Abstract
The postrhinal cortex (POR) of the rat is homologous to the parahippocampal cortex of the primate based on connections and other criteria. Postrhinal cortex provides the major visual and visuospatial input to the hippocampal formation, both directly to CA1 and indirectly through connections with the medial entorhinal cortex. Although the cortical and hippocampal connections of the postrhinal cortex are well described, the physiology of postrhinal neurons has not been studied. Here we examined the electrical and morphological characteristics of layer 5 neurons from postrhinal cortex of 14-16 day-old rats using an in vitro slice preparation. Neurons were subjectively classified as regular-spiking (RS), fast-spiking (FS) or low threshold-spiking (LTS) based on their electrophysiological properties and similarities with neurons in other regions of neocortex. Cells stained with biocytin included pyramidal cells and interneurons with bitufted or multipolar dendritic patterns. Similarity analysis using only physiological data yielded three clusters that corresponded to FS, LTS, and RS classes. The cluster corresponding to the FS class was composed entirely of multipolar nonpyramidal cells, and the cluster corresponding to the RS class was composed entirely of pyramidal cells. The third cluster, corresponding to the LTS class, was heterogeneous and included both multipolar and bitufted dendritic arbors as well as one pyramidal cell. We did not observe any intrinsically bursting pyramidal cells, which is similar to entorhinal cortex but unlike perirhinal cortex. We conclude that POR includes at least two major classes of neocortical inhibitory interneurons, but has a functionally restricted cohort of pyramidal cells.
Keywords: parahippocampal, temporal cortex, perirhinal, entorhinal, hippocampal
The hippocampus was the primary focus of research on episodic memory for decades, but more recent research indicates that the surrounding cortical regions, including the postrhinal (POR), perirhinal (PER), and entorhinal (ENT) cortices, also contribute to memory. Connectional studies indicate that these parahippocampal structures serve as an interface for the bidirectional flow of information between neocortex and the hippocampus (Burwell and Amaral, 1998b; Dolorfo and Amaral, 1998; Naber et al., 1999; Pinto et al., 2006; Witter et al., 2000). Input from neocortical association regions arrives in the PER and POR and is transmitted to the ENT, which in turn projects directly to the dentate gyrus and field CA3 of the hippocampus. The PER provides multimodal information preferentially to the lateral ENT and the POR provides visual and visuospatial information preferentially to the medial ENT (Burwell and Amaral, 1998a; Burwell and Amaral, 1998b). Both PER and POR also project directly to field CA1 of the hippocampus. Functional studies of these structures indicate that they are not merely conduits for sensory input. Rather, neocortical information is integrated and further processed at every level, such that information likely becomes increasingly more complex and abstract as it progresses from unimodal and polymodal associational regions, to the PER and POR, to the ENT, and through the hippocampal trisynaptic circuit (reviewed in Lavenex and Amaral, 2000). In order to understand how neocortical input is processed at each level, it is necessary to understand the electrophysiological properties of the cells in each component of the hierarchy.
There are published studies of the electrophysiological properties of neurons in many regions of neocortex, including PER and ENT, but almost nothing is known about the neuronal physiology of POR. Here we used an in vitro slice preparation and whole-cell recording techniques to study layer 5 neurons in POR of young rats. Layer 5 was targeted because it is the main output layer to the hippocampus and other parahippocampal areas, and because prior studies of layer 5 cells in the PER and ENT are available for comparison (e.g. Hamam et al., 2002; Hamam et al., 2000; Moyer et al., 2002). A neuron's electrophysiological characteristics are the complex product of its membrane properties and dendritic morphology (Llinas, 1988), and previous studies have demonstrated that morphology can be correlated with electrophysiology (e.g.Beierlein et al., 2003; Chagnac-Amitai et al., 1990; Fanselow et al., 2008; Kasper et al., 1994a; Kawaguchi, 1993; Kawaguchi and Kubota, 1997; Markram et al., 2004; McCormick et al., 1985). Thus, we labeled a subset of recorded cells with biocytin in order to characterize the morphology of POR cells with known electrophysiological properties.
This is the first study of the electrical and morphological characteristics of neurons in the POR, and one of the few to examine both the morphology and electrophysiology of inhibitory interneurons in the parahippocampal region.
Methods and Materials
Subjects
Subjects were Sprague-Dawley rats with ages ranging from postnatal days 14 to 16. All procedures involving animals were carried out according to protocols approved by Brown University's Institutional Animal Care and Use Committee and conforming to the NIH guidelines.
Electrophysiology
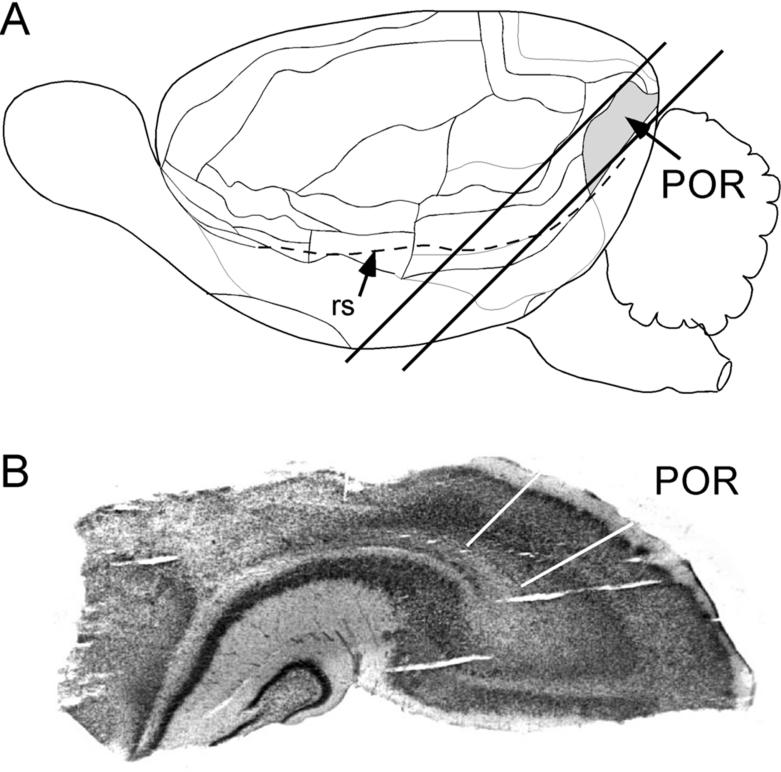
Animals were decapitated and the brains taken immediately for preparation of slices. Because the POR wraps obliquely about the caudal pole of the rodent brain, it was necessary to devise a unique slice angle to gain the best access to POR layer 5 (Figure 1). After the brain was bisected, each hemisphere was laid on a diagram with its lateral side facing upwards. This diagram was used as a guide to cut along a 45° line through the parahippocampal areas. The caudal part of the hemisphere was then glued onto a horizontal block and sectioned parallel to the newly cut surface. Slices were taken along this axis such that each slice captured the rostrocaudal extent of the POR. Beginning at the oblique dorsal surface of the POR, the tissue was sectioned at 400 μm with a vibratome (Model VSL, World Precision Instruments Inc.) while immersed in ice-cold artificial cerebral spinal fluid (ACSF). Slices were immediately transferred to 32°C ACSF for 30 min. Slices were then transferred to the recording chamber and maintained at room temperature (20-22°C) in order to enhance the stability of the tissue for long experiments. ACSF was saturated with 95% O2/ 5% CO2 and was composed of (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 dextrose, and 2 CaCl2.
