Abstract
Background
Diazoxide maintains myocyte volume and contractility during stress via an unknown mechanism. The mechanism of action may involve an undefined (genotype unknown) mitochondrial adenosine triphosphate-sensitive potassium (mKATP) channel and is dependent upon the KATP channel subunit SUR1. KATP channel openers have been shown to inhibit succinate dehydrogenase (SDH) and a gene for a portion of SDH has been found in the SUR intron. Thus, diazoxide may be cardioprotective via inhibition of SDH, which may form part of a KATP channel or share its genetic material. This study investigated the role of inhibition of SDH by diazoxide and its relationship to the SUR1 subunit.
Study Design
Mitochondria were isolated from wild type and SUR1 knockout mice. Succinate dehydrogenase activity was measured by spectrophotometric analysis of 2,6-Dichloroindophenol reduction for 20 minutes as the relative change in absorbance over time. Mitochondria were treated with succinate (20 mM), succinate + 1% dimethylsulfoxide, succinate + malonate (8 mM) (competitive inhibitor of SDH), or succinate + diazoxide (100 μM).
Results
Both malonate and diazoxide inhibit SDH activity in mitochondria of wild type mice and in mice lacking the SUR1 subunit (p<0.05 vs control).
Conclusions
The ability of DZX to inhibit SDH persists even after the deletion of the SUR1 gene. Therefore, the enzyme complex SDH is not dependent on the SUR1 gene. The inhibition of SDH by DZX may play a role in the cardioprotection afforded by DZX, however, this role is independent of the KATP channel subunit SUR1.
INTRODUCTION
The cardioprotective mechanism of action of mitochondrial adenosine triphosphate sensitive potassium (mKATP) channel opener, diazoxide (DZX), remains elusive. We and others have demonstrated the cardioprotective properties of DZX (1-5). In an isolated myocyte model of myocardial stunning, DZX maintained myocyte volume and contractility during exposure to stress in 3 different species (6-10).
Diazoxide is generally believed to be more selective for a purported mKATP channel (1). KATP channels are composed of a potassium inward rectifier channel forming subunit (Kir) and a sulfonylurea regulatory (SUR) subunit (11). There are 2 proposed types of cardiac KATP channels: a sarcolemmal KATP (sKATP) and a purported mKATP channel. The sKATP channel is composed of SUR2A and Kir 6.2 subunits in mouse ventricle and SUR1 and Kir 6.1 subunits in mouse atria (12). However, both SUR1 and SUR2A subunits have been identified in mouse heart (12) and in neonatal rat ventricular tissue (13).
Unlike the sarcolemmal KATP channel, the mitochondrial KATP channel has not been cloned and its genetic material is undefined. In addition, measuring ion flux across a mitochondrial membrane to confirm mitochondrial KATP channel activity is not feasible. Therefore, investigation of the mechanism of action of diazoxide requires indirect methods.
Previously, the cardioprotection afforded by DZX was localized to a non-sarcolemmal KATP channel location as DZX failed to generate a potassium current via the sKATP channel and by the evidence that DZX provides no cardioprotective benefit to mouse myocytes lacking the SUR1 subunit (14).
Interestingly, diazoxide is also a known inhibitor of the mitochondrial enzyme complex II, succinate dehydrogenase (SDH), which is a component of the electron transport chain (15-17). SDH inhibition by DZX has been shown to decrease reactive oxygen species generation, decrease ATP breakdown, and preserve ATP concentration during stress and has been proposed to be a non-KATP channel mechanism of cardioprotection (16-17). Malonate and 3-nitropropionic acid (3-NPA), both inhibitors of SDH, are also cardioprotective, mimic ischemic preconditioning, and decrease oxygen radical production (18-20).
