Abstract
Background
Limited data are available on the characteristics, clinical management, and outcomes of patients with atrial fibrillation at risk of stroke, from a worldwide perspective. The aim of this study was to describe the baseline characteristics and initial therapeutic management of patients with non-valvular atrial fibrillation across the spectrum of sites at which these patients are treated.
Methods and Findings
The Global Anticoagulant Registry in the FIELD (GARFIELD) is an observational study of patients newly diagnosed with non-valvular atrial fibrillation. Enrollment into Cohort 1 (of 5) took place between December 2009 and October 2011 at 540 sites in 19 countries in Europe, Asia-Pacific, Central/South America, and Canada. Investigator sites are representative of the distribution of atrial fibrillation care settings in each country. Cohort 1 comprised 10,614 adults (≥18 years) diagnosed with non-valvular atrial fibrillation within the previous 6 weeks, with ≥1 investigator-defined stroke risk factor (not limited to those in existing risk-stratification schemes), and regardless of therapy. Data collected at baseline included demographics, medical history, care setting, nature of atrial fibrillation, and treatments initiated at diagnosis. The mean (SD) age of the population was 70.2 (11.2) years; 43.2% were women. Mean±SD CHADS2 score was 1.9±1.2, and 57.2% had a score ≥2. Mean CHA2DS2-VASc score was 3.2±1.6, and 8,957 (84.4%) had a score ≥2. Overall, 38.0% of patients with a CHADS2 score ≥2 did not receive anticoagulant therapy, whereas 42.5% of those at low risk (score 0) received anticoagulant therapy.
Conclusions
These contemporary observational worldwide data on non-valvular atrial fibrillation, collected at the end of the vitamin K antagonist-only era, indicate that these drugs are frequently not being used according to stroke risk scores and guidelines, with overuse in patients at low risk and underuse in those at high risk of stroke.
Trial Registration
ClinicalTrials.gov TRI08888
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disorder, with approximately one-quarter of individuals over 40 years of age developing this arrhythmia [1]. The risk of stroke – including ischemic stroke, hemorrhagic stroke and cerebral bleeds – increases fivefold among patients with AF [2]. AF is also associated with a twofold excess risk of cardiovascular death and stroke within 1 year of observation [3].
Vitamin K antagonists (VKAs) have served as the cornerstone of stroke prevention in AF for several decades. Comprehensive evidence-based management guidelines [4], [5], [6], [7] advocating the use of risk scores to identify patients most (or least) at risk of thrombotic or bleeding events are widely available. VKAs have a number of drawbacks, however, including a narrow therapeutic window, multiple food and drug interactions [8], and substantial inter-patient variability due to genetic or other factors, making their long-term use in clinical practice a challenge [9]. Physicians remain reluctant to prescribe anticoagulant prophylaxis in a large proportion of the population at risk for stroke, in part due to the limitations of VKAs, misperception of thrombotic risk [10], and concern about bleeding complications, especially among the elderly [11].
International observational studies have provided insights into the characteristics, risk profiles, management, and clinical outcomes of patients with various cardiovascular diseases [XPATH ERROR: unknown variable "start2".], [13]. Less is known about individuals newly diagnosed with AF and perceived to be at risk of stroke by their physicians, and few data are available that reflect the broad range of healthcare settings for AF from a worldwide perspective.
The Global Anticoagulant Registry in the FIELD (GARFIELD) was initiated to describe everyday antithrombotic treatment patterns in patients newly diagnosed with non-valvular AF and one or more additional investigator-defined stroke risk factor across the spectrum of care settings at which these patients are treated, and to understand the burden of thromboembolic and bleeding complications in this population. This article presents the baseline characteristics and initial management of the first of five cohorts of over 10,000 patients enrolled in the GARFIELD Registry.
Methods
Ethics Statement
Independent ethics committee and hospital-based institutional review board approvals were obtained, as necessary, for the registry protocol. (See Ethics List S1) The registry is being conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and the International Conference on Harmonisation–Good Pharmacoepidemiological and Clinical Practice guidelines. All patients provided written informed consent to participate.
Trial Design and Participants
The GARFIELD Registry is an ongoing, observational, multicenter, worldwide study of adults (≥18 years) with non-valvular AF diagnosed according to standard local procedures within the past 6 weeks (electrocardiogram confirmation was not mandated) and ≥1 additional factor judged by the clinician to increase the patient’s risk of stroke; such factors were not prespecified in the protocol, nor were they limited to the factors in risk-stratification schemes such as CHADS2 (cardiac failure, hypertension, age, diabetes, stroke [doubled]) [14] or CHA2DS2-VASc (cardiac failure, hypertension, age ≥75 [doubled], diabetes, stroke [doubled]-vascular disease, age 65–74 and sex category [female]) [15].