Figure 1.
Postrhinal (POR) slice preparation. A. Schematic of the lateral surface view of a rat brain showing the location of POR (grey), the rhinal sulcus (rs), and the angle of the slice. B. Nissl-stained section from a re-sectioned POR slice. POR borders are indicated by white lines.
Patch micropipettes were made from 1.5 mm borosilicate glass (Sutter Instrument Co.) and filled with a solution of (in mM): 135 K-gluconate, 4 KCl, 2 NaCl, 10 HEPES, 0.2-4 EGTA, 4 ATP-Mg, 0.3 GTP-Tris, and 0.5-10 phosphocreatine-Tris; pH adjusted to 7.25 with 1 M KOH and osmolarity to 295 mOsm. Whole-cell recordings were obtained under IR-DIC optics using a Nikon E600-FN microscope. Current-clamp recordings were obtained using Axoprobe 1A amplifiers (Axon instruments). Data were collected and analyzed using programs written in LabVIEW 5.0.
Cells were not selected randomly. Rather cells were selected by the morphology of the soma as seen under IR-DIC in order to accumulate a sample of neurons with diverse electrical properties that included both principal cells and interneurons. That said, we tended to avoid the extremes such that most cells were likely taken at midrostrocaudal levels. Each cell was injected with at least one set of current steps with +/-50 or 100 pA increments beginning at -400 pA, and ending after the cell reached its maximum degree of spike frequency adaptation. A subset of cells was filled with 0.2-0.4% biocytin for 30 min, or for as long as the recording could be held. When biocytin was used, only one cell was recorded from each slice. After a cell was recorded and filled with biocytin, the slice was placed in 4% paraformaldehyde / 0.1 M phosphate buffer (PB) at a pH of 7.2-7.3 overnight. Slices were then transferred to 30% sucrose / 4% paraformaldehyde / 0.1 M PB for cryoprotection and storage until processed for visualization of the recorded cell.
Visualization of Filled Cells
The 400 μm slices were resectioned at 80 μm with a freezing microtome (American Optical Corp.). These sections were washed four times with 0.1 M PB, incubated in 0.3% H2O2 / 0.1 M PB for 30 min, and then washed three times with 0.1 M PB. Sections were then incubated in 0.25% Triton X / 2% bovine serum albumin (BSA) / 0.1 M PB for 45 min, followed by a five min wash in 2% BSA / 0.1 M PB before transfer to a dilute solution of avidin and a biotinylated enzyme, prepared from an ABC Elite Kit (Vector Laboratories Inc.). Sections were incubated in this solution overnight at 4°C on an orbital shaker, and were then washed four times in 0.1 M PB. Sections were transferred to a 0.05% diaminobenzidine (DAB) / 0.1 M PB solution for 35 min on an orbital shaker with as little exposure to light as possible. After DAB preincubation, the sections were moved to a 0.02% H2O2 / 0.05% DAB / 0.1 M PB solution for 5 min on an orbital shaker. This reaction was stopped by washing the sections three times in 0.1 M PB. The sections were transferred to a 0.01 M PB solution and mounted onto gelatin-coated slides. After drying, the mounted tissue was dehydrated in successive ethanol (ETOH) solutions (70%, 95%, and 100% for three min each), transferred to xylene (2 × 5 min), and cover-slipped with Krystalon. For each stained cell, the dendrites, axonal processes, and somata of representative neurons in each physiological class were drawn using a camera lucida apparatus at a magnification of 40X.
Analysis
The spike half-width was defined as the duration of an action potential at the amplitude halfway between the threshold and peak of the first spike evoked by a depolarizing current step. The frequency adaptation index was calculated as the ratio of the 10th interspike interval (if available) to the 1st interspike interval. Occasionally, the 7th through the 9th were used if the spike train was shorter. For consistency in obtaining this value, the repetitive spiking traces that were selected for analysis had an initial spiking frequency between 20 and 45 Hz. The afterhyperpolarization (AHP) following a spike train was measured as the voltage difference from resting membrane potential (Vrest) to the nadir of the trough after a train of spikes evoked by a positive current stimulus. Input resistance was calculated with Ohm's law, using the voltage measured at the end of a –50 pA current step. Spike amplitude was recorded as the voltage difference between Vrest and the peak of the first evoked spike during a positive current step, and threshold was also taken from this first spike. Membrane time constant was estimated from the best fit of a single exponential to the initial hyperpolarization during a –50 pA step. Data are means +/- standard deviations unless otherwise noted.
Recorded cells were classified upon examination of suprathreshold voltage traces as regular-spiking (RS), fast-spiking (FS), or low threshold-spiking (LTS), using previously published criteria (Beierlein et al., 2000; Beierlein et al., 2003; Gibson et al., 1999; McCormick et al., 1985). Biocytin-stained cells were morphologically classified as bitufted, multipolar, or pyramidal cells based on the shape of the soma and the pattern of the proximal dendritic processes.
