Abstract
We investigated intraguild interactions between two egg parasitoids of Nezara viridula (L.) (Heteroptera: Pentatomidae), Ooencyrtus telenomicida (Vassiliev) (Hymenoptera: Encyrtidae) and Trissolcus basalis (Wollaston) (Hymenoptera: Platygastridae), as the former has the potential to be a facultative hyperparasitoid of the latter. We assessed the suitability of N. viridula eggs for the development of O. telenomicida as a function of egg age when they were unparasitized, or had been attacked by T. basalis at different times prior to exposure to O. telenomicida females. Ooencyrtus telenomicida can exploit healthy N. viridula host eggs up to 5 days of age, just prior to the emergence of N. viridula. This window of opportunity can be extended for an additional 6–7 days through interspecific competition or facultative hyperparasitism. While there are minor fitness costs for O. telenomicida as the result of interspecific larval competition, those costs are greater with facultative hyperparasitism. In choice assays O. telenomicida females discriminated between different quality N. viridula eggs, avoiding those where their progeny would have to develop as facultative hyperparasitoids of T. basalis. Results are discussed with respect to the possible effects that the costs of intraguild parasitism might have on biological control programmes.
Introduction
Intraguild interactions occur among organisms sharing a common resource [1] and “intraguild predation” (IGP), which is common in natural populations [2], occurs when two species that share a common host, under certain circumstances, prey upon each other [3]. Most IGP studies have focused on prey-predator interactions but recently it has been recognized that similar ecological interactions occur between host-parasitoid and host-pathogen interactions [4].
In parasitoid guilds there can be interspecific competitive interactions, either between adult parasitoids searching/exploiting hosts (extrinsic competition) or between parasitoid larvae developing within the same host (intrinsic competition) [5]–[7]. However, Rosenheim et al. [8] noted that intraguild parasitism can occur when one guild member is a facultative hyperparasitoid. Such species can act either as a primary parasitoid utilising some life stage of an herbivorous insect as a host, or as a hyperparasitoid where it uses a primary parasitoid as a host. Thus a facultative hyperparasitoid can exploit a healthy host but if it oviposits in a common host that has been already attacked by another species there are two possible outcomes: interspecific larval competition will occur if the competitor's offspring has not yet consumed all of the host resources, but if it has then hyperparasitism will occur [8], [9].
The evolution of facultative hyperparasitism is poorly understood [10] but may be key to the trophic shift from primary parasitism to obligatory hyperparasitism [11].
There are several documented cases of facultative hyperparasitism but this phenomenon is probably underestimated [10] and a real understanding of parasitoid trophic structure will only be achieved by very careful examination and dissection of host remains [12] and through the use of molecular techniques [13]. For example, Trissolcus spp. and Ooencyrtus spp. are parasitoids that exploit the eggs of the same stink bugs species and the latter group can develop as facultative hyperparasitoids of the former [14], [15]. Given that egg parasitoid guilds composed of Ooencyrtus and Trissolcus spp. have been reported in North America [16]–[19], South America [20], [21], Europe [22] and Japan [23], it is possible that both interspecific competition and facultative hyperparasitism occur and deserve to be investigated further.
This is not only important from a purely theoretical perspective, but also with respect to using parasitoids as biological control agents of important pests. There are benefits for a parasitoid that has the ability to be a facultative hyperparasitoid, such as an extended window of opportunity when it can successfully attack its host [24], [25], as well as gaining additional food resources [26]. However, there could also be associated fitness costs. It has been well documented that interspecific competition may result in longer development times, as well as smaller adults with reduced longevity and fecundity [27]: It is also possible that similar fitness costs may be associated with facultative hyperparasitism, due to the greater conversion costs when developing on entomophagous hosts [26], [28], [29].
While several studies have investigated intraguild predation [30]–[36], few have experimentally looked at intraguild parasitism [8], [37]–[40]. We, therefore, undertook a study to investigate interspecific interactions between Trissolcus basalis (Wollaston) (Hymenoptera: Platygastridae) and Ooencyrtus telenomicida (Vassiliev) (Hymenoptera: Encyrtidae), two idiobiont egg parasitoids of the Southern Green Stink Bug, Nezara viridula (L.) (Heteroptera: Pentatomidae) that co-occur in cultivated crops grown in Sicily. These parasitoid species differ in their host location and larval competitive abilities, with T. basalis being more efficient in host location [22], [41]–[43] while O. telenomicida largely dominates interspecific larval competition regardless of the order/time interval between oviposition events. Furthermore, O. telenomicida has the ability to develop as a facultative hyperparasitoid [15], [44].