The two proposed cardioprotective mechanisms of diazoxide (KATP channel opening and SDH inhibition) may be associated or linked (20-22). Specifically, 4 specific mitochondrial proteins (mitochondria ATP-binding cassette 1, phosphate carrier, adenine nucleotide translocator, ATP synthase) have been identified that associate with SDH (22). This multi-protein complex was capable of generating a potassium current and potassium influx upon exposure to DZX. This potassium current was diminished in the presence of ATP and 5-hydroxydecanoate (5-HD), both mKATP channel inhibitors; but not with HMR-1098, a sKATP channel inhibitor. Malonate, a competitive inhibitor of SDH, has also been shown to generate a potassium current leading to mitochondrial matrix swelling (a proposed consequence of mitochondrial KATP channel activity) and is inhibited by ATP and 5-HD (20). In addition, a genetic link between a KATP channel and SDH has been proposed (21). A gene encoding an anchoring protein (CII-3) of the SDH enzyme has been identified in an intron of the SUR1 gene.
These proposed associations between the SDH enzyme complex and the SUR1 subunit taken together with the knowledge that the SUR1 subunit is required for DZX cardioprotection and the suggestion that the inhibition of SDH underlies the cardioprotection afforded by diazoxide (3,16-17) led to the hypothesis for the present study. We hypothesized that the genetic deletion of the SUR1 subunit would result in the the loss of SDH activity..
METHODS
All animal procedures were approved by the Animal Studies Committee at Washington University School of Medicine and all animals received humane care in compliance with the National Institute of Health's Guide to Care and Use of Laboratory Animals (23).
Mitochondrial Succinate Dehydrogenase Activity
Mitochondria were isolated from hearts of wild type C57BL/6 mice and SUR1(-/-) mice. SUR1 (-/-) mice were created by removal of the 1-kbp gene segment containing both promoter and exon 1 sequences of SUR1 gene by re-mediated recombination (24). SUR1(-/-) mice were originally generated on a 129Sv background and then backcrossed >6X onto C57BL/6. Genotype was confirmed by polymerase chain reaction (24).
Mice (either sex, 6-15 weeks old, average 25 grams) were anesthetized with 3% Avertin (0.3 grams 2,2,2 tribromoethanol, 1.86 μL 2-methyl-2-butanol, 9.841mL sterile water) intraperitoneally and rapid cardiectomy was performed. Ventricular tissue was rapidly minced and homogenized with a 7mL Dounce homogenizer containing cold buffer (in mM/L: 10 HEPES (N-[2-hydroxyethyl]piperazine-N-[4-butanesulfonic acid]), 1 EDTA-K2 (ethylene diamine tetraacetic acid potassium), 250 Sucrose, adjusted to a pH of 7.1 with 20% potassium hydroxide. The homogenate was transferred to microcentrifuge tubes and centrifuged at 900 × g for 10 minutes at 4°C. Supernatant was then centrifuged at 5000 × g for 15 minutes. Supernatant was discarded and 300 μL homogenization buffer was added to each pellet. A Bradford protein assay (Thermo Scientific; Rockford, IL) was utilized to determine and normalize total protein per each pellet. Mitochondria were stored in -20°C freezer and thawed on ice just before use and kept on ice throughout each assay. All other solutions were kept at room temperature.
Mitochondria, at a concentration of 1.8 μg, were exposed to one of the following solutions (in a 1mL reaction): 20mM succinate (control) (Sigma, St. Louis) (N=11 WT, N=8 SUR1(-/-)), succinate + 1% Dimethylsulfoxide (DMSO) (Sigma; St. Louis, MO)(N= 10 WT, N=8 SUR1(-/-)), Succinate + 8mM malonate (competitive inhibitor of SDH) (Sigma; St. Louis, MO)(N=11 WT, N=8 SUR1(-/-)), and succinate + 100 μM DZX (KATP channel opener, DZX) (Sigma; St. Louis, MO)(N=10 WT, N=8 SUR1(-/-)). DZX was dissolved in DMSO.
Each reaction additionally contained 2 mM potassium cyanide (KCN), 50 uM 2,6 Dicholoroindophenol (DCIP), 1.625 mM phenazine methosulfate (PMS) and was brought to 1mL volume with 0.1M potassium phosphate buffer, pH 7.4. Reactions were prepared in disposable cuvettes covered with parafilm and allowed to activate for 20 minutes at room temperature before the final addition of KCN, DCIP and PMS.
SDH activity was measured by spectrophotometric (UV-1700 Spectrophotometer, Shimadzu Scientific Instruments; