Abstract
Molecules containing damage-associated molecular patterns (DAMP) play an important role in many pathogenic processes. Our aim was to investigate the role of IL-33, a DAMP molecule, in adenovirus (Ad)-induced liver inflammation. Ad-infected mice exhibited a steadily increased IL-33 and its receptor ST2 expression in the liver during the first week of the infection. Treatment of exogenous IL-33 resulted in a great decrease in the serum alanine aminotransferase (ALT) levels and the number of Councilman bodies in the liver. Attenuated liver injury by IL-33 correlated with an increase in T regulatory (Treg) cells but with a decrease in macrophages, dendritic cells and NK cells in the liver. IL-33 enhanced both type 1 (IL-2 and IFN-γ) and type 2 (IL-5 and IL-13) immune responses in infected mice. However, IL-33 inhibited TNF-α expression in hepatic T cells and macrophages, and significantly reduced TNF-α levels in the liver. We found that in addition to its direct effects, IL-33 strongly induced novel nuocytes in the livers and spleens of infected mice. When co-cultured with nuocytes, hepatic T cells and macrophages expressed lower levels of TNF-α. The IL-33-treated mice also demonstrated a slight delay, but no significant impairment, in eliminating an intrahepatic infection with Ad. In conclusion, this study reveals that IL-33 acts as a potent immune stimulator and a hepatoprotective cytokine in acute viral hepatitis. Its direct immunoregulatory functions and ability to induce novel nuocytes further suggest to us that it may be a potentially promising therapeutic candidate for the management of viral hepatitis.
Introduction
Viral hepatitis is a major public health problem affecting millions of people in the world. There is no vaccine to prevent hepatitis C virus (HCV) infection or to treat the disease. Recently, experimental vaccines based on adenovirus (Ad) vectors have been shown to induce sustained protective immunity against HCV in humans (1). In addition to its potential values in vaccine development (2), Ad is also commonly used for gene delivery and cancer therapy (3, 4). Despite its utilities, Ad infection can also induce strong CD8+ CTL, CD4+ Th and B lymphocyte responses (5, 6), a common characteristic among a number of hepatotropic viruses, including HAV, HBV, cytomegalovirus, herpes simplex, and Epstein-Barr virus. When i.v. injected, Ad preferentially targets the liver. Although the majority of the invading Ad is eliminated by the innate immune mechanisms, the clearance of the remnant virus is slow and variable dependent on the virus-specific CTL and Th responses (5, 7, 8). Failure to constrain immune responses can turn a self-limiting infection to necroinflammatory hepatitis, and/or result in treatment failure, or even patient death (9, 10). While the liver injury associated with Ad-infection is known to be mediated by hepatotoxic cytokines such as TNF-α (11, 12), the key events that set off hepatic inflammation and regulate immune responses in the liver are still not totally clear.
Immune responses are often initiated by recognition of pathogen-associated molecular pattern (PAMP) molecules (13). Engagement of PAMPs in macrophages, γδ T cells, and dendritic cells (DCs) triggers their phagocytic or endocytotic activities as well as cytokine and chemokine secretion. In the case of autoimmune diseases, ischemia and reperfusion injury and chronic organ rejection events, strong immune responses are initiated and perpetuated by damage-associated molecular pattern (DAMP) molecules (14, 15). They are also known to synergize with PAMPs in infectious diseases, and further enhance immune responses (16).
Among DAMPs, IL-33 is a member of the IL-1 superfamily and binds to IL-1 receptor-like 1 (ST2), which signals the NF-κb pathway (17). IL-33 is released from necrotic cells during tissue injury, and is closely correlated with serum alanine aminotransaminase (ALT) and aspartate aminotransferase (AST) levels in chronic hepatitis patients (17, 18). IL-33 is a crucial amplifier of the innate immunity and drives antiviral CD8+ cell responses (19, 20). Yet IL-33 can also induce the expression of Th2-like cytokines (e.g. IL-5 and IL-13) as well as T regulatory (Treg) cells, and prolong cardiac allograft survival (21, 22). Collectively, these reports indicate a broad function of IL-33 in infectious and non-infectious diseases, as well as its multifunctional and enigmatic mechanisms of action. IL-33 can be released from endothelial and epithelial cells (23). Lately, it has been reported that epithelial cells, hepatocytes, and hepatic stellate cells are the main sources of IL-33 in the liver (24–26), and that IL-33 protects the liver from Con A- and ischemia/reperfusion-induced liver injury (27, 28). However, it is unclear whether such a protective mechanism is due to a direct, Th2-mediated immune response, or, alternatively, to the induction of other cell populations, such as novel nuocytes first reported in 2010 (29). Nuocytes are often referred to as innate type 2 cells, innate type 2 helper cells, or nature helper cells (29–32). These cells belong to a heterogeneous family of innate cells that do not express T or B lymphocyte markers. Nuocytes are present in human and mouse lungs, gut, and fat-associated lymphoid clusters, as well as in the liver (32–34), and contribute to type 2 immune responses and tissue repair in asthma and parasitic diseases in an antigen-non-specific fashion (29, 35). However, their role in viral hepatitis and hepatic inflammation is not understood.
In this study, we found a steady increase of IL-33 and ST2 expression in the liver in parallel with inflammatory cytokines during the first week of Ad infection in mice. While the administration of IL-33 enhanced both type 1 and type 2 immune responses, it increased Treg cell frequencies and significantly reduced liver injury. IL-33 inhibited the TNF-α levels in the liver-derived CD4+, CD8+ T and CD11b+ cells. More importantly, IL-33 induced a strong expansion of nuocytes in vivo, which further suppressed TNF-α expression in the hepatic lymphocytes. In conclusion, this study indicates that DAMP molecule IL-33 can act as both a potent immune stimulator and a hepatoprotective cytokine in viral hepatitis. Its direct immunoregulatory functions and ability to induce novel nuocytes further suggest it as a potentially promising therapeutic candidate for the management of viral hepatitis.
Materials and methods
Animals and treatment
Female C57BL/6 (B6) mice were purchased from the Jackson Laboratory. All mice were maintained under specific pathogen-free conditions at the UTMB animal care facility and used at 7–10 wk of age, according to the NIH Guidelines and with the approval of the IACUC. Mice were i.v. injected with 3 × 109 PFU Ad carrying the lacZ gene (AdLacZ) that expresses a reporter β-gal, to induce hepatitis. Cytokines from Biolegend were used in our studies, unless specified otherwise. Recombinant mouse IL-33 (0.8 μg/mouse) or PBS was administered i.p. daily from 1 to 5 days post-infection (dpi) and mice were sacrificed at the indicated time points. For the isolation of innate type 2 cells, naïve mice were i.p. injected daily with IL-33 (0.8 μg/mouse) for 5 days and euthanized at 6 dpi.
H&E and histological scores
Liver specimens were fixed in 10% buffered formalin. Paraffin-embedded sections were stained with H&E for histological evaluation by using a modified Knodell score system (36). Briefly, normal liver architecture without remarkable injury or cellular infiltration is scored as 0. Score 1 represents limited infiltration of inflammatory cells in the portal triad without significant involvement in the lobular and pericentral regions. In addition to these pathological changes, score 2 depicts moderate involvement in the portal areas, accompanied by isolated apoptosis and necrosis in the lobular and pericentral areas. Score 3 involves extensive lymphocyte infiltration in the portal area with widespread apoptosis and bridging necrosis throughout the liver.
Detection of AdLacZ in liver
The detection was performed as previously described (37). Briefly, following fixation with 0.5% glutaraldehyde for 30 min, frozen liver sections were incubated with 0.2 mg of X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml at 37°C for 120 min. Infected cells expressing β-gal activity were stained blue, whereas uninfected cells were counterstained red (neutral red). Eight images of each liver section were randomly selected and captured with the Olympus BX51 microscope equipped with the Olympus DP70 video camera (Olympus Optical, Tokyo, Japan). The images were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). Infectivity of the hepatocytes was expressed as the average ratio of infected to total cells.
Isolation of cell populations
For intrahepatic lymphocyte (IHL) isolation, livers were perfused, digested and purified by density gradient centrifugation with 30% and 70% Percoll (Sigma). For splenic lymphocyte isolation, the spleen was pressed through a 75-μm strainer. After treatment with the red blood cell lysing buffer (Sigma), mononuclear cells were collected. For lineage-negative (Lin−) and lineage-positive (Lin+) cell isolation, spleen cells were prepared from the IL-33-treated mice, blocked with FcR blocker (clone 2.4G2, eBioscience), and then incubated with FITC-conjugated anti-CD3, CD4, CD8, CD11b, CD11c, B220, NK1.1, Ter-119 and Gr-1 (eBioscience) for 30 min at 4°C. After washing, cell suspensions were incubated with anti-FITC MicroBeads, followed by a negative selection on the LD column (Miltenyi Biotec). The purities of the Lin− and Lin+ cells were 92% and 93%, respectively.
Cell culture
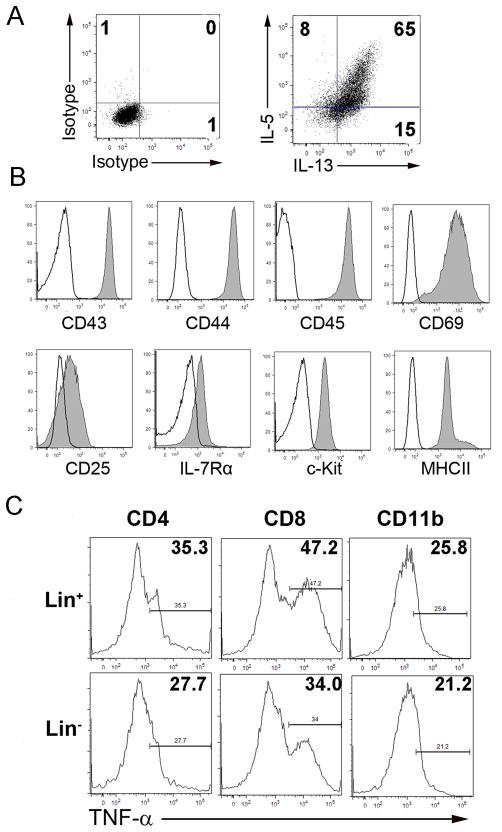
Lymphocytes isolated from 6 day-infected mice were seeded at 1 × 106/ml in 24-well plates incomplete RPMI medium at 37°C and with 5% CO2. In some cultures, IL-2 and IL-7 (10 ng/ml) were used together with various concentrations of IL-33. After 48 h, cells were collected for FACS analysis. In some experiments, Lin− cells were seeded at 1 × 105/ml in 6-well plates in complete RPMI medium at 37°C and with 5% CO2. To expand the cells, IL-2, IL-7 and IL-33 (10 ng/ml) were added respectively to the culture system. The medium was changed every 2 days, and the cytokines were supplemented. Cultured cells were analyzed for the surface markers CD43, CD44, CD45, CD69, CD25, IL-7Rα, c-Kit and MHC II, as well as the intracellular cytokines IL-5 and IL-13, by flow cytometry to confirm the characteristics of nuocytes as reported (29).
Flow cytometry
Cells were blocked with FcγR blocker first and stained with fluorochrome-labeled antibodies or biotinylated mAbs, followed by fluorochrome-conjugated streptavidin. The specific Abs and their corresponding isotype controls were purchased from Biolegend and eBioscience. The following Abs were used in combinations: FITC-anti-CD3 ε (145-2C11), PE-Cy7-anti- CD3 ε (145-2C11), FITC-anti-CD4 (GK1.5), Pacific Blue-anti-CD4 (RM-4.5), allophycocyanin-Cy7-anti-CD4 (RM-4.5), FITC-anti-CD8a (53.6.7), allophycocyanin-Cy7-anti-CD8a (53.6.7), Pacific Blue-anti-CD8 (53.6.7), FITC-anti-CD11b (M1/70), Percp-Cy5.5-anti-CD11b (M1/70), FITC-anti-CD11c (N418), allophycocyanin-anti-CD25 (PC61.5), Biotin-anti-CD43 (1B11), PE-anti-CD44 (IM7.8.1), PE-anti-CD45 (30-F11), FITC-anti-B220 (RA3-6B2), PE-anti-CD80 (16-10A1), Biotin-anti-CD90.2 (30-H12), PE-anti-CD127 (A7R34), FITC-anti-NK1.1 (PK136), PE-Cy7-anti-NK1.1 (PK136), FITC-anti-Gr-1 (RB6-8C5), FITC-anti-Ter-119 (TER-119), PE-anti-IL-4 (BVD4-1D11), PE-anti-IL-5 (TRFK5), PE-anti-IL-6 (MP5-20F3), Alexa Flour 647-anti-IL-13 (ebio13A), PE-anti-TNF-α (MP6-XT22), allophycocyanin-anti-IFN-γ (XMG1.2), allophycocyanin-anti-Foxp3 (FJK-16s), PE-anti-IgG2a isotype (m2a-15F8), allophycocyanin-anti-IgG2b isotype (eBMG2b) and FITC-anti-IgG2b isotype (eBMG2b).
For intracellular cytokines staining, cells were incubated for 4 h with PMA (50 ng/ml, Sigma), ionomycin (750 ng/ml, Sigma) and GolgiStop (1μl/ml BD Bioscience). At the end of the incubation, cells were collected and blocked with FcγR blocker (1 μg/106 cells, eBioscience) before extracellular staining for the corresponding fluorochrome-labeled monoclonal antibodies for markers. After surface staining, cells were fixed, permeabilized and stained for intracellular cytokines by using a fixation/permeabilization kit (eBioscience). Data were collected by LSRII FACS Fortessa (Becton Dickinson, San Jose, CA), and analyzed by using FlowJo software 8.86 (TreeStar, Ashland, OR).
Quantitative RT-PCR
Snap-frozen liver tissues were used to extract genomic DNA and total RNA. DNA was extracted with a DNeasy Blood & Tissue kit (Qiagen) and total RNA was extracted with an RNeasy mini kit (Qiagen) and digested with DNase I (Ambion). The concentrations of DNA and RNA were assessed by spectrophotometer (Eppendorf). cDNA was synthesized by using a SuperScript III First-Strand Synthesis System (Invitrogen). The quantitative RT-PCR (qRT-PCR) assays were performed with iQ SYBR Green Supermix and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The PCR assays were denatured for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. Melt-curve analysis was also used to check the specificity of the amplification reaction. Relative quantity of mRNA expression was calculated by using the 2−ΔΔCT method. The primers are listed in Table S1.