We conducted experiments to determine: 1) the suitability of N. viridula eggs as a host for O. telenomicida as a function of time since they had been parasitized by T. basalis females; 2) the potential fitness costs, by comparing life history parameters of O. telenomicida when it developed in unparasitized N. viridula eggs, under interspecific competitive conditions (eggs containing a 1st instar T. basalis larva) or as a facultative hyperparasitoid (where all host resources had been totally exploited by a mature T. basalis larva); 3) the preferences of O. telenomicida females when provided unparasitized N. viridula eggs, and host eggs previously exploited by T. basalis that would result in either interspecific competition or facultative hyperparasitism.
Materials and Methods
Insect rearing
The Nezara viridula colony, augmented regularly with field collected material, was maintained at 24±1°C, 70±5% RH, 16 h∶8 h L∶D on a diet of sunflower seeds and seasonal fresh vegetables that was changed every 2–3 days. All used insects were collected in the surroundings of Palermo, Italy. No specific permits were required for collection of insects. The collection sites were not privately owned or protected in any way and field samplings did not involve endangered or protected species.
Immatures and adults were kept in separate cages. Adult cages had paper towels as an ovipositional substrate and eggs were collected daily. The O. telenomicida and T. basalis colonies were established using wasps that emerged from naturally laid N. viridula egg masses or sentinel egg masses placed in the field. Colonies of each species were maintained at 24±2°C, 80±5% RH, 16 L∶8 D in 16-ml glass tubes and fed with a solution of honey–water. To maintain the colonies, newly laid N. viridula egg masses were exposed to five parasitoid females for 48 h, and the resulting male and female parasitoids were kept together to ensure mating. In all the bioassays 4–5 day old, mated females of O. telenomicida and T. basalis were used, and in all cases, parasitoids were naive with respect to oviposition. The wasps were isolated in small vials (1.5×5 cm) with a drop of honey–water solution one day before bioassays and transferred to the assay room at 24±1°C, 60±10% RH 1 h before being tested. Tests were conducted from 8:30 to 14:00 h and females were only used once.
Bioassays
To test the window of opportunity of parasitism for O. telenomicida females a series of experiments was carried out. A female O. telenomicida was released at the center of a vertical, cylindrical Plexiglas® arena (diameter: 1.8 cm, height: 0.5 cm) with an egg mass (5 N. viridula eggs on a small piece of Parafilm®) located centrally on the floor. There were three different treatments: (I) unparasitized 1, 2, 3, 4 or 5 day old eggs; (II) 1 day old eggs parasitized by T. basalis and then exposed once to O. telenomicida 1, 2, 3, 4, 5, 6, 7 or 8 days later; and (III) 3 day old egg masses parasitized by T. basalis and then exposed once to O. telenomicida 1, 2, 3, 4, 5, 6, 7 or 8 days later. Each assay was observed and the O. telenomicida female was removed after she had parasitized all of the eggs. There were 10 replicates for each time interval of all three treatments and all egg masses held at 24±1°C, 70±5% RH, 16L∶8D so the number of O. telenomicida adults emerging from each egg mass could be recorded. The few host eggs that produced T. basalis adults or no parasitoid at all were not included in the subsequent analyses.
The possible effects of interspecific larval competition and facultative hyperparasitism on O. telenomicida were determined by comparing the number and sex ratio (% males) of emerging adults, as well as the developmental time, and size (estimated from the length of the hind tibia as done by Wajnberg et al. [45]) of both sexes when females were allowed to oviposit in host egg masses that were: (I) 1 day old and unparasitized (II) 2 or (III) 4 days old that had been parasitized by T. basalis 24 h earlier, or (IV) 10 days old that had been parasitized by T. basalis 7 days earlier. When O. telenomicida oviposited 24 h after T. basalis, the latter is at the stage of young 1st instar larva but when O. telenomicida oviposited 7 days after T. basalis, the mature 3rd instar larva of T. basalis has consumed all ooplasm and is ready to pupate. Thus treatments (II) and (III) represent natural situations of interspecific larval competition, while (IV) would be facultative hyperparasitism. Egg masses were held at 24±1°C, 70±5% RH, 16L∶8D and checked daily. Adults were frozen upon emergence (−18°C) then preserved in ethanol (70%) until the different measurements were taken.
