance on a face‐name paired associate task, which allows for the dissociation of encoding and recall‐related memory processes. Fifteen clinically remitted bipolar I disorder patients and 24 demographically matched healthy comparison subjects were scanned during task performance. At the voxel level, bipolar patients showed reduced cortical activation, relative to controls, in multiple task‐related brain regions during encoding. During recognition, bipolar patients under‐activated left hippocampal and parahippocampal regions, despite adequate task performance. Region of interest analyses indicated that, during encoding, bipolar patients had greater bilateral dorsolateral prefrontal (DLPFC) activity than healthy subjects. In contrast, during recognition patients showed hypo‐activation relative to controls in the right, but not the left, DLPFC. Although hippocampal activity did not differ between groups during encoding, bipolar patients failed to activate hippocampal regions to the same extent as healthy subjects during recognition. Finally, while better task performance was associated with recognition‐related hippocampal activity in healthy subjects, bipolar patients showed an inverse relationship between task performance and hippocampal activity. Remitted bipolar patients over‐engaged dorsolateral prefrontal regions when learning face‐name pairs, but relative hypoactivation in both prefrontal and medial temporal regions during recognition. These findings suggest a neural basis for the long‐term memory deficits consistently observed in patients with bipolar disorder; further, as these patterns appear in symptomatically remitted patients, they are unlikely to be an artifact of mood symptoms. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: Hippocampus, declarative memory, long‐term memory, bipolar disorder, fMRI, frontal cortex
INTRODUCTION
Bipolar affective disorder is associated with declarative memory disturbances, even in the absence of overt mood symptomatology [Bearden et al.,2006]. Given that mild memory impairment is also observed in unaffected relatives of patients with bipolar illness [Arts et al.,2007], declarative memory dysfunction may represent a vulnerability marker for the disorder. Nevertheless, the neural basis of these deficits is not well understood.
Evidence from neurophysiological, neuroimaging, and lesion studies indicate that a distributed network of brain regions, including the medial temporal lobes (e.g., hippocampus, parahippocampus, amygdala) and regions of the prefrontal cortex subserve declarative memory. The hippocampus is putatively involved in the “binding” of previously unrelated information [Eichenbaum,1997; Jackson and Schacter,2004], and recollection, while the parahippocampal gyrus and perirhinal cortex are involved in familiarity‐based recognition [Eichenbaum et al.,2007]. In contrast, prefrontal regions have been implicated in cognitive control processes, with the ventrolateral (VLPFC; Brodmann areas (BA) 45 & 47) regions contributing to the selection of goal‐relevant information [Blumenfeld and Ranganath,2007] and the dorsolateral (DLPFC; BA 9 & 46) portions involved in the monitoring, organization and contextual processing of information placed into memory [Murray and Ranganath,2007; Petrides et al.,1995]. The relative contribution of each region to successful recollection appears to be dependent upon the exact demands of the cognitive task employed.
Consistent with theories that postulate a critical role of hippocampal dysfunction in bipolar disorder [Frey et al.,2007; Strakowski et al.,2005], postmortem studies have reported decreased density of non‐pyramidal neurons in region CA2 of the hippocampus in bipolar patients [Benes et al.,1998], as well as decreased hippocampal expression of GABA‐synthesizing messenger RNA [Heckers et al.,2002]. In contrast, in vivo neuroimaging results have generally failed to observe hippocampal volume deficits [McDonald et al.,2004], suggesting subtle hippocampal pathology may be present in bipolar disorder, in the absence of global volume changes. Behavioral research supports this notion, as patients with bipolar disorder have deficits on paired associate memory paradigms [Thompson et al.,2005], which are believed to be particularly dependent upon intact hippocampal function [Cohen and Eichenbaum,1993; Ryan et al.,2000]. However, memory impairments in bipolar disorder may also be linked to poor organizational or cognitive control processes attributed to frontal regions of the working memory network, such as the DLPFC [Deckersbach et al.,2005; Glahn et al.,2006]. Functional neuroimaging studies examining working memory in bipolar disorder have reported both reduced and increased task‐associated DLPFC activity in bipolar disorder [Adler et al.,2004; Lagopoulos et al.,2007; Monks et al.,2004; Robinson et al.,2009], depending on the particular task demands, raising questions about the explicit organizational demands of the tasks employed.
Such confounds have made delineating the sources of bipolar memory impairment a challenge. In a study specifically designed to elucidate this issue, Bearden et al. (1) report that impairments are more consistent with encoding deficits, rather than increased forgetting. However, other behavioral studies have reported pronounced delayed recall and recognition deficits in bipolar patients, suggesting difficulties maintaining information over time or increased rates of forgetting [Goodwin and Jamison,2007]. Together, these findings leave doubt about the nature of declarative memory impairment in bipolar disorder, and its underlying neural mechanisms. One hypothesis is that memory impairment in bipolar disorder may reflect limited capacity of attentional mechanisms for complex, effortful processing [Goodwin and Jamison,2007]. Alternately, encoding deficits in bipolar disorder may reflect poor organization of material, likely to affect short‐term and working memory performance, as well as longer‐term memory consolidation. To disentangle these components, neuroimaging investigations of declarative memory in bipolar disorder should allow for the independent assessment of encoding and recall/recognition processes.