Finally, we addressed the issue of whether statistical classification based on electrophysiological characteristics corresponded with morphology. We used classification data analysis techniques (Gordon, 1999) to examine the relationship between electrophysiological parameters and morphology in the subset of recorded cells that were filled with biocytin and stained. Cluster analysis was used to determine how recorded cells would be grouped based on electrophysiological parameters. The cluster algorithm was Ward's method, a hierarchical agglomerative method that employs a coefficient of Euclidian distance. The appropriate cluster solution was chosen by plotting the number of clusters against the semi-partial R-squared (SPRSQ) and then selecting the number of clusters at which the curve flattened markedly. The cluster analysis was evaluated by using an external validation technique; the cluster solution was statistically compared with morphological classification of cells by using Rand's (1971) statistic ® such that a value of 0 is obtained when two classifications exhibit no similarity and a value of 1 is obtained when two classifications are identical (Hubert and Arabie, 1986). Canonical discriminant analysis was employed to determine the magnitude and direction of effects of electrophysiological variables on cluster groupings (Gordon, 1999). This analysis permitted a graphical presentation to illustrate how the electrophysiological data contributed to cluster separation and how clusters corresponded to morphological classification.
Class differences in electrophysiological properties were analyzed using analysis of variance (ANOVA). Differences in frequency distributions were assessed using the Kolmogorov-Smirnov statistic. All analyses were conducted in SAS (SAS Institutes, Inc., Cary, NC) and interpreted with significance level of p<0.05.
Results
Because the ratio of principal cells to interneurons in cortical regions is high, and in order to have sufficient numbers of interneurons for statistical comparisons, we intentionally targeted a population of cells for whole cell recordings that, based on soma morphology, were more likely to be interneurons. This procedure yielded a sample of recordings from 111 cells in layer 5 of POR that was diverse in electrophysiological and morphological characteristics. Based on firing patterns in response to injected current steps, cells were classified as RS (47%), FS (36%), or LTS (17%). ANOVA revealed a significant effect of Class on all electrophysiological variables except input resistance and spike threshold. There was, however, a trend toward a class difference in spike threshold (p<0.08). Post-hoc pairwise comparisons also revealed a number of differences (Table 1).
Table1.
All Recorded Cells Classified by Electrophysiological Properties
| Significant Tests | RS | FS | LTS | |
|---|---|---|---|---|
| N | 52 | 40 | 19 | |
| Spike half-width (ms) | * # ‡ † | 2.2±0.1 | 1.3±0.1 | 1.5±0.1 |
| Frequency adaptation index | * # ‡ † | 0.36±0.02 | 0.95±0.01 | 0.66±0.02 |
| AHP of spike train (mV) | * # ‡ | 3.7±0.4 | 2.4±0.4 | 1.4±0.3 |
| Vrest (mV) | * # ‡ | -60.3±0.6 | -63.9±0.8 | -63.2±0.9 |
| Input resistance (MΩ) | 197.4±11.0 | 168.7±11.1 | 206.1±22 | |
| Spike amplitude (mV) | * # † | 104.2±1.3 | 98.7±1.2 | 106.9±1.8 |
| Spike threshold (mV) | † | -40.2±0.8 | -39.1±1.4 | -43.7±1.6 |
| Time constant (ms) | * # † | 57.3±1.9 | 39.8±3.0 | 53.2±5.1 |
Values are means±S.E. See Methods for calculations.
Significant main effect of class.
Significant post-hoc comparisons:
RS vs FS
RS vs LTS
FS vs LTS.
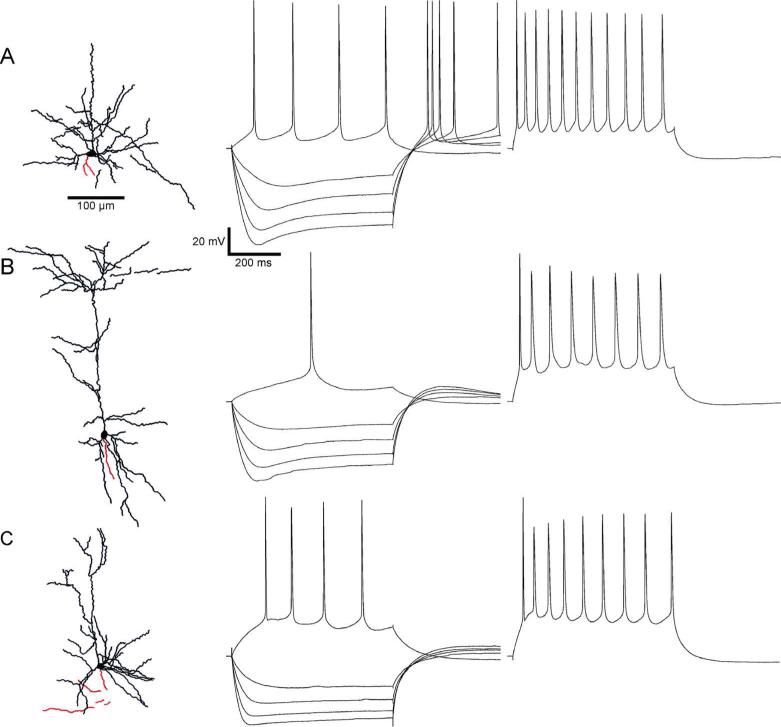
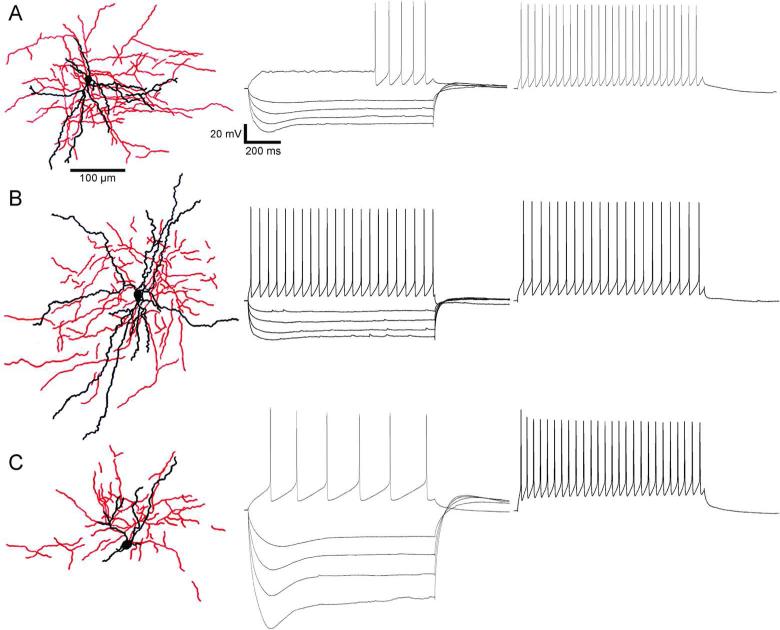
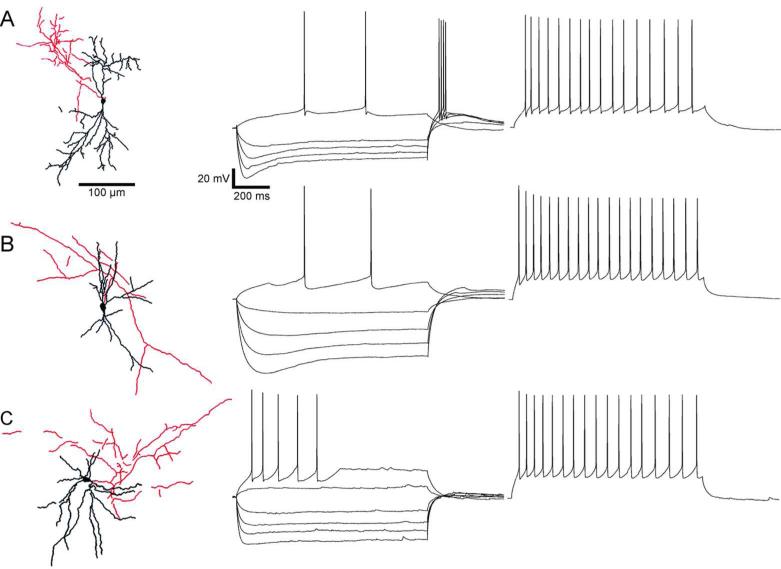
We recorded from 52 RS neurons (Figure 2, right). These cells fired relatively broad spikes and showed strong adaptation during repetitive firing. A distinguishing feature of RS neurons was an initial spike doublet in a spike train, with an elevated first AHP between the first two spikes (Figure 2A-B). Pyramidal cells in deep layers of perirhinal cortex generate a prolonged latency to the first spike when stimulated near threshold (“late-spiking” cells)(McGann et al., 2001; Moyer et al., 2002). We did not explicitly attempt to quantify this property, but some of our RS cells did exhibit this behavior (Figure 2, middle). We recorded 40 neurons that were classified as FS cells. FS firing patterns were characterized by high frequency, brief action potentials, and little or no adaptation of spiking rate (Figure 3, right). Nineteen cells had properties suggesting that they were LTS cells. LTS cells responded at just-suprathreshold stimulation with no delayed spikes and exhibited moderate spike adaptation (Figure 4, right). The spike half-widths and frequency adaptation indexes of neurons with LTS patterns tended to fall between those of RS and FS cells (Table 1).
Figure 2.
Regular-spiking (RS) cells with pyramidal morphologies (left) are shown with their firing patterns and responses to hyperpolarizing current steps in A, B, and C. Pyramidal cells were injected with steps in 100 pA increments, from -400 pA until the first spikes were evoked by a depolarizing step (middle traces). The resting membrane potentials of these traces were adjusted to -68.0 mV +/- 0.4 (A), -61.5 mV +/- 0.4 (B), and -64.3 mV +/- 0.9 (C). Traces with repetitive spiking (right) show prominent frequency adaptation. The average initial frequency for these traces is 23.6 Hz +/- 1.1.
Figure 3.
Fast-spiking (FS) cells with multipolar morphologies (left) are shown with their respective hyperpolarization and firing patterns in A, B, and C. Cells were injected with current steps in 50 (A,B) or 100 pA (C) increments until the first spikes were evoked (middle). The resting membrane potentials of these traces were adjusted to -61.8 mV +/- 0.4 (A), -67.3 mV +/- 2.0 (B), and -70.2 mV +/- 0.7 (C). Traces with repetitive spiking (right) showed little or no adaptation in firing rate. The average initial frequency for these traces is 26.0 Hz +/- 4.5.
Figure 4.
Low-threshold spiking (LTS) cells with bitufted (A, B) and multipolar (C) morphologies (left) are shown with their respective hyperpolarization and firing patterns. Cells were injected with current steps in increments of 50 pA until the first spikes were evoked (middle). The resting membrane potentials of these traces were adjusted to -65.3 mV +/- 3.5 (A), -67.6 mV +/- 0.6 (B), and -58.7 mV +/- 1.9 (C). Traces with repetitive spiking (right) show modest adaptations of firing rate. The average initial frequency for these traces is 28.8 Hz +/- 4.3.
Comparing the physiological properties of the three classes of neurons revealed a number of significant differences. The AHPs that followed single spikes in cells with RS firing patterns were relatively long-lasting and shallow compared to those of cells with FS firing patterns, which were much deeper and reached a nadir more quickly after the action potential. LTS cells often exhibited a brief afterdepolarization following a fast and small AHP. During a just-suprathreshold depolarizing stimulus, spikes in FS cells would occasionally be delayed for tens of milliseconds, preceded by a membrane potential plateau (Figure 3A). Under similar stimulus conditions, some LTS cells would spike at short latency and then cease spiking entirely, followed by a voltage plateau (Figure 4C). Many LTS cells also exhibited a slow depolarization following the termination of a hyperpolarizing pulse (i.e. a “low threshold spike”, which originally defined the cell class in frontal neocortex (Kawaguchi and Kubota, 1997)), which was strong enough to trigger an action potential or two (Figure 4A, middle).
Forty three of the recorded cells were successfully stained with biocytin and were classified by morphology. Cells with a prominent, vertically oriented apical dendrite, and a skirt of basal dendrites were identified as pyramidal cells (Figure 2, left); many also had pyramidal shaped somata, although some somata were more spherical or oblate. Many pyramidal cells had thick apical dendritic shafts, similar to those observed in layer 5 of occipital and parietal cortex (Chagnac-Amitai et al., 1990; Kasper et al., 1994b; Kim and Connors, 1993). Axons usually emerged from the base of the soma. Nonpyramidal cells were classified as multipolar or bitufted types based on differences in the size and extent of dendritic trees. Multipolar cells were characterized by primary dendrites emerging at multiple orientations from the cell body (Figure 3, left). Axons of multipolar cells emerged from the soma and formed a dense plexus near or surrounding the cells’ dendritic arbors. Bitufted neurons had primary dendrites that emerged from opposite vertical poles of the cell body before branching. The axons of bitufted cells either formed a dense local plexus (Figure 4A) or meandered across the breadth of the dendritic structure and beyond (Figure 4B,C). Of the 43 well-stained neurons, 13 were identified as pyramidal, 27 as multipolar, and three as bitufted.
