Abstract
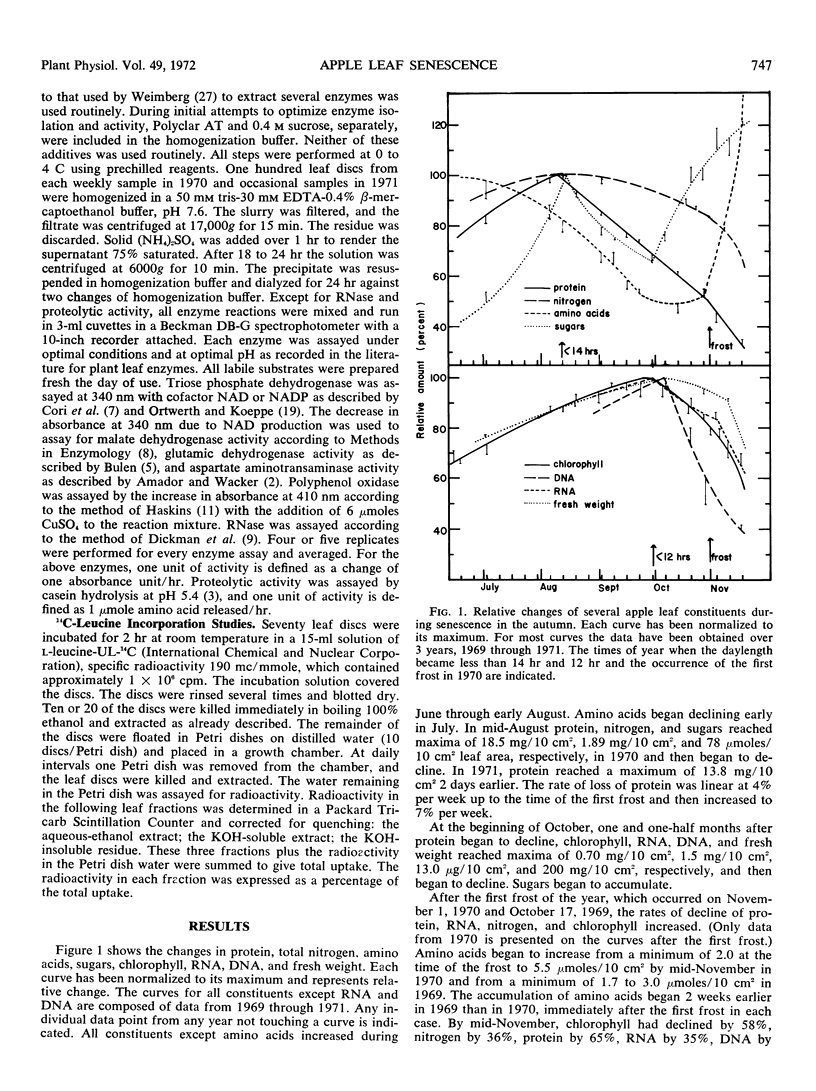
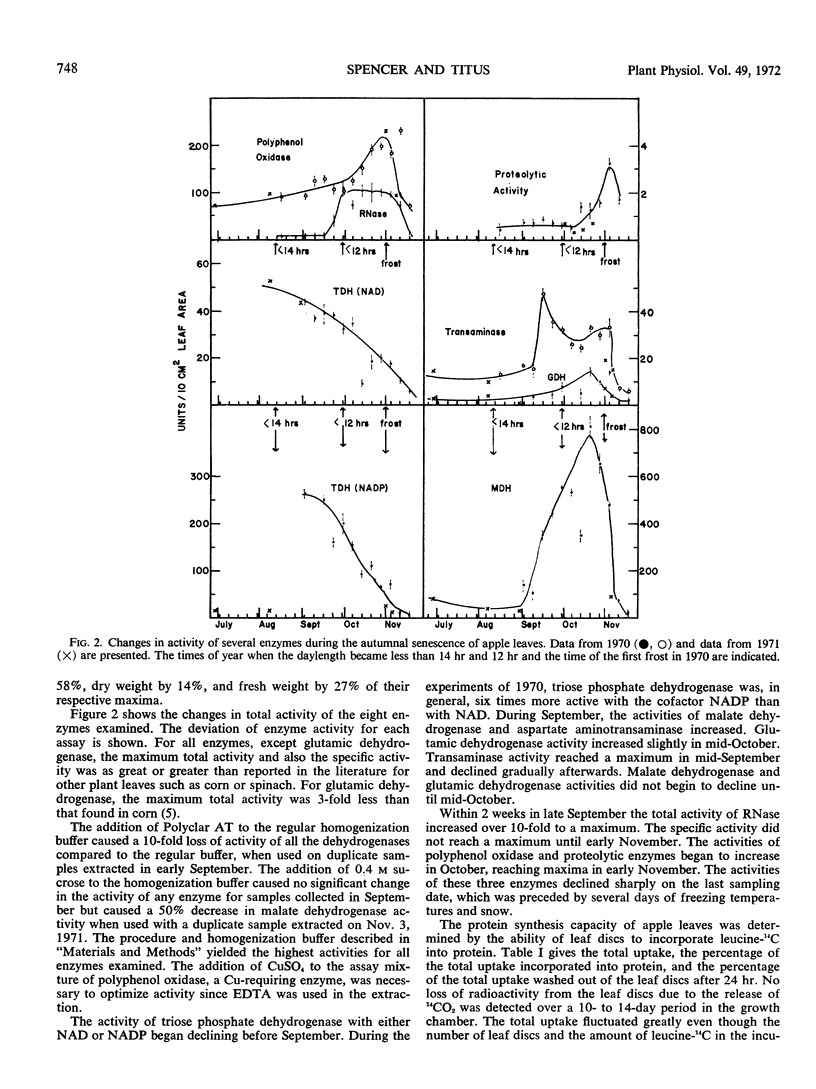
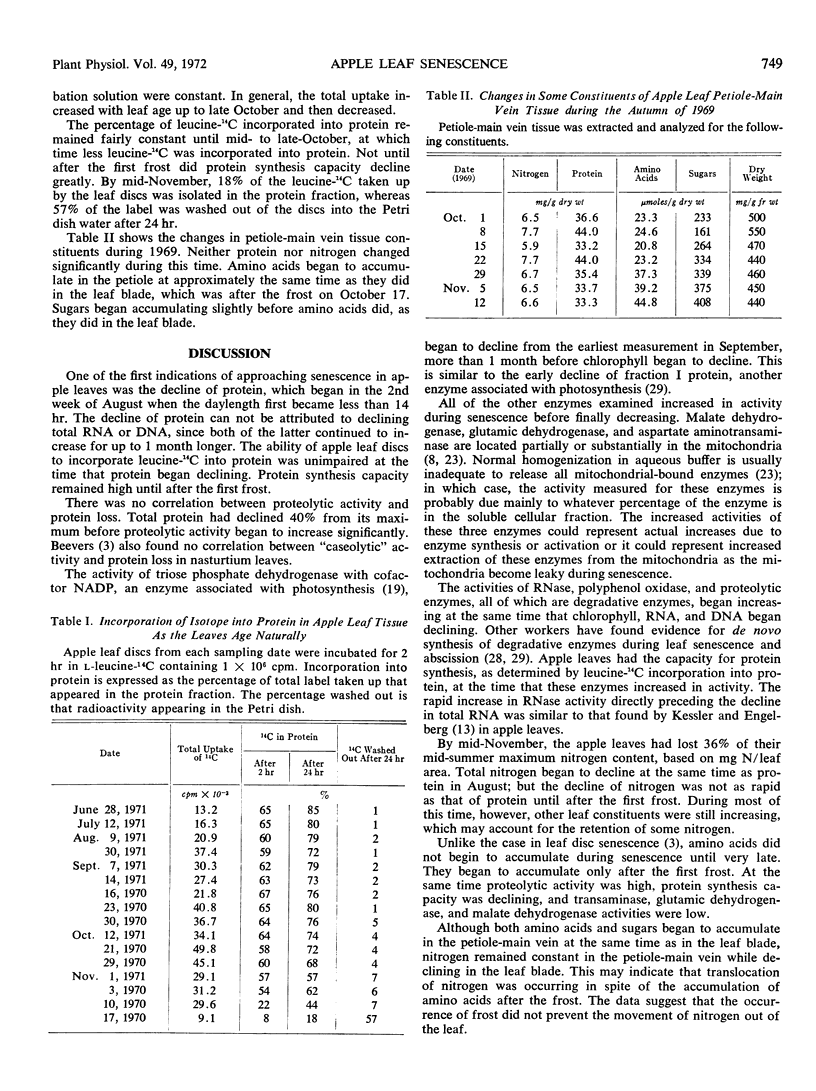
The biochemical changes occurring during the natural senescence of apple leaf tissue (Pyrus malus L., Golden Delicious) coincided with specific changes in the environment. Protein, sugars, and total nitrogen began declining in leaf tissue when the daylength first became less than 14 hours in the second week of August. The activity of triose phosphate dehydrogenase declined shortly afterwards, while the activities of malate dehydrogenase, glutamic dehydrogenase, and aspartate aminotransaminase increased. Chlorophyll, DNA, RNA, and fresh weight began declining when the daylength first became less than 12 hours at the end of September. At the same time sugars and the activities of RNase, polyphenol oxidase, and proteolytic enzymes began increasing. Protein synthesis, total nitrogen, and the activities of malate dehydrogenase, glutamic dehydrogenase, and aspartate aminotransaminase