In the current study, we applied a face‐name paired associate task during functional MRI to assess declarative memory in remitted bipolar patients and demographically matched healthy comparison subjects. Paired‐associate paradigms index performance during both the encoding and retrieval stages of memory processes, because individuals are required to create a new association between two previously unrelated stimuli, and remember that association over time [Bookheimer et al.,2000]. The face‐name paired‐associate task employed here is similar to those previously shown to evoke distinct hippocampal, DLPFC and VLPFC activity [Sperling et al.,2001; Zeineh et al.,2003], and allows for independent modeling of activation during the encoding/learning and during the recall/recognition of face‐name pairs. On the basis of our prior findings with list‐learning [Bearden et al.,2006] and delayed match to nonsample [Glahn et al.,2006] behavioral tests, we hypothesize that individuals with bipolar disorder will have encoding deficits associated with poor organizational abilities, and that these impairments will be manifest in aberrant prefrontal (DLPFC) and hippocampal encoding‐related activity.
SUBJECTS AND METHODS
Sample
Fifteen remitted bipolar I disorder patients and 24 healthy comparison subjects, group‐matched for age (M ± SD, 38.00 ± 13.1 vs. 34.91 ± 10.4 years, respectively; F = 1.3, P = 0.29), gender (64% vs. 58% female; χ2 = 0.13, P = 0.71), education (14.57 ± 2.3 vs. 14.96 ± 1.9; F = 0.03, P = 0.58), parental education (13.54 ± 3.6 vs. 13.47 ± 3.8; F = 0.01, P = 0.94), racial/ethnic distribution (χ2 = 4.13, P = 0.25), and estimated full‐scale IQ (108.21 ± 7.8 vs. 107.72 ± 12.1; F = 0.02, P = 0.90) participated in the functional MRI experiment. Two additional patients and one comparison subject participated, but were excluded for excessive motion (>2.0 mm). All participants provided written informed consent for the study, as approved by the institutional review board at the University of Texas Health Science Center at San Antonio (UTHSCSA).
Patients were recruited from UTHSCSA outpatient clinics and community mental health facilities. Inclusion criteria for patients included (1) a diagnosis of bipolar I disorder, as determined by the Structured Clinical Interview for DSM‐IV (SCID); (2) no current comorbid Axis I disorder (with the exception of anxiety disorders); (3) no history of a medical or neurological condition that might affect brain function. Healthy comparison subjects were recruited through advertisements in the community, according to the same exclusion criteria. In addition, control participants had no history of Axis I disorder based on direct SCID interview, and no history of mood disorder in first‐degree relatives.
Bipolar patients were asymptomatic at the time of assessment: the maximum Hamilton Depression Rating Scale score [Hamilton,1967] was 6.0 (average 2.67 ± 1.67; range, 0–6) and maximum Young Mania Rating Scale score [Young et al.,1978] was 2.0 (average 1.00 ± 0.85; range, 0–4). On average, patients had been remitted for 4.2 ± 3.0 (range, 2–16) months. The mean number of hospitalizations was 2.78 ± 3.3 (range, 0–12) and mean duration of illness was 7.64 ± 7.4 (range, 1–26) years. Eleven of the patients (73%) were taking mood‐stabilizing medications (lamotrigine and/or valproate; 1 on lithium); 5 patients (33%) were taking antidepressants; and 7 (47%) were on atypical antipsychotics. One of the patients was drug‐free at the time of assessment. Eight patients (72%) had a history of lifetime DSM‐IV anxiety disorders, with six currently meeting DSM‐IV criteria for anxiety disorder [4 with post‐traumatic stress disorder (PTSD)]. Six of the bipolar patients (46%) had a previous history of alcohol or substance abuse that was in full remission for at least the past 6 months.
Declarative Memory Paradigm
During the fMRI protocol, subjects performed a face‐name paired‐associate task [Sperling et al.,2001; Zeineh et al.,2003] that included three distinct conditions: encoding/learning, distractor (active baseline), and recognition. Conditions were interleaved and repeated 8 times within the imaging experiment, for a total duration of 8 min and 24 s (see Fig. 1). During encoding, pairs of faces and common gender‐appropriate first names (four male, four female) were presented serially every 3 s and subjects were asked to remember each face‐name pairing. The 15‐s distractor (active baseline) task required subjects to press a button when the fixation changed to an arrow (randomly within a 3‐s interval). During the recognition condition, each face was shown with four gender‐appropriate names and subjects were asked to indicate, via button press, which name was previously paired with that face. Twenty‐four unique face‐name pairs were used during the experiment, and each paring was presented twice. Stimuli were back‐projected via an LCD projector onto a screen placed above the subjects' head, and behavioral responses (button presses and reaction times) were recorded through a hand‐held response box.
Figure 1.
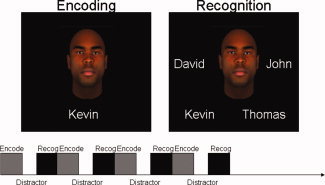
Face‐Name Paired Associate Paradigm. Subjects were asked to learn eight pairs of faces and common gender‐appropriate first names (four male, four female). After a 15‐s distractor task, each face was shown with four gender‐appropriate names and subjects were asked to indicate, via button press, which name was previously paired with that face. Twenty‐four unique face‐name pairs were used during the experiment, and each paring was presented twice.
