Abstract
Purpose
To determine if automated continuous distraction osteogenesis at rates > 1mm/day would result in clinical and radiographic bone formation in a minipig model.
Materials and Methods
An automated, continuous, curvilinear distraction device was placed across a mandibular osteotomy in 10 minipigs. After 12 mm of distraction and 24 days fixation, animals were sacrificed and bone healing evaluated. The continuous distraction rates were 1.5 (n=5) and 3 mm/day (n=5). A semiquantitative scale was used to assess ex-vivo clinical appearance of the distraction gap (3= osteotomy not visible; 2= <50%; 1= >50%; 0= 100% visible); stability (3 = no mobility; 2 and 1 = mobility in 2 or 1 plane respectively; 0= mobility in 3 planes); radiographic density (4 = 100% gap opaque, 3= >75%, 2 = 50% – 75%, 1= <50%, or 0 = radiolucent). Groups of 4 minipigs distracted discontinuously at 1, 2, and 4 mm/day served as controls.
Results
The continuous DO 1.5 mm/day group had significantly higher scores for appearance and radiographic density compared to the discontinuous 4 mm/day group. The continuous DO 3mm/day group had significantly higher scores for appearance and radiographic density compared to the discontinuous 4 mm/day group, and higher stability compared to the discontinuous 2 and 4 mm/day groups.
Conclusions
Results of this preliminary study indicate that continuous DO at rates of 1.5 and 3.0 mm/day produces better bone formation when compared to discontinuous DO at rates faster than 1mm/day.
Keywords: continuous distraction osteogenesis, craniofacial surgery, micrognathia, mandible
Introduction
The first clinical application of distraction osteogenesis for correction of craniofacial deformities was published in 1992 by McCarthy et al.1 Since that report, distraction osteogenesis (DO) has become a commonly used technique for expansion of the craniomaxillofacial skeleton. An osteotomy is created, a rigid distraction device fixed across the gap and the bone gradually lengthened by activation of the device. DO utilizes the body’s natural healing process to form new bone within a slowly expanding osteotomy gap.2–5 It eliminates the need for bone and soft tissue grafts and complications associated with donor site operations.6, 7
As a result of extensive studies in a canine long bone model and evaluation of a large number of clinical cases, Ilizarov established the ideal rate of distraction at 1 mm/day with faster rates resulting in inadequate bone formation and non-union.3–5 A rate of 1 mm/day has also been shown to be the most predictable rate of distraction in the craniofacial skeleton, based on experimental and clinical data.8, 9 After a variable latency period, 1 mm/day lengthening is achieved by manually turning an activation screw 1 to 4 times/day.8, 10 This is followed by a consolidation period typically twice the amount (millimeters) of distraction in days.3, 4 As DO is generally employed for large movements, the overall treatment is long and fraught with complications.11
Currently, cumbersome distraction devices require considerable patient and caregiver cooperation and skill to manage the activation process. This leads to difficulties with treatment acceptance and compliance. Frequent office visits and radiographs are required to monitor compliance, gap size and distraction vector. An automated distraction device would eliminate the reliance on patient compliance. Continuous distraction has been reported to improve bone fill and may allow distraction at rates up to 2 mm/day.4, 12–16 An automated, continuous device for distraction osteogenesis (United States Patent #8177789) has been developed through a collaboration between the Department of Oral and Maxillofacial Surgery at Massachusetts General Hospital and Physical Sciences Incorporated (Andover, MA USA) funded by NIH SBIR grant #5R44DE014803-03. A preliminary feasibility study served to test the design and safety of the device.17 The purpose of the current study was to assess whether automated continuous DO at rates greater than 1 mm per day would result in bone formation and clinical union in a standardized minipig model. Our hypothesis was that automated, continuous distraction at rates greater than the standard 1 mm/day would allow equivalent bone fill.
The specific aims of this study were: 1) To further validate the safety and efficacy of a device developed for automated continuous DO in minipigs, 2) To assess bone healing of the distraction gap using clinical and radiographic criteria at continuous distraction rates of 1.5 and 3 mm/day.
Materials and Methods
An automated, continuous, semiburied distraction device for mandibular DO was tested in Yucatan minipigs. The predictor variables were the rates (mm/day) and type (continuous or incremental) of DO. Group 1 was programmed to distract continuously at 1.5 mm day while Group 2 was programmed at 3 mm/day. Control groups consisted of minipigs that underwent mandibular DO discontinuously at 1, 2, and 4 mm/day. The assessment of the DO wound included the evaluation of clinical appearance, stability, and radiographic density of the distraction wound, on a semiquantitative scale, at end-fixation.
The Automated Device
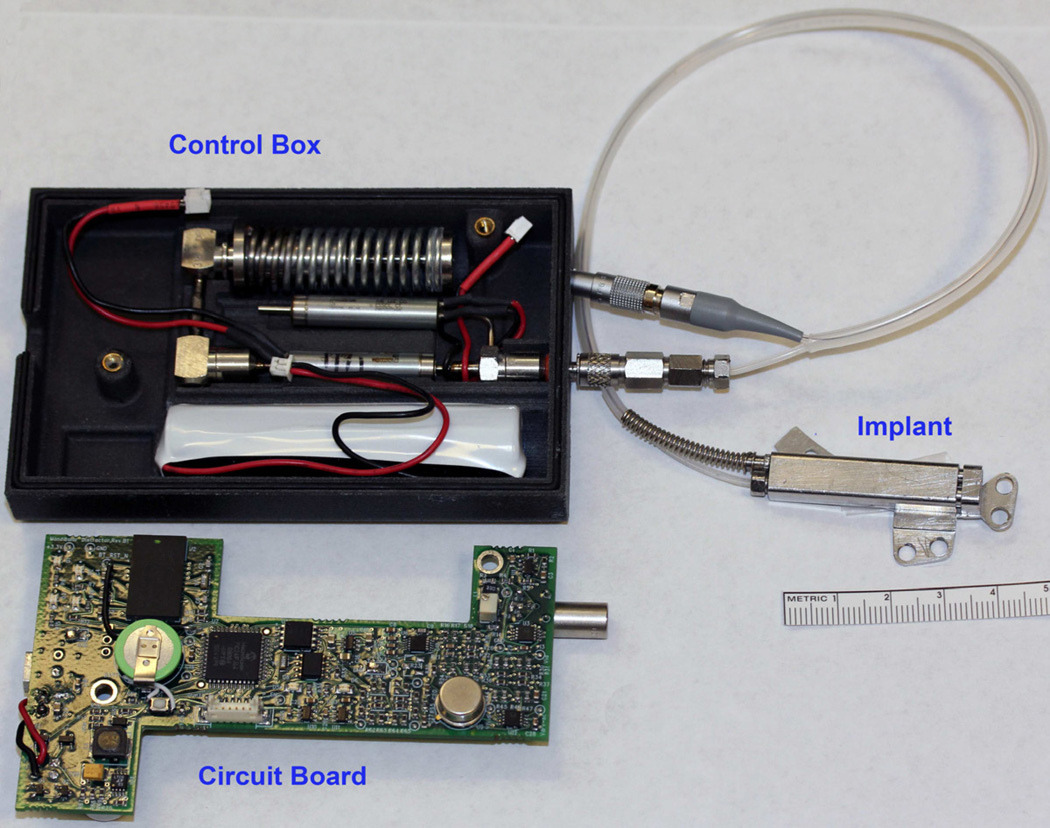
A semiburied, automated, continuous distraction device powered by a battery was used in these experiments (NIH SBIR grant #5R44DE014803-03, and U.S. Patent #8177789). (Figure 1) The distraction device is hydraulically driven by a digital controller with position feedback. A spring-powered, hydraulic reservoir supplies 2.0 Megapascals (MPa) at full spring extension to 3.4 MPa (full spring compression) of pressurized water to the distractor through a microdispensing solenoid valve. The hydraulic system can provide 25N–40N of force to expand the distraction site. Based on feedback from an inductive position sensor in the distractor, a digital controller opens the valve for a period of 100 µs-10 ms at 15 minute intervals. This results in continuous movement along the curvilinear slider-rail to the desired position.