Enrollment will take place in five independent, sequential cohorts [16]. Patient enrollment into Cohort 1 took place between 21 December 2009 and 26 October 2011. In parallel with prospectively enrolled patients, a validation group (part retrospective and part prospective) was enrolled, comprising patients with established AF (i.e., AF first diagnosed ≥6 months and ≤24 months before enrollment) and ≥1 additional risk factor for stroke, regardless of therapy; in these patients, data were collected retrospectively to the time of first AF diagnosis, and prospectively up to 2 years after diagnosis. The rationale for the inclusion of the retrospective cohort was to evaluate, by comparing retrospective with prospective data, whether initiation of the GARFIELD Registry influenced AF management patterns on a site level; if the effect was zero or minimal, the data from both cohorts would to be combined.
Patients for whom follow-up up to 2 years was unlikely and those with a transient reversible cause of AF were excluded. Data were collected using an electronic case report form (eCRF) [16].
Investigator sites are representative of the distribution of AF treating care settings in each country. Sufficient sites were identified from the spectrum of care settings (office-based practice, hospital departments [neurology, cardiology, geriatrics, internal medicine, emergency], anticoagulant clinics, and general or family practice) to ensure proportional representation in all countries, and the lists and ratios were validated by national coordinators [16]. Sites were selected randomly and recruited following a qualification call. Before site initiation, investigators were required to complete a training program that provided guidance on patient screening, enrollment, and follow-up. Patients were enrolled consecutively, as stipulated in the protocol.
Procedures
Data collected at baseline included patient and clinical characteristics at diagnosis, medical history (including cardiovascular and bleeding history), care setting at diagnosis, type of AF, date and method of diagnosis, symptoms, antithrombotic treatment at diagnosis (VKAs, factor Xa inhibitors, thrombin inhibitors, and heparins), and reasons for not providing VKAs (when applicable). Ethnicity was classified by the investigator, in agreement with the patient, to investigate ethnic differences in the prevalence of AF [17].
Heart failure, hypertension (blood pressure >140/90 mmHg or treated hypertension), age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack were used to calculate, retrospectively, stroke risk according to the CHADS2 risk index [14]. Additionally, left ventricular ejection fraction <40%, prior thromboembolism, vascular disease (acute coronary syndrome, peripheral artery disease), age 65–74 years, and female gender were used to determine stroke risk using the CHA2DS2-VASc score [15].
Registry data were captured in electronic CRFs (designed by Dendrite Clinical Systems Ltd, Henley-on-Thames, UK, who are also responsible for ongoing database programme management). Data collection and entry are managed by Quintiles (Durham, NC, USA), who oversee all operational aspects of the programme, apart from in the UK where these aspects are undertaken by The University of Birmingham Department of Primary Care Clinical Sciences. Submitted data are examined by the coordinating center (Thrombosis Research Institute, London) to ascertain their completeness and accuracy, and data queries are sent to participating sites. Data are extracted for each analysis and analyzed by an independent statistician (PW). Confidentiality and anonymity of all patients enrolled into this registry was maintained at all times.
Statistical Analysis
Continuous variables are expressed as mean±standard deviation (SD). Categorical variables are expressed as frequencies and percentages. Differences between cohorts were tested for statistical significance using the Chi-squared test for categorical variables and the unpaired t-test for continuous variables. Statistical analysis was performed using SAS® software version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
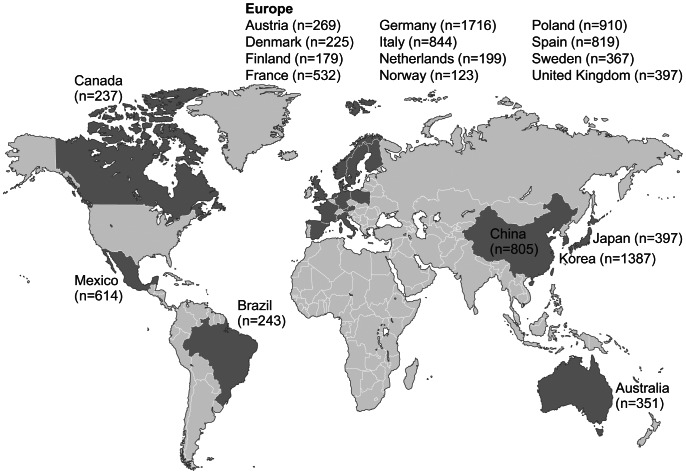
A total of 10,614 patients were enrolled into Cohort 1 at 540 sites in 19 countries in Asia-Pacific (n = 2,940, 27.7%; Australia, China, Korea, Japan), Canada (n = 237, 2.2%), Europe (n = 6,580, 62.0%; Austria, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Spain, Sweden, UK), and Central/South America (n = 857, 8.1%; Brazil, Mexico) (Figure 1). More than half of the patients (n = 6,262, 59.0%) were enrolled by cardiologists, 20.8% (n = 2,208) by internal medicine specialists, 17.7% (n = 1,880) by primary care/general practice physicians, 2.1% (n = 218) by neurologists, and 0.4% by geriatricians (n = 40) (data for six patients unknown). Each site recruited 20 consecutive patients on average. Baseline data were locked in 99.9% of the patients.