Image Acquisition
Scanning was carried out on a 3T Siemens MRI scanner in the UTHSCSA Research Imaging Center. Functional imaging used a gradient echo, echoplanar sequence sensitive to the blood‐oxygen‐level‐dependent (BOLD) signal [Kwong1995], acquiring 26 slices parallel to the AC‐PC plane (TR/TE 3000/31 ms, 90° flip angle, voxel size = 2.0 × 2.0 × 4.0 mm, FOV = 256). After the functional study, a coplanar T1‐weighted sequence (TR/TE 500/20 ms, 90° flip angle) and a high‐resolution MPRAGE series (TR/TE 2.2/3.04 s, 13° flip angle, 0.8 mm isotropic) were obtained for each subject and used for anatomic reference and normalization.
Functional Imaging Analysis
Functional image analyses were performed with tools available as part of the FSL software package (http://www.fmrib.ox.ac.uk/fsl/) [Smith et al.,2004] and supplemented with utilities developed in‐house. To combat potential motion artifacts, each image in a time series underwent spatial registration to the middle data point in the time series. Data were smoothed with a non‐linear algorithm designed to preserve image structure by only smoothing voxels thought to be of the same tissue type (5‐mm kernel). Each data set was subjected to a multiple‐regression analysis, using a prewhitening technique to account for the intrinsic temporal autocorrelation of BOLD imaging [Bullmore et al.,1996]. For each intracranial voxel, least‐squares coefficients were generated independently reflecting the encoding and recognition conditions. By contrasting these conditions with the distractor task (active baseline), statistical images were created that reflect changes in BOLD signal associated with encoding or recognition of face‐name pairs.
To facilitate multisubject voxel level analyses and based on the parameters created from the higher resolution anatomical images, statistical images were spatially normalized to a standard stereotactic space [Kochunov et al.,2002]. Higher‐level multi‐subject analyses utilized a mixed effects model, where subject was represented as a random factor, providing z‐images for each diagnostic group separately for each contrast described above. Group maps were thresholded based on the magnitude (z ≥ 2.3) and extent (cluster significance, corrected for multiple comparisons, P < 0.001) of activation [Forman et al.,1995; Poline et al.,1997].
Given prior evidence for engagement of the hippocampus, DLPFC, and VLPFC in this task paradigm, a region of interest (ROI) analysis was conducted. Right and left hippocampal ROIs were manually drawn on each subject's high‐resolution anatomic image, according to published procedures [Brambilla et al.,2003]. The dorsolateral and ventrolateral regions were based on regions delinitated by the Harvar Center for Morphometric Analysis (CMA) and distributed by the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas-descriptions.html). The dorsolateral and ventrolateral regions were based on regions delineated by the Harvard Center for Morphometric Analysis (CMA) and distributed by the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas-descriptions.html). The Harvard‐Oxford regions of interest (ROIs) are probabalistic ROIs developed from individually segmented, high‐resolution T1‐weighted images from 21 healthy male and 16 healthy female subjects (ages, 18–50). T1‐weighted images were affine‐registered to a common space, and the transforms then applied to the individual labels. Finally, these were combined across subjects to form population probability maps for each label. In the current analysis, the DLPFC region was restricted to BA 9, BA 46 and the junction of these regions (see Fig. 2). The VLPFC region included portions of BA 47 (see Fig. 2). These prefrontal ROIs were affine‐registered into each subject's space and edited to ensure anatomic accuracy. Each ROI was overlaid upon statistical data to determine the percent signal change for the encoding and recognition conditions above baseline (distractor task). These values, after winsorization to reduce the effects of outliers and ensure normality, were entered two‐sample t‐tests and signifncace was set at 5% FDR. Pearson correlations between ROI‐based signal change and behavioral performance were calculated.
Figure 2.
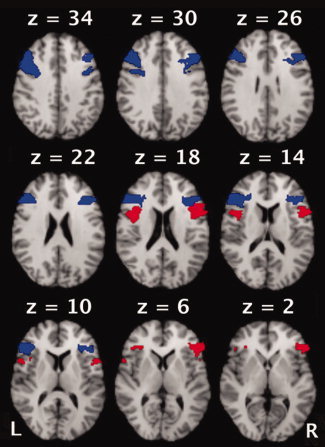
Probabilistic Regions of Interest. A priori regions of interest were anatomically defined and based on the set of probabilistic regions segmented by the Harvard Center for Morphometric Analysis and distributed by the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain. The dorsolateral prefrontal (blue) and ventrolateral prefrontal (red) regions of the Harvard‐Oxford ROI sets are shown.
RESULTS
Behavioral Performance
Task performance (% correctly recalled face‐name pairs) did not significantly differ between patients with bipolar disorder and healthy comparison subjects (59.50% ± 5.9 vs. 63.29% ± 6.7; t(1,36) = −1.76, P = 0.09). Within the bipolar group, memory performance was not significantly correlated with duration of illness or number of hospitalizations, and task performance of patients with history of comorbid substance usage did not differ from patients without history of substance abuse (all P > 0.30).
Imaging Results
All subjects, regardless of diagnostic group, showed significant activation during encoding/learning and recognition, relative to the baseline distractor task. The estimated absolute and relative motion during scanning did not differ between groups (F[1,38] = 2.41, P = 0.14; F[1,38] = 0.69, P = 0.42, respectively).