Figure 1.
Automated continuous distraction device attached to opened control box with circuitry and spring driven hydraulic reservoir.
Current position, motion time history, battery level and other parameters can be monitored through a computer program using Bluetooth® connection with the control box. The controller produces alarms in the form of blinking LEDs on the external box in response to a range of device or tracking errors, including deviations from the desired distraction distance or low batteries. Through this same software, the parameters of distraction are set and can be adjusted during the course of treatment.
Animals
Female Yucatan minipigs (n=10) in the mixed-dentition stage (4–6 months old; weighing 25–35 kg) were used in this study. This standardized model has been the focus of a series of mandibular distraction experiments since 1997.8, 9, 18–26 The Yucatan minipig model was selected because it has mandibular size, morphology and chewing patterns similar to humans.27, 28 It also has ginglymo-arthrodial function of the temporomandibular joint, as well as similar bone turnover rates as compared to humans.28–30
The animals were acclimated to living quarters, a cloth jacket containing the control unit and a pureed diet for 9–10 days prior to distractor placement. After implantation, the device’s control-unit was housed within the jacket and was directly connected to the hydraulic and sensor lines. The pigs were assessed twice daily for signs of infection, weight, activity level and general appearance. The care and use of the minipigs met the requirements of the Accreditation of Laboratory Animal Care standards and was approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (SRAC 2009N0000073). The authors have read and are in full compliance with the Declaration of Helsinki.
Surgical Procedure
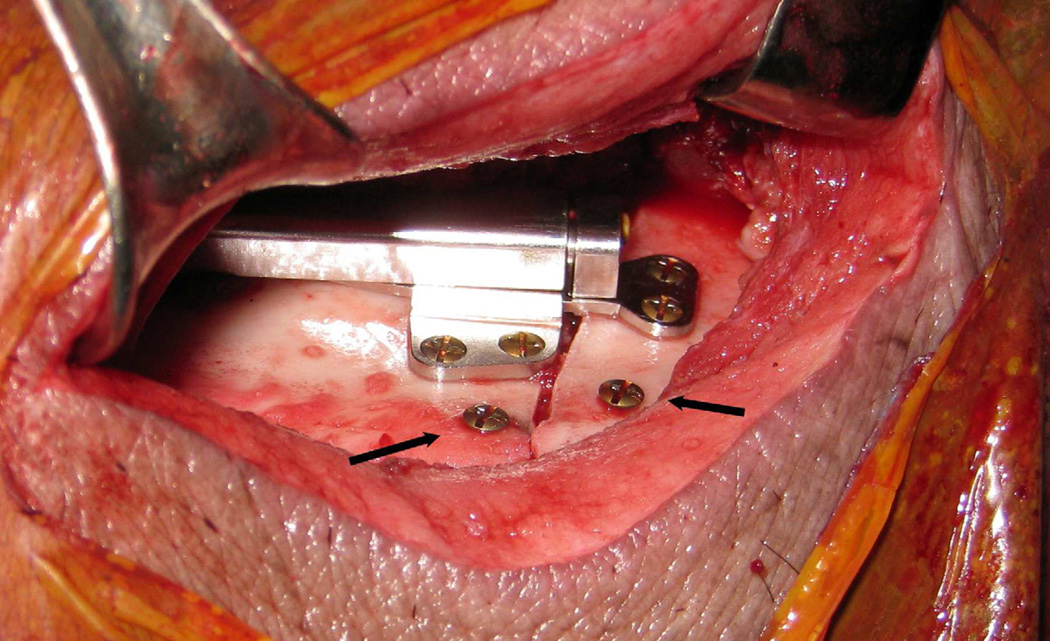
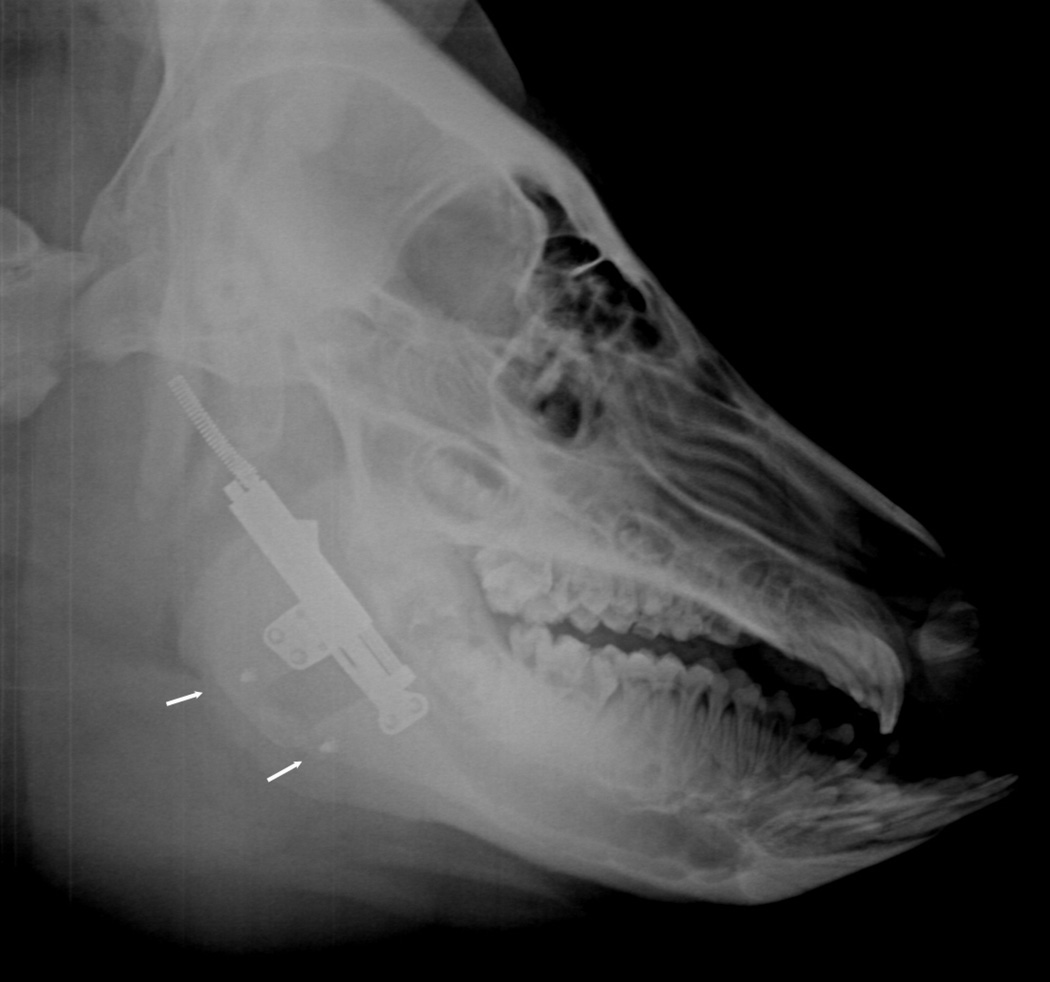
The animals were sedated with 4.4 mg/kg telazol and 2.2 mg/kg xylazine and administered 0.04 mg/kg atropine intramuscularly. After oral intubation, anesthesia was maintained with isofluorane. Pre- and postoperative photographs, overjet measurments and lateral cephalograms were obtained on a standardized porcine cephalostat.8 Through a submandibular incision and standard dissection, the mandible was exposed and an osteotomy along a line extending from a point 1 cm anterior to the mandibular angle to the retromolar region in front of the anterior ramus was marked on the bone. The corticotomy was carried out with a reciprocating saw and the distraction device placed along the lateral aspect of the mandible centered over the corticotomy. The distractor was then secured with Synthes® (West Chester, PA, USA) 2.0 mm diameter titanium screws 8 and 10 mm in length. Two screws were placed 10–12 mm apart at the inferior border of the mandible for measurement of the amount of distraction. The osteotomies were then completed. The hydraulic and sensor lines of the device were tunneled subcutaneously (20 cm) to exit from the dorsal skin in an area not accessible to the animal. The device was activated to confirm function, reversed to baseline and the distance between marker screws measured. (Figure 2) The wounds were closed in layers.
