Abstract
Cancer vaccines, so far, have shown limited effect to control the growth of established tumors due largely to effector failure of the antitumor immune responses. Tumor lesion is characterized as chronic indolent inflammation in which the effector function of tumor infiltrating lymphocytes (TILs) is severely impaired. In this study, we investigated whether the effector function of CD8 TILs could be rescued by converting the chronic inflammation milieu to acute inflammation within tumors. We found that injection of TLR3/9 ligands (PolyI:C/CpG) into a tumor during the effector phase of lentivector (lv) immunization effectively rescued the function of lv-activated CD8 TILs and decreased the percentage of Treg within the tumor, resulting in a marked improvement in the antitumor efficacy of lv immunization. Mechanistically, rescue of the effector function of CD8 TILs by TLR3/9 ligands is likely dependent on production, within a tumor, of type-1 IFN that can mature and activate tumor infiltrating dendritic cells (TIDCs). The effector function of CD8 TILs could not be rescued in mice lacking intact type-I IFN signaling. These findings have important implications for tumor immunotherapy, suggesting that type-I IFN mediated activation of TIDCs within a tumor will likely restore/enhance the effector function of CD8 TILs and thus improve the antitumor efficacy of current cancer vaccines.
Keywords: Lentivector, genetic immunization, tumor immunology, effector function of TIL, TLR ligands
Introduction
To exploit the host immune system for cancer treatment, the rare tumor Ag specific immune cells must be activated and expanded by cancer vaccines in lymphoid tissue (priming phase). These vaccine-activated tumor specific immune cells must then migrate to the tumor where they can exert their effector functions of cytokine production and tumoricidal activity (effector phase). Efforts directed at the priming phase have yielded many forms of peptide- (1), DC- (2), and gene based (3–9) cancer vaccines that can break tolerance to self/tumor Ags and activate tumor specific T cells. We have previously found that immunization with a recombinant lentivector (lv) is a powerful approach to activate tumor specific CD8 T cells and generate life-long prevention of autochthonous melanoma in tumor-prone mice (10). However, despite the remarkable expansion and increase in Ag specific immune cells observed in tumor infiltrates, the therapeutic effect of cancer vaccines remains limited in treating established tumors (11–14). Recent studies revealed that one of the main reasons why vaccine-induced immune responses fail to exhibit antitumor activity in the treatment of established tumors is failure of the effector phase within the tumor (15). We (16) and others (17, 18) found that vaccine-activated CD8 T cells lost their effector functions within the tumor but retained function outside of the tumor environment, in the circulation and lymphoid tissues. This functional impairment of tumor infiltrating lymphocytes (TILs) has also been reported in human tumors (19). Thus, a critical barrier to the success of cancer immunotherapy is at the effector phase within the tumor (20). Measures to rescue the effector functions of TILs within a tumor will therefore be critical for enhancing the antitumor effect of cancer vaccines.
A tumor lesion is characterized as chronic indolent inflammation (21–23). A major characteristic shared by a chronically inflamed tumor lesion (16–18) and a chronic virus infection (24) is that the effector T cells in both settings are functionally impaired so are unable to either eradicate the tumor or resolve the infection. In contrast, acute virus infections can be quickly resolved when adaptive immunity is induced. Immunologically, the drastically different outcome between acute and chronic virus infections is likely due to the different functional status of Ag specific T cells (24, 25). While virus Ag specific T cells can effectively perform their function and eradicate pathogens in acute infection, the effector functions of Ag specific T cells are lost in chronic viral infection (24). Similar to T cells in chronic virus infection, TILs lose their effector function within the tumor. Inspired by the demonstration of functional efficacy of immune effectors in acute virus infection, we hypothesize that conversion of the chronic inflammation milieu within a tumor to one of acute inflammation may rescue the effector function of TILs and that some key molecules and signaling pathways in acute inflammation, but missing in tumors, are critical to rescue of the effector function(s) of TILs.
To test this hypothesis, we studied whether induction of acute inflammation by intra-tumor (i.t) injection of Toll-like receptor ligands (TLR-Ls) could rescue the effector function of CD8 TILs and enhance the antitumor effect of lv-based cancer vaccines. TLR 3/9 ligands (PolyI:C/CpG) were selected because they have been shown to induce both an acute inflammatory response and high level of type I IFN (26, 27) thus mimicking acute viral infection. Although it may not be clinically applicable, induction of acute inflammation within a tumor provides a platform to investigate the key molecules involved in rescue of the function of TILs. We found that i.t injection of TLR3/9 ligands effectively rescued the effector function of TILs primed by cancer vaccine and decreased the percentage of Treg, resulting in marked enhancement of the antitumor effect of lv immunization. Rescue of TILs function was associated with effective Ag presentation by the tumor infiltrating DCs (TIDCs), which were matured by local administration of TLR-Ls in a type-I IFN dependent manner. Type-I IFN signaling within the tumor milieu is critical for efficient activation of TIDCs, the rescue of the effector function of TILs and the outcome of tumor immunotherapy of cancer vaccines.
Materials and Methods
Mice, cell lines, and tumor
Pmel transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice were obtained from the National Cancer Institute (Frederick, MD) and the Jackson Laboratory. IFN-α/β receptor knockout (IFNα/βR−/−) mice were obtained from Dr. Moskophidis (28) of Georgia Regents University. All mice were housed under specific pathogen-free conditions within the Laboratory Animal Services. Animal care protocols were approved by the Institutional Animal Care and Use Committee of Georgia Regents University.
B16F10 melanoma cells and 293T kidney cells were cultured in DMEM medium supplemented with 10% FBS. Tumors were generated by subcutaneous injection of B16F10 cells (2×105/50μl) into the shaved flank of C57BL/6 mice.
