Abstract
Background
Superoxide dismutases (SODs) cause dismutation of superoxide radicals to hydrogen peroxide and oxygen. Besides protecting the cells against oxidative damage by endogenously generated oxygen radicals, SODs play an important role in intraphagocytic survival of pathogenic bacteria. The complete genome sequences of Yersinia enterocolitica strains show presence of three different sod genes. However, not much is known about the types of SODs present in Y. enterocolitica, their characteristics and role in virulence and intraphagocytic survival of this organism.
Methodology/Principal Findings
This study reports detection and distribution of the three superoxide dismutase (sodA, sodB and sodC) genes in 59 strains of Y. enterocolitica and related species. The majority (94%) of the strains carried all three genes and constitutive expression of sodA and sodB was detected in 88% of the strains. Expression of sodC was not observed in any of the strains. The sodA, sodB and sodC genes of Y. enterocolitica were cloned in pET28a (+) vector. Recombinant SodA (82 kDa) and SodB (21 kDa) were expressed as homotetramer and monomer respectively, and showed activity over a broad range of pH (3.0–8.0) and temperature (4–70°C). SodA and SodB showed optimal activity at 4°C under acidic pH of 6.0 and 4.0 respectively. The secondary structures of recombinant SodA and SodB were studied using circular dichroism. Production of YeSodC was not observed even after cloning and expression in E. coli BL21(DE3) cells. A SodA− SodB− Escherichia coli strain which was unable to grow in medium supplemented with paraquat showed normal growth after complementation with Y. enterocolitica SodA or SodB.
Conclusions/Significance
This is the first report on the distribution and characterization of superoxide dismutases from Y. enterocolitica. The low pH optima of both SodA and SodB encoded by Y. enterocolitica seem to implicate their role in acidic environments such as the intraphagocytic vesicles.
Introduction
Y. enterocolitica is an important food- and water-borne enteropathogen. It is associated with a variety of gastrointestinal problems and clinical manifestations that include acute gastroenteritis, terminal ileitis, and mesenteric lymphadenitis [1]. Virulence of Y. enterocolitica is attributed to the presence of a 70 kb pYV (plasmid for Yersinia virulence) plasmid and many chromosomally-encoded virulence factors [2]. It is an extracellular pathogen that has the ability to survive inside macrophages [3]. Y. enterocolitica can survive at low temperatures where its growth is governed by polynucleotide phosphorylase (PNPase) encoded by pnp gene [4]. Ability of Y. enterocolitica to grow at low temperatures and isolation from vacuum-packed frozen foods makes it an important pathogen associated with food-borne infections and poses a significant risk to the processed-food industry [5], [6]. Recently, Champion et al. [7] have reported that Y. pseudotuberculosis sodC mutant showed increased susceptibility to superoxide and reduced virulence in murine infection model. Similarly, intraphagocytic survival of Y. enterocolitica suggests resistance to reactive oxygen species (ROS) produced by macrophages although the mechanism has not been well defined. Roggenkamp et al. [8] have previously reported that SodA played an important role in the survival of Y. enterocolitica 1B/O:8 in the spleen and liver of mice and its absence led to an increased susceptibility of the organism to killing by neutrophils. However, no further studies have been reported on the nature and the distribution of superoxide dismutases from Y. enterocolitica.
Superoxide dismutases (EC 1.15.1.1) are metalloenzymes that detoxify oxygen radicals through the dismutation of superoxide to oxygen and hydrogen peroxide which is further reduced to water and oxygen by catalase or peroxidase [9]. These enzymes are ubiquitously present in both prokaryotes and eukaryotes where they function to protect the cells from endogenously generated superoxide anions during aerobiosis. Reactive oxygen species (ROS) such as superoxide anions (O⋅ 2 ), hydroxyl radicals (OH⋅) and hydrogen peroxide (H2O2) are known to damage nucleic acids, proteins and lipids [10], [11]. In addition to detoxification of endogenously produced superoxides, bacterial SODs play a significant role in the pathogenicity of animal and plant pathogens [12], [13], [14], [15]. Secretion of SODs by strains of Brucella, Haemophilus, Legionella, Mycobacterium, and Nocardia, further seems to suggest their role in neutralizing exogenous oxygen radicals produced by the host immune system [16]. On the basis of the metal co-factor, SODs are classified into four types: manganese co-factored (Mn-SOD or SodA), iron co-factored (Fe-SOD or SodB), copper-zinc co-factored (Cu/Zn-SOD or SodC) and nickel co-factored (Ni-SOD or SodN) [9], [17]. Prokaryotic SodA and SodB are located in the cytoplasm of the bacterial cell whereas SodC is periplasmic [9]. Cells that do not produce superoxide dismutase, in general, show reduced cellular health and increased damage due to oxidative stress. An increase in the frequency of the mutations has been observed in E. coli lacking SodA and SodB [18], and Saccharomyces cerevisiae lacking Mn-SOD were found to be highly sensitive to oxygen [19], [20].
In Y. enterocolitica the role of SodB and SodC has not been studied as yet. Moreover, SODs of Y. enterocolitica have not been characterized, till date. In this study, the distribution of sod genes in different strains of Y. enterocolitica biovar 1A was assessed. Furthermore, the SODs of Y. enterocolitica biovar 1A were cloned and expressed in E. coli BL21 (DE3) and the purified recombinant superoxide dismutases (YeSODs) were extensively characterized biochemically and biophysically. This is the first report on the distribution and detailed characterization of novel SODs from strains of Y. enterocolitica biovar 1A.
Materials and Methods
2.1. Bacterial Strains and Vectors
A total of 54 strains of Y. enterocolitica were used in this study. Three strains of Yersinia intermedia and two of Yersinia frederiksenii were also included in the study. The details of these strains are given in supplementary data (Table S1 in File S1). The strains were grown overnight in tryptone soya broth (TSB) or tryptone soya agar (TSA) plates (HiMedia, Mumbai, India) at 28°C. E. coli strains were grown overnight in Luria Bertani (LB) broth at 37°C with shaking. Details of strains and vectors used in cloning and expression of sod genes are given in Table 1.
Table 1. Bacterial strains and plasmids used for cloning and expression in this study.
| Strains/Plasmids | Relevant characteristics | Reference/Source |
| Y. enterocolitica | ||
| IP27366 | Biotype 1A; serotype O:6,30–6,31, clinical isolate | [68] |
| E. coli | ||
| DH5α | F2 D(lac-argF)U169 recA1 endA1 hsdR (rK 2 mK 1) supE44 gyrA1 relA1 deoR thi-1 (F80dlacZDM15) | Invitrogen |
| BL21(DE3) | E. coli B F− dcm ompT hsdS(r− B m− B ) gal λ(DE3) | Stratagene |
| AB1157 | F- thr-1; leuB6; proA2; his-4; thi-1; argE2; lacY1; galK2; rpsL; supE44; ara-14; xyl-15; mtl-1; tsx-33 | Joan S. Valentinea |
| PN134 | AB1157 with (sodA::Mu d PR13)25 (sodB−kan) | Joan S. Valentinea |
| Plasmids | ||
| pGEM-T easy | T-A Cloning vector; Amp r | Promega |
| pGEMsodA | pGEM cloning vector with 624 bp fragment of sodA amplified from Y. enterocolitica | This study |
| pGEMsodB | pGEM cloning vector with 579 bp fragment of sodB amplified from Y. enterocolitica | This study |
| pGEMsodC | pGEM cloning vector with 525 bp fragment of sodC amplified from Y. enterocolitica | This study |
| pGEMsodCTrunc | pGEM cloning vector with 471 bp truncated fragment of sodC amplified from Y. enterocolitica | This study |
| pET28a+ | pET vectors are derived from pBR322 with opposite orientation of the cloning/expression region; Kanr | Novagen |
| pET28sodA | pET28 vector containing YesodA | This study |
| pET28sodB | pET28 vector containing YesodB | This study |
| pET28sodC | pET28 vector containing YesodC | This study |
| pET28sodCTrunc | pET28 vector containing truncated copy of YesodC | This study |
| pGFPuv | GFP gene cloned between the two MCSs of the pUC19 derivative pPD16.43, Amp r | Clontech |
| pGFPsodA | pGFP vector containing YesodA | This study |
| pGFPsodB | pGFP vector containing YesodB | This study |
Prof. Joan S. Valentine, University of California Los Angeles.
2.2. PCR Detection of sodA, sodB and sodC Genes in Yersinia spp
Genomic DNA from the strains was isolated using Pure Link Genomic DNA Minikit (Invitrogen, USA), as per manufacturer’s instructions and used as the template for amplification of sodA, sodB and sodC by PCR. The details of the primers and the respective PCR conditions used are given in Table 2. The primers were designed using the complete genome sequence of Y. enterocolitica strain 8081 (accession no. AM286415).
Table 2. Details of primers and the PCR conditions.
| Gene | Primers | Sequence (5'-3') | Amplicon(bp) | Annealing temperature | Restrictionsites | Position of primers on the Y.e. strain 8081 complete genome |
| sodA | SodA 624F | TTTGGATCCATGAGTTACTCACTGCCATCCCT | 624 | 58°C –40s | BamHI and SacI | 4530100–4530122 |
| SodA 624R | TTTGAGCTCCTTAGCTTGAGCGAAGCGC | 4530720–4530702 | ||||
| sodB | SodB 579F | TTTGAGCTCATGTCTTTTGAATTACCGGCGTT | 579 | 59°C –40s | SacI and NotI | 2356988–2357007 |
| SodB 579R | TTTGCGGCCGCCCGGCTAAGTTTTTCTCAACGAA | 2357563–2357543 | ||||
| sodC | SodC 525F | AAAGCGGCCGCATGAAACTGAAATACTTAGTGTTACCC | 525 | 50°C –40s | NotI and XhoI | 877988–878014 |
| SodC 525R | AAACTCGAGTTACTCAATCACACCACAAGC | 878514–878492 | ||||
| sodC | SodC Trun F | AAAGCGGCCGCATGGCTGCGGATATTACTGTGAC | 476 | 50°C –40s | NotI and XhoI | NA |
| SodC 524R | AAACTCGAGTTACTCAATCACACCACAAGC | 878514–878492 |
Nucleotide bases in bold represent restriction sites. NA: the primer was designed using the sequence of sodC (accession no. JX204785) from strain IP27366 as the template.
2.3. Whole Cell Protein Extraction and Zymogram Analysis
Overnight grown cultures of Yersinia spp. were harvested, washed and resuspended in lysis buffer (50 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA) containing 4 mM/mL PMSF (phenyl methane sulfonyl chloride). The cells were lysed by sonication on ice with 5 cycles of 1 min pulse (3 sec on/off) to obtain the cytoplasmic proteins. The periplasmic proteins were obtained by suspending the cell pellet in 20 mM TE buffer (pH 8.0) containing 25% (w/v) sucrose and 1 mM EDTA. The cell suspension was incubated at 30°C with mild shaking for 15 min. The cells were collected by centrifugation at 4°C followed by osmotic treatment for 10 min by suspending the cell pellet into 5 mM chilled MgSO4 solution to release the periplasmic fraction of the cells which was further collected as supernatant on centrifugation. The total protein concentration of each bacterial lysate was estimated using Bradford method [21] with bovine serum albumin (BSA) (0–100 mM) as standard.
The presence of active SODs was confirmed by zymogram analysis on native PAGE using the methodology described previously [22], with slight modifications. Crude protein extracts (cytoplasmic or periplasmic) were electrophoresed on non-denaturing 15% polyacrylamide (1∶29, bisacrylamide-acrylamide) gel using a mini-Protean III apparatus (Bio-Rad, USA). The gels were soaked in 2.4 mM nitrobluetetrazolium (Sigma) for 25 min in dark and later immersed in 0.03 mM riboflavin solution containing 210 µl tetramethylethylenediamine (TEMED) for 25 min followed by illumination of gels under a 15 W luminescent light source for 20 min.
2.4. Differentiation of SodA, SodB and SodC
Enzyme inhibitors were used to differentiate different SODs expressed by the Y. enterocolitica strains. Following separation of the total cell protein on non-denaturing PAGE, the gels were treated with H2O2 [23] and sodium azide [24] to inactivate Fe-SOD, whereas, diethyldithiocarbamate (DDTC) was used to inactivate the Cu/Zn-SOD [25]. After 30 min of incubation with different inhibitors, the gels were washed thoroughly to remove the inhibitors and processed for zymogram development.
To detect the presence of Cu/Zn-SOD, ice cold 2∶3 v/v chloroform: ethanol solution was added to the crude protein as described previously [26]. The protein-chloroform:ethanol mixture was kept at room temperature for 30 s and centrifuged at 2,500 g for 10 min. The aqueous phase, devoid of Mn- and Fe-SOD, was carefully loaded on the native gel and processed for zymogram development.
2.5. RT-PCR for SOD mRNA Transcripts
RNA was extracted from overnight cultures of Y. enterocolitica, Y. intermedia and Y. frederiksenii grown in the presence or absence of paraquat (Sigma, USA), using RNeasy Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. Paraquat (methyl viologen) (0.1 mM) was added to the culture media to induce oxidative stress conditions for the bacteria. 1 µg total RNA was used for single-strand cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad, USA), according to manufacturer’s instructions. The cDNA was further used to amplify sodA, sodB and sodC using specific primers (Table 2).
2.6. Expression and Purification of Recombinant Superoxide Dismutases
sodA, sodB and sodC genes were amplified from Y. enterocolitica strain IP27366, cloned in pGEM-T easy vector and propagated in E. coli DH5α cells. Cloning of sod genes was confirmed using colony PCR followed by sequencing using Sanger dideoxy sequencing method at the central instrumentation facility, University of Delhi South Campus using ABI 3700 genetic analyzer. Further, the respective sod genes were subcloned into pET28a (+) vector at specific restriction sites (sodA- BamHI-SacI; sodB- SacI-NotI and sodC- NotI-XhoI) using instant ligation mix (Clontech, Takara). Thereafter, the recombinant plasmids (pET28a-sodA/sodB/sodC) were transformed separately into E. coli BL21 (DE3) and confirmed by sequencing. The positive clones were grown overnight. Erlenmeyer flask (250 mL) containing 50 mL of LB broth supplemented with kanamycin (50 µg/mL) were inoculated with respective cultures grown overnight [1% (v/v)] and incubated till the optical density of 0.5 at 600 nm was achieved. The expression of recombinant SODs was induced by addition of 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) to the cultures which were incubated further. Cells were harvested periodically to study the expression of the recombinant proteins.
The recombinant Y. enterocolitica SODs (YeSodA and YeSodB) were purified by affinity chromatography using Ni2+-NTA agarose resins (Novagen, USA) according to manufacturer’s instructions. Purified recombinant proteins were eluted in 5 mL of 250 mM imidazole and analyzed on 15% SDS-PAGE to check the purity and titre of the recombinant SODs. Further, the molecular weights of the purified proteins were determined by SDS-PAGE and fast protein liquid chromatography (FPLC, AKTAPrime plus). FPLC was performed using a Sephacryl S-200 column (16/60, AKTA) pre-equilibrated with 100 mM potassium phosphate buffer (pH 7.5) [27].
2.7. Biochemical Characterization
Isoelectric focusing (IEF) of the purified enzymes (YeSodA and YeSodB) was carried out in 6% polyacrylamide gel containing 2% ampholyte (pH 5–7) (Biolyte, Ampholyte, Bio-Rad). 3–5 µL of purified protein containing ca. 5 µg of protein was loaded on the gel and focused using a Mini IEF cell (Bio-Rad, USA) at 4°C according to the manufacturer’s instructions. The gels were stained with coomassie brilliant blue stain to visualize the SOD bands against the pI ladder.
Thermostability and pH stability were measured by incubating purified YeSODs at different temperatures (4–70°C) and pH (2–10) for varying time intervals (5 min – 24 h) followed by determining the enzyme activity using superoxide dismutase kit (Cayman Chemicals, USA) according to manufacturer’s instructions.
2.8. Biophysical Characterization using Circular Dichroism (CD)
The secondary structures of YeSodA and YeSodB were analysed from the CD spectra in the ‘far-UV’ spectral region (190–240 nm) using a JASCO J-815 spectropolarimeter equipped with a peltier thermostatic cell holder (PTC-348 WI, JASCO, Japan). The far-UV CD spectrum was recorded as described previously [28]. The proteins in different buffers of pH (3.0–8.0) were scanned at different temperatures. Results were expressed as mean residue ellipticity by calculating mean residue weight per amino acid residue. The K2D2 software [29] was used for analyzing the data.
2.9. Growth of E. coli SodA− SodB− Strain Complemented with YeSodA and YeSodB under Oxidative Stress
The effect of paraquat was observed on the growth of SodA− SodB− E. coli strain PN134, complemented with YeSodA or YeSodB. Full length sodA and sodB genes from Y. enterocolitica strain IP27366 were cloned into pGFPuv vector (Clontech). The individual recombinant vectors (pGFPsodA or pGFPsodB) were transformed into E. coli PN134. The E. coli PN134, expressing YeSodA or YeSodB, was propagated aerobically at 37°C overnight in LB broth (25 ml) supplemented with 0.2% sucrose. The overnight cultures were subcultured into fresh LB broth (250 mL) and kept at 37°C with agitation at 200 rpm in an orbital shaker (New Brunswick Scientific, USA) to A600 of 0.2. The culture was divided equally into five 250 mL flasks, each containing a different concentration of paraquat and incubated at 37°C with agitation. The bacterial growth was monitored every 2 h by measuring A600 with a spectrophotometer. A wild type strain (E. coli AB1157) and E. coli PN134 (SodA− SodB−) were also included as controls.
2.10. Proposed 3D Structures of Y. enterocolitica SODs
Crystal structures of SODs (PDB ID: 1VEW and 1ISA) from E. coli were used to propose the secondary and tertiary structure of YeSODs [30], [31]. Preliminary studies on the secondary structures were carried out using online software ESpript 2.2. The deduced amino acid sequences of YeSods were submitted to online server ESyPred3D Web Server 1.0 [32] to obtain a PDB file. PyMol software was used to view the PDB files for constructing the 3D structure.
2.11. Nucleotide Sequence Accession Numbers
The Y. enterocolitica sodA, sodB and sodC sequences were submitted to GenBank database and assigned accession nos. JX204782, JX204783 and JX204785 respectively.
Results
3.1 Distribution and Expression of sod Genes
PCR amplifications revealed that sodA gene was present in all strains, sodB was absent only in one strain (Y. enterocolitica biovar 1A strain IP27430) whereas sodC was absent in two Y. intermedia strains (IP27388 and IP27389) (Table S2 in File S1).
Zymogram analysis showed two achromatic zones in each lane instead of the expected three clear zones (Figure 1a). Treatment of Y. enterocolitica crude lysate with specific SOD inhibitors (Figure 1b) revealed expression of SodA and SodB in 100% and 88% of the strains respectively, whereas SodC was not expressed by any of the strains (Table S2). Additionally, growth even under conditions of oxidative stress and enrichment of growth medium by incorporation of Cu/Zn (0.1–2 mM) did not induce the expression of SodC.
Figure 1. Zymogram analysis of superoxide dismutases:
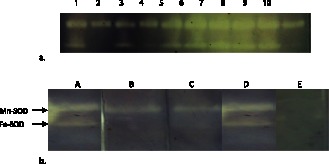
(a) Representative zymogram showing two acromatic bands of crude bacterial lysate from Yersinia spp. (b) Effect of different inhibitors on SOD isoforms of Y. enterocolitica. Lane A: Cell lysate without inhibitor; B: with 5 mM H2O2; C: with 5 mM NaN3; D: with DDTC; E: with chloroform:ethanol.
RT-PCR not only generated amplicons of sodA (624 bp) and sodB (579 bp) as expected, but also amplified a 525 bp (sodC) amplicon when primers specific for sodC were used. The sodC was amplified from cDNA from strains grown with or without paraquat in the culture medium.
3.2. PCR Amplification and Sequence Analysis
Full-length sod genes of Y. enterocolitica strain IP27366 were amplified using specific primers and cloned into pET28a(+) vector. The sizes of the Y. enterocolitica sodA, sodB and sodC genes were 624 bp, 579 bp and 525 bp with an overall G+C content of 51%, 46% and 50% respectively. The deduced amino acid sequences revealed presence of the signature sequences of the respective SOD families (Figure 2). Phylogenetic analysis showed proximate relationships of YeSODs and other bacterial species based on amino acid sequence of respective SOD enzymes (Figure S1).
Figure 2. Nucleotide and deduced amino acid sequences of the superoxide dismutase genes from Y. enterocolitica strain IP27366:

(a) SodA showed one signature sequence (D V W E H A Y Y) and four metal binding ligands (H-27, H-82, D-169 and H-173). (b) SodB also showed one signature sequence (D V W E H A Y Y) and four metal binding ligands (H-27, H-74, D-157and H-161). The metal binding ligands are shown in boxes. (c) SodC showed signature sequence I (G F H L H E N P S C T) and II (G G G G A R M A C G V I).
3.3. Expression and Purification of Recombinant YeSODs
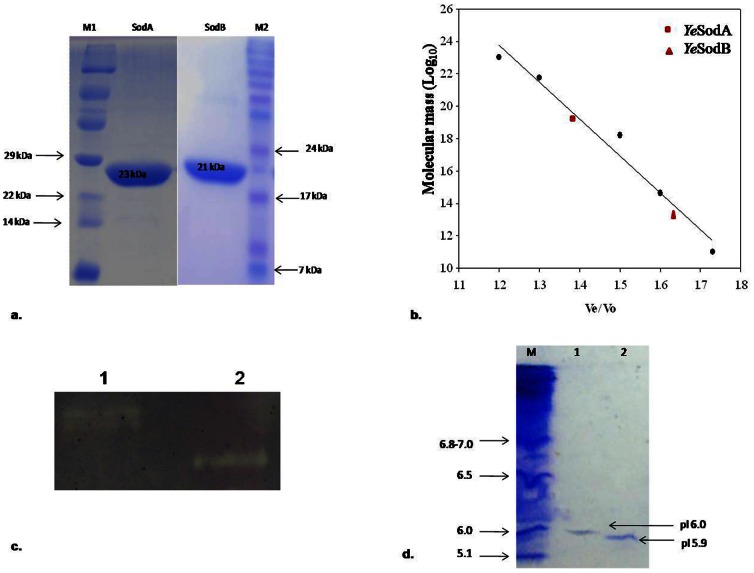
The expression of recombinant YeSodA and YeSodB was induced by addition of 1 mM IPTG in E. coli BL21 (DE3). These were purified using Ni2+-NTA resins and observed as single protein band each on 15% SDS-PAGE (Figure 3a). Molecular weights of YeSodA and YeSodB were observed to be 23 kDa and 21 kDa respectively on SDS-PAGE. Further, analysis of purified YeSodA and YeSodB by FPLC revealed molecular weight of 82 kDa and 21 kDa respectively (Figure 3b). This suggested that YeSodA and YeSodB were expressed as tetramer and monomer respectively. However, SodC was not expressed even after cloning in pET28a(+) vector.
Figure 3. Molecular weight, activity and pI analysis of recombinant SODs:
(a) SDS–PAGE of recombinant YeSodA and YeSodB expressed in pET 28a (+) (samples were resolved on 15% polyacrylamide gel and stained with Coomassie Brilliant Blue R-250). The purified SodA and SodB showed a single band each of 23 KDa and 21 kDa respectively. M1 and M2: Protein marker; Lane 1: SodA; Lane 2 SodB. (b) Molecular weight determination of YeSodA (82 kDa) and YeSodB (21 kDa) by Sephacryl S-200 molecular sieve chromatography. The molecular weight of marker proteins (SigmaAldrich) were as follows: β-Amylase (200 kDa), Alcohol dehydrogenase (150 kDa), BSA (66 kDa), Carbonic anhydrase (29 kDa) and Cytochrome C (12.4 kDa). (c) Zymogram analysis showing achromatic bands of YeSodA and YeSodB against a dark background. Lane 1: YeSodA; Lane 2: YeSodB. (d) Isoelectric point (pI) of purified recombinant YeSodA and YeSodB stained with coomassie brilliant blue. M: pI marker; Lane 1: YeSodA; Lane 2: YeSodB.
Multiple sequence alignment of the deduced amino acid sequence of SodC showed highly unconserved N-terminal region. Therefore, an attempt was made to truncate sodC for 54 nucleotides downstream of the initiation codon and was amplified using specific primers (Table 2). The truncated gene was cloned in pET28a (+) vector and transformed into E. coli BL21 (DE3). However, even the truncated recombinant sodC could not be expressed after IPTG induction.
The zymogram analysis of the purified YeSodA and YeSodB revealed clear bands against the dark background of the polyacrylamide gel which further confirmed the expression of active recombinant YeSodA and YeSodB (Figure 3c).
3.4. Biochemical Characterization
The isoelectric points (pI) of the native YeSodA and YeSodB were observed to be approximately 6.0 and 5.9 (Figure 3d), respectively. These values were quite similar to the theoretical pI’s of 6.2 and 5.8 respectively, which calculated by PROTPARAM software (http://web.expasy.org/protparam).
The recombinant YeSodA and YeSodB were active over a broad range of pH (3.0–8.0) and temperature (4–70°C) with optimum of YeSodA at pH 6.0 and 4°C and, that of YeSodB at pH 4.0 and 4°C. The specific activities of purified YeSodA and YeSodB at optimum temperature and pH were 12,800 U/mg and 24,400 U/mg of protein respectively. The activities of these enzymes decreased steadily with increase in temperature whereas change in pH also had significant effect on the enzyme activity (Figure 4).
Figure 4. Effect of physical parameters on recombinant SOD activity:
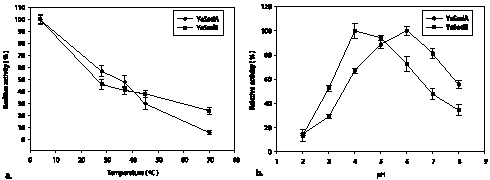
(a) Optimum temperature of YeSodA and YeSodB was 4°C (b) while optimum pH was 4.0 and 6.0 respectively. The results are expressed as percent change in the activity of the respective enzyme with the value at optimum temperature and pH taken as 100%.
3.5. Proposed 3D Structure
ESpript 2.2 analysis showed α-helix dominant structures of YeSodA and YeSodB. Preliminary structure of YeSodA comprised of 11 α-helix and 3 β-sheets, whereas, YeSodB showed a total of 9 α-helix and 3 β-sheets along with random coils and sharp turns in both enzyme structures (Figure 5).
Figure 5. Sequence homology:
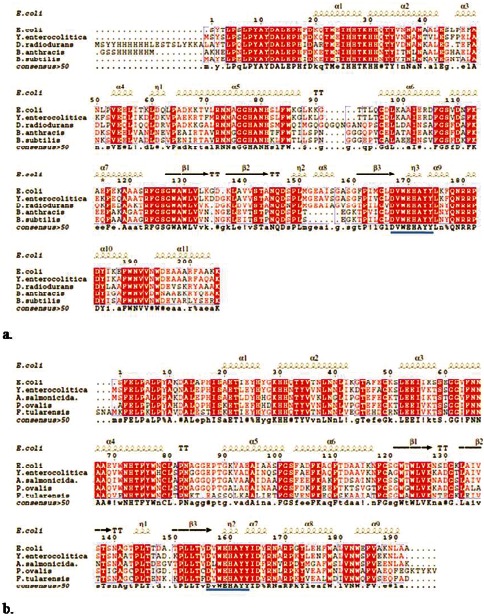
Multiple sequence alignment (MSA) of (a) YeSodA with E. coli (PDB id: 1VEW), Deinococcus radiodurans (PDB id: 2CDY), B. anthracis (PDB id: 1XUQ) and B. subtilis (PDB id: 2RCV); (b) YeSodB with E. coli (PDB id: 2NYB), Aliivibrio salmonicida (PDB id: 2W7W), Pseudomonas ovalis (PDB id: 1DT0) and Francisella tularensis (PDB id: 3H1S) drawn using ESPript 2.2. Symbols α and β indicate alpha helices and beta sheets, respectively; η represents turns and TT denotes sharp turns in the structure.
The proposed three dimensional structures of YeSodA and YeSodB imitate similar profile of α-helix and β-sheets. Sequence analysis revealed metal binding ligands in YeSodA as His27, His82, Asp169 and His173, and in YeSodB as His27, His74, Asp157 and His161. Two of these amino acids (Asp169/His173 for SodA and Asp157/His161 for SodB) were present in the highly conserved signature sequences. 3D structure analysis showed clustering of all four metal binding ligands which formed a groove for binding the respective metal ion (Figure 6).
Figure 6. Proposed three dimensional structure:
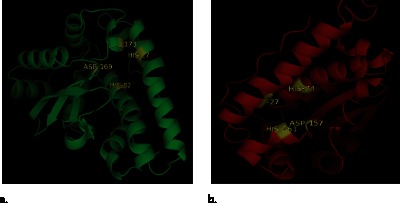
Predicted 3D structure of (a) SodA and (b) SodB showing metal binding ligands: His27, His82, Asp169 and His 173 in SodA and, His27, His74, Asp157 and His161 in YeSodB.
3.6. Biophysical Characterization
3.6.1. Secondary structure
The secondary structures of purified YeSodA and YeSodB were determined by circular dichroism spectroscopy (CD). The far-UV CD spectra indicating the secondary structures of YeSodA and YeSodB are presented in Figure 7. The secondary structure of SodA at 28°C and pH 7.0 showed 29% α-helices and 16% β-sheets whereas SodB consisted of 44% α-helices and 13% β-sheets (Figure 7a, b). The far-UV CD spectra of the purified proteins recapitulate the predictions from ESpript 2.2 and proposed 3D structure of the respective SODs. Although no significant changes were observed in the secondary structures of the SODs with increase in temperature (data not shown), change in pH had considerable impact on the α-helix and β-sheet content of the respective SODs (Figure 7c, d).
Figure 7. Secondary structure analysis using circular dichroism:
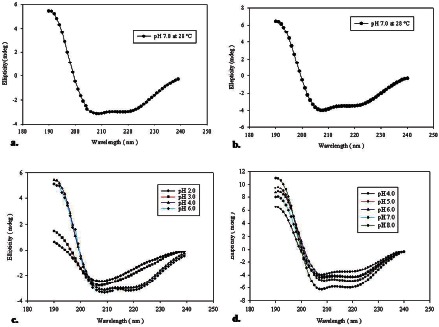
Far-UV spectra of (a) YeSodA and (b) YeSodB at pH 7 and 28°C. Far-UV spectra of (c) YeSodA and (d) YeSodB at different pH.
3.7. Significance of Y. enterocolitica SodA and SodB in Growth under Oxidative Stress
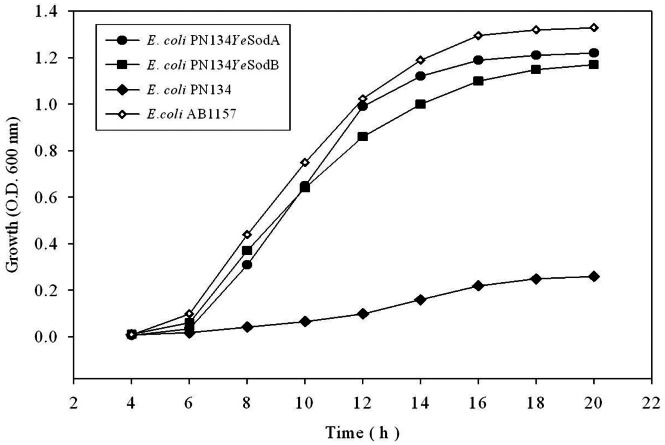
The effect of paraquat on growth of E. coli strain PN134 expressing YeSodA or YeSodB was studied. Figure 8 shows effect of paraquat on the survival of E. coli PN134 expressing YeSodA and YeSodB compared to a wild type strain of E. coli (AB1157) and the SOD double mutant (E. coli PN134 SodA− SodB−). The dose-dependent inhibition of growth indicated that all recombinant strains were inhibited by high concentration of paraquat (data not shown); however, at lower concentrations (0.1 mM) the strain expressing YeSODs showed resistance to paraquat-induced oxidative stress. E. coli PN134 SodA− SodB− strain showed high sensitivity even to low concentrations of paraquat (0.1 mM) whereas the wild type strain displayed normal growth (Figure 8).
Figure 8. Analysis of Growth profile:
Growth of E. coli PN134 (SodA− SodB−), wild type E. coli AB1157 (SodA+ SodB+) and E. coli PN134 complemented with YeSodA or YeSodB in the presence of paraquat (0.1 mM).
Discussion
In this study, we report PCR detection, distribution and expression of different superoxide dismutases in strains of Y. enterocolitica and related species. PCR amplification revealed presence of three sod genes in majority (95%) of the strains used in this study. The assays to detect superoxide dismutase activities revealed that each strain expressed at least one functional SOD. Specific inhibitors were used to distinguish the types of the superoxide dismutases expressed by the strains. The Mn- and Fe-SODs of Y. enterocolitica were inhibited by chloroform:ethanol solution as reported previously [26], [33]. Inhibition of the band with lower molecular weight by sodium azide and hydrogen peroxide confirmed the presence of Fe-SOD [23], [24]. The failure of diethyldithiocarbamate (DDTC) to inhibit any of the superoxide dismutases suggested absence of the active SodC and confirmed that the high molecular weight achromatic band was Mn-SOD [25].
Presence of identical signature sequences and similar conserved metal binding domains seemed to suggest that Y. enterocolitica SodA and SodB originated from a common progenitor gene as reported earlier by Smith and Doolittle [34]. The deduced amino acid sequence of YeSodA was the most conserved of the three SODs. Phylogenetic analysis of YeSodA indicated high (>82%) similarity with SodA from other gram-negative enteropathogens and shared 86% and 47% similarity with E. coli SodA and human mitochondrial Mn-SOD, respectively. BLAST analysis of YeSodA revealed 66% similarity with Deinococcus radiodurans [35], a highly radio resistant organism. However, the significance of this similarity is not clear and requires further investigation. YeSodB showed similarity with Fe-SODs encoded by members of the family Enterobactericeae and shared ca. 82% similarity with E. coli SodB. Analysis of deduced amino acid sequence of YeSodC indicated that it was the least conserved of the three YeSODs and showed low similarity with strains of other Yersinia spp. and only 58% similarity with the E. coli SodC. The N-terminal region of SodC was highly unconserved. Although, YeSodA and YeSodB showed high similarities to superoxide dismutases from members of gamma-proteobacteria only, YeSodC showed similarity to that of members of the alpha-proteobacteria such as Brucella spp.
Benov and Fridovich (1994) [36] reported that SodC activity in E. coli sodA − sodB − double mutant was ca. 2% of the total SOD activity of a wild type strain. To verify whether YeSodC was expressed at such low concentrations in our studies, the complete sodC gene was cloned and transformed in E. coli BL21 (DE3) cells. However, expression of SodC was not detected even after induction with IPTG. The 5′ region that encodes the signal sequence was highly unconserved in sodC and might be the reason for non-expression of SodC. The presence of SodC in the periplasmic space, as seen in other organisms, reiterates the importance of the signal peptide in guiding the enzyme to its required destination. Therefore, highly variable N- terminal region of SodC was truncated and tried to express in E. coli BL21 (DE3) cells however, no expression was detected. Enrichment of growth medium with incorporation of Cu/Zn also failed to express SodC. In addition, growing Y. enterocolitica in the presence of different concentrations of paraquat did not lead to “oxidative stress-induced” expression of SodC as reported for Brucella abortus [37], B. melitensis [38] and Caulobacter crescentus [39]. Nevertheless, RT-PCR revealed transcription of SodC mRNA when Y. enterocolitica was grown under normal conditions. It has also been previously reported that bacterial SodC showed wider variations in their amino acid sequences, compared to eukaryotic SodC which share a single structural model that has been preserved strictly throughout the evolution [40]. It has also been observed that bacterial SodC showed high degree of insertions and deletions in the major loops of the β-barrel that may cause disparity in the conformation of the active-sites and their subunit assembly. Substitutions of conserved metal ligands may also lead to significant alterations in the enzyme activity [41], [42]. Therefore, in the light of the constitutive transcription of sodC mRNA in Y. enterocolitica, we may hypothesize that the highly unconserved N-terminal region of the YeSodC may hinder proper folding of the protein leading to its degradation. Similarly, Bakshi et al. [43] reported that Francisella tularensis sodC does not encode a functional protein under any set of growth conditions, whereas, expression of SodB was observed throughout the growth phase. Interestingly, similar to the YeSodC, BLASTP analysis of F. tularensis SodC showed highly unconserved N- terminal region that, however, contains excellent signal peptide characteristics. These observation, although not conclusive, point towards an important role of N- terminal signal sequence in the expression of SodC.
The active recombinant YeSodA and YeSodB proteins were obtained in solublized form from the cytoplasmic fraction of the cells which was consistent with a previous report [44]. The predicted molecular masses deduced from amino acid sequences of YeSodA and YeSodB were 23 and 21 kDa respectively. However, FPLC analysis revealed molecular masses of 82 kDa and 21 kDa of YeSodA and YeSodB respectively, which suggested that YeSodA was expressed as tetramer whereas YeSodB was expressed as a monomer although its homologs in most prokaryotes are expressed as dimers [45], [46], [47] or tetramers [48], [49]. Similarly, prokaryotic SodC, which is dimeric in most organisms, has been reported to be expressed as monomers in E. coli [50] and Salmonella [51].
The optimal temperature and pH of YeSodA was observed to be 4°C and pH 6.0; for YeSodB it was 4°C and pH 4.0. At optimal pH and temperature YeSodA and YeSodB showed specific activities of 12,800 U/mg and 24,400 U/mg, respectively. The low temperature optima of YeSodA and YeSodB were exceptional as most SodA and SodB which have been characterized in the past showed temperature optima of 25–70°C. Fe-SOD has been reported to show optimum activity at 4°C in only a few bacteria such as Aliivibrio salmonicida [52] and Pseudoalteromonas haloplanktis [53]. Similarly, a low pH optimum for bacterial SODs has been reported only rarely. Wang et al. [54] reported that Rhodothermus Fe-SOD showed optimum activity at pH 5.0 and 50°C, whereas Lumsden et al. [55] characterized a Mn-SOD from Rhodopseudomonas that retained 70% of its activity after 30 min at pH 3.0. The low pH optima of both YeSODs may be of significance under acidic conditions such as those encountered inside the macrophages and eventually help in the dissemination of Y. enterocolitica in the host by sequestering the organism in these cells. At low temperatures, reduction in the cellular metabolic rate causes accumulation of electrons in the respiratory chain that leads to overproduction of reactive oxygen species [56]. Y. enterocolitica being a psychrotrophic food-borne pathogen, the low temperature optima of YeSODs may help in neutralizing the excess oxygen radicals produced during its growth at lower temperatures in refrigerated foods [57]. Therefore, we may hypothesize that the SODs produced by Y. enterocolitica with high specific activities are helpful in dismutating endogenously produced reactive oxygen species at low temperatures. Both the YeSODs were also endowed with significant overall thermal stability which is common for SODs belonging to this family.
The pIs of Y. enterocolitica SodA (6.0) and SodB (5.9) were almost similar to the theoretical pI of 6.2 and 5.8, respectively, and were in the range of pI values (3.8–6.0) for the known respective superoxide dismutases [58], [59].
The conservation of most of the amino acid residues of E. coli SODs involved at active site, substrate binding and catalysis in YeSodA and YeSodB as well, suggested similar structure and function. The metal binding residues- His27, His82, Asp169 and His173 in YeSodA were also conserved as reported earlier for Thermus thermophilus, Bacillus stearothermophilus and Bacillus subtilis [46], [49], [60]. Similarly, in YeSodB His27, His74, Asp157 and His161 were in accordance with conserved residues in E. coli, Helicobacter pylori and Pseudomonas ovalis [61], [62], [63].
Analysis of far-UV CD spectra of SodA and SodB revealed that the elements of secondary structures were more sensitive to pH than to temperature. At pH 7.0 and 28°C, SodA contained 29% α-helix and 16% β-sheets whereas SodB contained 44% α-helix and 13% β-sheets. The α-helix (29%) in SodA agreed with the 30% found in E. coli Mn-SOD than 40–50% seen in the crystal structures of the Mn-SODs from Thermus thermophilus and Bacillus stearothermophilus [45], [46], [49]. The far-UV CD spectra of YeSodB revealed high content of α-helix (44%) that was in agreement to the 50% α-helix found in Fe-SOD of Pseudomonas ovalis [47]. Changes in the optimum pH of the respective SODs resulted in significant loss of ordered secondary structure, which manifested as corresponding decrease in the enzyme activity.
NADPH-mediated reduction of paraquat generates an intracellular flux of O2 − that induces toxicity [64]. Farr et al. [18] have previously reported that E. coli SOD double mutant (strain PN134) showed high sensitivity to paraquat that was reversed by introducing a copy of functional sod gene. E. coli PN134 strains expressing YeSodA or YeSodB also showed increased resistance to paraquat when compared to the parental strain and these results were similar to those observed by Leclere et al. [12]. Maize Mn-SOD expressed in SOD-deficient S. cerevisiae has been shown to protect the cells against oxidative stress whereas its over-expression in Caenorhabditis elegans and Drosophila melanogaster augmented their life span [65], [66], [67]. In the same vein, the YeSODs almost normalized the growth of E. coli mutant strain under conditions of oxidative stress by scavenging the exogenous ROS.
Conclusions
This work represents the first report on the distribution and detailed characterization of superoxide dismutases from Y. enterocolitica biovar 1A. Most strains expressed both SodA and SodB, whereas, SodC was not expressed by any of the strains used in this study. The recombinant YeSodA and YeSodB were expressed as tetramer and monomer respectively. Complementation of an E. coli SOD double mutant with YeSodA and YeSodB enabled E. coli to grow under oxidative stress. This coupled with the stability of the YeSODs at low pH and temperature suggested their possible role in conditions such as acidic pH and oxidative stress that are encountered by the organism inside the phagolysosome and refrigerated food respectively.
Supporting Information
Phylogenetic (Neighbour Joining) tree: Constructed using YeSODs amino acid sequences (a. SodA, b. SodB and c. SodC) and other bacterial SOD sequences retrieved from NCBI database. The digit at each branch point represents percentage bootstrap support calculated from 1000 replicates. The trees were constructed by the neighbor joining method in MEGA 4.0 package.
(EPS)
Includes Table S1 and S2. Table S1. Details of Y. enterocolitica, Y. intermedia and Y. frederiksenii strains used in the study. Table S2. Distribution of sodA, sodB and sodC genes and their expression amongst strains of Yersinia spp. used in this study.
(DOCX)
Acknowledgments
The authors would like to thank Prof. Joan S. Valentine for kindly gifting the E. coli PN134 and AB1157 strains. The authors would like to thank Digvijay Verma for help in protein characterization.
