Abstract
Aims
To examine the association between duration and quality of sleep and the prevalence of undiagnosed and clinically identified diabetes mellitus and pre-diabetes in a nationally representative sample.
Methods
Cross-sectional study of 2285 participants ≥ 30 years old and without diagnosed sleep disorders from the National Health and Nutrition Examination Survey (2005–2008). The primary exposures were sleep duration and quality. Sleep quality was assessed by questionnaire using trouble initiating sleep, trouble maintaining sleep, and waking up too early. The primary outcomes were clinically identified and undiagnosed pre-diabetes and diabetes as defined by the American Diabetes Association using fasting plasma glucose (5.6–6.9 mmol/l = pre-diabetes; ≥ 7.0 mmol/l = diabetes). Multivariate logistic regression was used to test the association between sleep quality, sleep duration and glycaemic status.
Results
After adjustment for socio-demographic characteristics and health behaviors, sleeping ≤ 5 h/night was associated with clinically identified pre-diabetes (odds ratio 2.06, 95% CI 1.00–4.22 vs. 7 h). Both trouble maintaining sleep ≥ 5 times/month (odds ratio 3.50, 95% CI 1.30–9.45) and waking up too early ≥ 5 times/month (odds ratio 2.69, 95% CI 1.21–5.98) were also significantly associated with increased risk of clinically identified pre-diabetes. Trouble initiating sleep and sleeping ≥ 9 h/night were not found to be associated with having diabetes.
Conclusions
Only clinically identified pre-diabetes was associated with trouble maintaining sleep, waking up too early, and short sleep. No other relations were found to be significant. Findings suggest that poor sleep quality and short sleep duration were more strongly associated with clinically identified pre-diabetes than long sleep duration.
Introduction
Sleep plays an important role in the regulation of metabolism, appetite and immune function [1]. Inadequate sleep has been associated with obesity, insulin resistance and cardiovascular disease [1–4]. Multiple aspects of sleep (e.g. duration, latency, persistence) may be independently related to Type 2 diabetes mellitus risk [5–12]. Cappuccio et al. found that both short (≤ 6 h/night) and long (> 8 h/night) sleep duration were associated with risk of diabetes, but evidence is mixed [9,10]. There is a notable lack of research on the relationship between sleep and pre-diabetes. It is unresolved whether poor sleep is associated with risk for undiagnosed, as opposed to clinically identified, diabetes.
The purpose of this study was to examine the association between sleep duration and quality and undiagnosed and clinically identified diabetes mellitus and pre-diabetes.
Methods
Study population
Data come from the fasting blood sample subset of the 2005/2006 and 2007/2008 National Health and Nutrition Examination Survey (NHANES), part of a series of cross-sectional, nationally representative surveys and laboratory exams (n = 5024) [13]. Participants were excluded if they were < 30 years old (nexcluded = 1509); were diagnosed with cancer (other than non-melanoma skin cancer) in the past 3 years (nexcluded = 77); had chronic heart disease (nexcluded = 175); used sleeping medication (nexcluded = 626); had an established sleeping disorder (e.g. insomnia, sleep apnoea) (nexcluded = 146); or had missing data on sleep, BMI, education, race or fasting plasma glucose (nexcluded = 206). After these exclusions, the analytic sample size was 2285. NHANES is approved by the National Center for Health Statistics Research Ethics Review Board. All participants provided informed consent.
Sleep duration and quality
Sleep quality and duration were assessed by questionnaire. Sleep duration was assessed by the question: ‘How much sleep do you usually get at night on weekdays or workdays?’ categorized as: ≤ 5, 6, 7, 8 and ≥ 9 h. Sleep quality was assessed by asking: ‘In the past month, how often did you have trouble falling asleep?’; ‘How often do you wake up during the night?’ and ‘How often do you wake up too early in the morning?’ Each was categorized as never, 1–4 times/month and 5–30 times/month.