Viral vector preparation and injection
Recombinant lv, Hgp100-lv, was prepared and the titer was determined as we previously described (10). For immunization, Hgp100-lv (1.5×107 transduction units/50μl) was injected subcutaneously, in the footpad. Immunizations were carried out 5 days after tumor inoculation, when tumors were clearly visible.
I.t injection of TLR Ligands
To induce acute inflammation with TLR ligands, 100μl PBS containing 100μg TLR3 ligand, PolyI:C (Sigma, St Louis) and 50μg TLR9 ligand, CpG1668 (Fisher Scientific, Pittsburgh), individually or in combination, were injected into a tumor. In some experiments, 100 μl PBS containing 50μg TLR2 ligand, Pam3CSK4 (Invivogen, San Diego) and 100μg of TLR4 ligand, LPS (Sigma), were injected into a tumor.
Adoptive cell transfer
TCR transgenic hgp10025–33 specific Pmel T cells (29) were isolated from Pmel transgenic mice using magnetic beads (Miltenyi, Auburn, CA). Purified Pmel cells (5 × 105) were injected i.v. into mice one day prior to lv immunization.
Intracellular staining, flow cytometry, and DC sorting
Antibodies used in this study, anti-CD90.2 (Thy1.2), anti-CD90.1 (Thy1.1), anti-CD45, anti-CD8, anti-CD4, anti-CD11c, anti-CD40, anti-CD86, anti-FoxP3, anti IFNγ, and anti-TNFα, were purchased from BD Biosciences(San Diego, CA), Biolegend (San Diego, CA), or eBioscience (San Diego, CA). Tumors were collected, weighed and made into single cell suspensions. Briefly, 10–100mg tumor was cut into small pieces and incubated at 37°C for 0.5h in complete RPMI 1640 containing 1mg/ml type V collagenase, 1mg/ml hyaluronidase, and 100 U/ml DNase I. All enzymes were purchased from Sigma (St. Louis, MO).
To measure cytokines, cells were stimulated with peptides in vitro for 3.5h in the presence of GolgiStop (BD Biosciences) and stained for IFNγ and TNFα as previously described (10). Cells were collected using a FACScanto or LSR-II (BD Biosciences). Data were analyzed using FCS Express V3 software (De Novo Software, Los Angeles, CA).
For DCs, tumors and spleens were digested with collagenase to liberate DC cells. Briefly, tumor or spleen was cut into small pieces, incubated at 37°C for 0.5~1 h in RPMI 1640 containing 500U/ml type-IV collagenase and then made into a single-cell suspension for subsequent staining.
For TIDCs, single cell suspensions were generated from a tumor, enriched on a Percoll column and then sorted, using a BD FACSAria, based on expression of the CD11c and CD45 markers. 7AAD was used to exclude dead cells.
Ex vivo CTL assay
Lymphocytes were isolated from single cell suspensions of tumors using 40% Percoll (GE-Healthcare Bioscience AB, Uppsala, Sweden) as previously described (30). CD8 T cells were further purified using CD8 magnetic beads. Purified CD8 TILs (~80% of them were Pmel cells) were tested for cytotoxic capacity using gp100 peptide pulsed EL4 tumor cells as targets in a JAM assay (11).
In vitro proliferation of Pmel cells
Magnetic bead-isolated Pmel cells (5×104) were labeled with 0.5 μM CFSE and then co-cultured with TIDCs (5×103) previously pulsed with 5pg/ml hgp100 peptide. Two days later, Pmel cell proliferation was determined, using flow cytometry, according to the dilution of CFSE.
Type 1 IFN assay
Type-I IFN within the tumor was measured using an interferon bioactivity assay as described (31, 32). Briefly, NCTC929 cells were pre-treated overnight with serial dilutions of tumor homogenate in the presence of 4μg/ml anti-IFNγ Ab (clone XMG1.2, BD Biosciences) and then infected with VSV-Indiana. Infected cells were cultured for 3 days, and viable cells were stained with crystal violet, dissolved in isopropanol and quantified by light absorption at 595nm using a plate reader.
Quantitative RT-PCR
RNA was isolated from tumors. The level of IL-12 expression within the tumor after i.t injection was determined by quantitative RT-PCR as we previously described (33).
Statistical analysis
The statistical significance of the experimental data was analyzed using unpaired two-tailed t test and Log-rank test (GraphPad Software, La Jolla, CA).
Results
I.t injection of TLR3/9 ligands markedly improves the antitumor efficacy of lv immunization
TLR3/9 ligands (CpG/PolyI:C) have been shown to induce type-I IFN inflammatory responses (34, 35). Thus, i.t injection of TLR3/9 ligands would seem to be a valid approach to induce transient and localized acute inflammation within a tumor lesion. First, we studied whether i.t administration of TLR3/9 ligands could enhance the antitumor efficacy of lv immunization. C57BL/6 mice bearing 5-day B16 tumors were immunized with hgp100-lv (Fig. 1A). One week later, when an lv-induced CD8 response was detectable, TLR3/9 ligands were injected into the tumor to induce inflammatory responses. Injection of PBS was used as a control. We found that injection of either TLR3 or TLR9 ligand alone marginally (not significantly) enhance the antitumor effect of lv immunization (supplemental Fig. S1A). However, i.t injection of a combination of TLR3/9 ligands markedly increased the antitumor efficacy of lv immunization (Fig. 1B). Approximately 50% of the tumors in the mice receiving lv immunization plus a single i.t injection of TLR3/9 ligands underwent regression and 30% were completely eradicated. In addition, mice treated with this combined approach survived significantly longer (Fig. 1C). In contrast, although lv immunization alone significantly inhibited tumor growth, it was unable to eradicate a tumor, consistent with our previous data (11). A single injection of only TLR3/9 ligands slightly inhibited tumor growth (Fig. 1B). All of the mice in the groups receiving lv immunization alone or TLR3/9 ligand alone succumbed to tumor growth.