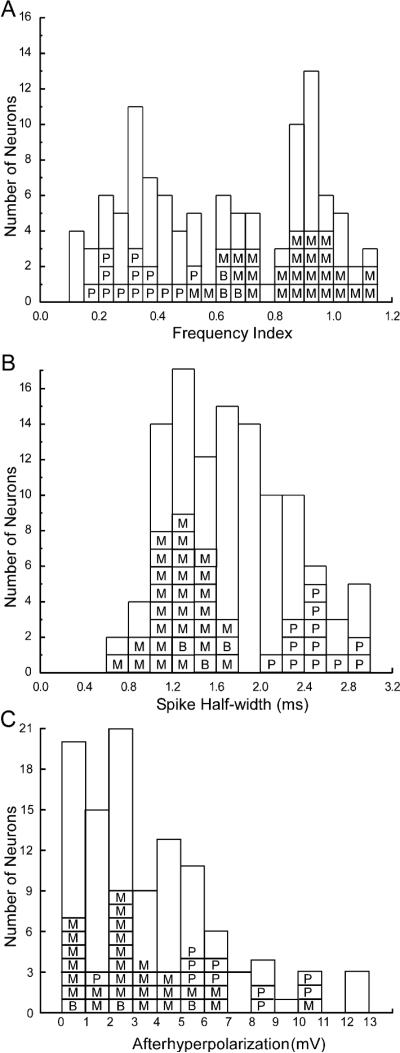
Some, but not all, electrophysiological properties differed by morphological class (Table 2). ANOVA revealed main effects of morphology on AHP spike train [F(2,38)=10.32, p<0.0003], spike half-width [F(2,40)=103.28, p<0.0001], and adaptation index [F(2,40)=43.20, p<0.0001]. There were no main effects of morphology on the remaining electrophysiological variables. Posthoc analysis indicated that pyramidal cells differed significantly from both bitufted and multipolar cells on all three measures (p-values ranged from 0.01 to 0.0001). Bitufted and multipolar cells were marginally significantly different on adaptation index (p=0.053), but not the other two measures. Although the general distribution of these physiological variables appears similar for stained and unstained cells (Figure 5), it was of interest to know whether the distributions differed statistically. The Kolmogorov-Smirnov asymptotic two-sample (KSa) test indicated that the two distributions were not different for AHP spike train (p>0.42) or frequency adaptation index (p>0.08), but that the distributions of spike half-width were statistically different [KSa(67,43)=1.8, p=0.003]. This suggests that the bimodal distribution of spike half-width for the stained cells is not representative of the whole population of layer 5 cells, i.e. the bimodal distribution might disappear with a larger sample of stained cells.
Table 2.
Electrophysiological Properties of Three Morphological Classes
| Significant Tests | Pyramidal | Multipolar | Bitufted | |
|---|---|---|---|---|
| n | 11 | 27 | 3 | |
| Spike half-width (ms) | * | ‡2.4±0.1 | 1.3±0.1 | 1.5±0.1 |
| Frequency adaptation index | * | ‡0.29±0.02 | †0.87±0.03 | 0.65±0.03 |
| AHP of spike train (mV) | * | ‡5.5±0.8 | 2.1±0.4 | 2.0±1.2 |
| Vrest (mV) | -62.4±1.2 | -64.0±1.0 | -66.5±1.1 | |
| Input resistance (MΩ) | 177.3±17.0 | 148.0±12.7 | 139.5±17.3 | |
| Spike amplitude (mV) | 102.8±2.2 | 101.7±1.7 | 107.3±4.0 | |
| Spike threshold (mV) | -42.2±1.8 | -40.7±1.6 | -46.7±1.4 | |
| Time constant (ms) | 56.2±4.3 | 41.9±4.3 | 43.3±13.6 |
Values are means±S.E. See Methods for calculations. Main effect of class:
p<0.0003.
Significant post-hoc comparisons: Pyramidal vs Multipolar, ; Pyramidal vs Bitufted:
p<0.01.
Multipolar vs Bitufted:
p<0.53.
Figure 5.
Frequency histograms of the spike frequency adaptation index (A), spike half-width (B), and spike-train afterhyperpolarization (C). Stained cells are identified by morphological classification as pyramidal (P), bitufted (B), or multipolar (M). Empty bars indicate frequencies of cells that were not stained.
To further examine the relationship between electrophysiological parameters and morphology of POR neurons, we conducted an exploratory analysis of the electrophysiological variables that were significantly different across morphological classes. AHP spike train, spike half-width, and frequency adaptation index for the stained POR cells were entered into a cluster analysis. There was a marked flattening of the SPRSQ curve at the 3-cluster solution, indicating that the variance explained by three clusters of cells would not be substantially improved by further subdivision of clusters. The three-cluster solution accounted for 72% of the variance. The first cluster division separated pyramidal cells from nonpyramidal cells with one exception. The second cluster included only multipolar cells. The third cluster was mixed, including the one remaining pyramidal cell, all three bitufted cells, and the remaining multipolar cells (about one-third).
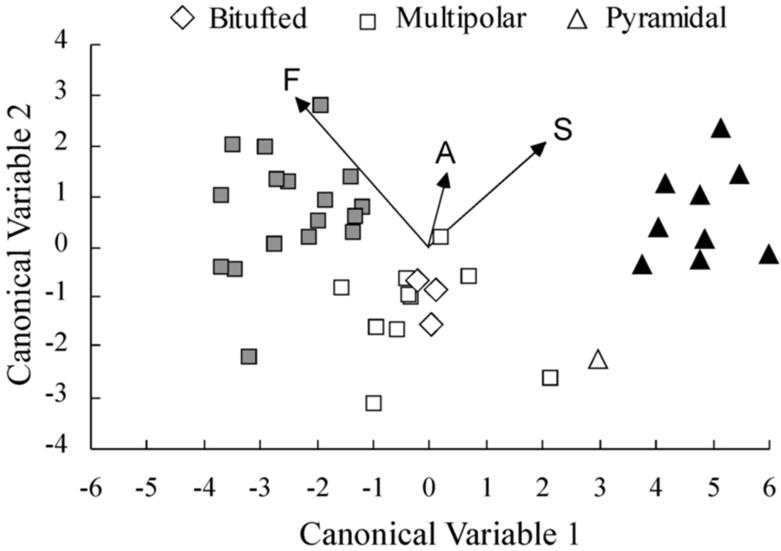
To determine how electrophysiological characteristics contributed to cluster membership, we conducted a canonical discriminant analysis of the cluster solution. The analysis yielded two canonical variables, i.e. linear combinations of the variables contributing to the cluster analysis. Plotting the scores of each cell on these variables permitted visualization of the cluster solution in two dimensions (Figure 6). Each of the electrophysiological variables is plotted as a vector from the origin to the point described by its canonical coefficients. The vectors show, visually, the degree to which frequency adaptation index, AHP spike train, and spike half-width contributed to cluster separation. Of the three variables, frequency adaptation index contributed relatively more and AHP contributed relatively less to the cluster solution.