Pre-diabetes and diabetes mellitus
Glycaemic status was defined according to the American Diabetes Association using fasting plasma glucose levels [1]. Participants were asked if they had ever been told by a physician that they had diabetes and their current medications were recorded. Diabetes and pre-diabetes were considered clinically identified if participants reported a physician diagnosis of diabetes or pre-diabetes, impaired fasting glucose, impaired glucose tolerance, borderline diabetes or blood sugar higher than normal but not high enough to be called diabetes, respectively. Normoglycaemia was defined as glucose < 5.6 mmol/l and no use of hypoglycaemic agents or physician diagnosis. Undiagnosed pre-diabetes was defined as glucose between 5.6 and 6.9 mmol/l and no physician diagnosis. Clinically identified pre-diabetes was defined as glucose between 5.6 and 6.9 mmol/l plus physician diagnosis. Undiagnosed diabetes was defined as glucose ≥ 7.0 mmol/l and no physician diagnosis. Clinically identified diabetes was defined as glucose ≥ 7.0 mmol/l plus physician diagnosis or use of hypoglycaemic agents. Hyperglycaemia was defined as having fasting glucose ≥ 5.6 mmol/l.
Covariates
Age was treated as a continuous variable. BMI was calculated using measured weight and height. Race was categorized as ‘non-Hispanic white’, ‘non-Hispanic black’ or ‘Hispanic’. Education was categorized as less than high school, high school graduate, some further education/college and further education/college graduate. Tobacco use, alcohol use and physical activity were assessed by self-report. Smoking was categorized as current, former or never smoker. Alcohol consumption was categorized as > 1 drinks/day vs. ≤ 1 drinks/day. Physical activity was categorized as vigorous, moderate or none.
Analysis
Initial comparisons of covariates by glycaemic status (normoglycaemic, undiagnosed pre-diabetes, clinically identified pre-diabetes, undiagnosed diabetes and clinically identified diabetes) were assessed using χ2-tests for categorical variables and F-tests for continuous variables. Multivariate logistic regression was used to examine the association between sleep characteristics as the independent variables and glycaemic status as the dependent variable. We examined several forms of glycaemic status: (1) any form of hyperglycaemia (clinically identified diabetes, undiagnosed diabetes, clinically identified pre-diabetes and undiagnosed pre-diabetes) vs. normoglycaemia; (2) diabetes (clinically identified and undiagnosed) vs. normoglycaemic; and (c) pre-diabetes (clinically identified and undiagnosed) vs. normoglycaemic. We fitted three nested regression models: adjusted for age; additionally adjusted for race, education and sex; and additionally adjusted for BMI, physical activity, cigarette use and alcohol intake. In order to assess dose–response relationships, linear tests for trends were estimated for the association between the sleep quality and glycaemic status; nonlinear tests for trends were used to assess the potential U-shaped relationship between sleep duration and glycaemic status [8]. Analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC, USA) using sample weights for the fasting glucose subset, and all P-values refer to two-tailed tests.
Results
Four in 10 (39.2%) participants had undiagnosed pre-diabetes (n = 878), 3.5% had clinically identified pre-diabetes (n = 103), 2.5% had undiagnosed diabetes (n = 74), 8.4% had clinically identified diabetes (n = 289) and the remaining 46.4% were normoglycaemic (n = 941). Sleep duration varied by glycaemic status (χ2 = 30.4, P < 0.002), but sleep quality did not (χ2 = 8.4, P = 0.40 for trouble initiating sleep; χ2 = 11.5, P = 0.18 for waking during the night; χ2 = 14.7, P = 0.08 for waking early).
Sleep duration
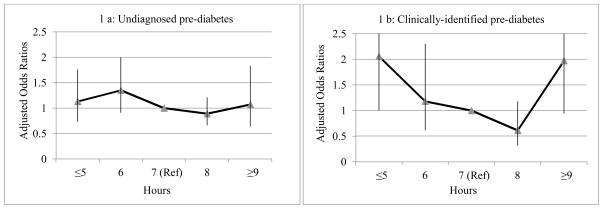
Short sleep duration (≤ 5 h/night) (odds ratio 2.06, 95% CI 1.00–4.22 vs. 7 h) was associated with clinically identified pre-diabetes, but long duration (odds ratio 1.97, 95% CI 0.94–4.14) was not significantly associated with clinically identified pre-diabetes. As shown by Fig. 1, the associations between sleep duration and either undiagnosed or clinically identified pre-diabetes appeared U-shape, but the P non-linear trend for both undiagnosed pre-diabetes and clinically identified pre-diabetes were not significant; findings were similar for undiagnosed and clinically identified diabetes mellitus.