ELISA assay
For extracting proteins from the liver, liver tissues were suspended in a RIPA lysis buffer (Cell signal) that contains 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4 and 1 μg/ml leupeptin in the presence of the protease inhibitor cocktail (Sigma). Protein concentrations were assayed by using a BCA kit (Pierce). The levels of IL-33 in serum and liver tissues were assayed by using an ELISA kit (eBioscience) according to the manufacturer’s instruction.
Bio-Plex assays
Serum IFN-γ and IL-2 were examined on a Bio-Plex platform (Bio-Rad). Briefly, colored beads coated with different antigens were mixed together with serum sample, and then allowed to incubate for overnight at 2–8°C. After two wash cycles, detection antibody was added and allowed to incubate for 1 h at room temperature (RT), followed by incubation with Streptavidin-Phycoerythrin for 30 min at RT. After removal of excess conjugate, 150 μl of sheath fluid was added to each well. The beads were read through a bead detector based on the fluorescence of the dyes. Raw data were measured as the relative fluorescence intensity and then converted to the concentration according to the standard curve. The Multiplex Assay Kit was purchased from Millipore.
Adoptive cell transfer
B6 mice were i.p. injected with IL-33 (0.8 μg/mouse) for 5 days. Lin− cells were isolated and expanded with cytokines IL-2, IL-7 and IL-33 (10 ng/ml) in vitro. Cells from 2–6 days’ culture were analyzed by flow cytometry and used for an adoptive transfer experiment as reported (29). The expanded nuocytes (2 × 106 cells in 200 μl PBS) were i.v. transferred into B6 mice at 1, 3 and 5 dpi (PBS was used as a control). All of the mice were sacrificed at 6 dpi.
Statistical analyses
Data were shown as mean ± SEM and analyzed by using the two-tailed Student’s T-test when compared between two groups. The P-value < 0.05 or < 0.01 is considered significant and marked as * or **, respectively. Graphs prepared from of flow cytometric findings were from 3 independent experiments with similar results. Findings were statistically analyzed by using GraphPad Prism software 5.0 (GraphPad Software Inc., San Diego, CA).
Results
IL-33 attenuated T cell-mediated liver injury in viral hepatitis
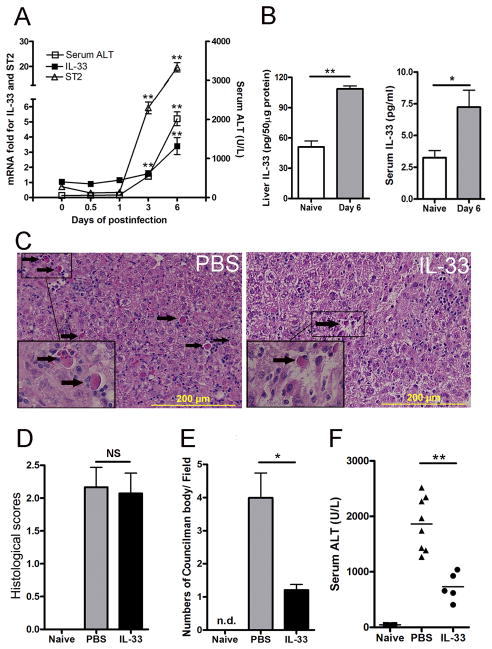
To determine the expression of DAMP molecule IL-33 in viral hepatitis, we i.v. injected B6 mice with AdLacZ that expresses a reporter β-gal, as described previously (8). When AdLacZ was injected through the tail vein, the hepatotropism of the virus was evidenced by β-gal staining of the liver but not the spleen, kidneys, or lungs (data not shown). Animals were sacrificed at 0, 0.5, 1, 3 and 6 dpi, and their hepatic injury as well as IL-33 and ST2 expression levels were measured. As shown in Fig. 1A, serum ALT levels began to rise on day 3 and continued on to day 6. Hepatic IL-33 and ST2 displayed the same pattern on these time points. By 6 dpi, the levels of IL-33 in both the liver and serum of infected mice were significantly higher than those in naïve animals (Fig. 1B). These data confirm a previous report (18), showing a positive association between the IL-33 levels and liver injury in viral hepatitis.
Figure 1. IL-33 was involved in viral hepatitis and attenuated liver damage.
B6 mice were i.v. injected with AdLacZ (3 × 109 PFU/mouse). A) Mice were sacrificed at the indicated time points. IL-33 and ST2 expression was analyzed by qRT-PCR. Serum was collected for ALT detection (5–7 mice per group). B) The liver and serum IL-33 levels in naïve mice and mice at day 6 post-infection were detected by an ELISA (6–7 mice per group). C) At 24 h post-infection, exogenous IL-33 (0.8 μg/ mouse in PBS) was i.p. injected into the infected mice daily until sacrifice at day 6. Representative microphotographs of hepatic H&E stain (6 dpi) are shown. The scale bars were 200 μm. D) Cumulative graphical representation of the histological scores. E) The number of Councilman bodies was counted in the H&E slides. F) Serum ALT was detected after IL-33 treatment (5–8 mice per group). Experiments were repeated for three times independently. Values were shown as mean ± SEM. A Two-tailed T-test was used for statistical analysis. * P < 0.05; ** P < 0.01.
To investigate the role of IL-33 in viral hepatitis, we treated mice with recombinant mouse IL-33 (0.8 μg/mouse, i.p.) or PBS daily from day 1–5 post-infection. Animals were sacrificed and analyzed for liver inflammation at 6 dpi (Fig. 1C). IL-33 treatment significantly up-regulated ST2 mRNA expression, although it didn’t change that of IL-33 (Fig. S1A). AdLacZ injection caused prominent portal and lobular lymphocytic infiltration. Bridging necrosis accompanied by many Councilman bodies was found in all three adjacent zones (Fig. 1C, arrows). The hepatic histopathological scores of the IL-33-treated mice were comparable to those of the PBS group (Fig. 1D). Further analyses revealed a significant reduction in Councilman bodies in the IL-33-treated mice (Fig. 1E). IL-33 treatment also reduced the serum ALT levels considerably in these animals (Fig. 1F). These results suggest to us that there is a protective role of IL-33 in Ad-induced liver injury in mice.