Using the same experimental setup described above a choice bioassay was conducted to determine if O. telenomicida would exhibit an oviposition preference when simultaneously presented with different quality hosts. An O. telenomicida female was introduced in the arena containing a mass of 4 N. viridula eggs, one each of the following treatments: (I) a 1 day old unparasitized egg; (II) a 2 day old; and (III) a 4 day old egg that had been parasitized 24 h previously by T. basalis; and (IV) a 10 day old egg that had been parasitized 7 days previously by T. basalis. The oviposition preference was assessed in terms of “first oviposition”, i.e. the first host egg that has been parasitized by O. telenomicida under multiple choice conditions. There were 50 replicates and each was terminated after the O. telenomicida female had oviposited once.
Statistical analysis
Data were tested for normality (Kolmogorov-Smirnov test) and if significantly different from a normal distribution were analyzed with non parametric tests. The effect of host age or time interval between oviposition by the two parasitoid species on the number of O. telenomicida adults that emerged, as well as the effect of different host quality on developmental time and hind tibia length were compared using a one-way ANOVA followed by Tukey test. The effect of host types on sex ratio was compared with the Kruskal-Wallis ANOVA and the Dunn test for multiple comparisons. The ability of O. telenomicida females to discriminate among hosts of different quality was tested with a χ2 test with Bonferroni correction. All statistical analyses were processed using STATISTICA7 software [46].
Results
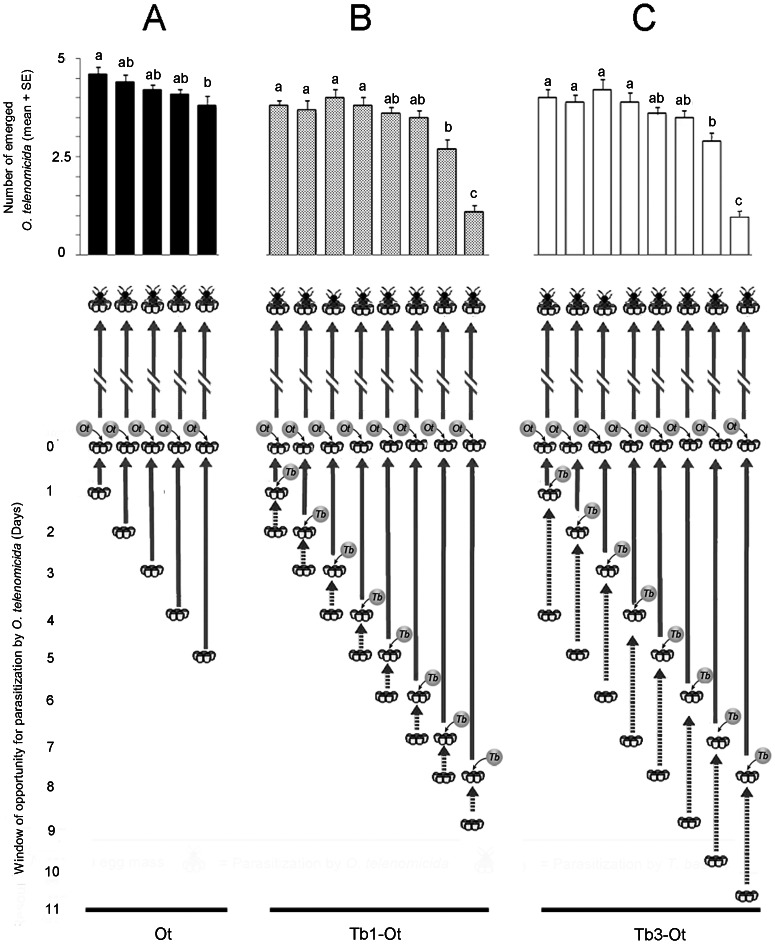
There was a significant effect of host age on the number of adult O. telenomicida emerging from unparasitized N. viridula eggs (Fig. 1A; F = 3.21, df = 4, 45, P<0.05;), being significantly lower from 5 than from 1 day old hosts. Similar temporal effects were observed when O. telenomicida oviposited in N. viridula eggs that had been attacked by T. basalis when the eggs were 1 day old (Fig. 1B; F = 20.26, df = 7, 72, P<0.001) or 3 days old (Fig. 1C; F = 23.41, df = 7, 72, P<0.001. In both cases there was a decrease in the number of O. telenomicida emerging from the oldest hosts.
Figure 1. Window of opportunity for Ooencyrtus telenomicida as function of host egg age and interspecific parasitism status.
