Abstract
Rearing cats from birth to adulthood in darkness prevents neurons in the superior colliculus (SC) from developing the capability to integrate visual and non-visual (e.g., visual-auditory) inputs. Presumably, this developmental anomaly is due to a lack of experience with the combination of those cues, which is essential to form associative links between them. The visual-auditory multisensory integration capacity of SC neurons has also been shown to depend on the functional integrity of converging visual and auditory inputs from ipsilateral association cortex. Disrupting these cortico-collicular projections at any stage of life results in a pattern of outcomes similar to those found after dark-rearing: SC neurons respond to stimuli in both sensory modalities, but cannot integrate the information they provide. Thus, it is possible that dark-rearing compromises the development of these descending tectopetal connections and the essential influences they convey. However, the results of the present experiments, using cortical deactivation to assess the presence of cortico-collicular influences, demonstrate that dark-rearing does not prevent association cortex from developing robust influences over SC multisensory responses. In fact, dark-rearing may increase their potency over that observed in normally-reared animals. Nevertheless, their influences are still insufficient to support SC multisensory integration. It appears that cross-modal experience shapes the cortical influence to selectively enhance responses to cross-modal stimulus combinations that are likely to be derived from the same event. In the absence of this experience, the cortex develops an indiscriminate excitatory influence over its multisensory SC target neurons.
Keywords: Vision, Audition, Multisensory Integration, Development
Introduction
The superior colliculus receives afferents from multiple unisensory sources (i.e., visual, auditory, somatosensory) that facilitate its role in detecting, localizing, and orienting to external events (Stein and Meredith, 1993). Many of its neurons are multisensory. They not only receive converging afferents from different senses, but they have the capability to integrate the information these senses provide, thereby enhancing their responses and the salience of the initiating event (Stein and Stanford, 2008). This, in turn, increases the probability that events registered by more than one sense will initiate overt SC-mediated behavioral responses and improve an organism’s likelihood of survival in a variety of ecological circumstances (Stein et al., 1989; Kadunce et al., 1997; Burnett et al., 2004). The visual-auditory SC neuron in the cat is the most common and best studied multisensory exemplar neuron; however, the principles derived from its study apply to other modality convergence patterns, brain areas, and species (Stein, 1998).
Using this neuron as a developmental model, it has also been found that the ability to integrate cross-modal cues develops gradually as animals gain cross-modal experience during postnatal life (Stein, 2012; Wallace and Stein, 1997; 2001; Xu et al., 2012). It does not develop in animals reared in darkness (Wallace et al., 2004; Yu et al., 2010), because the requisite visual-auditory experience is precluded. However, the development of SC multisensory integration also depends on converging influences derived from unisensory neurons in ipsilateral association cortex (the anterior ectosylvian sulcus, AES; and the rostral lateral suprasylvian sulcus, rLS). Thus, deactivation of these areas disrupts the development and expression of SC multisensory integration capabilities and the behaviors they facilitate (Wilkinson et al., 1996; Wallace and Stein, 2000; Jiang et al., 2001; Jiang et al., 2006; Alvarado et al., 2007b). A functional synergy between visual and auditory tectopetal afferents is required for SC neurons to synthesize this cross-modal information (Alvarado et al., 2009). Apparently, no other cortical areas can substitute for these regions. Thus, there is no compensation in this context even when these cortical regions are lost very early in life, during the period in which the brain is its most plastic (Jiang et al., 2006).
The similarities in the responses of SC neurons in dark-reared animals and in animals in which influences from association cortex are eliminated suggest a common etiology, in which dark-rearing interferes with the development of essential influences from these cortices. This possibility is consistent with the deleterious impact of dark-rearing on the integrity of association cortex (Carriere et al., 2007). Alternatively, these cortico-collicular influences may develop in dark-reared animals and may even be capable of exerting substantial control over the responses of multisensory SC neurons. They may simply lack the ability to facilitate the process of SC multisensory integration. The present experiments were designed to examine these possibilities by testing whether cryogenic deactivation of this cortex in dark-reared cats has any effect on the responses of SC multisensory neurons. If so, this would indicate that the pathway develops even in the absence of the relevant sensory experience.
Materials and Methods
All protocols used were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Animal Care and Use Committee of Wake Forest University School of Medicine, an AAALAC-accredited institution.