IL-33 had differential effects on liver-infiltrated cells
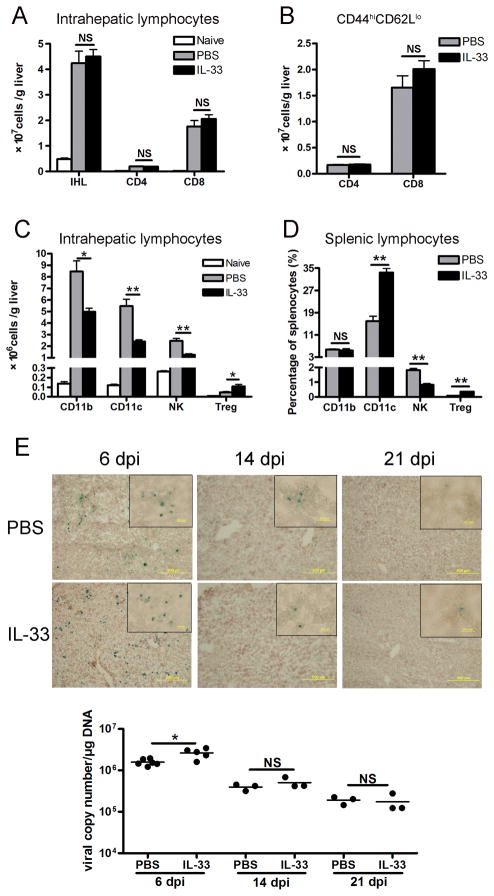
To understand the mechanism of liver protection by IL-33, we analyzed the infiltration and activation of immune cells in the liver of infected mice. As shown in Fig. 2A, injection of AdLacZ caused an approximately 8-folds increase in IHL recruitment at 6 dpi, and one-half of these IHL were CD8+ T cells. Consistent with the histological scores, the numbers of IHL were not altered by IL-33 treatment. Among the infiltrating CD4+ and CD8+ T cells in the liver, the number of activated T cell subpopulations (CD44hiCD62Llo) were comparable between the IL-33 and PBS groups (Fig. 2B), demonstrating that IL-33 did not influence the migration of effector T cells to the inflamed liver.
Figure 2. IL-33 had differential effects on liver-infiltrated cells.
B6 mice were i.v. injected with AdLacZ (3 × 109 PFU/mouse). At 24 h post-infection, exogenous IL-33 (0.8 μg/mouse in PBS) was i.p. injected into the infected mice daily until sacrifice at day 6 (5–6 mice per group). Livers were perfused and digested, and intrahepatic lymphocytes (IHL) were prepared by density gradient centrifugation. A) The numbers of IHL were counted under light microscopy. Hepatic CD4+ and CD8+ T cells were gated on CD3+ cells and assayed by flow cytometry. B) Hepatic CD4+ and CD8+ T cells were gated and analyzed for CD44 and CD62L expression by flow cytometry. The total numbers of CD4+CD44hiCD62Llo and CD8+CD44hiCD62Llo effector cells were shown. C) Hepatic infiltration of CD11b+, CD11c+, CD3−NK1.1+ and CD4+Foxp3+ T regulatory (Treg) cells was analyzed by flow cytometry. D) Percentages of CD11b, CD11c, NK and CD4+Foxp3+ Treg cells in the splenic lymphocytes were detected by flow cytometry. E) Viral clearance was assessed by the β-gal staining of liver frozen section as well as the viral copy numbers of liver tissues, respectively, at 6, 14, 21 dpi. Infected cells expressing β-gal activity were stained blue, whereas uninfected cells were counterstained red (3–5 mice per group). Original magnification: large panels 100 × (Scale bars: 200 μm); small panels 400 × (Scale bars: 20 μm). Experiments were repeated three times independently. Values were shown as mean ± SEM. A Two-tailed T-test was used for statistical analysis. * P < 0.05; ** P < 0.01.
In the liver, IL-33 treatment significantly reduced CD11b+, CD11c+ and NK cells, but increased Treg cells (Fig. 2C). The diminished and augmented frequencies of NK and Treg cells, respectively, were also observed in the spleen (Fig. 2D). IL-33 treatment repressed the expression of MHC II on CD11b+ and CD11c+ cells, as well as the expression of CD80 on CD11b+CD11c+ cells (Fig. S2), possibly indicative of subdued antigen-presentation in the IL-33 treated animals (38). To gauge the effect of IL-33 treatment on AdLacZ clearance in the liver, we stained samples for β-gal activity in the liver of both groups of mice (Fig. 2E). The results from these experiments showed that both IL-33- and PBS- treatments resulted in 10.6% ± 0.8% and 7.3% ± 0.7% infectivity, respectively, at 6 dpi. Both groups of mice followed similar courses of infection (0.8% ± 0.2% and 0.7% ± 0.3%, respectively, at 14 dpi), with the disappearance of most AdLacZ-infected hepatocytes by 21 dpi (< 0.1% in both groups). We speculated that at 21 dpi the viral loads might draw near the threshold of the β-gal assay and thus prohibited further identification of infected cells. To provide an independent measure of viral clearance, we also determined the viral load in the liver tissues by a highly sensitive quantitative PCR. Consistent with functional loss of viral-β-gal activity, there was a steady reduction of the viral genome in both groups of mice (Fig. 2E). Together, these results demonstrated a slight delay but no significant impairment in the ability of the IL-33-treated mice to eliminate an intrahepatic infection with Ad.
IL-33 induced IFN-γ but inhibited TNF-α in viral hepatitis
To further investigate the mechanism of mitigated liver injury after IL-33 administration, we examined the cytokines secreted by inflammatory cells. Mice were i.v. injected with AdLacZ followed by IL-33 or PBS treatment by i.p. injection daily and were sacrificed at 6 dpi. As shown in Fig. 3A, the percentages of hepatic IFN-γ + CD4+ and IFN-γ + CD8+ T cells were significantly increased in the IL-33 group compared with those of the PBS group. The serum IFN-γ and IL-2 were upregulated in IL-33 group (Fig. 3B), as well as the hepatic gene expression of IFN-γ, granzyme B and perforin (Fig. 3C), showing that IL-33 was able to enhance the type 1 immune responses in viral hepatitis.
Figure 3. IL-33 enhanced the CTL response in the liver of viral hepatitis.
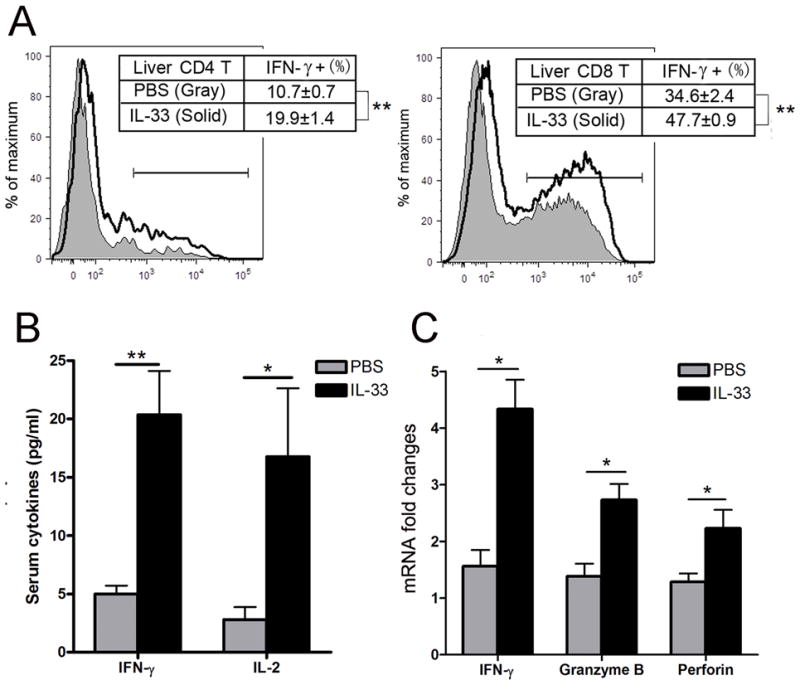
B6 mice were i.v. injected with AdLacZ (3 × 109 PFU/mouse) and at 24 h post-infection, exogenous IL-33 (0.8 μg/mouse in PBS) was i.p. injected into the infected mice daily until day 6 (5–6 mice per group). A) Mice were sacrificed at 6 dpi, and IHLs were isolated and stimulated with PMA and ionomycin for 4 h in the presence of GolgiStop. When analyzed by flow cytometry, IHLs were gated on CD3+ cells first. Percentages of hepatic IFN-expressing CD4+ and CD8+ T cells were further detected. B) Serum levels of IFN-γ and IL-2 of 6 dpi mice were detected by BioPlex assays. C) Liver tissues of 6 dpi mice were collected and hepatic gene expressions of IFN-γ, granzyme B and perforin were analyzed by qRT-PCR. Experiments were repeated twice independently. Values were shown as mean ± SEM. A Two-tailed T-test was used for statistical analysis. * P < 0.05; ** P < 0.01.