Figure 1. Number of patients enrolled per country in Cohort 1 (n = 10,614).
Overall, 29.7% of patients had new or unclassified AF, 27.5% had paroxysmal AF, 17.9% had persistent AF, and 24.9% had permanent AF. White patients represented the largest percentage of the population (62.2%), followed by Asians (7.6% Chinese and 17.0% other Asian ethnicities); the remaining patients were White–Hispanic/Latino (8.2%), mixed/other (1.2%), Afro-Caribbean (0.3%), or of unknown race (3.5%).
Of the overall cohort of 10,614 patients, 5,089 (47.9%) were enrolled retrospectively and 5,525 (52.1%) prospectively (see Figure S1 for year of diagnosis). No statistical or clinical concerns were apparent regarding any of the differences in baseline characteristics of the retrospectively and prospectively enrolled patients that would preclude combining the data from these two groups (Table S1, Figure S2), hence combined results are reported.
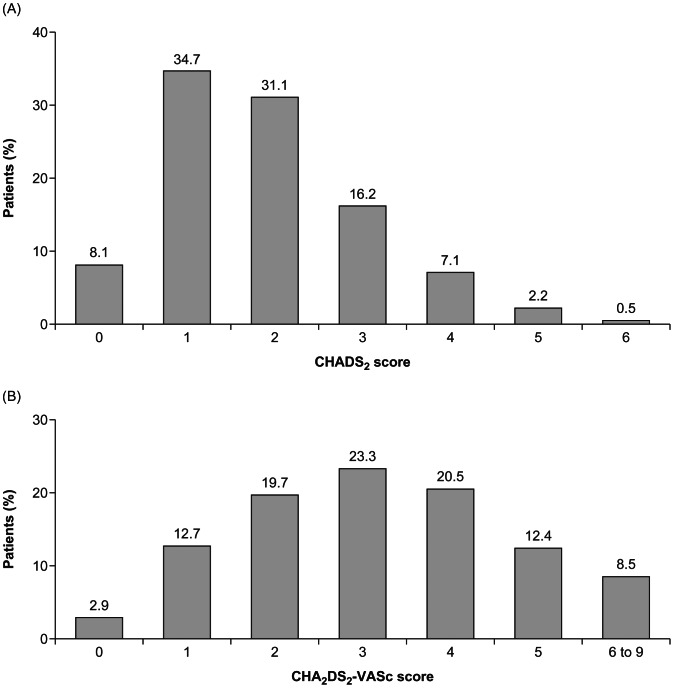
Baseline characteristics are given in Table 1. The mean±SD age was 70.2±11.2 years and 69.5% of patients were >65 years; 43.2% were women. More than three-quarters (77.8%) of the population had hypertension, 22.0% had diabetes mellitus, 21.0% had congestive heart failure, and 9.7% of patients had a history of stroke. Over one-third (35.2%) of patients were current or previous smokers. The mean±SD CHADS2 score was 1.9±1.2, and 57.2% (6,062/10,607) of patients had a score ≥2, indicating a moderate to high risk of stroke and guideline qualification for oral anticoagulant treatment. Mean±SD CHA2DS2-VASc score was 3.2±1.6, and 84.4% (8,957/10,607) had a score ≥2. The distributions of risk scores for stroke are shown in Figure 2.
Table 1. Patient baseline characteristics: Cohort 1 of the GARFIELD Registry.
| Variable | All patients (n = 10,614) |
| Age, mean (SD), years | 70.2±11.2 |
| Age group, n (%) | |
| >65 years | 7,374 (69.5) |
| ≥75 years | 4,091 (38.5) |
| 65–74 years | 3,540 (33.4) |
| Women, n (%) | 4,580 (43.2) |
| Body mass index,a mean (SD), kg/m2 | 27.5±5.3 |
| Smoking status (current/previous)b, n (%) | 3,504 (35.2) |
| Pulse,c mean (SD), bpm | 86.6±25.1 |
| Medical history, n (%) | |
| Acute coronary syndromes (myocardial infarction or unstable angina) | 1,060 (10.0) |
| Congestive heart failured | 2,229 (21.0) |
| Coronary artery diseased | 2,035 (19.2) |
| Hypercholesterolemiad | 4,159 (39.2) |
| Hypertensiond | 8,249 (77.8) |
| Family history of cardiac diseasee,f | 1,940 (18.3) |
| Diabetes mellitusf | 2,330 (22.0) |
| Stroked | 1,026 (9.7) |
| Stroke or transient ischaemic attackd | 1,528 (14.4) |
| Left ventricular ejection fraction <40%g | 586 (9.5) |
| Chronic renal diseaseh | |
| Mild renal dysfunction (GFR 60–89 mL/min) | 1,502 (19.6) |
| Moderate renal dysfunction (GFR 30–59 mL/min) | 871 (11.4) |
| Severe renal dysfunction or renal failure (GFR <30 mL/min) | 154 (2.0) |
| Peripheral artery diseased | 743 (7.0) |
| Carotid occlusive diseased | 368 (3.5) |
| Other thromboembolismd,i | 150 (1.4) |
| Systemic embolismd | 80 (0.8) |
| Pulmonary embolism or deep vein thrombosisd | 304 (2.9) |
| Bleedingd | 368 (3.5) |
| Heavy alcohol consumptionj | 215 (2.2) |
| Cirrhosisd | 55 (0.5) |
Abbreviation: GFR, glomerular filtration rate.