Figure 2.
Automated distraction device secured to the lateral aspect of the mandible across an osteotomy. Marker screws placed at the inferior border (arrows).
Distraction Protocol
The devices in Groups 1 and 2 were programmed to distract to 12 mm with distraction rates of 1.5 and 3 mm/day, respectively. No latency period was used and the fixation period was twice the distraction length in days (24 days).
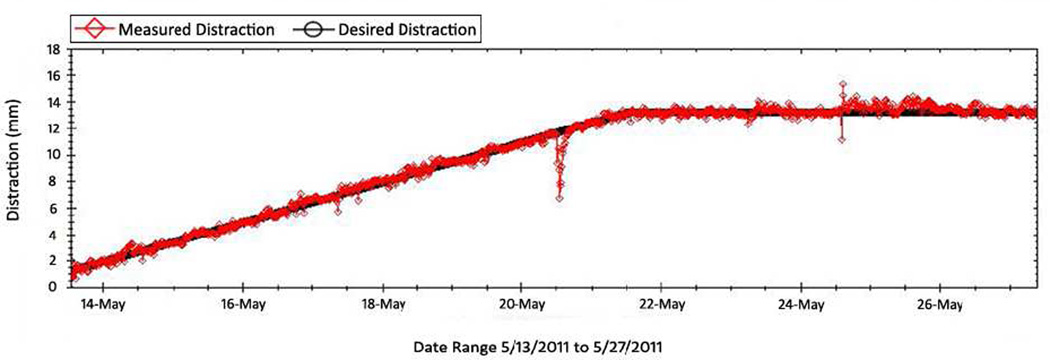
Distractor position was updated every 15 minutes and recorded remotely using a Bluetooth® connection to the control box. (Figure 3)
Figure 3.
Graph of projected (black line) and measured distraction (red). The device opened at 1.5 mm/day. The downward projection likely is from the force of the pig biting something solid causing the device to close slightly. The device then returns to programmed position.
Clinical Evaluation
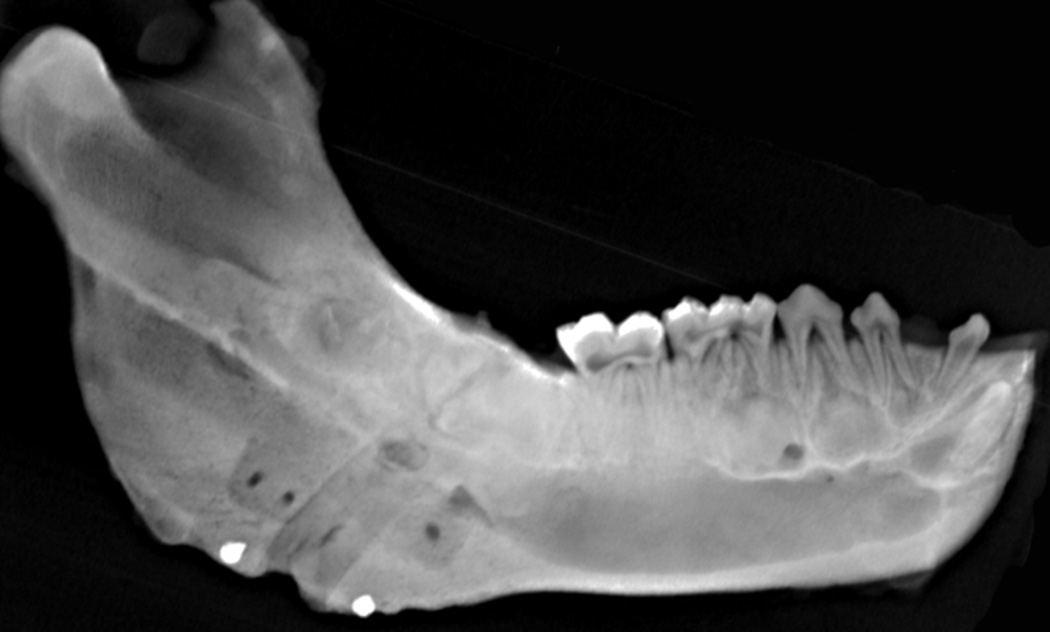
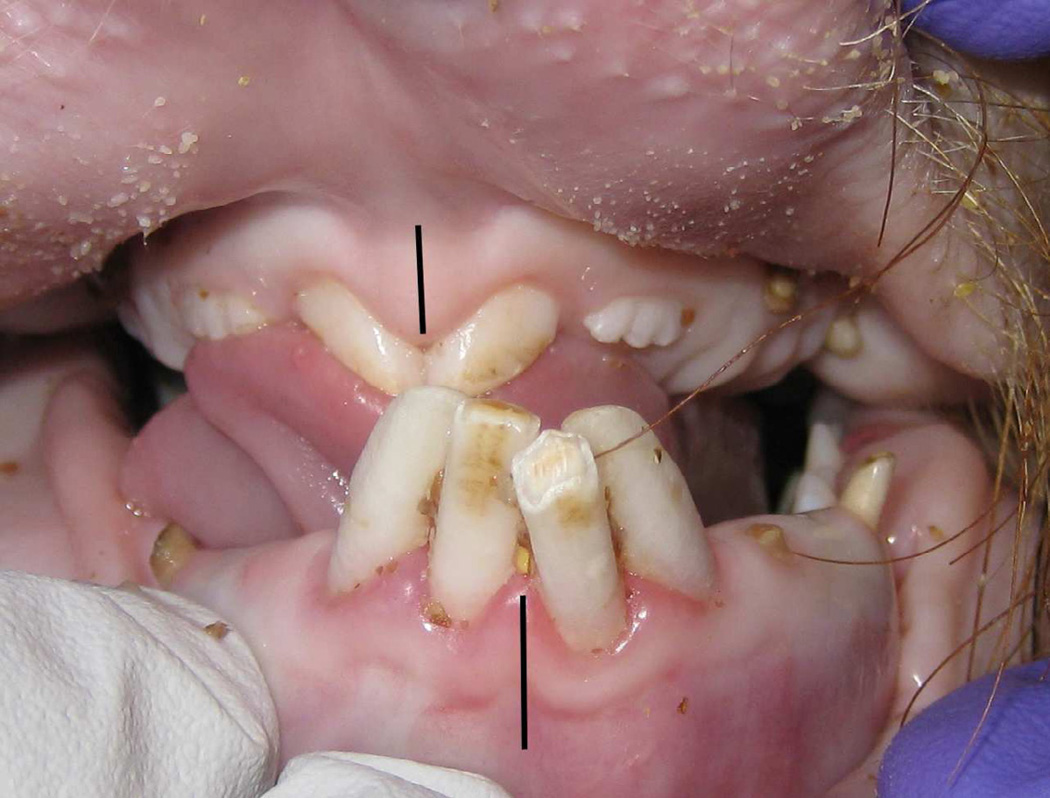
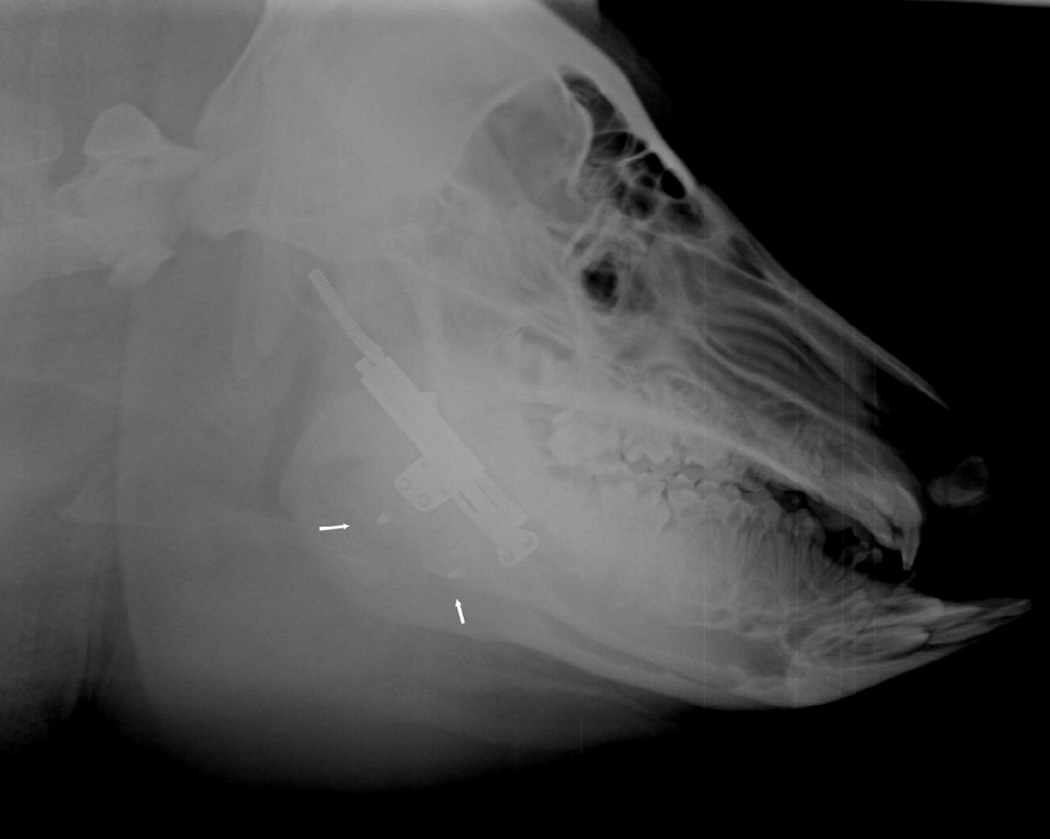
At mid-DO, end-DO, and end-fixation, the animals were again sedated with 4.4 mg/kg telazol and 2.2 mg/kg xylazine and administered 0.04 mg/kg atropine intramuscularly. The wounds were assessed and cleaned. Overjet was measured (Figure 4) and lateral cephalograms were obtained. (Figure 5A, B) At end-fixation, the pigs were sacrificed and the right hemi-mandible was removed. The ex-vivo appearance of the DO gap was graded using a semiquantitative scale: 3 - osteotomy not visible; 2 - osteotomy < 50% visible; 1 - osteotomy > 50% visible; 0 - osteotomy clearly visible. (Figure 6) Bimanual examination of the ex-vivo