Surgical preparation
All sterile surgeries were conducted using methods similar to those previously described (Jiang et al., 2001; Alvarado et al., 2009). Three adult cats (female) reared in a dark (light-tight) room from birth were used for this experiment. Each animal was pretreated with dexamethasone sodium phosphate (1 mg/Kg, i.m.) 12 hours prior to surgery. On the day of surgery, the animal was anesthetized in the dark room with ketamine hydrochloride (20mg/kg, i.m.) and acepromazine maleate (0.1 mg/kg, i.m.), transported to the surgical suite in a light-tight carrier, intubated, and placed on a heating pad and in a stereotaxic head-holder. Isoflurane (1.5–3%) was used to induce and maintain surgical anesthesia while the animal was artificially respired. Blood pressure, heart rate, CO2 level, and core body temperature were monitored continuously (Digital Vital Signs Moniotor, VetSpecs VSM7). Two craniotomies were made, one over AES and rLS (i.e., association cortex), and the other over the cortex overlying the SC. The dura over AES and rLS were reflected, and the walls of the sulci were carefully separated while cooling (i.e., deactivation) coils were gently inserted. The deactivation coils were prefabricated from 21-gauge steel stainless tubing (inner diameter: 0.023 inch; outer diameter: 0.032 inch) shaped appropriately to fit each sulcus (Lomber et al., 1999; Jiang et al., 2001; Alvarado et al., 2009). The area around the coils was tightly packed with moist gelfoam. A well providing access to the stems of the deactivation coils was attached to the skull using screws and orthopedic cement. A second well was attached to the skull over the SC craniotomy. Threaded openings on each side of the well were used to hold the head from posts during experimentation without wounds or pressure points (McHaffie and Stein, 1983). Both wells were sealed with removable caps. Postsurgical analgesics were administrated as needed (buprenorphine, 0.005–0.01 mg/Kg, every 12 h) and antibiotics were given for 7 days (ceftriaxone, 20 mg/Kg, twice daily). Once recovered from the surgery (10 days later), the animal was used for electrophysiological recordings in weekly sessions.
Experimental Procedures
Experimental procedures were as described previously (Yu et al., 2010). Briefly, the animal was anesthetized, intubated, and placed on a heating pad. The head-holding posts were used to attach the head well to the stereotaxic frame, and the animal was artificially respired. Paralysis was initiated with pancuronium bromide (0.1 mg/Kg) to prevent the eyes and ears from drifting during experimentation. Paralysis and anesthesia were maintained with continuous infusion (saline: 5% Dextrose and 0.9% sodium choloride and ketamine: 5.5–7.0 mg/Kg/h; pancuronium bromide: 0.05–0.07 mg/Kg/h) via the saphenous vein. Blood pressure, heart rate, respiratory CO2 level, and core body temperature were monitored continuously (VetSpecs VSM7). Body temperature was kept at 37–38°C and end-tidal CO2 was maintained at ~ 4.0%. The pupil of the eye contralateral to the SC being studied was dilated with ophthalmic atropine sulfate (1%) and covered by a contact lens to focus the eye on a translucent tangent screen placed in front of the animal, and upon which visual stimuli were projected. The other eye was covered with an opaque contact lens.
Electrophysiological recording
Conventional methods were used for single neuron electrophysiological recording. A glass-coated parylene-insulated tungsten electrode (impedance: 1–3MΩ at 1KHz), fixed to the stage of microdrive, was lowered to the surface of the SC manually. It was then advanced and retracted using a hydraulic microdrive. Neural signals were recorded, amplified and routed to an oscilloscope, audio monitor, and computer. The neural signal was evaluated visually on-line, and only well-isolated (e.g., 3:1 ratio of amplitude to noise) neurons whose shape did not change significantly during the recording period were analyzed. The signals were routed through a window discriminator for the construction of rasters and peristimulus time histograms. Upon terminating an experiment, all drugs were discontinued and the animal was returned to its home cage once stable respiration and coordinated locomotion were reinstated.
Apparatus and test stimuli
Visual stimuli consisted of moving bars of light (e.g., 6°×2°, 8°×2°) with different intensities (4.8–36.5 cd/m2). They were back-projected by an LC 4445 Philips projector onto a tangent screen placed 45 cm in front of the animal and which had a uniform gray background (0.62 cd/m2). The stimuli were moved across a neuron’s receptive field at different velocities (i.e., 50°–100°/sec). Auditory stimuli were brief (100–200ms) 55–75 dB SPL (Sound Pressure Level) broadband (20–20,000 Hz) noise bursts that were presented from moveable speakers against an ambient background of 48.4~52.7 dB SPL. All stimuli were controlled by custom software operating a NIDAQ digital controller (National Instruments) installed in a personal computer. Test stimuli were always placed within the neuron’s receptive fields. Cross-modal stimulus combinations consisted of the visual and auditory test stimuli in close spatial and temporal register and customized for each neuron (Jiang et al., 2001; Alvarado et al., 2007b; Yu et al., 2009; Yu et al., 2010). Each SC neuron’s responsiveness to visual, auditory, and cross-modal stimuli was examined prior to, during, and following cryogenic deactivation of both AES and rLS. Because the greatest proportionate response enhancement, even in normal animals, is obtained with poorly effective stimuli (i.e., the principle of inverse effectiveness) (Meredith and Stein, 1986), multiple levels of stimulus effectiveness were used when studying neurons to lessen the probability that cases of multisensory integration would be missed. In all cases weakly effective stimuli were included in the test battery.
To determine the generality of the observed effects, an additional cohort of somatosensory-responsive neurons were studied in the same manner as the visual-auditory neurons above. The somatosensory responses of these multisensory neurons are also only modestly affected by cortical deactivation in normal animals (Jiang et al., 2001). Somatosensory stimuli consisted of touching the skin or hairs with a cotton-tipped applicator or a probe with tips of variable sizes. The cotton-tipped applicator/probe was mechanically connected to and controlled by an electronically moving coil vibrator.