Data not available for 1,611 patients.
Data not available for 671 patients.
Data not available for 1,372 patients.
Data not available for 6 patients.
First-degree relative with premature cardiac history (age <55 years [male], <65 years [female]).
Data not available for 7 patients.
Data not available for 4,448 patients.
Data not available for 2,954 patients.
For example, central venous thrombosis, retinal occlusion.
Investigator defined; data not available for 1,048 patients.
Figure 2. Distribution of CHADS2 and CHA2DS2-VASc scores (n = 10,607) (scores not available for 7 patients).
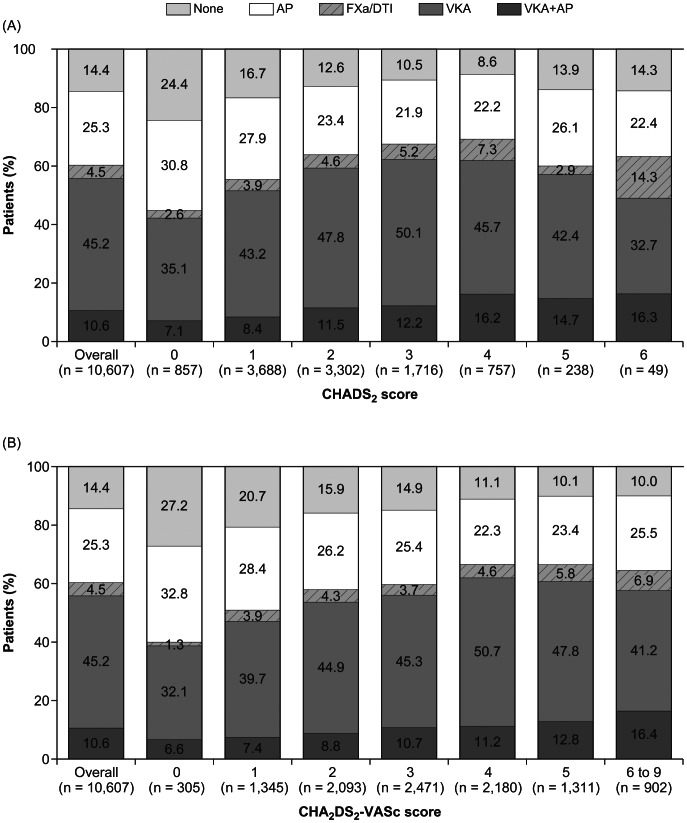
At diagnosis, 55.8% of patients overall were given a VKA for stroke prevention: 45.2% (n = 4,797) received a VKA alone and 10.6% (n = 1,128) received both a VKA and an antiplatelet drug (Figure 3). A minority of patients (4.5%, n = 475) received a novel oral factor Xa inhibitor or direct thrombin inhibitor. Just over one-quarter (25.3%, n = 2,681) of the patients received an antiplatelet drug alone and 14.4% (n = 1,533) received none of these antithrombotic drugs. Use of antithrombotic drugs at AF diagnosis and contraindications to anticoagulant therapy are detailed in Table 2. The most frequently given antiplatelet was aspirin. Use of all antithrombotic drugs was higher in patients with a CHADS2 score of 2–6 versus those with a score of 0 or 1. Contraindications to anticoagulant therapy were reported in 827 (7.8%) of patients.
Figure 3. Use of antithrombotic therapies, overall and according to (A) CHADS2 score and (B) CHA2DS2VASc score (n = 10,607).
AP indicates antiplatelet; FXa/DTI, activated coagulation factor X inhibitor/direct thrombin inhibitor (irrespective of AP use); VKA, vitamin K antagonist.