Figure 6.
Canonical discriminant analysis of the three cluster solution. Cluster membership is denoted by color (white, grey, or black). The morphology of the cells is indicated by the shape of the marker (see legend). Each cell is located by its score on the first two canonical variables. To show the degree to which physiological variables affected the cluster solution, each variable is plotted as a vector from the origin to the point described by its canonical coefficients. See text for further explanation. Abbreviations: F, frequency index; A, afterhyperpolarization; S, spike half-width.
Cluster 1, which was composed entirely of pyramidal cells (Figure 6, black markers), included all cells that exhibited an RS firing pattern, except one (the pyramid shown with open triangle). Cluster 2, which was composed entirely of multipolar cells, contained all cells that exhibited FS firing characteristics (Figure 6, grey markers) except one (cell in the middle cluster closest to the grey cluster). Cluster 3 contained predominantly multipolar cells, but also contained all three bitufted cells and one pyramidal (RS) cell (Figure 6, open markers). One of the multipolar cells had been classified as an FS cell.
Table 3 illustrates the electrophysiological properties across clusters. Not surprisingly, a number of the variables were significantly different across clusters. Indeed, there was a significant main effect of cluster for all variables except resting membrane potential and spike threshold (significance ranged from p<0.0001 for spike half-width, adaptation index, and AHP of spike train to p<0.05, 0.008, and 0.003 for input resistance, spike amplitude, and time constant, respectively. Post hoc paired comparisons revealed a number of between class differences. P values ranged from 0.0001 to 0.04.
Table 3.
Electrophysiological Properties of Clusters
| Significant Tests | Cluster 1 (RS) | Cluster 2 (FS) | Cluster 3 (LTS) | |
|---|---|---|---|---|
| n | 9 | 17 | 15 | |
| Spike half-width (ms) | * # ‡ † | 2.5±0.1 | 1.2±0.1 | 1.5±0.1 |
| Frequency adaptation index | * # ‡ † | 0.29±0.03 | 0.96±0.02 | 0.64±0.04 |
| AHP of spike train (mV) | * # ‡ | 6.3±0.7 | 2.52±0.58 | 1.5±0.4 |
| Vrest (mV) | -62.8±1.4 | -64.3±1.3 | -63.7±1.1 | |
| Input resistance (MΩ) | * # ‡ | 181.1±20.2 | 141.2±16.1 | 155.7±14.5 |
| Spike amplitude (mV) | * ‡ † | 103.3±2.1 | 97.9±1.6 | 107.0±2.2 |
| Spike threshold (mV) | † | -43.0±5.7 | -38.6±2.0 | -43.9±1.8 |
| Time constant (ms) | * # † | 53.7±3.0 | 34.4±4.0 | 54.0±6.6 |
Values are means±S.E. See Methods for calculations.
Significant main effect of Cluster.
Significant post-hoc comparisons:
RS vs FS
RS vs LTS
FS vs LTS.
Discussion
We recorded from layer 5 pyramidal and nonpyramidal cells in POR using whole-cell recording methods in an in vitro slice preparation. Using published electrophysiological criteria, the 111 recorded cells could be classified as RS cells (47%), FS cells (36%), or LTS cells (17%) (Amitai et al., 2002; Beierlein et al., 2003; Kawaguchi, 1993; McCormick et al., 1985). In order to examine the morphology of POR neurons, we stained 43 of the recorded cells with biocytin. Those were classified as pyramidal cells (35%), multipolar nonpyramidal cells (63%), or bitufted nonpyramidal cells (7%) cells.
A set of analyses addressed the associations between electrophysiology and morphology for recorded and stained cells. Consistent with such a relationship, the morphological classes of cells were significantly different on three physiological measures: frequency adaptation index, spike half-width, and afterhyperpolarization amplitude. A classification analysis using those three variables yielded three clusters that corresponded to RS, FS, and LTS cells with only two misclassifications – one previously characterized RS cell and one FS cell were joined with a cluster that was otherwise formed entirely of LTS cells. The cluster corresponding to the FS class was composed entirely of multipolar cells, and the cluster corresponding to the RS class was composed entirely of pyramidal cells. The LTS cluster contained the remaining multipolar cells, all three bitufted cells, and one pyramidal cell. The one pyramidal cell was the misclassified RS cell and one of the multipolar cells was the misclassified FS cell.
The classification analysis confirmed a close relationship between morphology and electrophysiology for RS and FS cells, but the LTS cell cluster was more morphologically diverse. All three bitufted cells were classified as LTS. Although the number of bitufted cells in our sample was very small, this result is consistent with studies from other neocortical regions suggesting that bitufted cells have the physiological properties of LTS cells (Deuchars and Thomson, 1995; Kawaguchi, 1993). Studies of LTS cells in some cortical areas and layers have shown that they can have either bitufted or multipolar nonpyramidal morphology (Amitai et al., 2002; Fanselow et al., 2008; Kawaguchi and Kubota, 1997). FS cells and LTS cells in other areas of neocortex have also been consistently distinguished by the proteins they express; almost universally, FS cells express parvalbumin but not somatostatin and LTS cells express somatostatin but not parvalbumin; both cell types are GABAergic (Gibson et al., 1999; Markram et al., 2004; Rudy et al., 2011). Although we did not test for these characteristics in our study, it seems very likely that they will hold for FS and LTS interneurons in POR. Indeed, POR does exhibit a topography of parvalbumin histochemistry such that dorsal POR stains for parvalbumin with an intensity similar to that of other neocortical regions, but ventral POR is nearly devoid of parvalbumin staining cells (Beaudin et al., 2012). It may be that the dorsal subregion was sampled more heavily than the ventral POR.