Cortical deactivation
Cortical deactivation was achieved by circulating refrigerated water (0°C) through the deactivation coils as in the past (Jiang et al., 2001; Alvarado et al., 2009). This lowers the temperature of the surrounding cortex to a stable level within 2–3 minutes. Rewarming and reactivation of cortex took place within a similar timeframe (generally 3–4 minutes). The temperature decrement with deactivation is greatest in the tissue adjacent to the coils, but is rapidly attenuated within 2 mms and has no effect on the surface temperature of the SC. These spatial and temporal gradients and the reversible deactivation effects on the physiology in the absence of anatomical changes in these cortices have been previously described in detail (see Jiang et al., 2001). To ensure that tests for SC multisensory integration would not begin in the present experiments before a stable cortical temperature decrement had been achieved, physiological testing did not begin until at least 5 min after the beginning of cortical deactivation. To ensure that rewarming and reactivation had reached a stable level after terminating the circulation of refrigerated water, post-deactivation tests did not begin until at least 8 minutes had passed. Both periods exceed those necessary in this context based on data from Jiang et al., (2001). However, to minimize the possibility of inducing long-term cortical damage by cortical deactivation, each period was kept to <30 minutes. In all cases the cortices were re-warmed passively by removing the refrigerated water from the coils.
Data analysis
A multisensory neuron was identified as one that responded to visual and auditory stimuli individually, and the multisensory response (i.e., to the combined cross-modal stimulus) was compared with the response evoked by the most effective modality-specific component stimulus to create the multisensory integration index (MSI): MSI = [(CM − SMmax)/ SMmax] × 100%, where CM represents the mean magnitude of multisensory response, SMmax represents the mean magnitude of the response evoked by the more effective modality-specific stimulus component (Meredith and Stein, 1983). MSI and SMmax are inversely correlated in normal animals (Stanford et al., 2005; Alvarado et al., 2007a), a well-established relationship used as a part of a standard in assessing “normal” multisensory integration. Data were compared statistically to determine significant differences using MATLAB R2011b and Sigmaplot10 using t-tests, paired t-tests, and Kolmogorov-Smirnov tests as noted. All of the data were expressed as mean ± standard deviation, and the criterion for statistical significance was p < 0.05.
Results
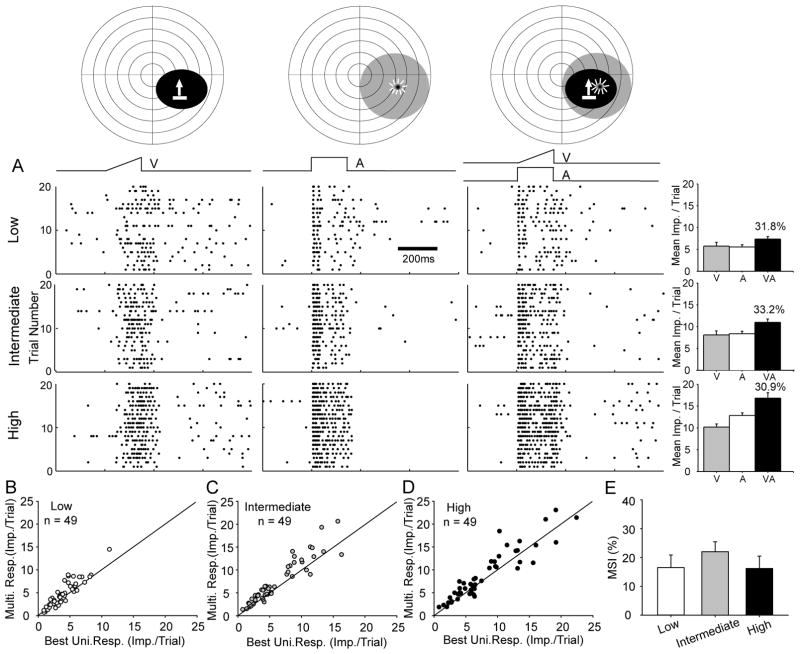
Consistent with prior observations (Wallace et al., 2004; Yu et al., 2010), the overwhelming majority (81.3%, 91/112) of neurons in dark-reared animals failed to exhibit a capacity to integrate their visual and auditory inputs despite tests with stimuli of varying degrees of effectiveness at multiple receptive field locations. Their responses to cross-modal stimulus combinations were not significantly different from their response to the most effective (“best”) of the component stimuli presented individually. An example is shown in Fig. 1A. Prior to deactivation, this neuron was not only unable to effectively integrate its visual and auditory inputs, but its MSI also failed to show the typical inverse correlation with stimulus effectiveness level (low: 31.8%; intermediate: 33.2%; high: 30.9%) that is evident in normal animals (Meredith and Stein, 1986; Stanford et al., 2005; Alvarado et al., 2007b).
Fig 1. SC multisensory neurons showed no multisensory integration capability at any level of stimulus effectiveness.
A: top, a representative neuron’s visual (black) and auditory (gray) receptive fields are shown on schematics of visual-auditory space. Icons identify the locations of the moving bar of light and the speaker that generated a broadband noise burst. Each concentric circle represents 10° of space. Below are shown raster plots of responses to the visual (V, left), auditory (A, middle), and cross-modal (VA, right) stimuli. Three levels of stimulus effectiveness (i.e., intensities) were used (top: low; middle: intermediate; bottom: high). Each dot represents one neuronal impulse, and each row represents one trial, with trials ordered from bottom to top. The visual and auditory stimuli are represented, respectively, by the ramp and square wave above the rasters. The horizontal line in the raster shows the time scale. Summary bar graphs on the right show the mean visual (gray), auditory (white), and multisensory (black) responses in terms of mean impulses per trial (error bars indicate s.e.m.). The number above each of the black bars indicates the MSI value. Note the absence of significantly elevated multisensory responses, and the lack of substantial differences in MSI as the effectiveness of stimuli changed. B-D: The comparison between the multisensory and largest (“best”) unisensory response for each neuron in the population studied was plotted for stimuli with low (B), intermediate (C) and high (D) effectiveness. Note the clustering of the points around the line of equality in each case. E: The summary bar graph shows that the population-averaged MSI did not change significantly or consistently as stimulus effectiveness increased.