Fig. 1. I.t injection of TLR3/9 ligands markedly enhances the antitumor effect of lv immunization.
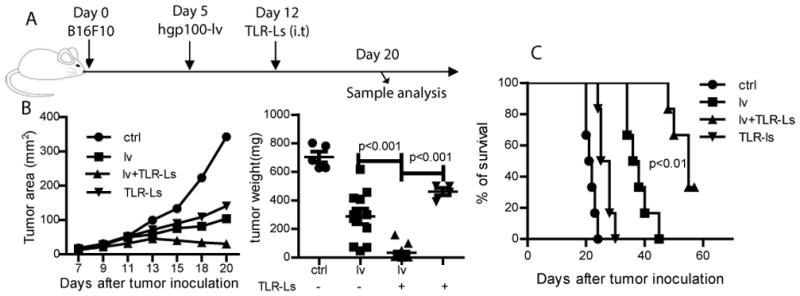
(A) Experimental plan - B16 mice bearing 5 day B16 tumors were immunized with hgp100-lv and then injected i.t. one week later with TLR3/9 ligands. (B) Tumor growth curves are shown as means. A summary of tumor weights at the end of experiment are based on three experiments. Non-paired two-tail t-test was used for statistical analysis. (C) Survival curve of mice treated with different regimes are shown. Two experiments were conducted with similar data. Log-rank test was used for statistical analysis.
I.t injection of TLR3/9 ligands significantly increases the Teff/Treg ratio in the tumor lesions
The remarkable synergistic antitumor effect of lv immunization plus i.t injection of TLR3/9 ligands prompted us to study immunological changes within the tumor. We speculated that the first reason for the enhanced antitumor effect of lv immunization by i.t injection of TLR3/9 ligands was due to an increase in the TILs. Compared to non-immunized control tumors, lv immunization increased the absolute numbers of CD4 and CD8 TILs, in agreement with our previous study (11). However, surprisingly, we found that i.t injection of TLR3/9 ligands decreased the absolute numbers of CD8 and CD4 TILs compared to lv immunization alone (Fig. 2A), indicating that enhancement of the antitumor effect by injection of TLR3/9 ligands was not due to an increase in the numbers of CD8 and CD4 TILs. However, one consistent observation was that, compared to lv immunization alone, the addition of an i.t. injection of TLR3/9 ligands further reduced the percentage of Treg within the tumors to <10% (Fig. 2B). Injection of either TLR3 or TLR9 ligand individually did not further decrease the percentage of Treg (Fig. S1B). Importantly, the absolute number of Treg within tumors was also reduced after injection of TLR3/9 ligands. Together, the ratio of CD8 Teff/Treg within the tumor was significantly increased (Fig. 2C). The increased Teff/Treg ratio within the tumors following i.t injection of TLR ligands may play an important role in enhancing the antitumor effect of lv immunization as previous studies found that the Teff/Treg ratio correlated to antitumor effect (36, 37).
Fig. 2. I.t injection TLR3/9 ligands significantly reduces the ratio and absolute numbers of Treg within the tumor.
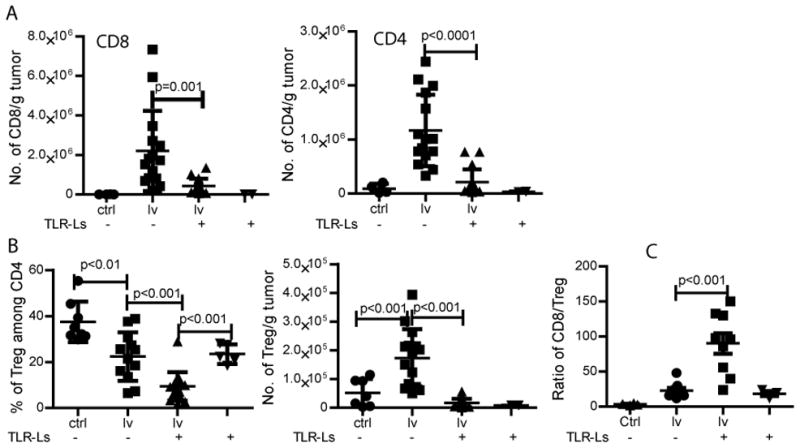
(A) One week after lv immunization, TLR3/9 ligands were injected into the tumors. Absolute numbers of CD8 and CD4 TILs were enumerated. (B) The percentage and absolute number of Treg within the tumor was summarized. (C) The CD8 Teff/Treg ratio was calculated from the absolute numbers of CD8 TILs and Treg within the tumor. Data are from three separate experiments. Non-paired t-test was used for statistical analysis.
I.t injection of TLR3/9 ligands rescues the function of TILs
We (16) and others (17, 18) previously showed that the function of CD8 TILs within tumors was severely impaired. We thus hypothesized that another reason for enhancing the antitumor effect of lv immunization by i.t injection of TLR3/9 ligands could be rescue of the effector function of TILs infiltrating the tumor. To test this hypothesis, we first examined the cytokine production of CD8 TILs by intracellular staining for IFNγ and TNFα. In order to gate on tumor specific T cells and to better characterize the effector function of Ag-specific CD8T cells within tumors, we adoptively transferred gp100 specific TCR transgenic Pmel cells into the tumor-bearing mice before lv immunization. We found that, while the percentage of Pmel cells in tumors did not change, the absolute numbers of Pmel cells within the tumors were decreased by i.t. injection of TLR3/9 ligands (Fig. 3A) due to a reduction in the total number of CD8 TILs (Fig. 2A). Importantly, IFNγ and TNFα cytokine production by Pmel TILs in the tumors of mice receiving lv immunization plus i.t injection of TLR-Ls was fully restored to the same, or even increased, levels compared with Pmel cells in the spleen (Fig. 3B). Even though the total number of Pmel TILs was reduced (Fig. 3A), the number of IFNγ and TNFα double positive Pmel cells in the tumor was slightly increased by TLR3/9 ligands (Fig. 3C). However, I.t injection of either TLR3 or TLR9 ligand individually did not significantly increase the cytokine production of Pmel TILs (Fig. S2).