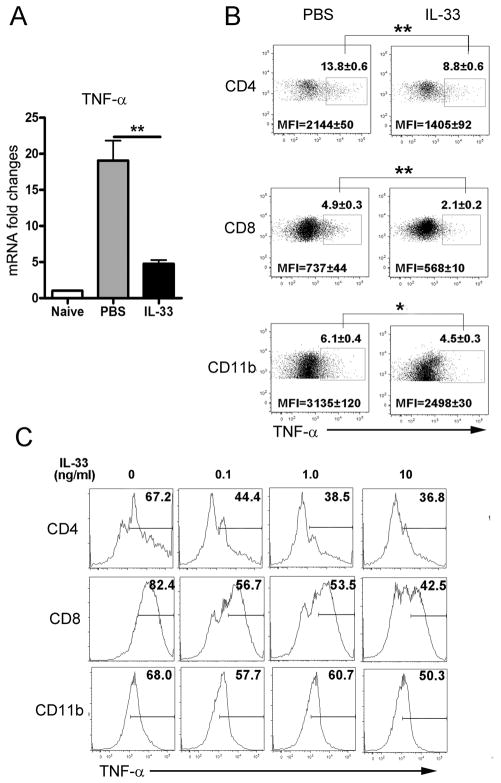
Next, we examined the expression of TNF-α, which is the crucial cytokine of hepatotoxicity and mediates liver damage in viral hepatitis (11). As indicated in Fig. 4A, the hepatic gene expression of TNF-α was markedly reduced in the IL-33 group. Then, we analyzed the intracellular TNF-α expression of hepatic lymphocytes from the IL-33 and PBS groups respectively. Consistently, IL-33 treatment significantly inhibited the expression of TNF-α on hepatic CD4+ T, CD8+ T and CD11b+ cells of infected mice (Fig. 4B). To further confirm the anti-inflammatory role of IL-33 in viral hepatitis, we isolated IHL from mice at 6 dpi and cultured in vitro with different concentrations of IL-33 (0, 0.1,1 and 10 ng/ml) together with IL-2 (10 ng/ml) and IL-7 (10 ng/ml) for 48 h. The results of the intracellular staining showed that the expression of TNF-α was inhibited by IL-33 in a dose-dependent manner (Fig. 4C). However, IL-33 by itself was unable to inhibit TNF-α expression in vitro (Fig. S3), indicating that IL-33 may function differently in the distinct microenvironments of inflammation and diseases.
Figure 4. IL-33 inhibited hepatic TNF-α in viral hepatitis.
B6 mice were i.v. injected with AdLacZ (3 × 109 PFU/mouse) and at 24 h post-infection, exogenous IL-33 (0.8 μg/mouse in PBS) was i.p. injected into the infected mice daily until sacrifice at day 6 (3–5 mice per group). Liver tissues were collected for analysis of the gene expression, and IHLs were prepared for flow cytometry detection. A) Gene expression of hepatic TNF-α was analyzed by qRT-PCR. B) Expressions of TNF-α on hepatic CD4+ T, CD8+ T and CD11b+ cells were detected by flow cytometry. Percentages and mean fluorescence intensities (MFI) were shown as mean ± SEM. C) The IHLs isolated at day 6 post-infection were cultured with IL-33 (0, 0.1, 1 and 10 ng/ml, respectively) in complete RPMI medium plus IL-2 and IL-7 (10 ng/ml) for 48 h. Each treatment was performed in triplicate. PMA/ionomycin and GolgiStop were added to the cells at the last 4 h of the culture. Expressions of TNF-α on CD4+ T, CD8+ T and CD11b+ cells were detected by flow cytometry at the end of the culture. Experiments were repeated three times independently, and represented graphs were shown. A Two-tailed T-test was used for statistical analysis. * P < 0.05; ** P < 0.01.
IL-33 induced nuocytes in Ad-infected mice
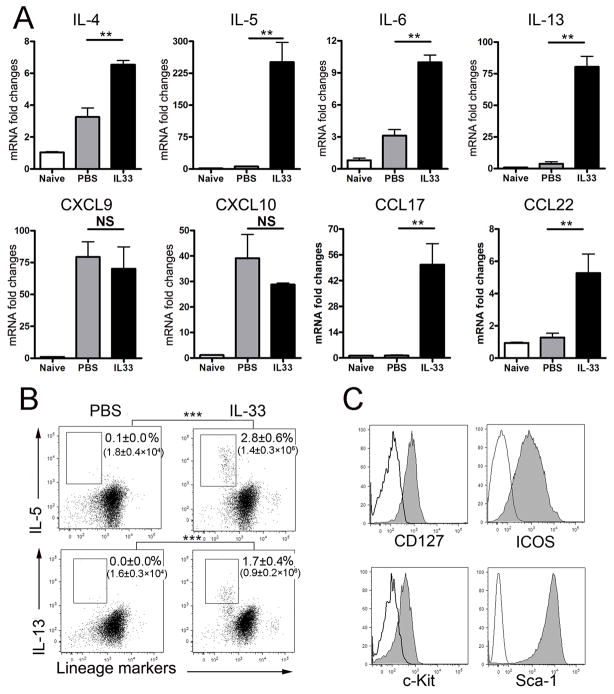
It is documented in autoimmune and parasitic diseases that administration of exogenous IL-33 not only promotes Th2 response but also initiates an unique innate type 2 immunity (39). It is unclear, however, whether IL-33 can promote such an innate type 2 immune response in viral hepatitis. Here, we infected the mice with AdLacZ and i.p. injected the IL-33 or PBS daily. Animals were sacrificed at 6 dpi and liver tissues were collected for cytokine gene detection. Lymphocytes were prepared from both spleens and livers for cytokine detection. As shown in Fig. 5A, IL-33 injection induced high levels of type 2 cytokines (especially IL-5 and IL-13) as well as the type 2 chemokines (CCL17 and CCL22), but not type 1 chemokines (CXCL9 and CXCL10) and anti-inflammatory cytokines IL-10 and TGF-β (Fig. S1A). These results demonstrated that IL-33 was indeed able to initiate the type 2 immune response in viral hepatitis. Moreover, as we examined AdLacZ-infected mice, we found that the splenic Lin− cells from these animals contained more IL-4+, IL-5+, IL-6+ or IL-13+ cells compared with those in uninfected mice, which indicated the presence of the innate type 2 cells or nuocytes (Fig. S1B). More interestingly, exogenous IL-33 was capable of inducing an expansion of both hepatic and splenic Lin− cells that expressed high levels of IL-5 and IL-13 (Figs. 5B and S3B). Further analyses of surface markers on the Lin−IL-13+ cells showed that they expressed CD127, ICOS, c-Kit, and Sca-1, which was consistent with the surface markers of nuocytes (Fig. 5C) (29). These results demonstrated that IL-33 induced nuocytes and facilitated an innate type 2 immune response in viral hepatitis.