The emergence of Ooencyrtus telenomicida from (A) unparasitized 1 to 5 day old Nezara viridula eggs (Ot); (B) 1day old N. viridula eggs parasitized by Trissolcus basalis that were then parasitized by O. telenomicida 1 to 8 days later (Tb1-Ot); and (C) 3 day old N. viridula eggs parasitized by T. basalis that were then parasitized by O. telenomicida 1 to 8 days later (Tb3-Ot).
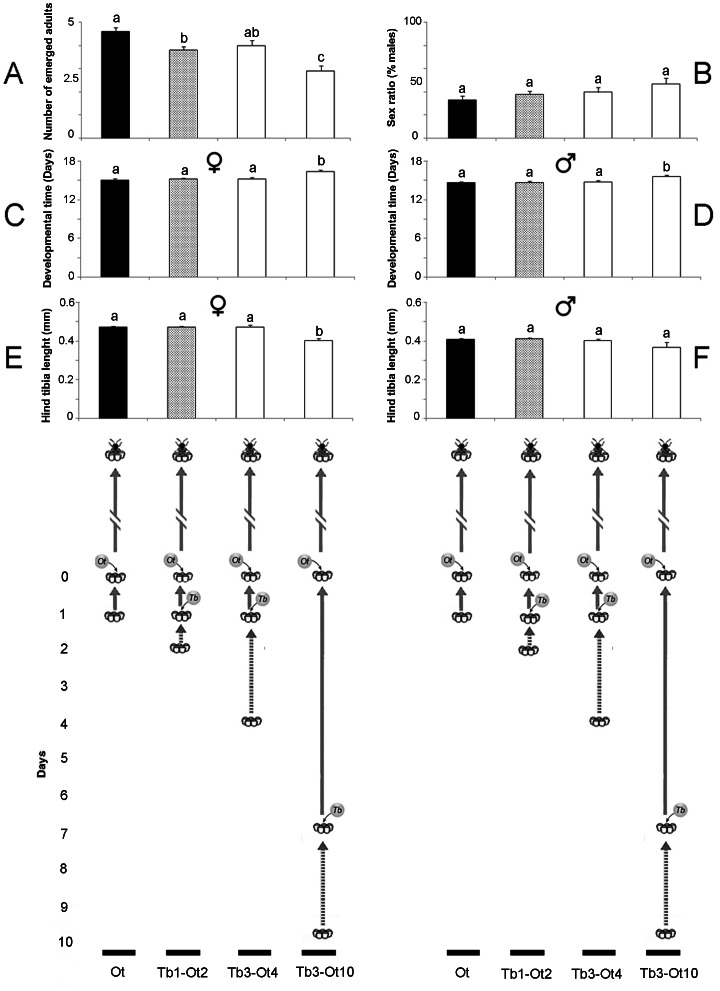
The average number of O. telenomicida adults produced was affected by the type of host exploited, (Fig. 2A; F = 13.84, df = 3, 36, P<0.001), generally being higher in previously unparasitized eggs than when in larval competition with, or as a facultative hyperparasitoid of T. basalis, although the proportion of males produced was similar in all treatments [Fig. 2B; H(3, N = 37) = 4.31, P = 0.229]. When O. telenomicida was a facultative hyperparasitoid of T. basalis the developmental time of both females (Fig. 2C; F = 20.67, df = 3, 36, P<0.001) and males (Fig. 2D; F = 5.51, df = 3, 33, P<0.001) was longer. Being a facultative hyperparasitoid also resulted in smaller females (Fig. 2E; F = 23.69, df = 3, 36, P<0.01), although male size was not affected (Fig. 2F; F = 2.53, df = 3, 33, P = 0.074).
Figure 2. Life history parameters of Ooencyrtus telenomicida when developing in different host types.
The number emerging (A), sex ratio (B), developmental time and size of female (C, E) and male (D, F) Ooencyrtus telenomicida adults developing in (I) 1 day old, unparasitized Nezara viridula eggs (Ot), (II) 2 day old N. viridula eggs that had been parasitized by Trissolcus basalis when they were 1 day old (Tb1 - Ot2), (III) 4 day old N. viridula eggs that had been parasitized by T. basalis when they were 3 days old (Tb3 - Ot4), or (IV) 10 day old N. viridula eggs that had been parasitized by T. basalis 7 days earlier (Tb3 - Ot10).