Table 2. Use of antithrombotic drugs at diagnosis and contraindications to anticoagulant therapy, overall and according to CHADS2 scores of 0 or 1 and 2–6.
| Drug | All patients (n = 10,614) | Patients with CHADS2score of 0 or 1 (n = 4,367) | Patients with CHADS2score of 2–6 (n = 6,240) |
| Antiplateleta,b, n (%) | |||
| Aspirin | 2,713 (26.5) | 1166 (28.4) | 1547 (25.2) |
| Thienopyridine | 713 (7.0) | 229 (5.6) | 484 (7.9) |
| Glycoprotein IIb/IIIa inhibitor | 16 (0.2) | 6 (0.1) | 10 (0.2) |
| Prostaglandin analogue | 18 (0.2) | 6 (0.1) | 12 (0.2) |
| Other antiplatelet | 102 (1.0) | 30 (0.7) | 72 (1.2) |
| Anticoagulant drugsa,c, n (%) | |||
| Vitamin K antagonist | 6,080 (58.2) | 2218 (52.8) | 3861 (61.9) |
| Heparin (unfractionated or low-molecular-weight) | 410 (3.9) | 151 (3.6) | 259 (4.2) |
| Factor Xa inhibitor (oral or injectable) | 312 (3.0) | 102 (2.4) | 210 (3.4) |
| Direct thrombin inhibitor (e.g., argatroban, dabigatran,bivalirudin, desirudin) | 128 (1.2) | 43 (1.0) | 85 (1.4) |
| Heparinoid (e.g., danaparoid, sulodexide, dermatan sulfate) | 89 (0.9) | 35 (0.8) | 54 (0.9) |
| Other anticoagulant (e.g., defibrotide, ramatroban,antithrombin III, protein C) | 31 (0.3) | 9 (0.2) | 22 (0.4) |
| Contraindication to anticoagulant therapy, n (%)a | |||
| Excessive bleeding risk | 289 (2.7) | 88 (2.0) | 201 (3.2) |
| Frequent falls or mechanical risk | 238 (2.2) | 38 (0.9) | 200 (3.2) |
| Risk of drug interaction | 39 (0.4) | 13 (0.3) | 26 (0.4) |
| Allergy | 11 (0.1) | 2 (<0.1) | 9 (0.1) |
| Other contraindication | 249 (2.3) | 76 (1.7) | 174 (2.8) |
Categories are not mutually exclusive.
Data not available for 360 patients.
Data not available for 171 patients.
The use of anticoagulant therapy by CHADS2 risk score is shown in Figure 3A. VKA use (alone or with an antiplatelet) increased as risk level increased, up to a maximum of 63% at a CHADS2 score of 3 and 4, then decreased thereafter. Use of novel factor Xa inhibitors and direct thrombin inhibitors was low across all risk categories. Overall, 38.0% (2,302/6,062) of patients with a CHADS2 score ≥2 did not receive anticoagulant therapy; conversely, 42.5% (364/857) of those at low risk (score of 0) received anticoagulant therapy. Similar patterns were observed when risk was assessed according to the CHA2DS2-VASc score: 40.7% (3,645/8,957) of the patients with a score ≥2 did not receive anticoagulant therapy, and 38.7% (118/305) of patients with a score of 0 received anticoagulant therapy (Figure 3B). For both risk scores, use of antiplatelet therapy showed an initial decline with rising risk level.
The reasons for not providing VKA therapy to patients at moderate to high risk of stroke are given in Table 3. Almost half of the reasons for not providing VKA related to physician choice.
Table 3. Main reasons why vitamin K antagonists were not given in patients with a CHADS2 score ≥2.
| Reason, n (%) | Patients with CHADS2≥2 (n = 2,302) |
| Alcohol misuse | 11 (0.5) |
| Already taking antiplatelet drug for another medical condition | 117 (5.1) |
| Patient refusal | 165 (7.2) |
| Previous bleeding event | 55 (2.4) |
| Taking medication contraindicated or cautioned for use with vitamin K antagonists | 16 (0.7) |
| Other | 239 (10.4) |
| Unknown | 587 (25.5) |
| Physician’s choice | 1,112 (48.3) |
| Bleeding risk | 170 (7.4) |
| Concern over patient compliance | 121 (5.3) |
| Guideline recommendation | 32 (1.4) |
| Fall risk | 150 (6.5) |
| Low risk of stroke | 95 (4.1) |
| Other | 544 (23.6) |
Discussion
This large, ongoing, international observational study of patients newly diagnosed with non-valvular AF and one or more additional risk factors for stroke provides a unique perspective of AF management at the end of the VKA-only era, transitioning into the period in which novel oral anticoagulants present an alternative treatment for stroke prevention. The data illustrate the high rate of comorbid conditions in this population, including hypertension, hypercholesterolemia, diabetes mellitus, heart failure, and coronary artery disease. A sizable proportion of the population had a history of stroke or transient ischemic attack. Nearly 6/10 patients presented with a CHADS2 score ≥2 and more than 8/10 with a CHA2DS2VASc score ≥2. These figures correlate with an annual adjusted stroke rate ranging from 3.4% for a CHADS2 score of 2 to 18.2% for a score of 6 [14], and from 2.2% for a CHA2DS2-VASc score of 2 to 15.2% for a score of 9 [15]. Despite this high level of thromboembolic risk, overall use of anticoagulant therapy was relatively low. A total of 40.7% of the patients with a CHA2DS2-VASc score ≥2 did not receive guideline-recommended anticoagulant prophylaxis [5], [6], [7], [18]. Conversely, 38.7% of the patients with a CHA2DS2-VASc score of 0 received anticoagulant therapy; these individuals are regarded as “truly” low-risk subjects, with a stroke/thromboembolism event rate of 0.84 (95% confidence interval [CI], 0.65–1.08) per 100 person-years in patients with AF, compared with 3.49 (95% CI, 3.31–3.68) per 100 person-years for AF patients with a CHADS2 score of 0 or 1 [19]. Our present study, in which risk stratification was done retrospectively through a review of data collected in the eCRFs, indicates that the identification in “real-world” practice of patients perceived to be at risk of stroke is often not based on evidence-based risk schemes and guidelines [6]. There appears to be overuse of anticoagulant therapy in patients at low risk of stroke or systemic embolism, and underuse in those at moderate to high risk. Physicians’ clinical judgment of stroke risk therefore appears to incorporate factors beyond those included in CHADS2 and CHA2DS2-VASc.