Figure 1.
Association between sleep duration and glycaemic status
Sleep quality
As shown by Table 1, for clinically identified diabetes only, trouble initiating sleep was marginally significant with glycaemic status relative to normoglycaemia (odds ratio 1.52, 95% CI 0.98–2.36) (P linear trend: 0.06). After adjustment for demographic characteristics and health behaviours, waking during the night was associated with any form of hyperglycaemia (odds ratio 1.39, 95% CI 1.00–1.93), as well as clinically identified pre-diabetes (odds ratio 3.50, 95% CI 1.30–9.45), relative to normoglycaemia. Waking too early was marginally associated with hyperglycaemia in any form relative to normoglycaemia (odds ratio≥ 5 times/month 1.41, 95% CI 1.00–1.99). Early waking was significantly associated with clinically identified pre-diabetes (odds ratio≥ 5 times/month 2.69, 95% CI 1.21–5.98). There was no association between waking too early and undiagnosed pre-diabetes and either undiagnosed or clinically identified diabetes. In post-hoc analyses, waking early was associated with clinically identified pre-diabetes among men but not women; waking at night was associated with hyperglycaemia (any type) in women.
Table 1.
Association between waking up during the night and waking up early and glycaemic status
| Glycaemic status | Waking up during the night | Waking up early | ||
|---|---|---|---|---|
|
| ||||
| No./normoglycaemic | Odds ratio (95% CI)* | No./normoglycaemic | Odds ratio (95% CI)* | |
| Hyperglycaemia | ||||
| Never (ref.) | 537/398 | 1.0 | 633/473 | 1.0 |
| 1–4 times/month | 565/410 | 1.06 (0.83–1.36) | 492/356 | 1.11 (0.84–1.45) |
| 5–30 times/month | 242/133 | 1.39 (1.00–1.93) | 219/112 | 1.41 (1.00–1.99) |
| Undiagnosed pre-diabetes | ||||
| Never (ref.) | 359/398 | 1.0 | 411/473 | 1.0 |
| 1–4 times/month | 369/410 | 1.03 (0.79–1.35) | 328/356 | 1.02 (0.82–1.49) |
| 5–30 times/month | 150/133 | 1.29 (0.94–1.78) | 139/112 | 1.42 (0.96–2.10) |
| Clinically identified pre-diabetes | ||||
| Never (ref.) | 32/398 | 1.0 | 39/473 | 1.0 |
| 1–4 times/month | 51/410 | 1.92 (0.87–4.22) | 48/356 | 2.06 (1.08–3.93) |
| 5–30 times/month | 20/133 | 3.50 (1.30–9.45) | 16//112 | 2.69 (1.21–5.98) |
| Undiagnosed diabetes | ||||
| Never (ref.) | 36/398 | 1.0 | 42/473 | 1.0 |
| 1–4 times/month | 30/410 | 0.56 (0.29–1.08) | 25/356 | 0.69 (0.36–1.31) |
| 5–30 times/month | 8/133 | 0.65 (0.22–1.97) | 7/112 | 0.59 (0.16–2.15) |
| Clinically identified diabetes | ||||
| Never (ref.) | 110/398 | 1.0 | 141/473 | 1.0 |
| 1–4 times/month | 115/410 | 1.29 (0.85–1.95) | 91/356 | 0.99 (0.70–1.41) |
| 5–30 times/month | 64/133 | 1.54 (0.84–2.84) | 57/112 | 1.41 (0.85–2.36) |
Adjusted for age, race, education, sex, BMI, physical activity, cigarette use and alcohol intake.
Discussion
In this large, cross-sectional study, we found that short sleep duration, frequently waking up during the night and waking up too early were associated with an increased likelihood of hyperglycaemia. This relationship was most evident among clinically identified pre-diabetes. To the best of our knowledge, this is the first study to examine the relationship between sleep and undiagnosed pre-diabetes. Our findings are partially consistent with extant studies of sleep and pre-diabetes. Hung and colleagues found that individuals with pre-diabetes and newly diagnosed diabetes had significantly worse sleep quality compared with those with normoglycaemia [14]. Chao et al. found that neither long nor short sleep duration was significantly associated with risk for pre-diabetes [12].