The population results were consistent with the results in this individual example. In Fig. 1B–D the multisensory response of each neuron in the population is plotted against its best unisensory response for each of three levels of stimulus effectiveness: low (B), intermediate (C), and high (D). The results of these comparisons are summarized in Fig. 1E. As noted above for the individual example, the population MSIs (low: 16.5 ± 30.5 %, intermediate: 22.0 ± 24.1 %, high: 16.2% ± 29.8%) failed to show the expected inverse relationship with stimulus effectiveness and were not significantly different from one another (paired t-test; p > 0.05). In the comparatively small sample of neurons (18.7%) that did show a statistically significant response enhancement, the level of enhancement (i.e., MSI) was marginal, and they too failed to show the normal inverse relationship between MSI and stimulus effectiveness. This suggests that these cases of marginal enhancement were clearly anomalous as opposed to examples of a normal integrative process that was simply poorly effective.
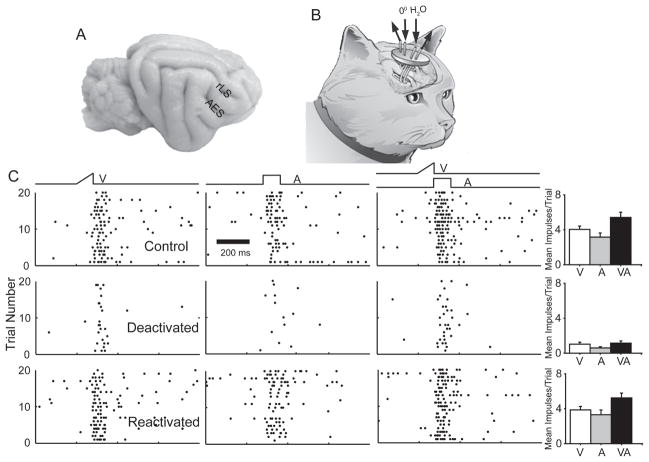
Despite the lack of multisensory integration capabilities in the majority of the visual-auditory SC neurons studied, their sensory responses proved to be significantly altered by deactivation of association cortex (AES and rLS) (Fig. 2A–B). This observation revealed that the cortico-collicular influences of association cortex had developed in these neurons despite the absence of visual and visual-auditory experience. In the vast majority (88%, 61/69) of neurons completing the full control-deactivation-reactivation series, both unisensory and multisensory response magnitudes were significantly reduced by cortical deactivation. This effect is illustrated by the example neuron in Fig. 2C. Prior to deactivation, the neuron’s mean visual, auditory, and multisensory responses were, respectively: 4.0, 3.2, and 5.3 impulses/trial. When association cortex was deactivated, the visual response decreased by 70% (to 1.2 impulses/trial), the auditory response by 81% (to 0.6 impulses/trial), and the multisensory response by 74% (to 1.4 impulses/trial). When the cortex was reactivated, each of the response magnitudes returned to approximately its pre-deactivation level.
Fig 2. Cortical deactivation significantly decreased the unisensory and multisensory responses of multisensory SC neurons.
A: The locations of AES and rLS are shown on a photograph of the cat brain. B: A diagram illustrates the location of the implanted deactivation coils through which refrigerated water was circulated. C: Raster plots from this representative visual-auditory neuron show its visual, auditory, and multisensory responses under 3 conditions: control (top), during deactivation of AES/rLS (middle), and following recovery from deactivation (bottom). The bar graphs on the right summarize the response magnitudes for each modality. Note the absence of significantly enhanced multisensory responses under any conditions, and the near equivalence of the effects of cortical deactivation on unisensory and multisensory responses. Conventions are the same as in Fig. 1.
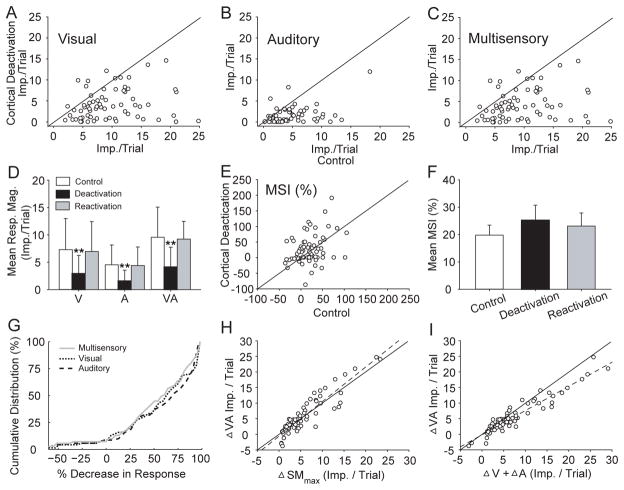
The effects of cortical deactivation were consistent across the population of neurons. To avoid the possible confounding floor effects of responses to weakly effective stimuli, the consequences of cortical deactivation presented in Fig. 3 were computed using modality-specific and cross-modal stimuli that were highly effective for each neuron studied. Here the visual (Fig. 3A), auditory (Fig. 3B), and multisensory (Fig. 3C) responses are compared before and during cortical deactivation across the sample population. In each case there was a substantial reduction in the responses elicited during cortical deactivation. Fig. 3D summarizes these data, showing the mean response magnitudes before deactivation (control), during deactivation, and after reactivation for each response category. Deactivation of association cortex decreased the mean visual response by more than half (from 7.66 ± 5.75 to 2.91 ± 3.29 impulses/trial), the mean auditory response by about two-thirds (from 4.49 ± 3.52 to 1.50 ± 1.96 impulses/trial), and the mean multisensory response by more than half (from 9.91 ± 5.56 to 4.09 ± 3.62 impulses/trial).