Comparison of GARFIELD with Other Registries in Non-valvular AF
One of the largest multinational studies in this field is the Euro Heart Survey on Atrial Fibrillation, conducted between 2003 and 2004 in more than 5,000 ambulatory and hospitalized patients [20]. The Euro Heart Survey provided a snapshot of the management of AF across 35 European Society of Cardiology member countries and revealed discordance between guidelines and everyday clinical practice. Oral anticoagulation was prescribed in 67% of patients considered eligible for VKA treatment according to guidelines [4], but also in 49% of ineligible patients. The Euro Heart Survey did indicate an increase in use of oral anticoagulant therapy with increasing stroke risk [21], in contrast to the results of a large nationwide retrospective US medical claims database study involving over 171,000 patients with AF (51,907 of whom had newly diagnosed AF), which indicated a low use of warfarin across all risk categories (overall rate 42.6%; 49.5% in patients newly diagnosed with AF) [22]. Both studies were consistent, however, in reporting underuse of oral anticoagulation in patients at elevated risk and overuse in those at low risk. Our present data show no apparent improvement in adherence to evidence-based guidelines [5], [6], [18] for AF in recent years.
Despite mandating the presence of one or more additional stroke risk factors in an effort to exclude patients with lone AF, the GARFIELD population fares as relatively low risk compared with other observational cohorts, and is substantially lower risk than the populations included in randomized trials of novel oral anticoagulant drugs (Table 4) [23], [24], [25], [26]. “Additional risk factors” were investigator defined in order to identify patients that physicians themselves perceived – during the course of their usual practice – to be at risk of stroke. This approach contrasts with other studies that mandated the presence of one or more specific risk factors, such as prior stroke or transient ischemic attack, hypertension, and heart failure, resulting in much higher-risk populations than typically seen in everyday clinical practice. Further, other data sets have been based on patients identified in emergency departments or hospitalized for another condition, who may be at high risk of a poor outcome [27]. In the nationwide US Outcomes Registry for Better Treatment of Atrial Fibrillation (ORBIT-AF) [28], for example, the mean±SD CHADS2 score was 2.3±1.3 [28], the mean age was 76 years, and approximately 30% of patients had diabetes and 30% heart failure. In contrast, in GARFIELD, the mean CHADS2 score was 1.9±1.2, mean age was 70 years, and only 22% patients had diabetes and 21% had heart failure. The differences in these two study populations may be due the fact that GARFIELD enrolled patients newly diagnosed with AF whereas ORBIT-AF enrolled patients with prevalent or incident AF.
Table 4. Baseline characteristics: Randomized clinical trials versus the GARFIELD Registry.