Fig. 3. I.t injection of TLR3/9 ligands rescues the effector function of CD8 TILs.
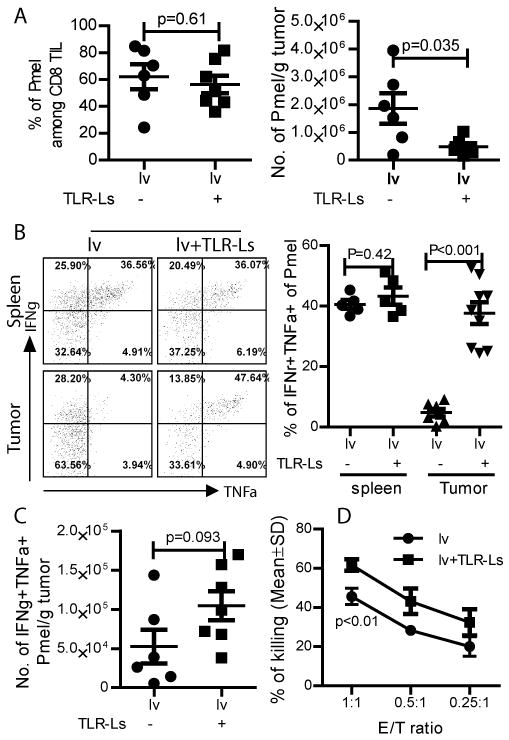
The experiment was conducted similar to Figure 1 except Pmel cells were adoptively transferred into mice 1 day prior to lv immunization. (A) The percentage and absolute number of Pmel cells within the tumor are summarized from 2 experiments. (B) Single cell suspensions from spleens and tumors were stained for Thy1.1, CD8, IFNγ, and TNFα. Only the Pmel (Thy1.1+) CD8 T cells were gated and were shown in the dot plot. Data are a summation of 2 experiments. (C) The absolute numbers of IFNγ and TNFα double positive Pmel cells from 2 experiments are summarized. (D) The data of in vitro CTL assay is shown. T-test was used for statistical analysis at each E/T ratio.
Enhancement of CD8 TIL function by i.t injection of TLR3/9 ligands was further studied by examining their cytotoxicity. Our data demonstrated that CD8 TILs isolated from TLR3/9 ligands injected tumors exhibited significantly higher cytotoxicity (Fig. 3D).
TLR2/4 ligands do not rescue the cytokine production of lv-activated CD8 TILs
Next, we determined whether other TLR ligands would have similar effects i.e. reducing the percentage of Treg within tumors and restoring the effector function of TILs. To this end, we utilized TLR2 (Pam3Cys) and TLR4 (LPS) ligands since previous studies had shown that they did not stimulate type-I IFN production (26, 27, 38) and did not generate significant antitumor activity (27). Our data show that i.t injection of TLR2/4 ligands did not restore cytokine production of CD8 TILs (Fig. 4A) and did not further decrease the percentage of Treg within the tumors (Fig. 4B) even though acute inflammation (a swollen and red appearance) was observed in the tumors. Not surprisingly, i.t. injection of TLR2/4 ligands did not enhance the antitumor effect of lv immunization (Fig. 4C). Our data suggest that TLR3/9 activation may result in distinct pro-inflammatory responses within the tumors that are able to rescue the effector function of TILs.
Fig. 4. I.t injection of TLR2/4 ligands does not affect the percentage of Treg and the function of TIL.
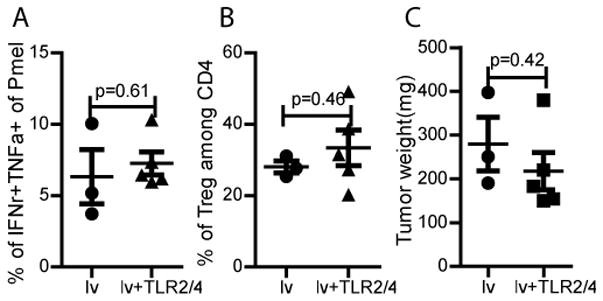
Mice bearing B16 tumors were adoptively transferred with Pmel cells and then immunized with hgp100-lv. One week later, TLR2/4 ligands were injected into the tumor. The percentage of Treg (A) and cytokine production (B) of TILs are analyzed and presented. (C) Tumor weights at the time of sacrifice are presented. Two experiments were conducted with similar results.
Rescue of the effector function of TILs by TLR3/9 ligands is dependent on intact type-1 IFN signaling
We next investigated the mechanism involved in rescue of TIL function. Previous reports showed that different TLR ligands could induce different levels of type-I IFN production (26, 27, 38): the type-I IFN inducing TLR 3/9 ligands generated strong antitumor effects whereas the type-I IFN non-inducing TLR2/4 ligands generated only weak antitumor effects (27). Therefore, type-I IFN produced after administration of TLR3/9 ligands may be the key molecule in the rescue of TIL function. To test this hypothesis, we first examined levels of type-I IFN within tumors after i.t injection of TLR3/9 or TLR2/4 ligands. We found that injection of TLR3/9 ligands, but not TLR2/4 ligands (LPS/Pam3Cys), could induce high levels of type-I IFN in the tumors (Fig. 5), consistent with previous reports (26, 27).
Fig. 5. Local acute inflammation increases the type I IFN within the tumor.