mandible was graded as: 3 - no mobility; 2 - mobility in 1 plane; 1 - mobility in 2 planes; 0 - mobility in 3 dimensions (unstable). Ex-vivo lateral radiographs, using a previously designed porcine cephalostat,8 were obtained to assess the density of bone fill within the osteotomy gap. Bone density was graded on a 5 point scale: 4 = entire gap opaque, 3= >75% and < 100% opacity, 2 = 50%–75% opacity, 1= <50% opacity, or 0 = completely radiolucent. (Figure 7)
Figure 4.
At end-DO, the reverse overjet increases and shifts the mandibular midline (black line) away from the side of distraction and maxillary midline (black line).
Figure 5.
A. End-DO lateral cephalograms showing the trapezoidal distraction gap measured with marker screws (white arrows). There is increased radiodensity at B. End-Fixation.
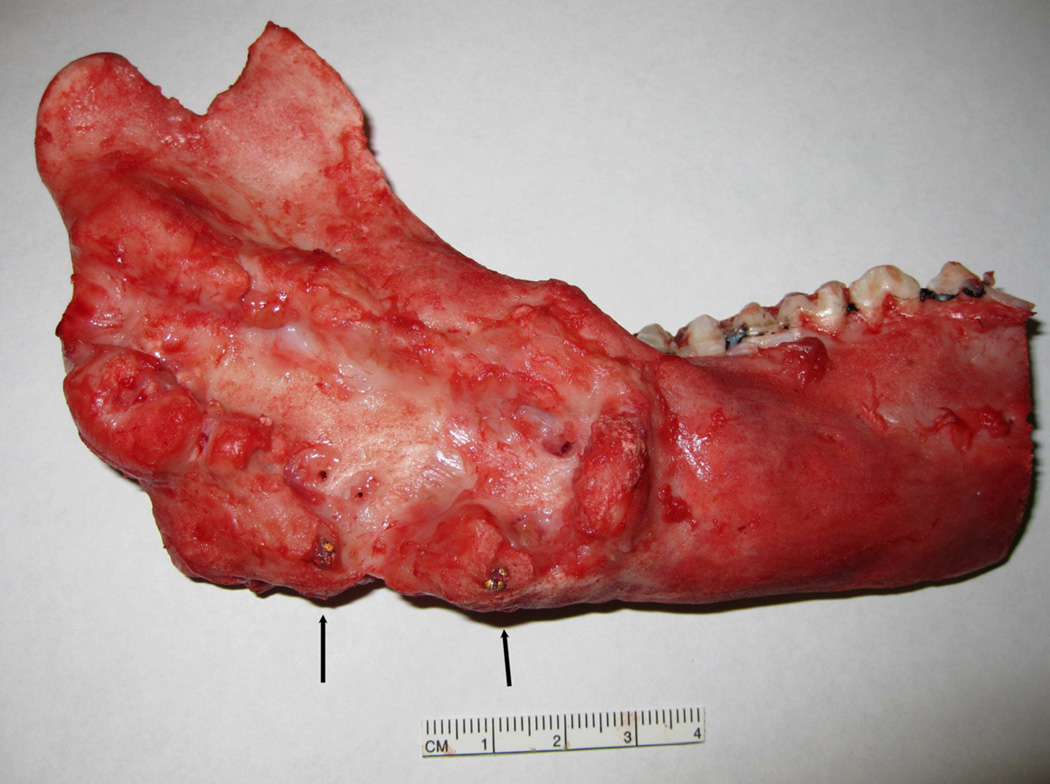
Figure 6.
Clinical photograph of ex-vivo mandible with complete fill of distraction gap of an animal distracted continuously at 3 mm/day. The regenerate is between the marker screws (arrows)
Figure 7.
Ex-vivo mandibular radiograph after sacrifice at end-fixation after continuous DO at 3 mm/day. The radiographic density of the distraction wound is higher at the superior border where the rate of distraction is less than at the inferior border.
Statistical Evaluation
Statistical evaluation was calculated using statistical software (IBM® Statistical Package of Social Sciences, version 20.0; SPSS Chicago, IL USA). Independent 2-sample t tests (2-tailed) were performed for each group with unequal variance assumed. P values were considered significant if less than 0.05.