Voxel‐Level Analysis of Encoding/Learning Activation
Across groups, encoding‐associated activation was observed in six spatially distinct regions, including bilateral medial temporal lobe regions (e.g., anterior hippocampus and parahippocampal gyri, extending to fusiform gyrus), bilateral prefrontal cortical regions (e.g., dorsal prefrontal cortex, lateral prefrontal cortex, and a medial prefrontal region including the anterior cingulate gyrus), bilateral posterior parietal cortex, bilateral occipital cortex, and cerebellum (see Table I and Fig. 3). In addition, the bipolar patient group activated a region of left lateral temporal cortex. Each of these regions has been previously associated with performance on learning tasks, particularly those that require encoding strategies for good performance [Blumenfeld and Ranganath2006; Weber et al.,2007].
Table I.
Activation foci for encoding/learning vs. distractor task
| Activation area | Healthy subjectsa | Bipolar disorder | ||
|---|---|---|---|---|
| z | x, y, z | z | x, y, z | |
| Left and right medial temporal regions | ||||
| Left Parahippocampal Gyrus (BA 28, 29 and 35) | 5.64 | −24, −28, −8 | 4.28 | −26, −30, −6 |
| Left Hippocampus | 3.69 | −34, −18, −20 | 4.59 | −26, −32, −2 |
| Left Amygdala | 3.31 | −32, −4, −16 | – | |
| Right Parahippocampal Gyrus (BA 27, 28 and 35) | 6.49 | 24, −30, −6 | 5.01 | 18, −32, −6 |
| Right Hippocampus | 3.18 | 26, −10, −20 | – | |
| Right Amygdala | 3.03 | 24, −6, −20 | – | |
| Left lateral prefrontal cortex | ||||
| Middle Frontal Gyrus (BA 46) | 5.67 | −46, 28, 20 | 4.73 | −44, 18, 26 |
| Middle Frontal Gyrus (BA 9) | 5.21 | −38, 4, 26 | 3.74 | −48, 14, 22 |
| Middle Frontal Gyrus (BA 6 & 8) | 4.75 | −48, 4, 46 | 4.28 | −44, 2, 42 |
| Inferior Frontal Gyrus (BA 47) | 4.81 | −50, 36, −2 | 4.51 | −46, 42, −2 |
| Right Lateral Prefrontal Cortex | ||||
| Middle Frontal Gyrus (BA 46) | 5.58 | 42, 28, 14 | 4.42 | 44, 32, 18 |
| Inferior and Middle Frontal Gyrus (BA 9) | 3.62 | 42, 16, 28 | 3.94 | 46, 6, 30 |
| Precentral Gyrus (BA 6) | 4.99 | 38, 6, 22 | 3.89 | 36, 6, 26 |
| Left medial prefrontal cortex | ||||
| Cingulate Gyrus (BA 32) | 5.36 | −8, 24, 36 | 4.09 | −6, 16, 44 |
| Superior Frontal Gyrus (BA 6 & 8) | 6.01 | −6, 14, 46 | 3.85 | −8, 18, 58 |
| Left and right parietal cortex | ||||
| Left Precuneus (BA 19) | 5.24 | −30, −72, 36 | 3.28 | −34, −68, 38 |
| Left Inferior and Superior Parietal Lobule (BA 7 and 39) | 4.29 | −28, −68, 24 | 3.51 | −36, −64, 40 |
| Right Precuneus (BA 19) | 5.67 | 34, −64, 42 | – | |
| Right Angular Gyrus (BA 39) | 5.46 | 32, −62, 34 | – | |
| Bilateral occipital lobe and cerebellum | ||||
| Middle and Inferior Occipital Gyri (BA 18) | 6.47 | 30, −86, −14 | 5.15 | −32, −96, 2 |
| Lingual Gyrus (BA 17 and 18) | 6.18 | −18, −102, −16 | 5.82 | 0, −76, −4 |
| Fusiform Gyrus (BA 18 and 19) | 6.03 | 26, −96, −14 | 5.33 | 22, −88, −14 |
| Cerebellum (Declive, Culmen and Tuber) | 6.68 | 34, −84, −22 | 6.01 | −28, −72, −22 |
| Left lateral temporal cortex | ||||
| Middle Temporal Gyrus (BA 21) | – | 4.39 | −58, −36, −2 | |
Value and Talairach coordinate for the maximum z‐statistic in that area for each group separately.
Figure 3.
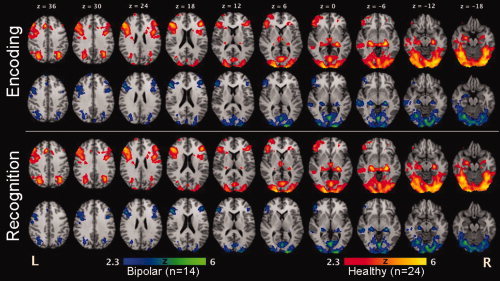
Voxel‐Level Analysis. Both patients with bipolar disorder (blue–green) and healthy comparison subjects (red–yellow) activated regions associated with declarative memory performance, including bilateral medial temporal regions (e.g., anterior hippocampus and parahippocampal gyri), bilateral prefrontal cortical regions (e.g., dorsal prefrontal cortex, lateral prefrontal cortex, and a medial prefrontal region including the anterior cingulate gyrus), bilateral posterior parietal cortex, bilateral occipital cortex, and cerebellum.