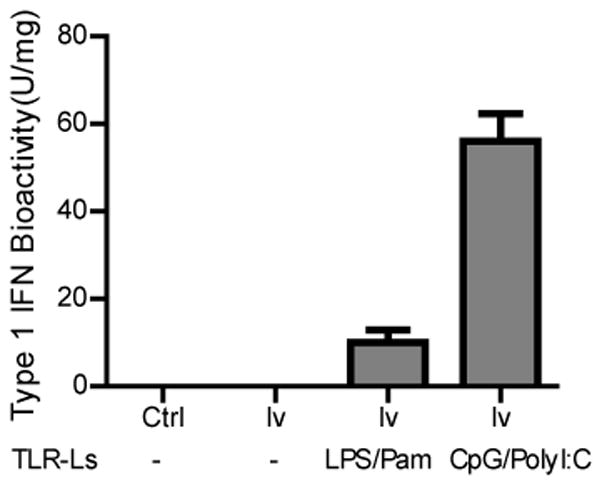
Tumors were collected one day after injection of different TLR ligands. Three tumors from each group were pooled and lyzed. The bioactivity of type-I IFN was analyzed in triplicate using a biological assay. The bioactivity of type-I IFN is shown.
To study if type-I IFN signaling was important for the rescue of TIL function, we utilized IFNαβR−/− mice in which the type-I IFN signaling is impaired. The experimental design is depicted in Fig. 6A. We first found that Pmel CD8 T cells could be activated, though to a lesser extent, in IFNαβR−/− mice (Fig. 6B). Approximately 20% of Pmel CD8 T cells in the spleens of IFNαβR−/− mice were able to produce both IFNγ and TNFα, compared to ~35% of Pmel CD8T cells in the spleens of wt mice. However, i.t injection of TLR3/9 ligands failed to enhance the antitumor effect of lv immunization in IFNαβR−/− mice (Fig. 6C). Furthermore, while i.t injection of TLR3/9 ligands rescued the effector function of TILs in wt mice, the same approach failed to improve the effector function of tumor-infiltrating Pmel cells in the IFNαβR−/− mice (Fig. 6D). In addition, i.t injection of TLR3/9 ligands did not decrease the percentage of Treg within the tumors of IFNαβR−/− mice (Fig. 6E). These data suggest that type-1 IFN signaling plays a key role in the restoration of cytokine production by CD8 TILs and in the decrease of Treg percentage within tumors.
Fig. 6. Local injection of TLR3/9 ligands cannot rescue the effector function of TILs or decrease the percentage of Treg in the absence of appropriate type-I IFN mediated signaling.
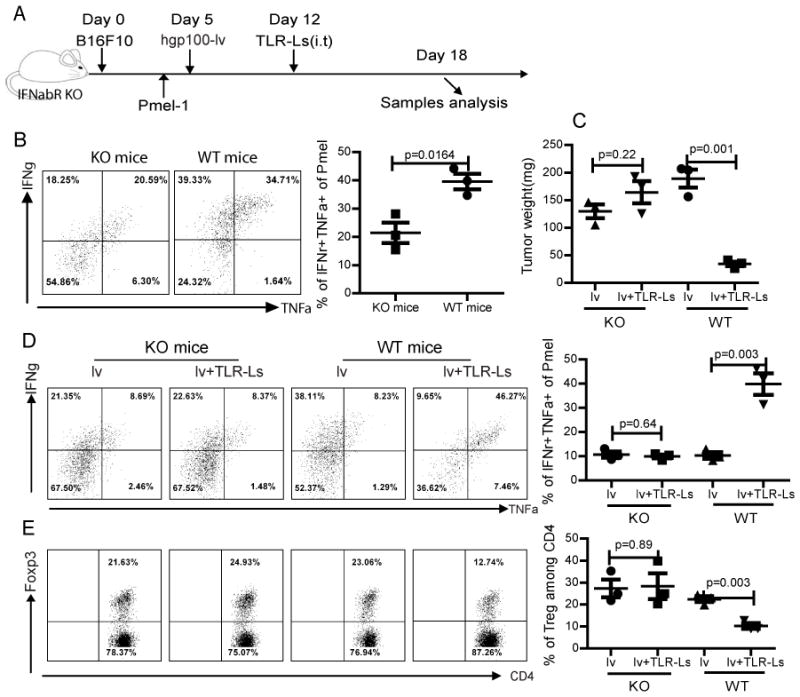
(A) The experimental scheme was shown. Both of wt and IFNαβR−/− mice were used in the study. (B) The ability to activate Pmel CD8 T cells by lv immunization in the IFNαβR−/− mice is demonstrated by the production of IFNγ and TNFα by the Pmel cells in the spleen. (C) Tumor weights at the time of sacrifice were recorded and presented. (D) Rescue of the effector function of CD8 TILs was determined by the production of IFNγ and TNFα. Only the Pmel cells were gated and are shown. (E) The percentage of Treg within the tumor is shown based on gating only the CD4 T cells. The experiment was repeated three times with similar results.