Ooencyrtus telenomicida females clearly discriminated between the different host egg types, avoiding host eggs that contained well developed T. basalis larvae where they would have to develop as a facultative hyperparasitoid (Table 1; χ 2 = 17.68, df = 3 P<0.001). Interestingly, there was a marginal preference for eggs that had been attacked by T. basalis when they were 1 day old over unparasitized eggs (χ 2 = 3.46, df = 1, P = 0.06), or those attacked by T. basalis when they were 3 days old (χ 2 = 2.78. df = 1, P = 0.09).
Table 1. The proportion of Ooencyrtus telenomicida females selecting a (I) 1 day old, unparasitized Nezara viridula eggs (Ot), (II) 2 day old N. viridula eggs that had been parasitized by Trissolcus basalis when they were 1 day old (Tb1 - Ot2), (III) 4 day old N. viridula eggs that had been parasitized by T. basalis when they were 3 days old (Tb3 - Ot4), or (IV) 10 day old N. viridula eggs that had been parasitized by T. basalis 7 days earlier (Tb3 - Ot10) as their first oviposition site in a choice bioassay.
| O. telenomicida ovipositing in N. viridula egg mass assembled using 4 different egg types | ||||
| Egg types | Ot | Tb1-Ot2 | Tb3-Ot4 | Tb3-Ot10 |
| Egg age | 1 | 2 | 4 | 10 |
| Egg age when parasitized by Tb | - | 1 | 3 | 3 |
| Choice (%± SE) | 24.0±6.0 a | 46.0±7.1 a | 26.0±6.2 a | 4.0±2.8 b |
Discussion
In Sicily, more T. basalis adults emerge from parasitized field-collected N. viridula eggs than O. telenomicida, (Cusumano personal observations), which is not particularly surprising given the superior abilities of the former to locate suitable hosts [22]. Females of both T. basalis and O. telenomicida exploit volatile cues emitted by N. viridula virgin males and pre-ovipositing females [22], [41]. In addition, T. basalis females use contact kairomones in host footprints and volatile oviposition-induced synomones [22], [41], [42], [47]–[49], so foraging females not only utilize more cues than O. telenomicida, but also ones that are more reliable indicators of the presence of host eggs. Furthermore, T. basalis females also have a higher total lifetime fecundity than O. telenomicida so the chances that O. telenomicida females find unparasitized egg masses may be quite low under field conditions.
However, as seen from the results of this study, O. telenomicida has evolved several strategies that increase the window of opportunity to exploit host eggs. For example, N. viridula eggs hatch after 5 days under our laboratory conditions and while T. basalis can only successfully develop on unparasitized N. viridula eggs that are <4 days old [50], O. telenomicida successfully exploits unparasitized N. viridula eggs up to the time of host emergence (Fig. 1A), similar to the congeneric, O. nezarae Ishii, an egg parasitoid of the bean bug Riptortus clavatus Thunberg (Heteroptera: Alydidae) [51]. Furthermore, O. telenomicida is clearly superior under the conditions of interspecific larval competition, whether the eggs that have been attacked by T. basalis were 1 or 3 days old (Fig. 1), as in all of our experiments, <15% of all parasitoid adults were T. basalis. In addition, when acting as facultative hyperparasitoid (Fig. 1c), O. telenomicida can effectively exploit eggs for at least 10 days after they are laid by N. viridula females.
There are fitness costs for O. telenomicida, associated with both interspecific competition and facultative hyperparasitism (Fig. 2). In the case of competition the only significant effect observed was a lower number of O. telenomicida adults emerging when there was early-stage interspecific larval competition (Fig. 2A). Interestingly, in the choice bioassays, O. telenomicida showed a marginally significant preference for 2 day old eggs recently parasitized by T. basalis, over unparasitized ones and 4 day old eggs that T. basalis had attacked 1 day earlier, even though fewer adults emerged (Table 1, Fig. 2A). At oviposition, T. basalis injects substances that arrest embryonic development of the host and when the parasitoid's egg hatches teratocytes are released that alter the ooplasm [52]. To what extent these two events associated with the development of T. basalis affects the suitability of the eggs for O. telenomicida, when interspecific competition occurs, remains to be clarified.