Relative to individuals with bipolar disorder, healthy subjects showed statistically greater activation during the encoding condition in left inferior frontal gyrus (maximum z‐value 2.34; Talairach coordinates −46, 26, 36; Brodmann area (BA) 9), left cingulate gyrus (2.37; −12, 14, 40; BA 8), left superior parietal lobule (2.66; −24, −76, 42, BA 7), right insular cortex (2.75; 36, −4, 20, BA 13), the lentiform nucleus of the left (3.01; −22, 2, 6) and right (2.60; 22, 0, 6) putamen, and bilateral occipital and cerebellar cortex (z = 3.05; −28, −96, −16 and 3.32; 30, −96, −12). In contrast, patients with bipolar disorder showed greater activation during encoding than healthy subjects in left middle frontal gyrus (2.14; −2, 28, 44, BA 8 & 9), bilateral precuneus (3.12, −16, −72, 24 and 3.42; 14, −64, 22), and the left superior temporal gyrus (3.15 −68, −32, −10, BA 22).
Voxel‐Level Analysis of Activation during Recognition
In general, a similar network of regions was engaged during the recognition condition as was observed for the encoding condition (see Table II and Fig. 3). Across groups, seven brain areas were engaged during recognition relative to baseline: bilateral medial temporal regions including the hippocampus and parahippocampal gyrus, extending into the left and right lateral prefrontal cortical regions (BA 9, 46 & 47); cingulate cortex (BA 32); bilateral parietal regions, including the inferior and superior parietal lobules; bilateral occipital cortex and cerebellum, and bilateral thalamus and putamen.
Table II.
Activation foci for recognition vs. distractor task
| Activation area | Healthy subjectsa | Bipolar disorder | ||
|---|---|---|---|---|
| z | x, y, z | z | x, y, z | |
| Left and right medial temporal regions | ||||
| Left Parahippocampal Gyrus (BA 27, 28 and 35) | 5.52 | −22, −28, −8 | 4.15 | −24, −26, −8 |
| Right Parahippocampal Gyrus (BA 27 and 28) | 5.59 | 20, −28, −8 | 4.49 | 22, −32, −4 |
| Left lateral prefrontal cortex | ||||
| Inferior Frontal Gyrus (BA 47) | 6.5 | −34, 20, −10 | 4.72 | −30, 22, −6 |
| Inferior Frontal Gyrus (BA 9) | 6.62 | −50, 6, 28 | 4.67 | −50, 8, 30 |
| Middle Frontal Gyrus (BA 9, 44, 46) | 5.26 | −48, 20, 30 | 5.36 | −44, 14, 28 |
| Precentral Gyrus (Baa 6 and 8) | 4.14 | −26, −24, 66 | 5.03 | −44, 0, 32 |
| Right lateral prefrontal cortex | ||||
| Inferior Frontal Gyrus (BA 47) | 6.18 | 34, 20, 4 | 4.12 | 32, 22, −6 |
| Middle Frontal Gyrus (BA 9) | 5.43 | 46, 24, 28 | 4.01 | 40, 28, 26 |
| Middle Frontal Gyrus (BA 46) | 4.91 | 42, 34, 24 | 3.82 | 36, 28, 24 |
| Precentral Gyrus (BA 6) | 6.01 | 40, 4, 32 | 4.21 | 44, −6, 32 |
| Medial prefrontal cortex | ||||
| Cingulate Gyrus (BA 32) | 7.09 | 2, 14, 40 | 6.43 | −2, 18, 38 |
| Middle Frontal Gyrus (BA 6 and 8) | 6.88 | 0, 14, 42 | 6.78 | −4, 18, 42 |
| Bilateral parietal cortex | ||||
| Left Precuneus (BA 7 and 19) | 7.46 | −24, −70, 40 | 5.61 | −26, −68, 34 |
| Left Inferior and Superior Parietal Lobule (BA 7 and 40) | 8.44 | −36, −52, 48 | 6.01 | −32, −58, 46 |
| Left Angular Gyrus (BA 39) | 6.93 | −32, −60, 36 | 5.63 | −34, −60, 38 |
| Left Postcentral Gyrus (BA 1, 2 and 3) | 7.26 | −52, −28, 40 | 4.47 | −54, −26, 46 |
| Right Precuneus (BA 7) | 6.23 | 20, −72, 46 | 4.02 | 32, −68, 30 |
| Left Inferior and Superior Parietal Lobule (BA 7 and 40) | 6.12 | 30, −54, 46 | 4.45 | 42, −34, 38 |
| Bilateral occipital lobe and cerebellum | ||||
| Middle and Inferior Occipital Gyri (BA 18) | 7.22 | −34, −88, −14 | 5.69 | 30, −80, 18 |
| Lingual Gyrus (BA 17 and 18) | 7.53 | −10, −102, −6 | 6.85 | 0, −94, −4 |
| Cuneus | 7.46 | 12, −102, 2 | 6.48 | 12, −102, 2 |
| Cerebellum (Declive, Culmen, Uvula and Tuber) | 7.26 | −6, −30, −14 | 4.27 | −44, −64, −18 |
| Left and right subcortical regions | ||||
| Left Thalamus | 6.43 | −14, −22, 8 | 4.67 | −16, −8, 10 |
| Left Putamen | 5.46 | −22, −2, 4 | 3.08 | −24, −4, 2 |
| Right Thalamus | 6.6 | 14, −6, 10 | 4.92 | 16, −6, 10 |
| Right Putamen | 6.37 | 14, 2, 8 | 2.54 | 20, 6, −2 |
Value and Talairach coordinate for the maximum z‐statistic in that area for each group separately.