TIDCs are activated and matured by i.t injection of TLR3/9 ligands via type-I IFN receptor mediated signaling pathway
Two recent studies suggest that type-I IFN receptor mediated signaling in the DCs is critical for generating antitumor effects (39, 40). In one study, Schreiber’s lab demonstrated that mice lacking type-I IFN receptor (IFNαβR−/−) on their DCs could not reject highly immunogenic tumor cells. Thus, DCs are functionally relevant targets of endogenous type-I IFN. Dunn et al found that the relevant target of type-I IFN was the host cells derived from hematopoietic cells (39, 41) and type-I IFN was required by DCs for immune rejection of tumors (33). We, thus, reasoned that appropriate Ag presentation by DCs was required to rescue the effector function of TILs. To further study the mechanisms involved in the rescue of functional CD8 TILs, we investigated the phenotypes and Ag presenting function of TIDCs following injection of TLR3/9 ligands into the tumors. We found that i.t injection of TLR3/9 ligands markedly increased CD86 and CD40 expression on TIDCs (an indication of DCs maturity) (Fig. 7A). In addition, IL-12 production within the tumors after treatment with TLR3/9 ligands was significantly increased (Fig. 7B). However, TIDCs from IFNαβR−/− mice showed no increase of CD86 and CD40 by i.t injection of TLR3/9 ligands even though they may have higher basal levels of CD40 and CD86. No IL-12 production was detected within the tumors of IFNαβR−/− mice. Furthermore, the Ag presentation function of TIDCs from the TLR3/9 ligands treated tumors was significantly more potent (Fig. 7C). In contrast, TIDCs in IFNαβR−/− mice could not be matured by i.t injection of TLR3/9 ligands and failed to increase CD8 T cell proliferation. These data suggest that type-I IFN signaling may play a critical role in the activation of TIDCs to become more capable of re-activating CD8TILs to exert their effector function within the tumor.
Fig. 7. The maturation, cytokine production, and Ag presentation of TIDCs is impaired in the absence of proper type-I IFN signaling.
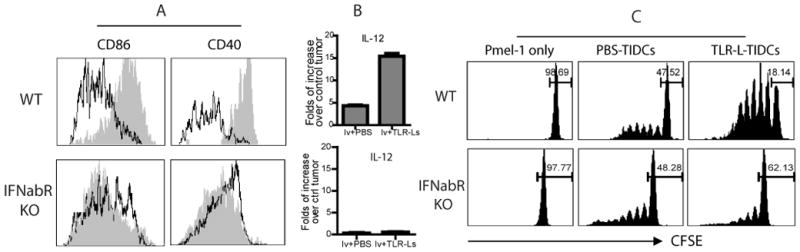
(A) One day after i.t injection of TLR3/9 ligands or PBS (control), the maturation phenotypes of the TIDCs in wt and IFNαβR−/− mice were analyzed. TIDCs of control (open) and TLR3/9 ligand injected (shade) mice were gated and analyzed for CD86 or CD40 expression. (B) IL-12 levels within the tumor after local induction of acute inflammation in wt mice (top panel) and IFNαβR−/− mice (lower panel) was determined by quantitative RT-PCR. Data are presented as fold increases in IL-12 levels over non-treated tumors. (C) TIDCs were isolated from wt and IFNαβR−/− mice one day after i.t injection of TLR3/9 ligands. Ag presentation function of TIDCs isolated from different tumors treated with various agents, as indicated, was determined by induction of Pmel proliferation in vitro.
Discussion
In this study, we found that i.t. injection of TLR3/9 ligands effectively rescued the function of CD8 TILs, significantly reduced the numbers and percentage of Treg within tumors, and improved the antitumor effect of lv immunization. Mechanistically, local administration of TLR3/9 ligands increased IL-12 level within tumors and induced TIDCs to mature thereby enhancing Ag presenting function. These favorable immunological changes, including rescue of the effector function of TILs and increases in Teff/Treg ratios within tumors, are dependent on appropriate type-I IFN signaling.
For decades, vaccine researchers have paid little attention to the effector phase of immune responses because it has been assumed that immune cells, once activated, will be on autopilot to exercise their function in target tissues. Failure to generate significant antitumor effects by most cancer vaccines, despite the apparent induction of potent immune responses, illustrates the need to study the effector phase of antitumor immunity. Teleologically, vaccine-activated and LN-derived effector cells should remain quiescent during transit to the target organ to avoid collateral damage (42). Upon reaching the target tissue, they will then require stimulation to exercise their effector function. Recent studies in infectious disease models began to shed light on this important issue (43, 44). Wakim et al found that memory T cell re-activation by DCs in the non-lymphoid tissues was critical to the control of HSV infection (43). Another study discovered that, after initial LN priming, CD8 T cells needed additional interaction with DCs resident in the lung in order to develop full strength immune responses against influenza virus (44). Similarly, in an autoimmune diabetes model, onset and progression of autoimmune diseases (i.e. autoimmune destruction) was determined by the “inflammatory status” of the target organ (45, 46). Thus, the conventional paradigm that immune effectors will exercise their effector function automatically in target organs may need revision. Re-activation of tissue infiltrating immune effector cells appears to be required in order for them to exert their effector function. The virus infected lesion can provide all of the signals required by tissue resident DCs to function effectively as APCs leading to reactivation of incoming T cells thus empowering them to exert their effector function. But, the scenario is quite different within the tumor in which Treg and other suppressor cells may inhibit function of TILs and as a consequence TIDCs in the late-stage tumor cannot stimulate TIL (47). The results from our study indicate that induction of inflammatory responses and type I IFN within tumors may create the appropriate environment to allow for TILs to exert their function.
Repeated i.t injection of CpG was reported to eradicate mouse lymphomas possibly by direct activation of TIDCs that engender Ag presentation in situ and initiate tumor specific responses (48, 49). In addition, repeated delivery of PolyI:C into tumor led to reactivation of tumor-resident T cells in situ (27). However, it is not clear how the TLR ligands will affect the effector function of the vaccine-activated and LN-derived TILs. Here, we found that the effector function of CD8 TILs primed by lv immunization could be rescued by a single injection of TLR3/9 ligands into the tumors (Fig. 3). Type-I IFN mediated signaling was a critical factor in TIL effector function (Fig. 6). TLR2/4 ligands without significant induction of type-I IFN did not rescue the effector function of TILs (Fig. 4). We also found that type-I IFN signaling was required for TIDC maturation and Ag presenting function (Fig. 7), in agreement with recent finding that DCs are the relevant targets of type-I IFN during lymphocyte-mediated tumor rejection (39). While we focused on the role of type-I IFN on host DCs, activation and expansion of Ag specific CD8 T cells requires appropriate type I IFN signaling on the T cells (50), which may activates cytokine expression in T cells. However, with an intact type-I IFN receptor, the adoptively transferred Pmel cells in this study cannot be rescued to exert their function without appropriate signals from host APCs in IFNαβR−/− mice, further suggesting that type-I IFN signaling of host cells plays an important role in rescue of TIL’s effector function.