| GARFIELD(Cohort 1) | RELY-AF [31] (dabigatran) | ROCKET AF [32] (rivaroxaban) | AVERROES [26] (apixaban) | ARISTOTLE (apixaban) [33] | ||||||
| (n = 10,614) | D 110(n = 6015) | D 150(n = 6076) | Warf(n = 6022) | Rivarox(n = 7131) | Warfarin(n = 7133) | Apixaban(n = 2808) | Aspirin(n = 2791) | Apixaban(n = 9120) | Warfarin(n = 9081) | |
| Age in years | 70±11 | 71±9 | 72±9 | 72±9 | 73 (65,78) | 73 (65,78) | 70±9 | 70±10 | 70 (63,76) | 70 (63,76) |
| Women | 43 | 36 | 37 | 37 | 40 | 40 | 41 | 42 | 36 | 35 |
| BMI (kg/m2) | 28±5 | – | – | – | 28 (25,32) | 28 (25,32) | 28±5 | 28±5 | – | – |
| Age ≥75 years | 39 | – | – | – | – | – | – | – | 31 | 31 |
| Prior stroke/TIA | 14 | 20 | 20 | 20 | – | – | 14 | 13 | – | – |
| Diabetes | 22 | 23 | 23 | 23 | 40 | 40 | 19 | 20 | 25 | 25 |
| Prior myocardial infarction | 10* | 17 | 17 | 16 | 17 | 18 | – | – | 15 | 14 |
| Hypertension | 78 | 79 | 79 | 79 | 90 | 91 | 86 | 87 | 87 | 88 |
| Heart failure | 21 | 32 | 32 | 32 | 63 | 62 | 40 | 38 | 36† | 35† |
| Classification of AF | ||||||||||
| Paroxysmal | 28 | 32 | 33 | 34 | 18 | 18 | 27 | 27 | 15 | 16 |
| Persistent | 18 | 32 | 31 | 32 | 81 | 81 | 21 | 21 | 85 | 84 |
| Permanent | 25 | 35 | 36 | 34 | – | – | 52 | 52 | ||
| Newly diagnosed or new onset | 30 | – | – | – | 1.4 | 1.4 | – | – | – | – |
| CHADS2 score | 1.9±1.2 | 2.1±1.1 | 2.2±1.2 | 2.1±1.1 | 3.5±0.9 | 3.5±1.0 | 2.0±1.1 | 2.1±1.1 | 2.1±1.1 | 2.1±1.1 |
Data given as %, mean±SD or median (IQR).
History of acute coronary syndrome.
Heart failure or reduced left ventricular ejection fraction.
AF, atrial fibrillation; BMI, body mass index; CHADS2, Cardiac failure, Hypertension, Age, Diabetes, Stroke (Doubled), COPD, chronic obstructive pulmonary disease; D, dabigatran; SD, standard deviation; TIA, transient ischaemic attack.
RealiseAF was a large, international, contemporary, cross-sectional study conducted in 10,523 outpatients in 26 countries between 2009 and 2010 [29], with the aim of investigating the success of rhythm versus rate control, and the impact of control on patients’ clinical symptoms and quality of life. The patients in this registry were slightly younger than those in GARFIELD (67 vs. 70 years), and they had higher rates of heart failure (46% vs. 21%), coronary artery disease (32% vs. 19%) and hypercholesterolemia (46% vs. 39%); these differences in baseline characteristics likely reflect differences in the treatment settings. In RealiseAF, for example, all of the patients were enrolled by cardiologists or internists (hospital- and office-based), whereas in GARFIELD the aim was to reflect the spectrum of care settings in each country; consequently 80% of the patients were enrolled by cardiologists or internists, 18% were enrolled by primary care/general practice physicians, and 2.1% were enrolled by neurologists. Furthermore, 62% of the RealiseAF population was diagnosed with AF >12 months previously, whereas patients in GARFIELD were newly diagnosed, a finding supported by the higher rate of permanent AF in RealiseAF (46% vs. 25%).
Several other large registries in AF have been launched recently, including the international Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF), and the nationwide US Practice INNovation And Clinical Excellence (PINNACLE-AF) registry – part of the American College of Cardiology National Cardiovascular Data Registry. Physician participation in PINNACLE-AF is voluntary, and the study will report on outcomes in 121,000 patients with AF [30]. Like GARFIELD, PINNACLE-AF will rigorously assess current and evolving practice patterns; it will also help US providers evaluate and improve adherence to guidelines and performance measures through the provision of checklists of guideline-recommended care and provision of quarterly reports. These national US data will be complementary to GARFIELD, which will be conducted in up to 35 countries throughout the world.
Future Insights from the GARFIELD Registry
The second GARFIELD cohort was initiated in October 2011, and an additional 11 countries (Argentina, Belgium, Chile, Czech Republic, Hungary, India, Russia, Singapore, South Africa, Thailand, Ukraine) joined the registry. Owing to its unique methodology, the GARFIELD Registry will continue to provide prospective and rigorous global and national data on “real-world” risk stratification, AF management, and clinical outcomes in patients newly diagnosed with AF and at risk of stroke.
Study Limitations
As an observational study, GARFIELD is subject to certain limitations inherent in all such studies, such as the collection of non-randomized data and missing or incomplete information. Given the wide spectrum of care settings, the presence of missing or incomplete data is not surprising, particularly as site selection was random, encompassed the spectrum of care settings, and included sites with no clinical research experience. The study does, however, provide broader insights into the management of AF patients, with the inclusion of care environments not normally included in such studies. The data collected will provide a “snapshot” of anticoagulant use at the point of evaluation, which may change over time. GARFIELD is currently the largest ongoing international academic registry in patients with non-valvular AF; it represents a novel approach to outcomes research through the recruitment of unselected patients in five consecutive cohorts. In addition, through random selection of nationally representative sites, consecutive patient enrolment, and inclusion of patients perceived by their physicians to be at risk of stroke, the population will reflect those treated in everyday clinical practice. The contemporary data reported here provide a benchmark against which subsequent data, incorporating new therapies for AF, can be compared.