Fig 3. Cortical deactivation dramatically decreased response magnitude but did not significantly change the underlying response relationships among the unisensory and multisensory responses of SC neurons.
A–C: The visual, auditory, and multisensory responses of multisensory SC neurons were compared in control and cortical deactivation conditions. D: The bar graph summarizes these comparisons across the population of neurons studied showing the mean visual, auditory and multisensory responses before (Control), during (Deactivation) and after (Reactivation) cortical deactivation (error bars indicate s.d., **p<.001). E–F: Although mean MSI was skewed positively in control and cortical deactivation conditions, deactivation did not induce a statistically significant change (error bars indicate s.e.m.). G: The cumulative population distributions reveal the close correspondence in the pecentage changes that were induced by cortical deactivation in multisensory (gray), visual (dot) and auditory (dash) responses. Change % = 100*(control - deactivation)/control. H: The multisensory response changes induced by cortical deactivation (ΔVA) were not significantly different from those induced in the best unisensory responses (ΔSMmax) as indicated by the clustering of points around the line of equality (solid) and the parallel regression line (dashed). I: However, the sum of the unisensory response changes (ΔV + ΔA) significantly exceeded changes in the multisensory responses.
However, deactivation of cortex did not consistently change the relationship between the multisensory and its unisensory comparator responses (i.e., MSI) as it does in normal animals (Wallace and Stein, 1994; Jiang et al., 2001; Alvarado et al., 2007b). Fig. 3E summarizes the distribution of MSIs of the population before and during cortical deactivation. The MSI values were not significantly different (paired t-test; p > 0.05) before deactivation (19.9 ± 30.4%), during deactivation (25.2 ± 46.2%), and after reactivation (23.1 ± 39.7%) (Fig. 3F).
Thus, the effects of cortical deactivation on SC responses in dark-reared animals differed from the effects on normal animals in two ways. First, the unisensory responses in dark-reared animals appeared to be highly dependent on influences from association cortex, while in normal animals this dependence was noted as marginal in most cases (Jiang et al., 2001; Alvarado et al., 2007b). Second, cortical deactivation in normal animals disproportionately decreases the multisensory response, whereas in this case, the cortex appeared to have roughly the same degree of influence over the visual, auditory, and visual-auditory responses. Put another way, the influence of the cortex on SC sensory responses in the present neuronal sample appeared to be non-selective, whereas in the SC neurons of normal animals its influence is expressed as a selective enhancement of their multisensory responses.
The nature of this non-selective cortico-collicular influence is summarized in Fig. 3G, where the effect of cortical deactivation (quantified as % decrement in response magnitude) on the multisensory and two unisensory responses is shown in a cumulative distribution plot. The distributions of the effects on the unisensory and multisensory responses showed a great deal of overlap. Furthermore, they are smooth and continuous, with no notable discrete subpopulations that appeared to be completely dependent or independent of the cortical influence.
This lack of selectivity was confirmed by the strong correlations between the effect of cortical deactivation on an individual neuron’s unisensory and multisensory responses. Indeed, the raw decrease in the magnitude of the multisensory response was closely matched to the raw decrease in the magnitude of the best (i.e., the comparator) unisensory response (paired t-test, p = 0.86) (Fig. 3H). This observation contrasts with what would be the simplest predicted relationship, that the decrement in the multisensory response would approximate the sum of the decrements in the two unisensory responses. Importantly, this was not observed (Fig. 3I): the decrease in the magnitude of the multisensory response (5.83 ± 6.05 impulses/trial)was significantly smaller than the linear sum of the decreases in the two unisensory responses (7.73±7.48 impulses/trial; paired t-test, p < 0.001). Thus, the pre-deactivation relationship among these SC sensory responses is preserved. This underscores the utility of using the best unisensory response as the comparator for multisensory integration (Stein et al., 2009; Stein et al., 2010), and highlights the non-selective nature of the cortical deactivation effect in these dark-reared animals.