Funding Statement
The authors have no support or funding to report.
References
- 1. Bottone EJ (1999) Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect 1: 323–333. [DOI] [PubMed] [Google Scholar]
- 2. Revell PA, Miller VL (2001) Yersinia virulence: more than a plasmid. FEMS Microbiol Lett 205: 159–164. [DOI] [PubMed] [Google Scholar]
- 3. Grant T, Bennett-Wood V, Robins-Browne RM (1998) Characterization of the interaction between Yersinia enterocolitica biotype 1A and phagocytes and epithelial cells in vitro . Infect Immun 67: 4367–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goverde RL, Huis in't Veld JH, Kusters JG, Mooi FR (1998) The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5 degrees C). Mol Microbiol 28: 555–569. [DOI] [PubMed] [Google Scholar]
- 5. Doherty A, Sheridan JJ, Allen P, McDowell DA, Blair IS, Harrington D (1995) “Growth of Yersinia enterocolitica O:3 on modified atmosphere packaged lamb.”. Food Microbiol 12: 251–257. [DOI] [PubMed] [Google Scholar]
- 6. Hanna MO, Stewart C, Carpenter L, Anderzant C (1977) “Effect of heating, freezing and pH on Yersinia enterocolitica-like organisms from meat,”. J Food Protect 40: 689–692. [DOI] [PubMed] [Google Scholar]
- 7. Champion OL, Cooper IA, James SL, Ford D, Karlyshev A (2009) Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis . Microbiology 155: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 8. Roggenkamp A, Bittner T, Leitritz L, Sing A, Heesemann J (1997) Contribution of the Mn-cofactored superoxide dismutase (SodA) to the virulence of Yersinia enterocolitica serotype O8. Infect Immun 65: 4705–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97–112. [DOI] [PubMed] [Google Scholar]
- 10. Storz GA, Tartaglia LA, Farr SB, Ames BN (1990) Bacterial defenses against oxidative stress. Trends Genet 6: 363–368. [DOI] [PubMed] [Google Scholar]
- 11. Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 12. Leclere V, Chotteau-Lelievre A, Gancel F, Imbert M, Blondeau R (2001) Occurrence of two superoxide dismutases in Aeromonas hydrophila: molecular cloning and differential expression of the sodA and sodB genes. Microbiology 147: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 13. Lefebre M, Valvano M (2001) In vitro resistance of Burkholderia cepacia complex isolates to reactive oxygen species in relation to catalase and superoxide dismutase production. Microbiology 147: 97–109. [DOI] [PubMed] [Google Scholar]
- 14. Santos R, Franza T, Laporte ML, Sauvage C, Touati D, Expert D (2001) Essential role of superoxide dismutase on the pathogenicity of Erwinia chrysanthemi strain 3937. Mol Plant Microbe Interact 14: 758–767. [DOI] [PubMed] [Google Scholar]
- 15. Smith SG, Wilson TJ, Dow JM, Daniels MJ (1996) A gene for superoxide dismutase from Xanthomonas campestris pv. campestris and its expression during bacterial-plant interactions. Mol Plant Microbe Interact 9: 584–593. [DOI] [PubMed] [Google Scholar]
- 16. Lynch M, Kuramitsu H (2000) Expression and role of superoxide dismutases (SOD) in pathogenic bacteria. Microbes Infect 2: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 17. Youn HD, Kim EJ, Roe JH, Hah YC, Kang SO (1996) A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem J 318: 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farr SB, Dari R, Touati D (1986) Oxygen-dependent mutagenesis in E. coli lacking superoxide dismutase. Proc Natl Acad Sci USA 83: 8268–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Loon AP, Pesold HB, Schatz G (1986) A yeast mutant lacking mitochondrial manganese superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci USA 83: 3820–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guidot DM, McCord JM, Wright RM, Repine JE (1993) Absence of electron transport (Rho-0 state) restores growth of a manganese superoxide dismutase-efficient Saccharomyces cerevisiae in hyperoxia: evidence for electron transport as a major source of superoxide generation in vivo . J Biol Chem 268: 26699–26703. [PubMed] [Google Scholar]
- 21. Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 22. Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287. [DOI] [PubMed] [Google Scholar]
- 23. Holovská K, Lenártová V, Holovská K, Javorský P (2002) Characterization of superoxide dismutase in the rumen bacterium Streptococcus bovis . Vet Med 47: 38–44. [Google Scholar]
- 24. Misra HP, Fridovich I (1978) Inhibition of superoxide dismutases by azide. Arch Biochem Biophys 189: 317–322. [DOI] [PubMed] [Google Scholar]
- 25. Heikkila RE, Cabbat FS, Cohen G (1976) In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem 251: 2182–2185. [PubMed] [Google Scholar]
- 26. Tarhan L, Tuzmen NM (2000) Some properties of Cu, Zn-Superoxide dismutase from sheep erythrocyte. Turk J Chem 24: 109–116. [Google Scholar]
- 27.Bollag DM, Edelstein SJ (1996) Protein methods, 3rd edn. Wiley, New York.
- 28. Merlino A, Krauss IR, Rossi A, Vergara A, De Vendittis A, Marco S (2012) Identification of an active dimeric intermediate populated during the unfolding process of the cambialistic superoxide dismutase from Streptococcus mutans . Biochimie 94: 768–775. [DOI] [PubMed] [Google Scholar]
- 29. Perez-Iratxeta C, Andrade-Navarro MA (2008) K2D2: Estimation of protein secondary structure from circular dichroism spectra. BMC Struc Biol 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edwards RA, Baker HM, Whittaker MM, Whittaker JW, Jameson GB (1998) Crystal structure of Escherichia coli manganese superoxide dismutase at 2.1-angstrom resolution. J Biol Inorg Chem 3: 161–171. [Google Scholar]
- 31. Lah MS, Dixon M, Pattridge KA, Stallings WC (1995) Structure-function in Escherichia coli iron superoxide dismutase: comparisons with the manganese enzyme from Thermus thermophilus . Biochemistry 34: 1646–1660. [DOI] [PubMed] [Google Scholar]
- 32. Lambert C, Leonard N, De Bolle X, Depiereux E (2002) ESyPred3D: Prediction of proteins 3D structures. Bioinformatics 18: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 33. Zhuanhua W, Rufa L, Zheng Z, Mingde Z (1993) Purification and characterization of superoxide dismutase from tertiary buckwheat leaves. Fagopyrum 13: 31–34. [Google Scholar]
- 34. Smith MW, Doolittle RF (1992) A comparison of evolutionary rates of the two major kinds of superoxide dismutase. J Mol Evol 34: 175–184. [DOI] [PubMed] [Google Scholar]
- 35. Markillie LM, Varnum SM, Hradecky P, Wong KK (1999) Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J Bacteriol 181: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benov LT, Fridovich I (1994) Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem 269: 25310–25314. [PubMed] [Google Scholar]