Results
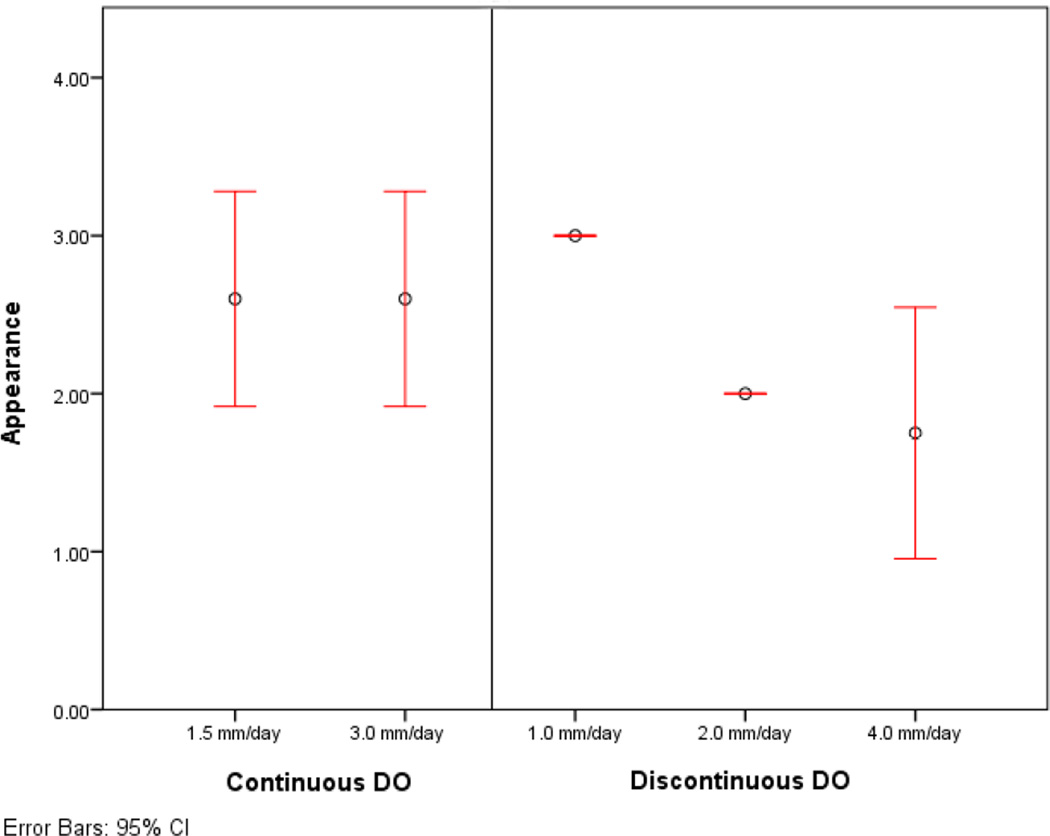
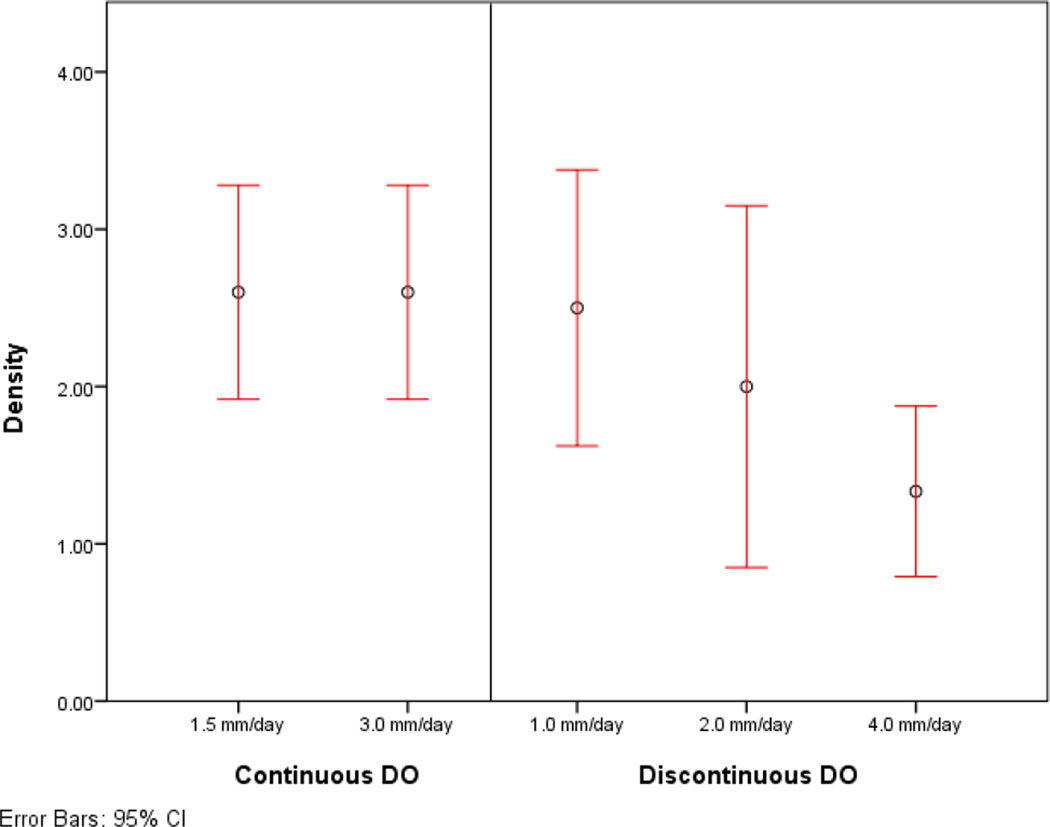
All mini-pigs survived the operation, distraction, and fixation periods. (Table 1) Group 1 (n=5) averaged 1.4 mm/day of distraction and 25 days of fixation. Mean scores for appearance, stability, and radiographic densities were 2.6, 2.4 and 2.6, respectively. Group 2 (n=5) averaged 2.4 mm/day of distraction and 28 days of fixation. Mean scores for appearance, stability, and radiographic densities were 2.6, 3, and 2.6, respectively. (Table 2) The historical control groups using discontinuous distraction rates of 1, 2 and 4mm/day had mean scores for appearance of 3, 2, and 1.75, stability: 3, 1.75 and 1.5, and radiographic density: 2.5, 2, and 1.33. Group 1 had significantly higher scores for appearance and radiographic density when compared to the incremental distraction groups at 4 mm/day (p = 0.046, p=0.004). Appearance and radiographic density were significantly higher in group 2 when compared to discontinuous DO at 4 mm/day (p = 0.046, p = 0.004). The scores for stability of the mandible in group 2 were significantly higher than both the groups of discontinuous DO at 2 and 4 mm/day groups (p = 0.015, p = 0.014). (Table 3–4, Figure 8)
Table 1.
Device assessment – distraction period, rate of distraction for each group
| Protocol | Subject Number |
Distraction Period |
Average Rate |
Total Distraction |
Complications |
|---|---|---|---|---|---|
| Group 1 | |||||
| 1.5 mm/d | 2–294 | 8 Days | 1.4 mm/d | 11.5 mm | Exit port infection |
| 1.5 mm/d | 3–079 | 8 Days | 1.0 mm/d | 8.0 mm | Wound and exit port infection |
| 1.5 mm/d | 4–088 | 8 Days | 1.5 mm/d | 12.0 mm | None |
| 1.5 mm/d | 4–067 | 8 Days | 1.5 mm/d | 7.0 mm | Torn hydraulic line |
| 1.5 mm/d | 5–040 | 8 Days | 1.4 mm/d | 11.5 mm | None |
| Group 2 | |||||
| 3.0 mm/d | 5–117 | 4 Days | 3.0 mm/d | 12.0 mm | None |
| 3.0 mm/d | 6–037 | 5 Days | 2.4 mm/d | 12.0 mm | Exit port infection |
| 3.0 mm/d | 7–105 | 9 Days | 1.4 mm/d | 13.0 mm | None |
| 3.0 mm/d | 8–056 | 6 Days | 2.1 mm/d | 12.5 mm | None |
| 3.0 mm/d | 8–028 | 4 Days | 3.0 mm/d | 12.0 mm | Minor wound infection |
Table 2.