Figure 5. Nuocytes were induced by IL-33 in the liver of viral hepatitis.
B6 mice were i.v. injected with AdLacZ (3 × 109 PFU/mouse) and at 24 h post-infection, exogenous IL-33 (0.8 μg/mouse in PBS) was i.p. injected into the infected mice daily until sacrifice at day 6 (3–4 mice per group). A) Liver tissues were collected, and hepatic gene expression of type 2 cytokines (IL-4, -5, -6, -13) and chemokines (CXCL9, CXCL10, CCL17 and CCL22) were detected by qRT-PCR. B) IHL were isolated and stimulated in the complete RPMI medium with PMA/ionomycin in the presence of GolgiStop for 4 h. Cells were collected, and lineage markers (CD3, CD4, CD8, CD11b, CD11c, NK1.1, B220, Gr-1 and Ter-119) as well as intracellular IL-5 and IL-13 were analyzed by flow cytometry. The numbers respectively represent the percentages and the absolute numbers of detected cells in the boxes. C) The lineage-negative (Lin−) IL-13+ cells in the IL-33 group were gated for the further detection of surface markers (CD127, ICOS, c-Kit and Sca-1). Solid lines represent the isotype control, and gray lines represent target antibody staining.
Nuocytes exhibited a potential protective role in hepatitis
To further confirm the function of the IL-33-induced nuocytes in viral hepatitis, we isolated both Lin+ and Lin− cells from the spleens at day 5 post-injection with IL-33. The purities of Lin+ and Lin− cells were up to 92% and 93%, respectively. The Lin− cells expanded robustly in the medium containing IL-2, IL-7 and IL-33 for 2–6 days (data not shown), as previously described (29). These expanded cells secreted high levels of IL-5 and IL-13 (Fig. 6A), and expressed CD43, CD44, CD45, CD69, CD25, CD127, c-Kit, and MHC II, characteristic of nuocytes (Fig. 6B). Importantly, when the Lin− or Lin+ cells were co-cultured with hepatic lymphocytes of infected mice in medium containing IL-2, IL-7 and IL-33 for 48 h, intracellular expressions of TNF-α in CD4+ T, CD8+ T and CD11b+ cells in the Lin− group were suppressed compared with those in Lin+ group (Fig. 6C).
Figure 6. Nuocytes inhibited TNF-α expression by CD4+ T, CD8+ T and CD11b+ cells in vitro.
A) Lin− cells were isolated with magnetic beads from the spleens of IL-33-treated non-infected mice and cultured in complete RPMI medium plus IL-2, IL-7 and IL-33 (10 ng/ml, respectively) in vitro. The medium was changed every 2 days, and the cytokines were supplemented. Lin− cells were cultured for 5 days followed by stimulation with PMA/ionomycin plus GolgiStop for 4 h. Intracellular IL-5 and IL-13 were detected by flow cytometry. Isotype antibodies were used as the controls. B) Surface markers of cultured Lin− cells (Solid lines represent the isotype control, and gray lines the target antibody) C) Freshly isolated Lin+ or Lin−cells (5 × 104 cells/well) were co-cultured, respectively, with IHL (2 × 106 cells/well) derived from day 6 post-infected mice in complete medium plus IL-2, IL-7 and IL-33 (10 ng/ml, respectively) for 48 h. PMA/ ionomycin and GolgiStop were added during the last 4 h of the culture. Percentages of TNF-α-expressingCD4+ T, CD8+ T and CD11b+ cells were detected by flow cytometry. The experiment was repeated three times independently, and representative graphs were shown.
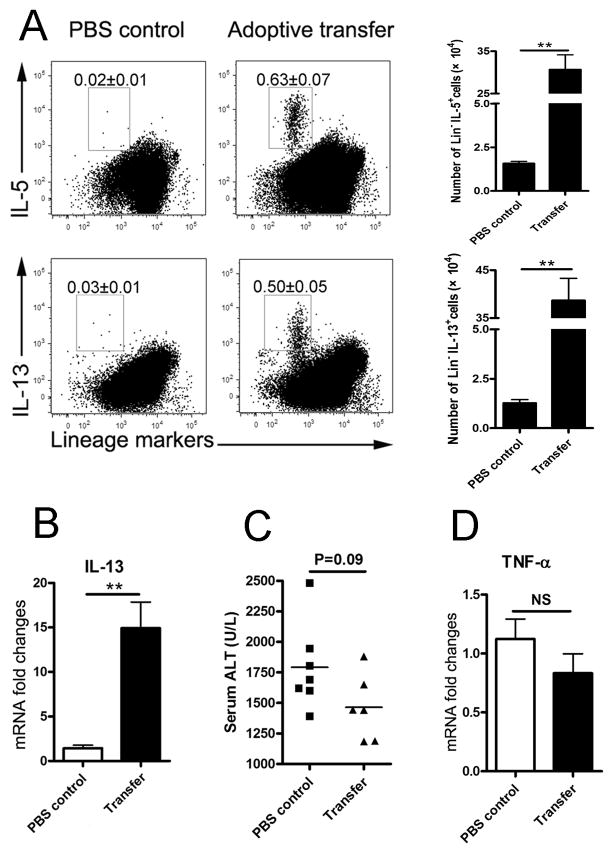
To explore the role of nuocytes in vivo, we adoptively transferred 2 × 106 of the cultured nuocytes into the AdLacZ-infected mice at 1, 3 and 5 dpi. At 6 dpi, we found increased numbers of Lin−IL-13+ or Lin−IL-5+ cells in the livers of transferred mice (Fig. 7A). Consistent with this observation of increased nuocytes, qRT-PCR results also showed the significant upregulation of hepatic IL-13 in the transferred group (Fig. 7B). Both the serum ALT and hepatic TNF-α expression of the transferred group presented a downward trend, but no statistical significance (Figs. 7C and D).
Figure 7. Adoptive transfer of nuocytes promoted type 2 immune responses and liver protection.
B6 mice were first infected with Ad, and nuocytes (2 × 106 cells in PBS) were i.v. transferred into the mice at 1, 3 and 5 dpi. All mice were sacrificed at 6 dpi (6–7 mice per group). A) IHLs were prepared for intracellular staining. After adoptive transfer of the nuocytes, the Lin−/IL-5+ or Lin−/IL-13+ cells were detected in the liver. The percentages of cells are shown in the flow cytometry figures, and the absolute numbers of the cells are shown in the histogram. B) Liver tissues were collected for gene expression analysis by qRT-PCR. C) Serum ALT levels of the adoptively transferred group and PBS control group were examined. D) Hepatic gene expression of TNF-α was detected in the transferred group and PBS control group. The experiment was repeated twice independently. Values were shown as mean ± SEM. A Two-tailed T-test was used for statistical analysis. ** P < 0.01.