The increase of IL-12 production within tumors after TLR3/9 administration may also play an important role in shaping the tumor milieu and generating antitumor effect as previous studies demonstrated that IL-12 delivery into tumors could initiate regression (51, 52). Based on the current data, we propose a model for how TILs can be rescued and exert their effector function to achieve antitumor outcomes (Fig. 8). To generate an antitumor effect, tumor specific immune effector cells, activated by vaccines in the lymphoid tissue, need to be appropriately re-activated to exert their function after migration to the tumor. However, the tumor milieu is immunologically suppressive and the TIDCs are therefore unable to present Ag to re-activate TILs. The importance of TIDCs was further emphasized in a recent study showing that TIDCs could change their function from immune stimulating to immune tolerogenic, resulting in conversion from tumor equilibrium into immune escape (47). Proper function of TIDCs within the tumor was reported to be important in reversing T cell tolerance in situ (53). Local injection of TLR ligands into the tumor stimulates production of type-I IFN, which then activates TIDCs. These activated TIDCs or other innate cells within the tumor produce IL-12 and become effective APCs to re-activate TILs, which together lead to a potent antitumor effect.
Fig. 8. A model of how TILs are rescued to exert their effector functions within a tumor.
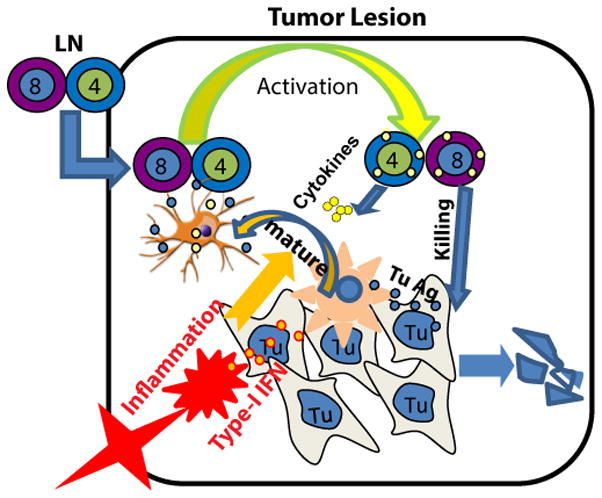
The vaccine-activated and LN-derived T cells require in situ activation by tumor infiltrating DCs (TIDCs) upon migration into a tumor. Unlike microbe-infected sites, the TIDCs are functionally suppressed. TLR3/9 ligands in the tumor activate TIDCs through type-I IFN dependent signaling to become effective APCs. The activated TIDCs are capable of acquiring, processing and presenting tumor Ag to re-activate TILs to exert their functions.
Type-I IFN dependent activation of TIDCs is probably the most critical step because of its role in rescuing the effector function of CD8 TILs. Recently, several other approaches were found to be capable of activating tumor associated APCs, leading to an improved antitumor effect. For example, radiation (54) and chemotherapy (55) were recently found to increase the antitumor efficacy of immune cells after adoptive transfer, by increasing the cross-priming capacity of TIDCs. In another study, CD40 agonist Ab was found to activate tumor associated macrophages (56), which, as professional APCs, may re-activate TILs in addition to killing tumor cells directly. Whether these approaches may result in rescue of the effector function of vaccine-primed TILs is an interesting topic for further study.
Recent studies by Sierro et al have shown that PD1 blockade can increase the antitumor effect of lv immunization (57). We also found that administration of PD1/PD-L1 Abs was partially capable of rescuing the cytokine production of CD8 TILs (16). Thus, PD1/PD-L1 blockade may further increase the antitumor efficacy of lv immunization and TLR3/9 ligand administration.
Tregs play an important role in immune suppression within the tumor. Our data show that i.t injection of TLR3/9 ligands reduced the percentage and number of Treg and thus increased the ratio of Teff/Treg within the tumor. The mechanism of how TLR3/9 ligands reduce the numbers of Treg remains to be studied. However, two studies have shown that Th1/Th2 T cells can inhibit peripheral Treg induction in vitro and in vivo (58) in an IFNγ dependent manner (59). Addition of exogenous IFNγ was also shown to markedly decrease Treg generation in vitro. Thus, it is possible that successful execution of the effector function of CD8TILs within the tumor increases the level of IFNγ, which leads to reciprocal inhibition of expansion of Treg. It is conceivable that the rescue of CD8 TIL function of secreting IFNγ leads to the decrease in Treg within the tumor.
In summary, we have demonstrated that loss of effector function by TILs within a tumor can be rescued by local administration of TLR3/9 ligands possibly through type-I IFN mediated activation of TIDCs. Although the application of this approach is limited by the difficulties of accessing each individual tumor, the identification of type-I IFN as a critical cytokine in the rescue of TIL effector function points to the potential that co-delivery of type-I IFN to tumors may contribute significantly to rescue of TIL effector function and increase the efficacy of current tumor immunotherapies.
Supplementary Material
Acknowledgments
We would like to thank our colleagues, Dr. Phillip Chandler and Davies Agyekum (B.S), for editing the manuscript.
This study was supported by NIH grant R01 CA16444 and Distinguished Investigator Fund from Georgia Research Alliance to YH.