Automated Continuous Distraction Osteogenesis: Appearance, stability and radiographic assessment
| Protocol | Subject Number |
Appearance | Stability | Radiographic Density |
|---|---|---|---|---|
| Group 1 | ||||
| 1.5 mm/d | 2–294 | 3 | 3 | 3 |
| 1.5 mm/d | 3–079 | 2 | 2 | 2 |
| 1.5 mm/d | 4–088 | 3 | 3 | 3 |
| 1.5 mm/d | 4–067 | 2 | 1 | 2 |
| 1.5 mm/d | 5–040 | 3 | 3 | 3 |
| Average | ~ | 2.6 | 2.4 | 2.6 |
| Group 2 | ||||
| 3.0 mm/d | 5–117 | 3 | 3 | 3 |
| 3.0 mm/d | 6–037 | 3 | 3 | 3 |
| 3.0 mm/d | 7–105 | 3 | 3 | 3 |
| 3.0 mm/d | 8–056 | 2 | 3 | 2 |
| 3.0 mm/d | 8–028 | 2 | 3 | 2 |
| Average | ~ | 2.6 | 3 | 2.6 |
Table 3.
Control Group: Incremental Distraction Osteogenesis. Appearance, stability and radiographic assessment
| Protocol | Appearance | Sample Size |
Stability | Sample Size |
Radiographic Density |
Sample Size |
|---|---|---|---|---|---|---|
| 1.0 mm/d | ||||||
| 0-day Latency | 3 | n = 2 | 3 | n = 2 | 2.25 | n = 4 |
| 4-day Latency | 3 | n = 2 | 3 | n = 2 | 3 | n = 2 |
| Combined | 3 | n = 4 | 3 | n = 4 | 2.5 | n = 6 |
| 2.0 mm/d | ||||||
| 0-day Latency | 2 | n = 2 | 1.5 | n = 2 | 1.75 | n = 4 |
| 4-day Latency | 2 | n = 2 | 2 | n = 2 | 2.5 | n = 2 |
| Combined | 2 | n = 4 | 1.75 | n = 4 | 2 | n = 6 |
| 4.0 mm/d | ||||||
| 0-day Latency | 1.5 | n = 2 | 1.5 | n = 2 | 1.5 | n = 4 |
| 4-day Latency | 2 | n = 2 | 1.5 | n = 2 | 1 | n = 2 |
| Combined | 1.75 | n = 4 | 1.5 | n = 4 | 1.33 | n = 6 |
Table 4.
Statistical Group Comparison
| Appearance | Stability | Radiographic Density | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol | P1.5 | P3.0 | P1.0 | P2.0 | P4.0 | P1.5 | P3.0 | P1.0 | P2.0 | P4.0 | P1.5 | P3.0 | P1.0 | P2.0 | P4.0 |
| 1.5 mm/d | ~ | 1.000 | 0.178 | 0.070 | 0.046 | ~ | 0.208 | 0.208 | 0.214 | 0.112 | ~ | 1.000 | 0.818 | 0.275 | 0.004 |
| 3.0 mm/d | 1.000 | ~ | 0.178 | 0.070 | 0.046 | 0.208 | ~ | 1.000 | 0.015 | 0.014 | 1.000 | ~ | 0.818 | 0.275 | 0.004 |
Figure 8.
Mean and 95% confidence intervals for (A) appearance, (B) stability and (C) radiographic density of the experimental and control groups.
Discussion
The purpose of this study was to validate an automated continuous distraction device in a minipig model and to assess whether continuous distraction would allow for bony healing of the distraction gap at rates faster than typically used in clinical settings. We hypothesized that the device would function safely and effectively, and that continuous distraction would produce bone fill and osseous union at faster rates of bony expansion than with incremental distraction. Specifically, a continuous and curvilinear distraction device was used to assess distraction rates up to 3 mm/day for clinical and radiographic bone fill of the distraction gap.
Results of this study validate a novel automated device for DO and demonstrate that continuous distraction allows bone fill at faster distraction rates than discontinuous DO. The distraction gap appeared to have near complete bone fill (average scores of 2.6 on the semiquantitative scale) in animals continuously distracted at 1.5 and 3 mm/day. This compared to scores of 2 and 1.75 in the previous study of incremental distraction at 2 and 4 mm/day, respectively.8 Clinical stability across the distraction gap was 2.4 for the 1.5 mm/day group and completely stable in all 5 animals distracted at 3 mm/day (scores of 3) compared to 1.75 and 1.5 for incremental distraction at 2 and 4 mm/day, respectively. Radiographic density of the distraction wound was 2.6 for both continuous distraction groups compared to 2 and 1.33 for discontinuous distraction at 2 and 4 mm/day.
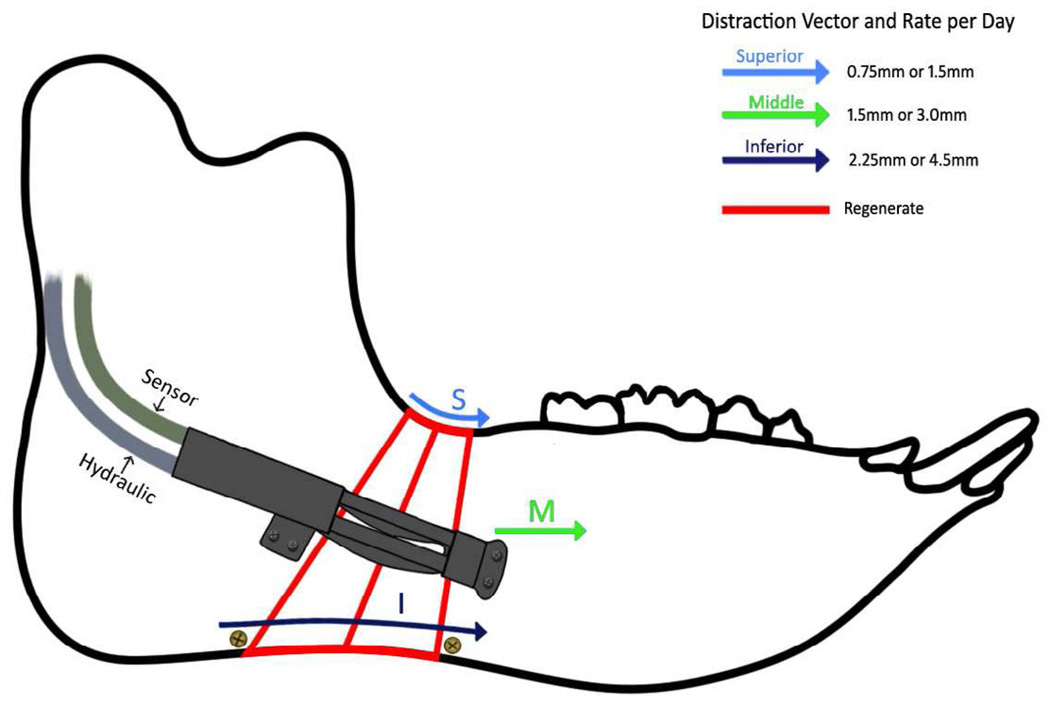
The curvilinear technique of DO lengthens the mandible at different rates from the superior border to the inferior border. The distraction device was placed at the center of the mandible, midway between the superior and inferior borders. Using the height of the mandible and the 5 cm radius of curvature, the theoretical rates at the superior and inferior borders can be calculated. At the superior border DO occurs at 0.75 mm/day and 1.5 mm/day in Groups 1 and 2, respectively. The inferior border is distracted at 2.25 mm/day and 4.5 mm/day respectively. (Figure 8) Continuous distraction allowed for clinical and radiographic bone fill in Group 2 to the inferior border, which theoretically opened at 4.5 mm/day in 3 of the 5 animals. This rate of distraction has not been shown to produce bone healing in previous studies of incremental or continuous distraction of the craniofacial skeleton.