Although the direction of most of the relationships tested were consistent with previous research, the majority of these associations were not statistically significant. This may be attributable to our study population, which was limited to people who did not have sleep disorders or take sleep medication. Of individuals excluded because of medical sleep problems, a higher percentage came from the clinically identified group with diabetes relative to the group with normoglycaemia (30.7 vs. 22.6%, respectively). However, in a post-hoc analysis including people with sleep disorders, our results were similar.
We expected to observe similar relationships among all forms of hyperglycaemia, regardless of clinical diagnosis. However, we found suggestive evidence that the relationship between sleep and hyperglycaemia varies by clinical identification for pre-diabetes. The variation may attributable to undetected medical conditions leading the diagnosis of pre-diabetes or certain biological processs of diabetes development.
These findings should be interpreted in light of study strengths and limitations. Participants were selected from a nationally representative survey and we used multiple indicators of sleep behaviour. We used plasma fasting glucose, rather than self-report, to determine glycaemic status; however, only one sample was obtained per participant. This is a cross-sectional study and therefore the causal direction of the relationship between sleep and diabetes cannot be inferred. Prospective studies are needed to further understand the relationship between sleep duration and quality and pre-diabetes, as well as the role co-morbidities may play in that relationship. Sleep, medical co-morbidities and health behaviours were assessed by self-report and may have resulted in residual confounding. Although we excluded cases of known sleep anpoea from the analysis, this condition is often undiagnosed and has been associated with sympathetic nervous activity and insulin insensitivity. The NHANES did not assess age of diabetes onset or diabetes type, which would have allowed us to distinguish between Type 1 and Type 2 diabetes.
Supplementary Material
What’s new?
Use of fasting plasma glucose data and self-report to assess clinically identified and undiagnosed diabetes and pre-diabetes status.
Clinically identified pre-diabetes was associated with trouble maintaining sleep, waking up too early, and short sleep.
Acknowledgments
Funding sources
BM was supported by a Career Development Award from the National Institute of Mental Health (K01-MH093642-A1).
The authors would like to thank the Post-Baccalaureate Research Experience Program (PREP) at VCU for providing the opportunity to work with the Department of Epidemiology and Community Health. We would also like to thank Kristin R. Austin for guidance on the statistical analysis.
Footnotes
Competing interests
None declared.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Participant inclusion/exclusion flow chart.
Table S1. Association between sleep quality and duration and all pre-diabetes (clinically identified and undiagnosed) vs. all diabetes (clinically identified and undiagnosed).
Table S2. Association between sleep quality and duration and all clinically identified diabetes (pre-diabetes and diabetes) vs. all undiagnosed diabetes (pre-diabetes and diabetes).
Table S3. Association between duration and diabetes status, adjusting for hypertension.
Table S4. Association between trouble initiating sleep and diabetes status, adjusting for hypertension.
Table S5. Association between waking up during the night and diabetes status adjusting for hypertension.
Table S6. Association between waking up early and diabetes status, adjusting for hypertension.
Table S7. Association between sleep characteristics and diabetes status in men.
Table S8. Association between sleep characteristics and diabetes status in women.
Table S9. Association between sleep characteristics and hyperglycaemia among participants with sleep problems.
Table S10. Association between duration and plasma glucose among those with undiagnosed hyperglycaemia (pre-diabetes and diabetes).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.XXXXXXXX. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35:S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 3.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab. 2007;92:3044–3051. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- 4.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 6.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 7.Meisinger C, Heier M, Loewel H MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 8.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 9.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H, et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao CY, Wu JS, Yang YC, Shih CC, Wang RH, Lu FH, et al. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 2011;60:799–804. doi: 10.1016/j.metabol.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 13.CDC. NCHS. National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol) Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; [Last accessed 23 September 2011]. p. XXXX. Available at http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/overviewbrochure_0708.pdf. [Google Scholar]
- 14.Hung HC, Yang YC, Ou HY, Wu JS, Lu FH, Chang CJ. The relationship between impaired fasting glucose and self-reported sleep quality in a Chinese population. Clin Endocrinol (Oxf) 2012 May 2; doi: 10.1111/j.1365-2265.2012.04423.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.