began declining rapidly and amino acids began to accumulate after the first frost of the year. RNase, polyphenol oxidase, and proteolytic activity reached their highest specific activities after the first frost.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., WACKER W. E. Serum glutamic-oxaloacetic transaminase activity. A new modification and an anaytical assessment of current assay technics. Clin Chem. 1962 Aug;8:343–350. [PubMed] [Google Scholar]
- AROSKAR J. P., DICKMAN S. R., KROPF R. B. Activation and inhibition of beef pancreas ribonuclease. Biochim Biophys Acta. 1956 Sep;21(3):539–545. doi: 10.1016/0006-3002(56)90192-5. [DOI] [PubMed] [Google Scholar]
- Addicott F. T. Environmental factors in the physiology of abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1471–1479. [PMC free article] [PubMed] [Google Scholar]
- BULEN W. A. The isolation and characterization of glutamic dehydrogenase from corn leaves. Arch Biochem Biophys. 1956 May;62(1):173–183. doi: 10.1016/0003-9861(56)90100-x. [DOI] [PubMed] [Google Scholar]
- KESSLER B., ENGELBERG N. Ribonucleic acid and ribonuclease activity in developing leaves. Biochim Biophys Acta. 1962 Jan 22;55:70–82. doi: 10.1016/0006-3002(62)90932-0. [DOI] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwerth B. J., Koeppe O. J. Light dependent increase of triosephosphate dehydrogenase in pea leaves. Plant Physiol. 1966 Sep;41(7):1213–1217. doi: 10.1104/pp.41.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. W. Types of leaf senescence. Symp Soc Exp Biol. 1967;21:215–230. [PubMed] [Google Scholar]
- Weimberg R. Enzyme levels in pea seedlings grown on highly salinized media. Plant Physiol. 1970 Sep;46(3):466–470. doi: 10.1104/pp.46.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollgiehn R. Nucleic acid and protein metabolism of excised leaves. Symp Soc Exp Biol. 1967;21:231–246. [PubMed] [Google Scholar]
- Woolhouse H. W. The nature of senescence in plants. Symp Soc Exp Biol. 1967;21:179–213. [PubMed] [Google Scholar]