Healthy subjects engaged six brain areas significantly more than bipolar patients during recognition, including the left hippocampus (2.17; −32, −16, −18) and parahippocampal gyrus (2.07; −30, −16, −20), bilateral regions of the cerebellum (2.42; −24, −70, −20 and 2.34; 18, −66, −20) and bilateral sensory‐motor regions (1.56; −22, 6, 54, BA 6 and 2.83; 20, 4, 54, BA 6). During recognition, no significant regions of increased activation were found in the bipolar patients relative to controls.
ROI‐Based Analysis
Groups did not differ for either the encoding or recognition conditions in the VLPFC regions. Bipolar patients activated both the right DLPFC and left DLPFC regions more than healthy subjects during encoding (Fig. 4 and Table III). In contrast, during recognition patients showed relative hypo‐activation, as compared with controls, in the right, but not left, DLPFC. Although hippocampal activity did not differ between groups during encoding, bipolar patients failed to activate hippocampal regions to the same extent as healthy subjects during the recognition condition. This pattern of results did not differ when behavioral performance was included as a covariate.
Figure 4.
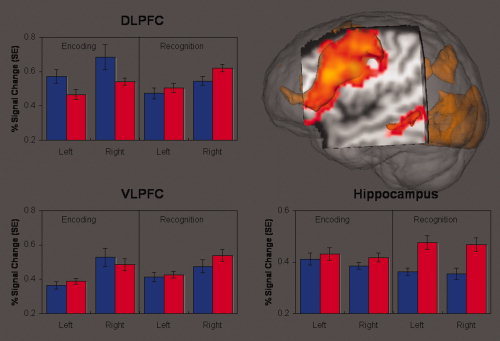
Region of Interest Analysis. A priori and anatomically defined regions of interest were examined in the hippocampus, and dorsolateral (DLPFC) and ventrolateral (VLPFC) prefrontal cortex. Bipolar patients (blue bars, n = 14) engaged the DLPFC more when encoding face name pairs than healthy comparison subjects (red bars, n = 24). In contrast, healthy subjects had significantly more hippocampal and right DLPFC activity when recognizing previously viewed stimuli.
Table III.
Means and group differences for the region of interest analysis
| Bipolar disorder (N = 14) | Healthy comparison (N = 24) | T‐testa (DF = 36) | P‐value | |
|---|---|---|---|---|
| Encoding/Learning | ||||
| Left Hippocampus | 0.412 (0.09) | 0.431 (0.12) | −0.52 | 0.604 |
| Right Hippocampus | 0.385 (0.05) | 0.418 (0.08) | −1.31 | 0.198 |
| Left VLPFC | 0.364 (0.08) | 0.387 (0.08) | −0.93 | 0.358 |
| Right VLPFC | 0.527 (0.20) | 0.485 (0.17) | 0.69 | 0.493 |
| Left DLPFC | 0.571 (0.14) | 0.466 (0.14) | 2.19 | 0.035 |
| Right DLPFC | 0.685 (0.28) | 0.540 (0.10) | 2.31 | 0.026 |
| Recognition | ||||
| Left Hippocampus | 0.363 (0.05) | 0.476 (0.13) | −3.16 | 0.003 |
| Right Hippocampus | 0.355 (0.08) | 0.468 (0.13) | −2.99 | 0.005 |
| Left VLPFC | 0.413 (0.10) | 0.427 (0.09) | −0.42 | 0.680 |
| Right VLPFC | 0.475 (0.14) | 0.539 (0.17) | −1.17 | 0.249 |
| Left DLPFC | 0.473 (0.12) | 0.504 (0.14) | −0.69 | 0.494 |
| Right DLPFC | 0.545 (0.10) | 0.620 (0.10) | −2.24 | 0.031 |
Significant group differences at 5% FDR.
Relationship between Activation and Behavior
Pearson correlations between memory performance and brain activation within the average left and right hippocampal, DLPFC and VLPFC regions of interest were performed separately in each group (Table IV). For healthy subjects, better behavioral performance was correlated with increased activation in each ROI during encoding and in the hippocampal region during recognition. In contrast, the only correlation to reach significance for the bipolar group was a negative correlation between hippocampal activity during recognition and accuracy.
Table IV.
Correlations between behavioral performance and brain activation (%signal change)
| Patients with bipolar disorder | Healthy volunteers | |||
|---|---|---|---|---|
| Encoding | Recognition | Encoding | Recognition | |
| VLPFC | 0.08 | −0.26 | 0.47∧ | 0.23 |
| DLPFC | 0.23 | −0.10 | 0.55* | 0.34 |
| Hippocampus | 0.24 | −0.62∧ | 0.46∧ | 0.45∧ |
P < 0.05;
P < 0.01.
Medication Effects on Activation
To model the effects of psychotropic medications on activation in hippocampal and DLPFC regions of interest in individuals with bipolar disorder, patients receiving the most commonly prescribed drugs were compared with the remaining patients (between group factor). Activation levels within the hippocampal and DLPFC ROIs in patients on mood stabilizers (n = 11) vs. those not on mood stabilizers (n = 4) were compared and found to be not statistically different (P = 0.3). Similar analyses were repeated for antidepressant (n = 5) and antipsychotic medications (n = 7), with similar nonsignificant results (P = 0.3 and P = 0.09, respectively).