Discussion
Many hepatotropic viruses can cause liver inflammation, and viral PAMPs are an important force driving CD8+ and CD4+ T cell priming through Toll- and/or Nod-like receptors (TLR and NLR) on APCs (13, 40, 41). However, it is now believed that, in addition to PAMP, the innate immune system can also recognize DAMPs, and both PAMP and DAMP can serve as the on-off switch of immunity (42, 43). DAMP molecules can initiate and perpetuate inflammatory processes not only in autoimmune processes but also in infectious diseases (14, 20, 44). Among them, IL-33 was initially considered a pro-Th2 cytokine (17, 45) but is now recognized as a crucial amplifier of innate immunity as well as Th1 and Th2 immune responses (19, 21, 45). IL-33 also plays a crucial role in driving antiviral CD8+ T cell responses in lymphocytic choriomeningitis virus (LCMV) infected mice (20). Moreover, IL-33 can synergize with other cytokines such as IL-12 to increase IFN-γ secretion by CD8+ T cells (46, 47). Although both the pro- and anti-inflammatory roles of IL-33 have been reported in Con A- and ischemia/reperfusion-induced liver injury, little is known about the mechanisms of IL-33 in viral hepatitis in humans or in mice (18, 27, 48).
In this study, we asked whether IL-33 is involved in Ad-induced hepatitis, and, if so, how it regulates T cell responses and inflammation in the liver. We measured cytokine expression on total T cell subpopulations, since a previous report has shown that most of the CD4+ and CD8+ T cells are virus-specific during the Ad infection (49). Our data suggest to us that IL-33 drove robust CD8+ and CD4+ T cells responses in the liver, which induced equally strong type-1 cytokine (IL-2 and IFN-γ) as well as type 2 cytokines (IL-4, IL-5, IL-6 and IL-13) in the liver and serum (Figs. 3–5). The local cytokine and chemokine microenvironment recruited large numbers of highly activated CD8+ and CD4+ T cells to the liver (Figs. 1 and 2). However, the enigmatic action of the IL-33/ST2 interaction did not exacerbate liver injury, but rather limited hepatic injury, as reflected by the greatly decreased numbers of Councilman bodies in the liver and serum ALT levels (Fig. 1). Based on our observations, we speculate that IL-33 mediates these potent hepatoprotective effects through direct and indirect mechanisms.
First, IL-33 induces strong, but divergent, immune effector functions. We have shown that IL-33 injection induced CD8+ and CD4+ T cells to secrete large amounts of IFN-γ in the liver as well as the serum; however, it greatly reduced TNF-α expression in hepatic T cells and macrophages (Figs. 3 and 4). In vitro IL-33 treatment inhibited TNF-α expression in the liver-derived CD8+, CD4+ and macrophages in a dose-dependent manner (Fig. 4). These data are consistent with previous reports, in which TNF-α was shown to play a central role in cellular necrosis and fulminant hepatitis (11, 50, 51). In addition, we have shown that IL-33 treatment resulted in fewer numbers of infiltrating NK cells and DCs with decreased levels of CD80 and MHC II expression in the liver (Figs. 2 and S2). In previous reports, IL-33-conditioned DCs were found to be less potent in priming naïve T cells (38). However, IL-33 has been reported to directly increase IFN-γ but decrease TNF-α in Th1 and Th2 cultures (52). Disruption of the IL-33/ST2 axis, on the other hand, enhanced NK functions and exacerbated Con A-induced hepatitis (53). Finally, we have demonstrated that IL-33 could modulate immune responses by expanding CD4+Foxp3+ Treg cells in the liver and spleen (Fig. 2). Disruption of the IL-33/ST2 signaling pathway prevented the generation of CD4+Foxp3+ Treg cells and exacerbated Con A-induced hepatitis (27). In addition, according to a previous report, the viral clearance of Ad was Fas and TNFR1-dependent (54). Our result showed that, although the viral clearance was delayed due to IL-33 treatment in the early stages, it was not compromised in the intermediate and late stages of Ad-infection (Fig. 2E). Collectively, these results suggested to us that IL-33 was able to directly engage multiple arms of immune mechanisms and limit liver injury in T cell-mediated hepatitis.
IL-33 is a potent inducer of a new population of innate type-2 leukocytes, namely nuocytes (29–32). These cells belong to a heterogeneous family of innate cells that do not express T or B lymphocyte markers (32–34). They are critically involved in intestinal parasite expulsion, influenza infection, and related airway hypersensitivity (29, 34, 35, 55, 56). In this study, we found that IL-33 strongly induced nuocytes in the liver and spleen of infected mice. In vitro, nuocytes expressed high levels of IL-5 and IL-13 and were capable of inhibiting TNF-α expression in liver-derived lymphocytes in infected mice (Figs. 5 and 6). This result is consistent with previous reports that IL-13 could inhibit TNF-α in mice (57) and reduced liver injury induced by ischemia/reperfusion (58). Furthermore, we found that IL-33 treatment-mediated ST2 up-expression (Fig. S1) and IL-33/ST2 pathway activation led to an enhanced expression of type 2 chemokines CCL17 and CCL22 in the liver (Fig. 5). In spite of its ability to induce a broad array of T cell effector molecules, cytokines, and chemokines, IL-33 did not induce IL-10 and TGF-β or CXCR3 ligands (CXCL9 and CXCL10) in the liver (Figs. S1 and 5). As previously reported (29), we used i.v. delivered Lin− cells or PBS in the adoptive transfer experiments, which may raise some concerns for an ideal control for nuocytes in such a study. Nevertheless, our adoptive transfer experiments clearly indicated that Lin− nuocytes persisted in the liver and secreted IL-5 and IL-13 in the liver of Ad-infected mice at 6 dpi (Fig. 7). Although the mice receiving nuocytes had lower serum ALT compared to the levels in the mock-transferred group, the difference between the two groups was not statistically significant (Fig. 7). These results suggested to us that nuocyte induction was merely one facet of the complex mechanisms of IL-33 action. More studies are needed to fully appreciate the direct and indirect actions of IL-33 in the pathogenesis of viral hepatitis.
In summary, we revealed that DAMP molecule IL-33 is a potent hepatoprotective cytokine in Ad-induced hepatitis. Exogenous IL-33 induced strong type 1, type 2 and regulatory T cell responses. However, it significantly inhibited TNF-α expression in T cells and macrophages and limited liver injury. In addition to its direct effect, IL-33 could also induce a strong expansion of IL-5/IL-13-expressing Lin− nuocytes, further down-modulated TNF-α. Its direct immunoregulatory functions and ability to induce novel nuocytes also may indicate that IL-33 is a potential therapeutic candidate for the management of liver injury and viral hepatitis.
Supplementary Material
Acknowledgments
We thank Dr. Yingzi Cong for critical review of the manuscript, Yixiao Sun for technical assistance and Ms. Mardelle Susman for assistance with manuscript preparation.
Financial Support: This work was supported partially by grants from the National Institutes of Health AI69142 (to J. S.) and AI078878 (to L. S.), as well as by the James W. McLaughlin Fellowship Fund (to Z. J.) and T35 fellowship (to D. V.).