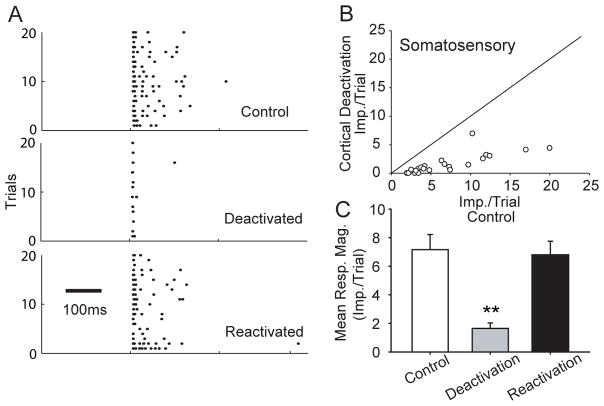
The possibility that the observed dependency on the cortex was not restricted to visual and auditory responses prompted an examination of an additional cohort of 22 somatosensory-responsive multisensory SC neurons. As can be seen in Fig. 4, the same striking depressive effect of cortical deactivation described above was also seen on somatosensory SC responses. These data speak to the generality of the dependence of multisensory neurons on association cortex for their sensory responses following dark-rearing.
Fig 4. Cortical deactivation also decreased the somatosensory responses of SC neurons.
A: Raster plots show somatosensory responses prior to cortical deactivation (top), during cortical deactivation (middle) and after cortical reactivation (bottom) in an individual exemplar neuron. B: The scatter plot demonstrates the comparison of somatosensory responses between control (x-axis) and cortical deactivation condition (y-axis) for the population of neurons studied. C: A summary of the mean somatosensory response for each condition. All conventions are the same as in previous figures.
Discussion
The present experiments were directed at understanding the relationship between the two major developmental events that normally establish multisensory integration capability in SC neurons: the acquisition of cross-modal (i.e., visual-auditory) experience, and the maturation of cortico-collicular influences from association cortex (i.e., AES/rLS). Of specific interest was whether disruption of the maturation of multisensory integration capabilities in SC neurons by precluding visual-auditory experience (i.e., by rearing animals in darkness) would be due to their inability to develop these cortico-collicular influences. The results demonstrate that the influences from association cortex not only developed in these animals, but appeared to have had an impact on their target SC neurons that was substantially greater than that seen in animals reared with normal visual and visual-auditory experience. But, unlike their influences in normal animals, the cortico-collicular influences observed in dark-reared animals were non-selective. They appeared to modulate SC sensory responses indiscriminately rather than preferentially enhancing their multisensory responses.
Preferential enhancement by cortex is a key component of SC multisensory integration and is a typical feature of the vast majority of multisensory SC neurons in normally-reared animals (Meredith and Stein, 1986; Perrault et al., 2005; Jiang et al., 2006; Alvarado et al., 2007a; Pluta et al., 2011). It ensures that SC responses to cross-modal stimuli significantly exceed those to the most effective of their component stimuli (sometimes exceeding the arithmetic sum of their unisensory component responses) and extend to the overt behaviors that these SC neurons mediate (Stein et al., 1989; Wilkinson et al., 1996; Jiang et al., 2002; Burnett et al., 2004; Burnett et al., 2007). The selectivity of cortically-induced response modulation of SC multisensory responses is evident not only in its marginal influence on responses to individual modality-specific stimuli, but also in the absence of its enhancement of neural responses to pairs of within-modal stimuli (Alvarado et al., 2007b), a stimulus configuration that has marginal differential effects on SC-mediated behaviors (Gingras et al., 2009).
While the present observations are consistent with the contention that cross-modal experience is critical for developing the link between senses that is essential for the maturation of SC multisensory integration capabilities, they also suggest that these cross-modal experiences operate in this circuit by altering the nature of the influences exerted by association cortical neurons over their SC multisensory target neurons. The cortex appears to serve as the portal for utilizing these experiences to craft the influences it needs to support the development of multisensory integration capabilities in SC neurons. In its absence, the cortex develops a general and more robust enhancement influence over all sensory responses. Whether the “abnormal” influences that it develops when not privy to relevant cross-modal events is due to its failure to create the required neuronal response properties in its constituent neurons and/or to properly craft their synaptic contacts in the SC is not yet known. But, the nondiscriminatory influences that its neurons do develop are quite unlike those seen in animals whose cortices are able to use cross-modal experience. The latter become less important for enhancing the overall sensory response magnitude of SC multisensory neurons and more important for the selective enhancement of their responses to cross-modal stimulus combinations that they have previously experienced (Xu et al., 2012).
The specific mechanism by which association cortex normally produces multisensory enhancement in the SC is unknown, but there are a number of possibilities being evaluated that are consistent with the results of the present experiments. These evaluations emphasize the importance of developing appropriate cortico-collicular synaptic convergence patterns. For example, a popular speculation is that the apparent specificity of this cortical influence on the SC multisensory product is due to achieving a particular weighting of inputs that depend on connectivity patterns (Patton and Anastasio, 2003; Rowland et al., 2007; Cuppini et al., 2010; Ohshiro et al., 2011). If the appropriate cortico-collicular projection pattern fails to develop in the absence of the directed influence of cross-modal experience, the resultant cortical influence would be predicted to be non-specific and unable to support enhanced multisensory responses. Deactivating cortex would be expected to alter all SC sensory responses in a roughly proportionate manner, so that the relative difference between responses evoked by modality-specific and cross-modal stimuli would be unchanged. These predictions correspond quite well with the empirical results obtained in the present experiments.