Conclusions
These data highlight a substantial gap between evidence-based risk stratification, management recommendations, and their application in everyday clinical practice. The long-term implications of these management decisions will become apparent in the follow-up data at 1 and 2 years. With the introduction of new anticoagulants for AF, GARFIELD will describe how management strategies, patient outcomes, and use of healthcare resources evolve over time on a global level and in participating countries.
Supporting Information
Ethics committee information.
(XLS)
Year of diagnosis of AF in retrospective (part prospective) and prospective patients.
(TIF)
Distribution of (A) CHADS2 and (B) CHA2DS2-VASc Scores in the GARFIELD Registry.
(TIF)
Patient baseline characteristics: retrospective (part prospective) validation group and prospective group.
(DOC)
GARFIELD Registry Investigators.
(DOC)
Acknowledgments
We thank the physicians, nurses, and patients involved in the GARFIELD Registry.
Funding Statement
The GARFIELD Registry is sponsored by the Thrombosis Research Institute, London, UK. Funding of the registry is provided through an educational research grant from Bayer Pharma AG, Berlin, Germany. The funding source had no involvement in the data collection, data analysis, or data interpretation. The authors had full access to all the data, and the corresponding author had final responsibility for the decision to submit for publication.
References
- 1. Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, et al. (2004) Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J 25: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, et al. (2007) Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J 28: 2803–2817. [DOI] [PubMed] [Google Scholar]
- 3.Goto S, Bhatt DL, Rother J, Alberts M, Hill MD, et al.. (2008) Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J 156: 855–863, 863 e852. [DOI] [PubMed]
- 4. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur Heart J 27: 1979–2030. [DOI] [PubMed] [Google Scholar]
- 5. Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M, et al. (2011) Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. The Canadian journal of cardiology 27: 74–90. [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, et al.. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation * Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. [DOI] [PubMed]
- 8. Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, et al. (2005) Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 165: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 9. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, et al. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 160S–198S. [DOI] [PubMed] [Google Scholar]
- 10. Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, et al. (2008) Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J 29: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 11. Tulner LR, Van Campen JP, Kuper IM, Gijsen GJ, Koks CH, et al. (2010) Reasons for undertreatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging 27: 39–50. [DOI] [PubMed] [Google Scholar]
- 12. Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA, et al. (2010) The Global Registry of Acute Coronary Events, 1999 to 2009 - GRACE. Heart 96: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 13. Antikainen RL, Moltchanov VA, Chukwuma C Sr, Kuulasmaa KA, Marques-Vidal PM, et al. (2006) Trends in the prevalence, awareness, treatment and control of hypertension: the WHO MONICA Project. Eur J Cardiovasc Prev Rehabil 13: 13–29. [DOI] [PubMed] [Google Scholar]
- 14. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, et al. (2001) Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 15. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 16.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, et al.. (2012) International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 163: 13–19 e11. [DOI] [PubMed]
- 17. Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, et al. (2008) Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc 100: 237–245. [DOI] [PubMed] [Google Scholar]
- 18. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2011) 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 123: e269–367. [DOI] [PubMed] [Google Scholar]
- 19.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY (2012) The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost 107. [DOI] [PubMed]
- 20. Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, et al. (2005) Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 26: 2422–2434. [DOI] [PubMed] [Google Scholar]
- 21. Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, et al. (2006) Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 27: 3018–3026. [DOI] [PubMed] [Google Scholar]
- 22. Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, et al. (2010) Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med 123: 446–453. [DOI] [PubMed] [Google Scholar]
- 23. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 24. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 25. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 26. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, et al. (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364: 806–817. [DOI] [PubMed] [Google Scholar]
- 27.Healey J, Oldgren J, Parekh A, Zhu J, Pais P, et al. Global variations in the 1-year rates of death and stroke in 15,432 patients presenting to the emergency department with atrial fibrillation in 47 countries: The RE-LY AF Registry; 2012; Munich, Germany.
- 28. Piccini JP, Holmes DN, Ollis DM, Fraulo ES, Thomas L, et al. (2011) Patterns of Atrial Fibrillation and Treatment Strategies Vary According to Provider Specialty Across Community Practice Settings: Findings From the ORBIT-AF Registry. Circulation 124: A16415. [Google Scholar]
- 29. Steg PG, Alam S, Chiang CE, Gamra H, Goethals M, et al. (2012) Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart 98: 195–201. [DOI] [PubMed] [Google Scholar]
- 30.American College of Cardiology (2012) Registry data shows early patterns for new atrial fibrillation treatments [press release].
- 31. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 32. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 33. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ethics committee information.
(XLS)
Year of diagnosis of AF in retrospective (part prospective) and prospective patients.
(TIF)
Distribution of (A) CHADS2 and (B) CHA2DS2-VASc Scores in the GARFIELD Registry.
(TIF)
Patient baseline characteristics: retrospective (part prospective) validation group and prospective group.
(DOC)
GARFIELD Registry Investigators.
(DOC)