Currently, DO for craniofacial reconstruction is limited by lengthy duration of treatment, the requirement for frequent activation of external or semi-buried devices, and reliance on the ability of the patient and/or family to activate the devices properly. Distraction protocols for the maxillofacial region have been adapted from studies in long bones.3, 4, 31 Ilizarov, showed the ideal rate of bone lengthening to be 1 mm/day in canine long bone experiments and in humans and demonstrated that faster rates (e.g. 2 mm/day) result in fibrous tissue or nonunion.4 Daily lengthening of limbs at 0.5 mm/day with a frequency of 0.125 mm every six hours led to premature consolidation. Lengthening at a rate of 2mm/day with a frequency of 0.5 mm every 6 hours gave rise to non-union and a reduced bone formation. DO at a rate of 1mm/day with frequencies up to 60 times/day produced the most active osteogenesis with little fibrous tissue.
As in long bones, distraction rates faster than 1 mm/day in the craniofacial skeleton have led to fibrous or nonunion.8 Treatment plans for correction of craniofacial deformities require lengthening of up to 30 mm requiring 30 days for distraction and at least 60 days for consolidation. The continuous distraction technique shown in this study and others may allow for bone fill at rates impossible in incremental distraction.12, 14, 32–37 It avoids the daily microtrauma and disruption of the healing process when activating an incremental device.14, 38 Continuous motion and strain on the mandibular wound result in improved bone regeneration and could allow for significantly decreased treatment times for craniofacial deformities.39
Several devices that use motors, springs, and hydraulic pressure for mandibular lengthening have been described.32–34, 40–42 Kessler et al describe a continuous device using hydraulic motion and showed that less force is required to open continuously at 1.5 mm/day and resulted in intra-membranous regeneration without chondroid ossification.14 The device used in this study has several advantages over previously developed continuous distraction devices. It is the first automated device that is small enough to allow widespread clinical use. Using a spring-loaded reservoir permits a small (4mm) piston diameter which allows the device to sit only 8 mm off the bone surface for curvilinear motion and 6mm if configured for linear motion. The device in the current study is the first to assess curvilinear continuous motion and to be tested at rates faster than 1.5 mm. This device can control and adjust position during the entire distraction process remotely. This could allow assessment and adjustment of the distraction without a clinical visit or radiograph. Also, it could allow for “pumping the regenerate” (i.e. advancing and reversing the device) in compromised wounds.43
The current study has several limitations. The sample size is limited with only 5 minipigs in each group limiting statistical comparisons. The device had been tested in 10 previous animals for optimization of safety and effectiveness prior to assessing the faster rates (unpublished data) and not all animals actually distracted at the set rate (i.e. 2 pigs in Group 2 had acute closure of the device requiring more time to stabilize at 12 mm despite opening 3mm/day). Despite the similarities in the minipig and human mandibles, it has not been demonstrated that continuous DO at faster rates would allow for osseous union in humans. Ilizarov also postulated that continuous distraction at rates faster than 1mm/day might result in successful bone formation. However, clinical trials will be necessary prior to widespread use of continuous distraction in humans. In addition, the use of zero latency in these and other experiments is not meant to recommend the same protocol in clinical protocols of discontinuous distraction.
The comparison to incremental DO rates was to historic controls using a different distraction device. The previous animals were distracted incrementally using single-vector distraction devices leading to a parallel DO wound as opposed to the trapezoidal wound with curvilinear DO. The exit port of the activating arm was closer to the exit port than for the automated distractor which potentially could lead to more infections at the site of distraction. Distraction was limited to 12 mm (10% of the minipig mandible) allowing comparison to previous studies using incremental distraction. DO would not usually be employed for only 12 mm of mandibular lengthening in humans. In the next phase of this project, the device will be tested with 20–30 mm of distraction.
The current automated device described in this paper has not yet met our goal of being completely buried. Two hydraulic lines run from the dorsal aspect of the pig to a control box mounted on a jacket. A completely buried device may reduce infection rates seen at the exit port and would be less prone to damage in an active child. The control box, in its current iteration, is analogous to Baja hearing aids or insulin pumps for patients with Treacher Collins syndrome or Type 1 diabetes mellitus, respectively, and likely would be similarly tolerated. Further refinement of the device will be geared toward making the control box smaller or integrated within a buried device.
Conclusion
Automated continuous distraction osteogenesis allows for faster rates of distraction in the minipig model when compared to discontinuous distraction. Continuous distraction rates up to 4.5 mm/day at the inferior border of the mandible produced clinical and radiographic bone fill and good clinical stability across the regenerate. This device and technique has the potential to shorten treatment time in the management of craniofacial deformities.
Figure 9.
Schematic of curvilinear automated continuous DO of the minipig mandible. Due to the curvilinear motion, distraction proceeds at faster rates at the inferior border compared to the superior border.
Acknowledgement
The authors acknowledge the help and expertise of Maria Papadaki, DDS, PhD for help in caring for the animals. Drs. Magill and Murphy are employed by Physical Sciences, Inc., with whom we collaborate with on our NIH SBIR grant.
Funding
This work was funded by NIH/NIDCR SBIR grant (5R44DE014803-03), the MGH Department of Oral and Maxillofacial Surgery Education and Research Fund, and the Hanson Foundation (Boston, MA, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented at the 94th Annual Meeting of the American Association of Oral and Maxillofacial Surgery on September 14, 2012 in San Diego, CA, USA
References
- 1.McCarthy JG, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1. [PubMed] [Google Scholar]
- 2.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. Am J Orthop Surg. 1905;2:353. doi: 10.1007/s11999-008-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989:249. [PubMed] [Google Scholar]
- 4.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989:263. [PubMed] [Google Scholar]
- 5.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990:8. [PubMed] [Google Scholar]
- 6.Steinbacher DM, Kaban LB, Troulis MJ. Mandibular advancement by distraction osteogenesis for tracheostomy-dependent children with severe micrognathia. J Oral Maxillofac Surg. 2005;63:1072. doi: 10.1016/j.joms.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kaban LB, Seldin EB, Kikinis R, et al. Clinical application of curvilinear distraction osteogenesis for correction of mandibular deformities. J Oral Maxillofac Surg. 2009;67:996. doi: 10.1016/j.joms.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troulis MJ, Glowacki J, Perrott DH, et al. Effects of latency and rate on bone formation in a porcine mandibular distraction model. J Oral Maxillofac Surg. 2000;58:507. doi: 10.1016/s0278-2391(00)90012-0. [DOI] [PubMed] [Google Scholar]