However, the response properties that association cortex neurons develop are also sensitive to disruptions in visual input, such as those induced by dark-rearing. This was previously noted by Rauschecker and Korte (1993), who showed that binocular lid suture decreases the incidence of neurons capable of responding to visual input in AES and allows an opportunistic expansion of its non-visual representations (Rauschecker and Korte, 1993). Also relevant here are the observations of Carriere et al. (2007) who showed that dark-rearing alters the excitatory-inhibitory balance in AES neurons to visual and auditory stimuli. Any changes in the visual and auditory response properties of association cortical neurons are likely to be reflected in the influences they exert over their SC target neurons.
Regardless of where the developmental failure lies, a final common feature that cross-modal experience normally induces in this circuit is missing: the synergy among the converging unisensory inputs that descend from association cortex to ensure its selectivity on multisensory integration (Alvarado et al., 2008; Alvarado et al., 2009). It is likely that there are many ways to induce this failure other than dark-rearing, and there is little reason to expect that the deficit would be any less compelling if cross-modal experience were compromised by auditory rather than visual deprivation. DELETION. Preliminary observations using auditory deprivation via a masking noise are consistent with this assumption (Xu et al., 2011). Similarly, if association cortex were rendered inactive during the period in which an animal is exposed to specific cross-modal stimulus combinations, it should also be unable to use those experiences to later foster multisensory integration in SC neurons. This assumption is also consistent with our recent preliminary observations (Stein 2012). Underscoring the widespread and nonspecific effect of the dark-rearing condition on association cortical influences were the changes induced by its deactivation on somatosensory SC responses. The vigor of these responses was compromised much like those evoked by visual and auditory stimuli.
Although beyond the scope of the present experiments, it would be of interest to compare the effects of different sensory deprivation paradigms on the responses of SC multisensory neurons. Visual deprivation may have had an exceptionally strong effect on the circuitry of the SC given that the primary role of this structure is visuomotor, and its sensitivity to visual input is greater than to any other modality. The strong, non-specific excitatory influence of association cortex on the unisensory (visual, auditory, somatosensory) and multisensory responses of its multisensory neurons observed in the present experiments may not be paralleled by depriving the brain of non-visual (i.e., somatosensory or auditory) inputs. The latter may have far less impact on the robustness of those responses. On the other hand, if, as hypothesized above, the development of multisensory integration capabilities depends on using cross-modal experiences to craft multisensory specificity in the cortico-collicular circuit, it is likely that different unisensory deprivation paradigms would also have at least one common effect. They would all eliminate the capability of neurons with the matching modality in their convergence pattern to engage in multisensory integration. The responses of neurons with other convergence patterns would be unaffected. Particularly helpful in evaluating this possibility are trisensory SC neurons. Each trisensory neuron has three possible convergence patterns by which multisensory integration can be assessed. Presumably, its deficits under this situation would be reflected in the loss of multisensory integration in response to only the two patterns that include the deprived modality. This would be an ideal candidate for helping us to understand these issues, and for evaluating possible rehabilitative strategies that facilitate sensorimotor function after cortical damage (Bolognini et al., 2005; Leo et al., 2008), or after the amelioration of congenital dysfunction by the removal of cataracts or the implantation of visual or auditory prostheses.
Acknowledgments
This research was supported by NIH grants EY016716 and NS036916. We thank Nancy London for technical assistance.
Abbreviations
- AES
the anterior ectosylvian sulcus
- SC
superior colliculus
- rLS
and the rostral lateral suprasylvian sulcus
Footnotes
No conflicts of interests, finances and otherwise are declared by the author(s).
References
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol. 2007a;97:3193–3205. doi: 10.1152/jn.00018.2007. [DOI] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 2007b;27:12775–12786. doi: 10.1523/JNEUROSCI.3524-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Rowland BA, Stanford TR, Stein BE. A neural network model of multisensory integration also accounts for unisensory integration in superior colliculus. Brain Res. 2008 doi: 10.1016/j.brainres.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci. 2009;29:6580–6592. doi: 10.1523/JNEUROSCI.0525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Rasi F, Coccia M, Ladavas E. Visual search improvement in hemianopic patients after audio-visual stimulation. Brain. 2005;128:2830–2842. doi: 10.1093/brain/awh656. [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Chaponis D, Wallace MT. Superior colliculus lesions preferentially disrupt multisensory orientation. Neuroscience. 2004;124:535–547. doi: 10.1016/j.neuroscience.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Burnett LR, Stein BE, Perrault TJ, Jr, Wallace MT. Excitotoxic lesions of the superior colliculus preferentially impact multisensory neurons and multisensory integration. Exp Brain Res. 2007;179:325–338. doi: 10.1007/s00221-006-0789-8. [DOI] [PubMed] [Google Scholar]
- Carriere BN, Royal DW, Perrault TJ, Morrison SP, Vaughan JW, Stein BE, Wallace MT. Visual deprivation alters the development of cortical multisensory integration. J Neurophysiol. 2007;98:2858–2867. doi: 10.1152/jn.00587.2007. [DOI] [PubMed] [Google Scholar]