DISCUSSION
Remitted patients with bipolar disorder had altered patterns of activation in brain regions critical for declarative memory, as compared to healthy comparison subjects, while performing a face‐name paired associate task. Region of interest analyses confirmed that the face‐name paired associate task activated the expected brain regions involved in encoding and retrieval of new visual information (i.e., the DLPFC, hippocampus, thalamus, fusiform gyrus, and visual association cortices) in healthy individuals. Although in general activation patterns in the patient group were similar to healthy subjects, bipolar patients engaged the DLPFC more than healthy subjects when learning face‐name pairs, and failed to activate the DLPFC and hippocampal/parahippocampal regions to the same extent as healthy comparison subjects during recognition. This pattern of hyperactivation during encoding, coupled with hypoactivation in both prefrontal and medial temporal regions during recognition, suggests that patients with bipolar disorder may have difficulty organizing information when creating memory stores, with subsequent difficulty retrieving those items from memory. Alternatively, it is possible that individuals with bipolar disorder have reduced prefrontal capacity coupled with increased hippocampal baseline activity. Indeed, increased tonic hippocampal activation in bipolar patients would be consistent with our finding of a negative correlation between signal change in this region and memory performance in the patient group. In either case, these data provide empirical evidence for the dysregulation of prefrontal and medial temporal brain regions in bipolar disorder and suggest that this decoupling is not a result of acute mood symptoms, as these patients were all remitted at the time of scanning.
Encoding‐related DLPFC hyperactivation among patients with bipolar disorder is consistent with previous evidence of prefrontal over‐activation during a working memory task in patients with major depressive disorder [Matsuo et al.,2007]. This region is commonly associated with the performance of working memory tasks and is thus implicated in the organization or manipulation of information [D'Esposito et al.,2000]. In our data, improved accuracy in remembering the face‐name pairs was positively correlated with DLPFC activity for healthy subjects (r = 0.552, P = 0.005). These correlations generally support the role of the DLPFC in complex encoding. Furthermore, DLPFC hyperactivation in bipolar disorder during encoding of face‐name pairs suggests that bipolar patients employ different, and potentially less effective, organizational strategies than healthy subjects.
Our finding of reduced DLPFC and hippocampal activation during recognition is consistent with dysregulation of medial temporal and prefrontal processing. While previous studies have reported diminished frontal activity in bipolar patients during working memory tasks [Frangou et al.,2007; Lagopoulos et al.,2007], they have not investigated medial temporal abnormalities, leaving the question of this potential aberrant circuitry unaddressed. The current experiment was designed to specifically examine the relationship between prefrontal and medial temporal functioning in bipolar disorder. Diminished hippocampal activity in this particular task may reflect limited access to information stored during encoding, conceivably due to the poor organization of this information (potentially as a result of over‐processing, given heightened DLPFC activity) during encoding. Alternatively, the pattern of findings in DLPFC and hippocampal regions may be due to the existence of more complex abnormalities in the reciprocal interconnections between these brain regions. Although it is tempting to interpret reduced task‐related hippocampal activity as reflecting poor encoding ability or reduced fronto‐temporal integration in patients with bipolar disorder, these interpretations must be tempered by the lack of significant between‐group performance differences, and the lack of a measurement of tonic medial temporal and frontal activation in the patient group.
Activation differences between patients with bipolar disorder and comparison subjects were observed, despite statistically similar between‐group behavioral performance, suggesting that these neural differences are not simply due to impaired performance in the patient group. Indeed, including behavioral performance as a covariate did not substantively change results. As has been reported in prior investigations, we found that better memory performance on the paired‐associate task was associated with increased hippocampal activation in healthy subjects [Murray and Ranganath,2007; Paller and Wagner,2002; Sperling et al.,2001; Staresina and Davachi,2006], particularly during the recognition condition. However, in bipolar patients, correlations between memory performance and activation were either non‐significant or negative. More accurate task performance was positively correlated with recognition‐related hippocampal activity in the healthy subjects, but negatively correlated in bipolar patients (Fisher's z‐test P = 0.001), indicating that remitted bipolar patients do not show the expected relationship between hippocampal activity and task performance. It is possible that poor performance on the face‐name paired associate task (∼60% accuracy) could have influenced the relationship between performance and hippocampal activity. However, the correlation between performance and hippocampal activity in healthy subjects was consistent with published reports [Blumenfeld and Ranganath2006; Weber et al.,2007]. Together, this pattern of behavioral correlations suggests that the bipolar patients may recruit other brain regions (e.g., overuse of DLPFC during encoding) to successfully complete the task, since behavioral performance was intact. Decreased integrity of hippocampal function in bipolar disorder is consistent with evidence for significant and stable memory impairments in this population.
Certain limitations of the present study must be noted. In particular, most of the individuals with bipolar disorder who participated in this study were on various psychotropic medications. However, as is common in bipolar disorder, few of these patients were on a single agent, making it difficult to determine the impact of particular medications. Nonetheless, in our secondary analysis of potential medication effects, we found no differences in activation level within the hippocampal and DLPFC ROIs between patients on mood stabilizers vs. those not on mood stabilizers, nor between patients on antidepressants and/or antipsychotic medications, when compared with those who were not.