In our study, layer 5 pyramidal cells of POR all demonstrated characteristics of RS cells. Studies of the electrophysiology and morphology of neurons done in the neighboring PER area proposed three distinct groups of pyramidal cells: RS, intrinsically bursting (IB), and late-spiking (LS) (Faulkner and Brown, 1999). RS and IB cells were observed in layer 5 (Moyer et al., 2002), while LS cells were recorded in all layers of PER (Faulkner and Brown, 1999; Moyer et al., 2002), although the LS cells may predominate in layers 2/3 and 6 (Beggs et al., 2000; McGann et al., 2001). In our study, some RS cells did display delayed spiking (e.g. Figure 2B), although we did not specifically test for that property during our experiments and did not characterize these cells as a separate class.
IB cells have been reported in layer 5 of prefrontal cortex and of PER in 4-6 week old rats (Kawaguchi, 1993; Moyer et al., 2002) as well as in layer 5 of the somatosensory, visual, and anterior cingulate areas (Connors et al., 1982; Kasper et al., 1994a; McCormick et al., 1985; Silva et al., 1991; Wang and McCormick, 1993). Thus, the absence of IB cells in our sample of 52 POR pyramidal cells is of some interest. In the PER study, the numbers of IB cells were low, about 9%, so in a sample of 52 pyramidal cells, we might expect to see four or five and could miss them entirely. We may also have failed to observe IB cells because their bursting phenotype matures later than RS firing patterns. Intrinsically bursting cells are reported to mature abruptly after postnatal day 14 in somatosensory cortex and in visual cortex (Franceschetti et al., 1993; Kasper et al., 1994a). Kawaguchi (1993), however, observed IB cells in layer 5 of prefrontal cortex in relatively young rats (16-22 days postnatal). Because roughly two-thirds of the RS cells we observed were recorded on postnatal days 15-16, we would expect to have seen IB cells if they were present.
We identified two types of interneurons in POR: FS cells and LTS cells. FS cells exhibited high firing frequency, brief action potentials, and little or no adaptation. FS interneurons have been identified in all layers of PER (Faulkner and Brown, 1999; Martina et al., 2001). About one-third of the interneurons in the present study exhibited characteristics of LTS cells, with low-threshold spikes and frequency adaptation. LTS cells with these characteristics were first described in rodent prefrontal cortex (Kawaguchi, 1993). As in the present study, LTS cells in that study were multipolar or bitufted. In addition, only 5% of the interneurons recorded in frontal layer 2/3 exhibited LTS properties. There are no reports of LTS cells in PER. This is probably due to differences in procedures, in particular the selective visual targeting of cells, but it is also possible that the interneurons populating layer 5 of the PER and POR are different.
The POR provides the main neocortical input to the ENT, targeting primarily its superficial layers. The electrophysiological and morphological characteristics of ENT neurons are well-described, especially cells in layers 2 and 3, which project to the dentate gyrus and hippocampus proper (Empson et al., 1995; Gloveli et al., 1997; Klink and Alonso, 1997; Tahvildari and Alonso, 2005). With respect to deeper layers, an earlier study applied criteria developed for cells of other regions of neocortex to ENT and classified layer 5/6 cells in medial ENT cortex as nonbursting, IB, or FS (Jones and Heinemann, 1988). Relatively few IB cells and FS cells were observed, however. There is some disagreement about whether criteria used for classifying neurons in other neocortical regions should be applied to cells in the ENT. Hamam and colleagues (Hamam et al., 2002; Hamam et al., 2000) argued that ENT cells were not easily classified according to these criteria.
Two studies have addressed the electrophysiology and morphology of cells in layer 5 of the lateral ENT(Hamam et al., 2002] and the medial ENT [Hamam, 2000 #18). In both subregions, three classes of cells were identified based on morphology, including pyramidal, horizontal, and polymorphic cells. The pyramidal cells were similar to those described for POR and the polymorphic cells were similar to what we have termed multipolar cells. The horizontal cells, however, seem to be a different class. Electrophysiological properties of the three classes were described as heterogeneous. Some pyramidal cells exhibited typical RS properties and some of the polymorphic cells demonstrated FS properties. It should be noted that Hamam and colleagues (Hamam et al., 2002; Hamam et al., 2000) saw no evidence for IB cells in layer 5 of either the lateral or medial ENT using sharp electrode intracellular recording methods.
Until the mid 1990s, there was no region in the rodent brain considered to be the homolog of the primate parahippocampal cortex. Burwell et al (1995) identified the POR in the rat brain and proposed homology with the primate parahippocampal cortex using principles outlined for establishing homology in the comparative anatomy of the nervous system (Campbell and Hodos, 1970). The proposed homology was based primarily on connectional criteria but also on topology, topography, electrophysiology, and behavioral evidence (Burwell and Amaral, 1998a; Burwell et al., 1995; Suzuki and Amaral, 1994a). Both the POR and the primate parahippocampal cortex have reciprocal connections with other parahippocampal areas as well as the hippocampus (Burwell and Amaral, 1998b; Suzuki and Amaral, 1994b). Given the importance of spatial and contextual information in episodic memory, it is particularly important to understand the properties of cells in these regions.
We recently demonstrated task-related theta rhythm modulation in POR (Ahmed et al., 2011). Theta power was significantly increased during task-related phases of the discrimination paradigm, including presentation of the stimuli and selection of the stimuli. Moreover, nearly half of the cells recorded were phase-locked to theta cycles of the local field potentials. The identification of LTS and FS neurons in POR is particularly interesting given that the results of recent studies suggest that inhibitory interneurons may contribute to the generation of synchrony and rhythms in neocortical regions. Both FS cells and LTS cells are extensively coupled by electrical synapses (Amitai et al., 2002; Gibson et al., 1999), and this allows both interneuron networks to synchronize their spikes and their inhibitory influences (Long et al., 2005; Mancilla et al., 2007). FS cells are strongly implicated in the generation of cortical and hippocampal gamma rhythms(Cardin et al., 2009; Whittington and Traub, 2003). LTS cells can effectively synchronize themselves and the local cortical network at lower frequencies (Beierlein et al., 2000; Cardin et al., 2009; Fanselow et al., 2008; Gibson et al., 1999; Long et al., 2005; Whittington and Traub, 2003). In addition, FS cells appear to be the target of excitatory feed-forward input from specific thalamic nuclei whereas LTS cells receive little direct thalamic input (Cruikshank et al., 2010). Rather, LTS cells appear to receive strong, facilitating synaptic input from local cortical networks, and help to synchronize their activity. Coordinated inhibition from the LTS network may result in the synchronous firing of principal (RS) cells observed in POR of rats performing complex tasks.