Abbreviations used in the paper
- lv
lentivector
- hgp100
human glycoprotein 100
- i.t
intratumoral
- TLR
toll-like receptor
- TIDCs
tumor infiltrating DCs
References
- 1.Elsawa SF, Rodeberg DA, Celis E. T-cell epitope peptide vaccines. Expert Rev Vaccines. 2004;3:563–575. doi: 10.1586/14760584.3.5.563. [DOI] [PubMed] [Google Scholar]
- 2.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RJ, Schneider J. Plasmid DNA and viral vector-based vaccines for the treatment of cancer. Vaccine. 2007;25(Suppl 2):B24–34. doi: 10.1016/j.vaccine.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv Drug Deliv Rev. 2006;58:931–947. doi: 10.1016/j.addr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 7.Dullaers M, Van Meirvenne S, Heirman C, Straetman L, Bonehill A, Aerts JL, Thielemans K, Breckpot K. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13:630–640. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- 8.Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Levy F, MacDonald HR. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111:1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Peng Y, Hong Y, Liu Y, Guo ZS, Bartlett DL, Fu N, He Y. Lentivector prime and vaccinia virus vector boost generate high-quality CD8 memory T cells and prevent autochthonous mouse melanoma. J Immunol. 2011;187:1788–1796. doi: 10.4049/jimmunol.1101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Peng Y, Mi M, Guevara-Patino J, Munn DH, Fu N, He Y. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J Immunol. 2009;182:5960–5969. doi: 10.4049/jimmunol.0900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh V, Ji Q, Feigenbaum L, Leighty RM, Hurwitz AA. Melanoma progression despite infiltrationby in vivo-primed TRP-2-specific T cells. J Immunother. 2009;32:129–139. doi: 10.1097/CJI.0b013e31819144d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 15.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Xiao H, Liu Y, Peng Y, Hong Y, Yagita H, Chandler P, Munn DH, Mellor A, Fu N, He Y. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J Immunol. 2010;185:5082–5092. doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8 T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey AB, Monu N. Effector-phase tolerance: another mechanism of how cancer escapes antitumor immune response. J Leukoc Biol. 2006;79:652–662. doi: 10.1189/jlb.1105628. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 24.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 25.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O’Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currie AJ, van der Most RG, Broomfield SA, Prosser AC, Tovey MG, Robinson BW. Targeting the effector site with IFN-alphabeta-inducing TLR ligands reactivates tumor-resident CD8 T cell responses to eradicate established solid tumors. J Immunol. 2008;180:1535–1544. doi: 10.4049/jimmunol.180.3.1535. [DOI] [PubMed] [Google Scholar]
- 28.Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Y, Peng Y, Mi M, Xiao H, Munn DH, Wang G, He Y. Lentivector expressing HBsAg and immunoglobulin Fc fusion antigen induces potent immune responses and results in seroconversion in HBsAg transgenic mice. Vaccine. 2011;29:3909–3916. doi: 10.1016/j.vaccine.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, McGaha TL, Ravishankar B, Lee JR, Munn DH, Mellor AL. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188:4913–4920. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyring S, Fleischmann WR, Jr, Baron S. A convenient microassay for cytolysis and cytostasis. Methods Enzymol. 1986;119:574–579. doi: 10.1016/0076-6879(86)19077-x. [DOI] [PubMed] [Google Scholar]
- 33.Hong Y, Peng Y, Xiao H, Mi M, Munn D, He Y. Immunoglobulin Fc fragment tagging allows strong activation of endogenous CD4 T cells to reshape the tumor milieu and enhance the antitumor effect of lentivector immunization. J Immunol. 2012;188:4819–4827. doi: 10.4049/jimmunol.1103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, Cheng G. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70:2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 38.Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, Harding CV. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol. 2012;188:1019–1026. doi: 10.4049/jimmunol.1102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required bydendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 42.Monsurro V, Wang E, Yamano Y, Migueles SA, Panelli MC, Smith K, Nagorsen D, Connors M, Jacobson S, Marincola FM. Quiescent phenotype of tumor-specific CD8+ T cells following immunization. Blood. 2004;104:1970–1978. doi: 10.1182/blood-2004-02-0525. [DOI] [PubMed] [Google Scholar]
- 43.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 44.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 46.Bach JF. A Toll-like trigger for autoimmune disease. Nat Med. 2005;11:120–121. doi: 10.1038/nm0205-120. [DOI] [PubMed] [Google Scholar]
- 47.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, Fiering S, Conejo-Garcia JR. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, Krieg AM, Levy R. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 50.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egilmez NK, Kilinc MO. Tumor-resident CD8+ T-cell: the critical catalyst in IL-12-mediated reversal of tumor immune suppression. Arch Immunol Ther Exp (Warsz) 2010;58:399–405. doi: 10.1007/s00005-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 52.Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Miyagi T, Jinushi M, Sakamori R, Kohga K, Uemura A, Ohkawa K, Storkus WJ, Hayashi N. Injection of IL-12 gene-transduced dendritic cells into mouse liver tumor lesions activates both innate and acquired immunity. Gene Ther. 2007;14:863–871. doi: 10.1038/sj.gt.3302941. [DOI] [PubMed] [Google Scholar]
- 53.Higham EM, Shen CH, Wittrup KD, Chen J. Cutting edge: delay and reversal of T cell tolerance by intratumoral injection of antigen-loaded dendritic cells in an autochthonous tumor model. J Immunol. 2010;184:5954–5958. doi: 10.4049/jimmunol.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salem ML, Cole DJ. Dendritic cell recovery post-lymphodepletion: a potential mechanism for anti-cancer adoptive T cell therapy and vaccination. Cancer Immunol Immunother. 2010;59:341–353. doi: 10.1007/s00262-009-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, Romero P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 58.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