In the case of facultative hyperparasitism the development time of both sexes was longer and females were significantly smaller (Fig. 2). This could be important as adult body size has been correlated with survival and reproductive success in many parasitoid species [11], [53], [54] although, as seen in the choice bioassays, O. telenomicida females will avoid hosts that result in facultative hyperparasitism if a choice is available (Table 1). If certain conditions resulted in high levels of facultative hyperparasitism this could impact on subsequent population dynamics at all trophic levels, and affect the efficacy of biological control programmes. As pointed out by Boivin and Brodeur [29], assessing the impact of a species that act simultaneously as primary parasitoid, interspecific competitor and facultative hyperparasitoid is a huge challenge, both theoretically and experimentally. However, the few studies examining the potential fitness costs of facultative hyperparasitsm have come up with quite varied findings, some showing there are fitness costs [26], [55], while others have found few or no effect [56], [57]. Therefore, it is clear that in order to understand the potential tradeoffs between the benefits accrued by a species that has the potential to be a facultative hyperparasitoid and the potential negative effects on all parasitoid species in the guild, both from basic and applied perspectives, considerably more information must be gathered from systems where interguild parasitism exists.
Acknowledgments
The authors would like to thank Ian C.W. Hardy (School of Biosciences, University of Nottingham) for his critical review of an early version of this manuscript.
Funding Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was partially supported by the project of the Italian Ministry of Education, University and Research (MIUR) 2007. The authors now declare that no additional external funding was received for this study. “Enhancing foraging behavior of insect egg parasitoids: the role of the volatile organic compounds and the epicuticular layers of the plants.”
References
- 1. Hawkins CP, MacMahon JA (1989) Guilds: the multiple meanings of a concept. Ann Rev Entomol 34: 423–451. [Google Scholar]
- 2. Arim M, Marquet PA (2004) Intraguild predation: a widespread interaction related to species biology. Ecol Lett 7: 557–564. [Google Scholar]
- 3. Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20: 297–330. [Google Scholar]
- 4. Borer ET, Briggs CJ, Holt RD (2007) Predators, parasitoids, and pathogens: a cross-cutting examination of intraguild predation theory. Ecology 88: 2681–2688. [DOI] [PubMed] [Google Scholar]
- 5.Zwolfer H (1971) The structure and effect of parasite complexes attacking phytophagous host insects. In: den Boer PJ, Gradwell GR, editors. Dynamics of populations: proceedings of the advanced study institute on dynamics and numbers in populations. Wageningen: Centre for Agricultural Publishing and Documentation. pp. 405–418.
- 6. De Moraes CM, Cortesero AM, Stapel JO, Lewis WJ (1999) Intrinsic and extrinsic competitive interactions between two larval parasitoids of Heliothis virescens . Ecol Entomol 24: 402–410. [Google Scholar]
- 7. Harvey JA, Poelman E, Tanaka T (2013) Annu Rev Entomol 58: 333–351. [DOI] [PubMed] [Google Scholar]
- 8. Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jafee BA (1995) Intraguild predation among biological control agents: theory and evidence. Biol Control 5: 303–335. [Google Scholar]
- 9. Cusumano A, Peri E, Vinson SB, Colazza S (2012a) Interspecific extrinsic and intrinsic competitive interactions in egg parasitoids. BioControl 57: 719–734. [Google Scholar]
- 10.Brodeur J (2000) Host specificity and trophic relationships of hyperparasitoids. In: Hochberg ME, Ives AR, editors. Parasitoid Population Biology. Princeton University Press. pp. 163–183.
- 11.Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton: Princeton University Press. 488 p.
- 12.Shaw M (1994) Parasitoid host ranges. In: Hawkins BA, Sheehan W, editors. Parasitoid Community Ecology. Oxford University Press. pp. 111–14.