- Cuppini C, Ursino M, Magosso E, Rowland BA, Stein BE. An emergent model of multisensory integration in superior colliculus neurons. Front Integr Neurosci. 2010;4:6. doi: 10.3389/fnint.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras G, Rowland BA, Stein BE. The differing impact of multisensory and unisensory integration on behavior. J Neurosci. 2009;29:4897–4902. doi: 10.1523/JNEUROSCI.4120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Two corticotectal areas facilitate multisensory orientation behavior. J Cogn Neurosci. 2002;14:1240–1255. doi: 10.1162/089892902760807230. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol. 2006;95:1380–1396. doi: 10.1152/jn.00880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 2001;85:506–522. doi: 10.1152/jn.2001.85.2.506. [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE. Mechanisms of within- and cross-modality suppression in the superior colliculus. J Neurophysiol. 1997;78:2834–2847. doi: 10.1152/jn.1997.78.6.2834. [DOI] [PubMed] [Google Scholar]
- Leo F, Bertini C, di Pellegrino G, Ladavas E. Multisensory integration for orienting responses in humans requires the activation of the superior colliculus. Exp Brain Res. 2008;186:67–77. doi: 10.1007/s00221-007-1204-9. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Horel JA. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 1999;86:179–194. doi: 10.1016/s0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. A chronic headholder minimizing facial obstructions. Brain Res Bull. 1983;10:859–860. doi: 10.1016/0361-9230(83)90220-4. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol. 1986;56:640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Angelaki DE, DeAngelis GC. A normalization model of multisensory integration. Nat Neurosci. 2011;14:775–782. doi: 10.1038/nn.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton PE, Anastasio TJ. Modeling cross-modal enhancement and modality-specific suppression in multisensory neurons. Neural Comput. 2003;15:783–810. doi: 10.1162/08997660360581903. [DOI] [PubMed] [Google Scholar]
- Perrault TJ, Jr, Vaughan JW, Stein BE, Wallace MT. Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J Neurophysiol. 2005;93:2575–2586. doi: 10.1152/jn.00926.2004. [DOI] [PubMed] [Google Scholar]
- Pluta SR, Rowland BA, Stanford TR, Stein BE. Alterations to multisensory and unisensory integration by stimulus competition. J Neurophysiol. 2011;106:3091–3101. doi: 10.1152/jn.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Korte M. Auditory compensation for early blindness in cat cerebral cortex. J Neurosci. 1993;13:4538–4548. doi: 10.1523/JNEUROSCI.13-10-04538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BA, Stanford TR, Stein BE. A model of the neural mechanisms underlying multisensory integration in the superior colliculus. Perception. 2007;36:1431–1443. doi: 10.1068/p5842. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci. 2005;25:6499–6508. doi: 10.1523/JNEUROSCI.5095-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE. Early Experience Affects the Development of Multisensory Integration in Single Neurons of the Superior Colliculus. In: Stein BE, editor. The new handbook of multisensory processing. Cambridge, MA: MIT Press; 2012. pp. 589–606. [Google Scholar]
- Stein BE. Neural mechanisms for synthesizing sensory information and producing adaptive behaviors. Exp Brain Res. 1998;123:124–135. doi: 10.1007/s002210050553. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA, Huneycutt WS, McDade L. Behavioral indices of multisensory integration: Orientation to visual cues is affected by auditory stimuli. J Cognit Neurosci. 1989;1:12–24. doi: 10.1162/jocn.1989.1.1.12. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Ramachandran R, Perrault TJ, Jr, Rowland BA. Challenges in quantifying multisensory integration: alternative criteria, models, and inverse effectiveness. Exp Brain Res. 2009;198:113–126. doi: 10.1007/s00221-009-1880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Burr D, Constantinidis C, Laurienti PJ, Alex Meredith M, Perrault TJ, Jr, Ramachandran R, Roder B, Rowland BA, Sathian K, Schroeder CE, Shams L, Stanford TR, Wallace MT, Yu L, Lewkowicz DJ. Semantic confusion regarding the development of multisensory integration: a practical solution. Eur J Neurosci. 2010;31:1713–1720. doi: 10.1111/j.1460-9568.2010.07206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol. 1994;71:429–432. doi: 10.1152/jn.1994.71.1.429. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 1997;17:2429–2444. doi: 10.1523/JNEUROSCI.17-07-02429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Onset of cross-modal synthesis in the neonatal superior colliculus is gated by the development of cortical influences. J Neurophysiol. 2000;83:3578–3582. doi: 10.1152/jn.2000.83.6.3578. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Sensory and multisensory responses in the newborn monkey superior colliculus. J Neurosci. 2001;21:8886–8894. doi: 10.1523/JNEUROSCI.21-22-08886.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Jr, Hairston WD, Stein BE. Visual experience is necessary for the development of multisensory integration. J Neurosci. 2004;24:9580–9584. doi: 10.1523/JNEUROSCI.2535-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA, Stein BE. The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp Brain Res. 1996;112:1–10. doi: 10.1007/BF00227172. [DOI] [PubMed] [Google Scholar]
- Xu J, Yu L, Rowland BA, Stanford TR, Stein BE. Incorporating cross-modal statistics in the development and maintenance of multisensory integration. J Neurosci. 2012;32:2287–2298. doi: 10.1523/JNEUROSCI.4304-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Stein BE, Rowland BA. Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci. 2009;29:15910–15922. doi: 10.1523/JNEUROSCI.4041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Rowland BA, Stein BE. Initiating the development of multisensory integration by manipulating sensory experience. J Neurosci. 2010;30:4904–4913. doi: 10.1523/JNEUROSCI.5575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]