- 9.Glowacki J, Shusterman EM, Troulis M, et al. Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone. Plast Reconstr Surg. 2004;113:566. doi: 10.1097/01.PRS.0000101061.99577.09. [DOI] [PubMed] [Google Scholar]
- 10.Troulis MJ, Padwa B, Kaban LB. Distraction osteogenesis: past, present, and future. Facial Plast Surg. 1998;14:205. doi: 10.1055/s-2008-1064346. [DOI] [PubMed] [Google Scholar]
- 11.Troulis MJ, Kaban LB. Complications of mandibular distraction osteogenesis. Oral Maxillofac Surg Clin North Am. 2003;15:251. doi: 10.1016/S1042-3699(02)00101-2. [DOI] [PubMed] [Google Scholar]
- 12.Kessler PA, Merten HA, Neukam FW, et al. The effects of magnitude and frequency of distraction forces on tissue regeneration in distraction osteogenesis of the mandible. Plast Reconstr Surg. 2002;109:171. doi: 10.1097/00006534-200201000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Shetsov VI, Popkov AV. Limb lengthening in automatic mode. Ortop Traumatol Rehabil. 2002;4:403. [PubMed] [Google Scholar]
- 14.Kessler P, Neukam FW, Wiltfang J. Effects of distraction forces and frequency of distraction on bony regeneration. Br J Oral Maxillofac Surg. 2005;43:392. doi: 10.1016/j.bjoms.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Djasim UM, Wolvius EB, Bos JA, et al. Continuous versus discontinuous distraction: evaluation of bone regenerate following various rhythms of distraction. J Oral Maxillofac Surg. 2009;67:818. doi: 10.1016/j.joms.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Djasim UM, Mathot BJ, Wolvius EB, et al. Histomorphometric comparison between continuous and discontinuous distraction osteogenesis. J Craniomaxillofac Surg. 2009;37:398. doi: 10.1016/j.jcms.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Magill JC, Byl MF, Goldwaser B, et al. Automating skeletal expansion: An implant for distraction osteogenesis of the mandible. J Med Device. 2009;3:14502. doi: 10.1115/1.3071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seldin EB, Troulis MJ, Kaban LB. Evaluation of a semiburied, fixed-trajectory, curvilinear, distraction device in an animal model. J Oral Maxillofac Surg. 1999;57:1442. doi: 10.1016/s0278-2391(99)90729-2. [DOI] [PubMed] [Google Scholar]
- 19.Thurmuller P, Troulis MJ, Rosenberg A, et al. Changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2002;60:1327. doi: 10.1053/joms.2002.35733. [DOI] [PubMed] [Google Scholar]
- 20.Kaban LB, Thurmuller P, Troulis MJ, et al. Correlation of biomechanical stiffness with plain radiographic and ultrasound data in an experimental mandibular distraction wound. Int J Oral Maxillofac Surg. 2003;32:296. doi: 10.1054/ijom.2002.0380. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann CE, Harris G, Thurmuller P, et al. Assessment of bone formation in a porcine mandibular distraction wound by computed tomography. Int J Oral Maxillofac Surg. 2004;33:569. doi: 10.1016/j.ijom.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann CE, Thurmuller P, Troulis MJ, et al. Histology of the porcine mandibular distraction wound. Int J Oral Maxillofac Surg. 2005;34:411. doi: 10.1016/j.ijom.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Thurmuller P, Troulis MJ, Rosenberg A, et al. Microscopic changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2006;64:249. doi: 10.1016/j.joms.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Lawler ME, Tayebaty FT, Williams WB, et al. Histomorphometric analysis of the porcine mandibular distraction wound. J Oral Maxillofac Surg. 2010;68:1543. doi: 10.1016/j.joms.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 25.Lawler ME, Hansen GM, Williams WB, et al. Serial histologic and immunohistochemical changes in anterior digastric myocytes in response to distraction osteogenesis. J Oral Maxillofac Surg. 2012;70:168. doi: 10.1016/j.joms.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Hansen GM, Lawler ME, Williams WB, et al. BMP4 localization and PCNA expression during distraction osteogenesis of the porcine mandible. Int J Oral Maxillofac Surg. 2012;41:867. doi: 10.1016/j.ijom.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Ciochon RL, Nisbett RA, Corruccini RS. Dietary consistency and craniofacial development related to masticatory function in minipigs. J Craniofac Genet Dev Biol. 1997;17:96. [PubMed] [Google Scholar]
- 28.de Vernejoul MC, Pointillart A, Golenzer CC, et al. Effects of iron overload on bone remodeling in pigs. Am J Pathol. 1984;116:377. [PMC free article] [PubMed] [Google Scholar]
- 29.Kuboki T, Shinoda M, Orsini MG, et al. Viscoelastic properties of the pig temporomandibular joint articular soft tissues of the condyle and disc. J Dent Res. 1997;76:1760. doi: 10.1177/00220345970760110701. [DOI] [PubMed] [Google Scholar]
- 30.Mosekilde L, Weisbrode SE, Safron JA, et al. Calcium-restricted ovariectomized Sinclair S-1 minipigs: an animal model of osteopenia and trabecular plate perforation. Bone. 1993;14:379. doi: 10.1016/8756-3282(93)90167-9. [DOI] [PubMed] [Google Scholar]
- 31.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1. [PubMed] [Google Scholar]
- 32.Schmelzeisen R, Neumann G, von der Fecht R. Distraction osteogenesis in the mandible with a motor-driven plate: a preliminary animal study. Br J Oral Maxillofac Surg. 1996;34:375. doi: 10.1016/s0266-4356(96)90090-x. [DOI] [PubMed] [Google Scholar]
- 33.Ploder O, Mayr W, Schnetz G, et al. Mandibular lengthening with an implanted motor-driven device: preliminary study in sheep. Br J Oral Maxillofac Surg. 1999;37:273. doi: 10.1054/bjom.1999.0115. [DOI] [PubMed] [Google Scholar]
- 34.Kessler P, Wiltfang J, Neukam FW. A new distraction device to compare continuous and discontinuous bone distraction in mini-pigs: a preliminary report. J Craniomaxillofac Surg. 2000;28:5. doi: 10.1054/jcms.2000.0101. [DOI] [PubMed] [Google Scholar]
- 35.Ayoub AF, Richardson W. A new device for microincremental automatic distraction osteogenesis. Br J Oral Maxillofac Surg. 2001;39:353. doi: 10.1054/bjom.2000.0659. [DOI] [PubMed] [Google Scholar]
- 36.Ayoub AF, Richardson W, Koppel D, et al. Segmental mandibular reconstruction by microincremental automatic distraction osteogenesis: an animal study. Br J Oral Maxillofac Surg. 2001;39:356. doi: 10.1054/bjom.2001.0658. [DOI] [PubMed] [Google Scholar]
- 37.Ayoub AF, Richardson W, Barbenel JC. Mandibular elongation by automatic distraction osteogenesis: the first application in humans. Br J Oral Maxillofac Surg. 2005;43:324. doi: 10.1016/j.bjoms.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Pesch HJ, Gunther CC, Strauss HJ. Diaphysial lengthening osteotomy performed on the femur of the cat. Histomorphological studies of callus formation (author's transl) Z Orthop Ihre Grenzgeb. 1980;118:768. doi: 10.1055/s-2008-1053537. [DOI] [PubMed] [Google Scholar]
- 39.Meyer U, Meyer T, Vosshans J, et al. Decreased expression of osteocalcin and osteonectin in relation to high strains and decreased mineralization in mandibular distraction osteogenesis. J Craniomaxillofac Surg. 1999;27:222. doi: 10.1016/s1010-5182(99)80033-x. [DOI] [PubMed] [Google Scholar]
- 40.Mofid MM, Inoue N, Tufaro AP, et al. Spring-mediated mandibular distraction osteogenesis. J Craniofac Surg. 2003;14:756. doi: 10.1097/00001665-200309000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Idelsohn S, Pena J, Lacroix D, et al. Continuous mandibular distraction osteogenesis using superelastic shape memory alloy (SMA) J Mater Sci Mater Med. 2004;15:541. doi: 10.1023/b:jmsm.0000021135.72288.8f. [DOI] [PubMed] [Google Scholar]
- 42.Zhou HZ, Hu M, Hu KJ, et al. Transport distraction osteogenesis using nitinol spring: an exploration in canine mandible. J Craniofac Surg. 2006;17:943. doi: 10.1097/01.scs.0000236437.74850.26. [DOI] [PubMed] [Google Scholar]
- 43.Greenwald JA, Luchs JS, Mehrara BJ, et al. "Pumping the regenerate": an evaluation of oscillating distraction osteogenesis in the rodent mandible. Ann Plast Surg. 2000;44:516. [PubMed] [Google Scholar]