- 13. Gariepy TD, Messing RH (2012) Development and use of molecular diagnostic tools to determine trophic links and interspecific interactions in aphid-parasitoid communities in Hawaii. Biological Control 60: 26–38. [Google Scholar]
- 14. Amarasekare P (2000) Spatial dynamics in a host–multiparasitoid community. J Anim Ecol 69: 201–213. [Google Scholar]
- 15. Cusumano A, Peri E, Vinson SB, Colazza S (2011) Intraguild interactions between two egg parasitoids exploring host patches. BioControl 56: 173–184. [Google Scholar]
- 16. Buschman LL, Whitcomb WH (1980) Parasites of Nezara viridula (Hemiptera: Pentatomidae) and other Hemiptera in Florida. Fla Entomol 63: 154–162. [Google Scholar]
- 17. Hoffmann MP, Davidson NA, Wilson LT, Ehler LE, Jones WA, et al. (1991) Imported wasp helps control southern green stink bug. Calif Agric 45: 20–22. [Google Scholar]
- 18. Shepard BM, Elsey KD, Muckenfuss AE, Justo HD Jr (1994) Parasitism and predation on egg masses of the southern green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae), in tomato, okra, cowpea, soybean, and wild radish. J Agric Entomol 11: 375–381. [Google Scholar]
- 19. Ehler LE (2002) An evaluation of some natural enemies of Nezara viridula in northern California. BioControl 47: 309–325. [Google Scholar]
- 20. Correa-Ferreira SB (1986) Natural occurrence of the egg parasitoid complex of stink bugs on soybean in Parana, Brazil. An Soc Entomol Bras 5: 189–199. [Google Scholar]
- 21. Correa-Ferreira SB, Moscardi F (1995) Seasonal occurrence and host spectrum of egg parasitoids associated with soybean stink bugs. Biol Control 5: 196–202. [Google Scholar]
- 22. Peri E, Cusumano A, Agro A, Colazza S (2011) Behavioral response of the egg parasitoid Ooencyrtus telenomicida to host related chemical cues in a tritrophic perspective. BioControl 56: 163–171. [Google Scholar]
- 23. Hokyo N (1965) Interspecific relations among egg parasites of Nezara viridula L. with special reference to Asolcus mitsukurii Ashmead and Telenomus nakagawai Watanabe (in Japanese). Nankiseibutu 7: 1–6. [Google Scholar]
- 24. Craig TP, Itami JK, Price PW (1990) The window of vulnerability for a shoot-galling sawfly to attack by a parasitoid. Ecology 71: 1471–1482. [Google Scholar]
- 25. Hayward A, Stone GN (2005) Oak gall wasp communities: Evolution and ecology. Basic Appl Ecol 6: 435–443. [Google Scholar]
- 26. Grandgirard J, Poinsot D, Krespi L, Nenon JP, Cortesero AM (2002) Costs of the secondary parasitism in the facultative hyperparasitoids Pachycrepoideus dubius: does host size matter? Entomol Exp Appl 103: 239–248. [Google Scholar]
- 27. Harvey JA, Gols R, Strand MR (2009a) Intrinsic competition and its effects on the survival and development of three species of endoparasitoid wasps. Entomol Exp Appl 130: 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Alphen JJM, Thunnissen I (1983) Host selection and sex allocation by Pachycrepoideus vindemiae Rondani (Pteromalidae) as a facultative hyperparasitoid of Asobara tabida Nees (Braconidae; Alysiinae) and Leptopilina heterotoma (Cynipoidae; Eucoilidae). Neth J Zool 33: 497–514. [Google Scholar]
- 29.Boivin G, Brodeur J (2006) Intra- and interspecific interactions among parasitoids: mechanisms, outcomes and biological control. In: Brodeur J, Boivin G, editors. Trophic and guild interactions in biological control. Springer. pp. 123–144.
- 30. Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96: 439–449. [DOI] [PubMed] [Google Scholar]
- 31. Lucas E, Coderre D, Brodeur J (1998) Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology 79: 1084–1092. [Google Scholar]
- 32. Nakashima Y, Birkett MA, Pye BJ, Powell W (2006) Chemically mediated intraguild predator avoidance by aphid parasitoids: interspecific variability in sensitivity to semiochemical trails of ladybird predators. J Chem Ecol 32: 1989–1998. [DOI] [PubMed] [Google Scholar]
- 33. Raak-van den Berg CL, De Lange HJ, Van Lenteren JC (2012) Intraguild Predation Behaviour of Ladybirds in Semi-Field Experiments Explains Invasion Success of Harmonia axyridis . Plos ONE 7 ((7)) e40681 doi:10.1371/journal.pone.0040681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgio G, Santi F, Maini S (2002) On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biol Control 24: 110–116. [Google Scholar]
- 35. Finke DL, Denno RF (2002) Intraguild predation diminished in complex-structured vegetation: implications for prey suppression. Ecology 83: 643–652. [Google Scholar]
- 36. Denno RF, Mitter MS, Langellotto GA, Gratton C, Finke DL (2004) Interactions between a hunting spider and a web-builder: consequences of intraguild predation and cannibalism for prey suppression. Ecol Entomol 29: 566–577. [Google Scholar]
- 37.Rosenheim JA, Harmon JP (2006) The influence of intraguild predation on the suppression of a shared prey population: an empirical reassessment. In: Brodeur J, Boivin G, editors. Trophic and guild interactions in biological control. Springer. pp. 1–20.