Understanding the local circuitry of the POR, including its interneurons, will be essential to appreciate how POR contributes to information processing in the mammalian brain (Freund and Buzsaki, 1996).
Acknowledgements
The authors would like to thank Jaime Mancilla for his patient and generous guidance in the electrophysiological methods and Saundra Patrick for her assistance in histological processing of filled cells. JBS is currently at the Tufts University School of Medicine.
Funding:
NSF IBN9875792 to RDB
NIH NS25983 to BWC
DARPA N66001-10-C-2010 to RDB and BWC
References
- Ahmed OJ, Furtak SC, Burwell RD. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. Theta modulation in postrhinal cortex during two-choice visual discrimination. [Google Scholar]
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22(10):4142–52. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin SA, Singh T, Agster KL, Burwell RD. Borders and Comparative Cytoarchitecture of the Perirhinal and Postrhinal Cortices in an F1 Hybrid Mouse. Cerebral cortex. 2012 doi: 10.1093/cercor/bhs038. DOI: 10.1093/cercor/bhs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Moyer JR, Jr., McGann JP, Brown TH. Prolonged synaptic integration in perirhinal cortical neurons. J Neurophysiol. 2000;83(6):3294–8. doi: 10.1152/jn.2000.83.6.3294. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3(9):904–10. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90(5):2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998a;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998b;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus. 1995;5(5):390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Campbell CB, Hodos W. The concept of homology and the evolution of the nervous system. Brain Behav Evol. 1970;3(5):353–67. doi: 10.1159/000125482. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296(4):598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982;48(6):1302–20. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65(2):230–45. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. Innervation of burst firing spiny interneurons by pyramidal cells in deep layers of rat somatomotor cortex: paired intracellular recordings with biocytin filling. Neuroscience. 1995;69(3):739–55. doi: 10.1016/0306-4522(95)00288-t. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398(1):25–48. [PubMed] [Google Scholar]
- Empson RM, Gloveli T, Schmitz D, Heinemann U. Electrophysiology and morphology of a new type of cell within layer II of the rat lateral entorhinal cortex in vitro. Neurosci Lett. 1995;193(3):149–52. doi: 10.1016/0304-3940(95)11684-o. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100(5):2640–52. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner B, Brown TH. Morphology and physiology of neurons in the rat perirhinal-lateral amygdala area. J Comp Neurol. 1999;411(4):613–42. [PubMed] [Google Scholar]
- Franceschetti S, Buzio S, Sancini G, Panzica F, Avanzini G. Expression of intrinsic bursting properties in neurons of maturing sensorimotor cortex. Neurosci Lett. 1993;162(1-2):25–8. doi: 10.1016/0304-3940(93)90551-u. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402(6757):75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Schmitz D, Empson RM, Dugladze T, Heinemann U. Morphological and electrophysiological characterization of layer III cells of the medial entorhinal cortex of the rat. Neuroscience. 1997;77(3):629–48. doi: 10.1016/s0306-4522(96)00494-0. [DOI] [PubMed] [Google Scholar]
- Gordon AD. In: Classification. Cox DR, Isham V, Keiding N, Reid N, Tong H, Louis T, editors. Chapman & Hall/CRC; London: 1999. p. 256. [Google Scholar]
- Hamam BN, Amaral DG, Alonso AA. Morphological and electrophysiological characteristics of layer V neurons of the rat lateral entorhinal cortex. J Comp Neurol. 2002;451(1):45–61. doi: 10.1002/cne.10335. [DOI] [PubMed] [Google Scholar]
- Hamam BN, Kennedy TE, Alonso A, Amaral DG. Morphological and electrophysiological characteristics of layer V neurons of the rat medial entorhinal cortex. J Comp Neurol. 2000;418(4):457–72. [PubMed] [Google Scholar]
- Hubert L, Arabie P. Comparing partitions. J. Classification. 1986;2:193–218. [Google Scholar]
- Jones RS, Heinemann U. Synaptic and intrinsic responses of medical entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol. 1988;59(5):1476–96. doi: 10.1152/jn.1988.59.5.1476. [DOI] [PubMed] [Google Scholar]
- Kasper EM, Larkman AU, Lubke J, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J Comp Neurol. 1994a;339(4):459–74. doi: 10.1002/cne.903390402. [DOI] [PubMed] [Google Scholar]
- Kasper EM, Lubke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol. 1994b;339(4):495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69(2):416–31. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7(6):476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13(12):5301–11. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Alonso A. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus. 1997;7(5):571–83. doi: 10.1002/(SICI)1098-1063(1997)7:5<571::AID-HIPO12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10(4):420–30. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242(4886):1654–64. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Long MA, Cruikshank SJ, Jutras MJ, Connors BW. Abrupt maturation of a spike-synchronizing mechanism in neocortex. J Neurosci. 2005;25(32):7309–16. doi: 10.1523/JNEUROSCI.0375-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci. 2007;27(8):2058–73. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol. 2001;86(6):2887–95. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGann JP, Moyer JR, Jr., Brown TH. Predominance of late-spiking neurons in layer VI of rat perirhinal cortex. J Neurosci. 2001;21(14):4969–76. doi: 10.1523/JNEUROSCI.21-14-04969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., McNay EC, Brown TH. Three classes of pyramidal neurons in layer V of rat perirhinal cortex. Hippocampus. 2002;12(2):218–34. doi: 10.1002/hipo.1110. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopez da Silva FH. Perirhinal cortex input to the hippocampus in the rat: evidence for parallel pathways, both direct and indirect. A combined physiological and anatomical study. Eur J Neurosci. 1999;11(11):4119–33. doi: 10.1046/j.1460-9568.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- Pinto A, Fuentes C, Pare D. Feedforward inhibition regulates perirhinal transmission of neocortical inputs to the entorhinal cortex: ultrastructural study in guinea pigs. J Comp Neurol. 2006;495(6):722–34. doi: 10.1002/cne.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand WM. Objective criteria for the evaluation of clustering methods. J. Amer. Statistical Assoc. 1971;66:846–50. [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71(1):45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251(4992):432–5. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994a;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994b;14(3 Pt 2):1856–77. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildari B, Alonso A. Morphological and electrophysiological properties of lateral entorhinal cortex layers II and III principal neurons. J Comp Neurol. 2005;491(2):123–40. doi: 10.1002/cne.20706. [DOI] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci. 1993;13(5):2199–216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26(12):676–82. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10(4):398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]