- 37. Kim J, Sha Z, Mayfield JE (2000) Regulation of Brucella abortus catalase. Infect Immun 68: 3861–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teixeira-Gomes AP, Cloeckaert A, Zygmunt MS (2000) Characterization of heat, oxidative and acid stress responses in Brucella melitensis. . Infect Immun 68: 2954–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinman HM (1993) Function of periplasmic copper-zinc superoxide dismutase in Caulobacter crescentus . J Bacteriol 175: 1198–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bordo D, Djinovic-Carugo K, Bolognesi M (1994) Conserved patterns in the Cu,Zn superoxide dismutase family. J Mol Biol 238: 366–368. [DOI] [PubMed] [Google Scholar]
- 41. Battistoni A (2003) Role of prokaryotic Cu,Zn superoxide dismutase in pathogenesis. Biochem Soc Trans 31: 1326–1329. [DOI] [PubMed] [Google Scholar]
- 42. Bordo D, Matak D, Djinovic-Carugo K, Rosano C, Pesce A (1999) Evolutionary constraints for dimer formation in prokaryotic Cu,Zn superoxide dismutase. J Mol Biol 285: 283–296. [DOI] [PubMed] [Google Scholar]
- 43. Bakshi CS, Malik M, Regan K, Melendez JA, Metzger DW (2006) Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol 188: 6443–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Storz G, Imlay JA (1999) Oxidative stress. Curr Opin Microbiol 2: 188–194. [DOI] [PubMed] [Google Scholar]
- 45. Wayne F, Beyer Jr, Fridovich I (1991) In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli . J Biol Chem 266: 303–308. [PubMed] [Google Scholar]
- 46. Parker MW, Blake CCF (1988) Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 Å resolution. J Mol Biol 199: 649–661. [DOI] [PubMed] [Google Scholar]
- 47. Ringe D, Petsko GA, Yamakura F, Suzuki K, Ohmori D (1983) Structure of iron superoxide dismutase from Pseudomonas ovalis at 2.9-A resolution. Proc Natl Acad Sci USA 80: 3879–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang SK, Jung YJ, Kim CH, Song CY (1998) Extracellular and cytosolic iron superoxide dismutase from Mycobacterium bovis BCG. Clin Vaccine Immunol 5: 6784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stallings WC, Pattridge KA Strong RK, Ludwig ML (1985) The structure of manganese superoxide dismutase from Thermus thermophilus HB8 at 2.4-A resolution. J Biol Chem 260: 16424–16432. [PubMed] [Google Scholar]
- 50. Battistoni A, Folcarelli S, Gabbianelli R, Capo C, Rotilio G (1996) The Cu,Zn superoxide dismutase from Escherichia coli retains monomeric structure at high protein concentration. Biochem J 320: 713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mori M, Jiménez B, Piccioli M, Battistoni A, Sette M (2008) The solution structure of the monomeric copper, zinc superoxide dismutase from Salmonella enterica: structural insights to understand the evolution toward the dimeric structure. Biochemistry 47: 12954–12963. [DOI] [PubMed] [Google Scholar]
- 52. Pedersen HL, Willassen NP, Leiros I (2009) The first structure of a cold-adapted superoxide dismutase (SOD): biochemical and structural characterization of iron SOD from Aliivibrio salmonicida . Acta Cryst 65: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Castellano I, Di Maro A, Ruocco MR, Chambery A, Parente A, Di Martino MT, Parlato G, Masullo M, De Vendittis E (2006) Psychrophilic superoxide dismutase from Pseudoalteromonas haloplanktis: biochemical characterization and identification of a highly reactive cysteine residue. Biochimie 88: 1377–1389. [DOI] [PubMed] [Google Scholar]
- 54. Wang X, Yang H, Ruan L, Liu X, Li F, Xu X (2008) Cloning and characterization of a thermostable superoxide dismutase from the thermophilic bacterium Rhodothermus sp. XMH10. J Ind Microbiol Biotechnol 35: 133–139. [DOI] [PubMed] [Google Scholar]
- 55. Lumsden J, Cammack R, Hall DO (1976) Purification and physicochemical properties of superoxide dismutase from two photosynthetic bacteria. Biochimica et Biophysica Acta 438: 380–392. [DOI] [PubMed] [Google Scholar]
- 56. Na J, Im H, Lee K (2011) Expression and purification of recombinant superoxide dismutase (PaSOD) from Psychromonas arctica in Escherichia coli . Bull Korean Chem Soc 32: 2405–2409. [Google Scholar]
- 57. Toora S, Budu-Amoako E, Ablett RF, Smith J (1994) Isolation of Yersinia enterocolitica from ready-to-eat foods and pork by a simple two step procedure. Food Microbiol 11: 369–374. [Google Scholar]
- 58. Ekanayake PM, Kang HS, de Zyosa M, Jee Y, Lee YH, Lee J (2006) Molecular cloning and characterization of Mn-superoxide dismutase from disk abalone (Haliotis discus discus) . Comp Biochem Physiol B Biochem Mol Biol 145: 318–324. [DOI] [PubMed] [Google Scholar]
- 59. Yun YS, Lee YN (2004) Purification and some properties of superoxide dismutase from Deinococcus radiophilus, the UV-resistant bacterium. Extremophiles 8: 237–242. [DOI] [PubMed] [Google Scholar]
- 60. Liu P, Ewis HE, Huang YJ, Lu CD, Tai PC, Weber IT (2007) Structure of Bacillus subtilis superoxide dismutase. Acta Crystallogr 63: 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Esposito L, Seydel A, Aiello R, Sorrentino G, Cendron L (2008) The crystal structure of the superoxide dismutase from Helicobacter pylori reveals a structured C-terminal extension. Biochim Biophys Acta 1784: 1601–1606. [DOI] [PubMed] [Google Scholar]
- 62. Bond CJ, Huang J, Hajduk R, Flick KE, Heath PJ, Stoddard BL (2000) Cloning, sequence and crystallographic structure of recombinant iron superoxide dismutase from Pseudomonas ovalis . Acta Crystallogr D Biol Crystallogr 56: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 63. Schinini ME, Maffey L, Barra D, Bossa F, Puget K, Michelson AM (1987) The primary structure of iron superoxide dismutase from Escherichia coli. . FEBS lett 221: 87–90. [DOI] [PubMed] [Google Scholar]
- 64. Hassan HM, Fridovich I (1979) Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem 254: 10846–10852. [PubMed] [Google Scholar]
- 65. Zhu D, Scandalius GJ (1995) The maize mitochondrial MnSODs encoded by multiple genes localized in the mitochondrial matrix of transformed yeast cells. Free Radic Biol Med 18: 179–183. [DOI] [PubMed] [Google Scholar]
- 66. Larsen PL (1993) Aging and resistance to oxidative damage in Caenorhabditis elegans . Proc Natl Acad Sci USA 90: 8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reveillaud I, Niedzwiecki A, Bensch KG, Fleming JE (1991) Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol 11: 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh I, Bhatnagar S, Virdi JS (2003) Isolation and characterization of Yersinia enterocolitica from diarrheic human subjects and other sources. Curr Sci 84: 1353–1355. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic (Neighbour Joining) tree: Constructed using YeSODs amino acid sequences (a. SodA, b. SodB and c. SodC) and other bacterial SOD sequences retrieved from NCBI database. The digit at each branch point represents percentage bootstrap support calculated from 1000 replicates. The trees were constructed by the neighbor joining method in MEGA 4.0 package.
(EPS)
Includes Table S1 and S2. Table S1. Details of Y. enterocolitica, Y. intermedia and Y. frederiksenii strains used in the study. Table S2. Distribution of sodA, sodB and sodC genes and their expression amongst strains of Yersinia spp. used in this study.
(DOCX)