- 38. Harvey JA, Pashalidou F, Soler R, Bezemer TM (2011) Intrinsic competition between two secondary hyperparasitoids results in temporal trophic switch. Oikos 120: 226–233. [Google Scholar]
- 39. Wang XG, Messing RH (2004) a Potential interactions between pupal and egg-or larval-pupal parasitoids of tephritid fruit flies. Environ Entomol 33: 1313–1320. [Google Scholar]
- 40. Wang XG, Messing RH (2004b) The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: host expansion and potential non-target impact. Biol Control 31: 227–236. [Google Scholar]
- 41. Colazza S, Salerno G, Wajnberg E (1999) Volatile and contact chemicals released by Nezara viridula (Heteroptera: Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol Control 16: 310–317. [Google Scholar]
- 42. Colazza S, Fucarino A, Peri E, Salerno G, Conti E, et al. (2004) Insect oviposition induces volatiles emission in herbaceous plant that attracts egg parasitoids. J Exp Biol 207: 47–53. [DOI] [PubMed] [Google Scholar]
- 43. Conti E, Colazza S (2012) Chemical Ecology of Egg Parasitoids Associated with True Bugs. Psyche doi:10.1155/2012/651015. [Google Scholar]
- 44. Cusumano A, Peri E, Vinson SB, Colazza S (2012b) The ovipositing female of Ooencyrtus telenomicida relies on physiological mechanisms to mediate intrinsic competition with Trissolcus basalis . Entomol Exp Appl 143: 155–163. [Google Scholar]
- 45. Wajnberg E, Curty C, Colazza S (2004) Genetic variation in the mechanisms of direct mutual interference in a parasitic wasp: consequences in terms of patch-time allocation. J Anim Ecol 73: 1179–1189. [Google Scholar]
- 46.StatSoft (2001) Statistica per Windows, User's Manual. StatSoft Italia, Vigonza, Padova, Italy.
- 47. Colazza S, Lo Bue M, Lo Giudice D, Peri E (2009) The response of Trissolcus basalis to footprint contact kairomones from Nezara viridula females is mediated by leaf epicuticular waxes. Naturwissenschaften 96: 975–981. [DOI] [PubMed] [Google Scholar]
- 48. Peri E, Sole MA, Wajnberg E, Colazza S (2006) Effect of host kairomones and oviposition experience on the arrestment behavior of an egg parasitoid. J Exp Biol 209: 3629–3635. [DOI] [PubMed] [Google Scholar]
- 49. Lo Giudice D, Riedel M, Rostas M, Peri E, Colazza S (2011) Host sex discrimination by an egg parasitoid on brassica leaves. J Chem Ecol 37: 622–628. [DOI] [PubMed] [Google Scholar]
- 50. Bin F, Vinson SB, Strand MR, Colazza S, Jones WA (1993) Source of an egg kairomone for Trissolcus basalis, a parasitoid of Nezara viridula . Physiol Entomol 18: 7–15. [Google Scholar]
- 51. Takasu K, Hirose Y (1993) Host acceptance behavior by the host-feeding egg parasitoid, Ooencyrtus nezarae (Hymenoptera: Encyrtidae): host age effects. Ann Entomol Soc Am 86: 117–121. [Google Scholar]
- 52. Volkoff N, Colazza S (1992) Growth patterns of teratocytes in the immature stages of Trissolcus basalis (Woll.) (Hymenoptera: Scelionidae), an egg parasitoid of Nezara viridula (L.) (Heteroptera: Pentatomidae). Int J Insect Morphol Embryol 21: 323–336. [Google Scholar]
- 53. Nicol CMY, Mackauer M (1999) The scaling of body size and mass in a parasitoid association: influence of host species and stage. Entomol Exp Appl 90: 83–92. [Google Scholar]
- 54. Ueno T (1999) Host-size-dependent sex ratio in a parasitoid wasp. Res Popul Ecol 41: 47–57. [Google Scholar]
- 55. Kfir R, Rosen D (1981) Biology of the hyperparasite Pachyneuron concolor (Förster) (Hymenoptera: Pteromalidae) reared on Microterys flavus (Howard) in brown soft scale. J Entomol Soc South Afric 44: 151–163. [Google Scholar]
- 56. Pérez-Lachaud G, Bachelor TP, Hardy ICW (2004) Wasp eat wasp: facultative hyperparasitism and intra-guild predation by bethylid wasps. Biol Control 30: 149–155. [Google Scholar]
- 57. Harvey JA, Wagenaar R, Bezemer TM (2009b) Interactions to the fifth trophic level: secondary and tertiary parasitoid wasps show extraordinary efficiency in utilizing host resources. J Anim Ecol 78: 686–692. [DOI] [PubMed] [Google Scholar]