Abbreviations
- Ad
adenovirus
- AdLacZ
Ad carrying the lacZ gene
- ALT
alanine aminotransaminase
- DAMP
damage-associated molecular pattern
- dpi
days post-infection
- ST2
IL-1 receptor-like 1
- IHL
intrahepatic lymphocytes
- Lin−
Lineage-negative
- Lin+
Lineage-positive
- PAMP
pathogen-associated molecular pattern
- qRT-PCR
Quantitative RT-PCR
References
- 1.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O’Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. Novel Adenovirus-Based Vaccines Induce Broad and Sustained T Cell Responses to HCV in Man. Science Translational Medicine. 2012;4:115ra111–115ra111. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, Hutnick NA, Betts MR, Dubey SA, Goudsmit J, Shiver JW, Robertson MN, Casimiro DR, Barouch DH. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15:873–875. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw AC, Baker AH. Gene therapy for cardiovascular disease: Perspectives and potential. Vascul Pharmacol. 2012 doi: 10.1016/j.vph.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK, Gerdemann U, Leen AM, Kairemo K, Oksanen M, Haavisto E, Holm SL, Karioja-Kallio A, Kauppinen S, Partanen KP, Laasonen L, Joensuu T, Alanko T, Cerullo V, Hemminki A. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 6.Jooss K, Ertl HC, Wilson JM. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. Journal of Virology. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Human Gene Therapy. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, Jie Z, Hou L, Wanderley JL, Soong L, Gupta S, Qiu S, Chan T, Sun J. Parenchymal expression of CD40 exacerbates adenovirus-induced hepatitis in mice. Hepatology. 2011;53:1455–1467. doi: 10.1002/hep.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raper SE. Gene therapy: The good, the bad, and the ugly. Surgery. 2005;137:487–492. doi: 10.1016/j.surg.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Nakatani T, Kuriyama S, Tominaga K, Tsujimoto T, Mitoro A, Yamazaki M, Tsujinoue H, Yoshiji H, Nagao S, Fukui H. Assessment of efficiency and safety of adenovirus mediated gene transfer into normal and damaged murine livers. Gut. 2000;47:563–570. doi: 10.1136/gut.47.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ZX, Govindarajan S, Okamoto S, Dennert G. Fas- and tumor necrosis factor receptor 1-dependent but not perforin-dependent pathways cause injury in livers infected with an adenovirus construct in mice. Hepatology. 2000;31:665–673. doi: 10.1002/hep.510310317. [DOI] [PubMed] [Google Scholar]
- 12.Oberholzer C, Oberholzer A, Tschoeke SK, Minter RM, Bahjat FR, LaFace D, Hutchins B, Moldawer LL. Influence of recombinant adenovirus on liver injury in endotoxicosis and its modulation by IL-10 expression. J Endotoxin Res. 2004;10:393–401. doi: 10.1179/096805104225005832. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 15.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zhao P, Guo H, Sun X, Jiang Z, Xu L, Feng J, Niu J, Jiang Y. Serum IL-33 Levels Are Associated with Liver Damage in Patients with Chronic Hepatitis C. Mediators of Inflammation. 2012;2012:Article ID 819636. doi: 10.1155/2012/819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The Alarmin Interleukin-33 Drives Protective Antiviral CD8+ T Cell Responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 21.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 22.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arshad MI, Piquet-Pellorce C, L’Helgoualc’h A, Rauch M, Patrat-Delon S, Ezan F, Lucas-Clerc C, Nabti S, Lehuen A, Cubero FJ, Girard JP, Trautwein C, Samson M. TRAIL but not FasL and TNFalpha, regulates IL-33 expression in murine hepatocytes during acute hepatitis. Hepatology. 2012;56:2353–2362. doi: 10.1002/hep.25893. [DOI] [PubMed] [Google Scholar]
- 25.Arshad MI, Rauch M, L’Helgoualc’h A, Julia V, Leite-de-Moraes MC, Lucas-Clerc C, Piquet-Pellorce C, Samson M. NKT cells are required to induce high IL-33 expression in hepatocytes during ConA-induced acute hepatitis. Eur J Immunol. 2011;41:2341–2348. doi: 10.1002/eji.201041332. [DOI] [PubMed] [Google Scholar]
- 26.Marvie P, Lisbonne M, L’Helgoualc’h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Theret N, Gascan H, Piquet-Pellorce C, Samson M. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, Pejnovic N, Arsenijevic N, Lukic ML. Protective Role of IL-33/ST2 Axis in Con A-Induced Hepatitis. J Hepatol. 2011;56(1):26–33. doi: 10.1016/j.jhep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Sakai N, Van Sweringen HL, Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ, Lentsch AB. Interleukin-33 is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology. 2012;56:1468–1478. doi: 10.1002/hep.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11:109–114. doi: 10.1097/ACI.0b013e3283448808. [DOI] [PubMed] [Google Scholar]
- 31.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 32.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 34.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol. 2011;41:1535–1538. doi: 10.1002/eji.201141668. [DOI] [PubMed] [Google Scholar]
- 36.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 37.Sun JR, Bodola F, Fan XG, Irshad H, Soong L, Lemon SM, Chan TS. Hepatitis C virus core and envelope proteins do not suppress the host’s ability to clear a hepatic viral infection. Journal of Virology. 2001;75:11992–11998. doi: 10.1128/JVI.75.24.11992-11998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol. 2009;39:3331–3342. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2011;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 41.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010:Article ID 672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 46.Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Duan L, Xiong A, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Blockade of IL-33 ameliorates Con A-induced hepatic injury by reducing NKT cell activation and IFN-gamma production in mice. J Mol Med (Berl) 2012;90(12):1505–15. doi: 10.1007/s00109-012-0938-4. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Zajac AJ, McPherson SA, Hsu HC, Yang P, Wu Q, Xu X, Wang X, Fujihashi K, Curiel DT, Mountz JD. Primary adenovirus-specific cytotoxic T lymphocyte response occurs after viral clearance and liver enzyme elevation. Gene Ther. 2005;12:1079–1088. doi: 10.1038/sj.gt.3302494. [DOI] [PubMed] [Google Scholar]
- 50.Trautwein C, Rakemann T, Brenner DA, Streetz K, Licato L, Manns MP, Tiegs G. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered by tumor necrosis factor in mice. Gastroenterology. 1998;114:1035–1045. doi: 10.1016/s0016-5085(98)70324-5. [DOI] [PubMed] [Google Scholar]
- 51.Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72–74. doi: 10.1016/s0140-6736(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 52.Blom L, Poulsen LK. IL-1 Family Members IL-18 and IL-33 Upregulate the Inflammatory Potential of Differentiated Human Th1 and Th2 Cultures. J Immunol. 2012;189:4331–4337. doi: 10.4049/jimmunol.1103685. [DOI] [PubMed] [Google Scholar]
- 53.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 54.Abougergi MS, Gidner SJ, Spady DK, Miller BC, Thiele DL. Fas and TNFR1, but not cytolytic granule-dependent mechanisms, mediate clearance of murine liver adenoviral infection. Hepatology. 2005;41:97–105. doi: 10.1002/hep.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE, DeKruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saglani S. Innate helper cells: a novel cell type essential in the initiation of asthma? Thorax. 2011;66:834–835. doi: 10.1136/thoraxjnl-2011-200510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Santo E, Meazza C, Sironi M, Fruscella P, Mantovani A, Sipe JD, Ghezzi P. IL-13 inhibits TNF production but potentiates that of IL-6 in vivo and ex vivo in mice. J Immunol. 1997;159:379–382. [PubMed] [Google Scholar]
- 58.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059–1064. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.