Although the interplay between prefrontal and hippocampal/parahippocampal regions during memory process are likely complex and task dependent [Blumenfeld and Ranganath,2007], the data presented here suggest that this relationship is disturbed in bipolar disorder. Improved characterization of the alterations in this system in bipolar disorder and how these changes are influenced by affective liability could significantly improve our understanding of the neurophysiologic basis of the illness.
Acknowledgements
The authors thank Sharon Primeaux for help recruiting and assessing patients. Subsets of these data were presented at the 2007 meeting of the Society for Biological Psychiatry and the Organization for Human Brain Mapping.
Potential conflict of interest: None declared.
REFERENCES
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM ( 2004): Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord 6: 540–549. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, Van Os J ( 2007): Meta‐analyses of cognitive functioning in euthymic bipolar patients and their first‐degree relatives. Psychol Med: 1–15. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Kaur S, Sanches M, Villarreal V, Bowden C, Soares JC ( 2006): Sources of declarative memory impairment in bipolar disorder: Mnemonic processes and clinical features. J Psychiatr Res 40: 47–58. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS ( 1998): A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 44: 88–97. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C ( 2006): Dorsolateral prefrontal cortex promotes long‐term memory formation through its role in working memory organization. J Neurosci 26: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C ( 2007): Prefrontal cortex and long‐term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13: 280–291. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW ( 2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC ( 2003): MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res 37: 287–295. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Brammer M, Williams SC, Rabe‐Hesketh S, Janot N, David A, Mellers J, Howard R, Sham P ( 1996): Statistical methods of estimation and inference for functional MR image analysis. Magn Resonance Med 35: 261–277. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H ( 1993): Memory, Amnesia and the Hippocampal System. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- D'Esposito M, Postle BR, Rypma B ( 2000): Prefrontal cortical contributions to working memory: Evidence from event‐related fMRI studies. Exp Brain Res 133: 3–11. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Dougherty DD, Bohne A, Loh R, Nierenberg A, Sachs G, Rauch SL ( 2005): Spontaneous and directed application of verbal learning strategies in bipolar disorder and obsessive‐compulsive disorder. Bipolar Disord 7: 166–175. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H ( 1997): How does the brain organize memories? Science 277: 330–332. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C ( 2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Resonance Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Frangou S, Kington J, Raymont V, Shergill SS ( 2008): Examining ventral and dorsal prefrontal function in bipolar disorder: A functional magnetic resonance imaging study. Eur Psychiatry 23: 300–308. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F ( 2007): The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol 18( 5–6): 419–430. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Barrett J, Bearden CE, Mintz J, Green MF, Serap Monkul E, Najt P, Soares JC, Velligan DI ( 2006): Dissociable mechanisms for memory impairment in bipolar disorder and schizophrenia. Psychol Med: 1–11. [DOI] [PubMed] [Google Scholar]
- Goodwin F, Jamison K ( 2007): Manic Depressive Illness. New York: Oxford University Press. [Google Scholar]
- Hamilton M ( 1967): Development of a rating scale for primary depressive illness. Br J Social Clin Psychol 6: 278–296. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM ( 2002): Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry 59: 521–529. [DOI] [PubMed] [Google Scholar]
- Jackson O,III , Schacter DL ( 2004): Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21: 456–462. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P ( 2002): An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Kwong KK ( 1995): Functional magnetic resonance imaging with echo planar imaging. Magn Resonance Quarterly 11: 1–20. [PubMed] [Google Scholar]
- Lagopoulos J, Ivanovski B, Malhi GS ( 2007): An event‐related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci 32: 174–184. [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC ( 2007): Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 12: 158–166. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe‐Hesketh S, Ellison‐Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N ( 2004): Meta‐analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56: 411–417. [DOI] [PubMed] [Google Scholar]
- Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SC, Simmons A, Giles N, Lloyd AJ, Harrison CL, Seal M, Murray RM, Ferrier IN, Young AH, Curtis VA ( 2004): A functional MRI study of working memory task in euthymic bipolar disorder: Evidence for task‐specific dysfunction. Bipolar Disord 6: 550–564. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C ( 2007): The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci 27: 5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Wagner AD ( 2002): Observing the transformation of experience into memory. Trends Cogn Sci 6: 93–102. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC ( 1995): Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc Natl Acad Sci USA 92: 5803–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ ( 1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Bearden CE, Monkul ES, Tordesillas‐Gutierrez D, Velligan DI, Frangou S, Glahn DC ( 2009): Fronto‐temporal dysregulation in remitted bipolar patients: An fMRI delayed‐non‐match‐to‐sample (DNMS) study. Bipolar Disord 11: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ ( 2000): Amnesia is a deficit in relational memory. Psychol Sci 11: 454–461. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS ( 2001): Encoding novel face‐name associations: A functional MRI study. Hum Brain Mapp 14: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L ( 2006): Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci 26: 9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM ( 2005): The functional neuroanatomy of bipolar disorder: A review of neuroimaging findings. Mol Psychiatry 10: 105–116. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH ( 2005): Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry J Mental Sci 186: 32–40. [DOI] [PubMed] [Google Scholar]
- Weber B, Kugler F, Elger CE ( 2007): Comparison of implicit memory encoding paradigms for the activation of mediotemporal structures. Epilepsy Behav 10: 442–448. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA ( 1978): A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry J Mental Sci 133: 429–435. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY ( 2003): Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science 299: 577–580. [DOI] [PubMed] [Google Scholar]


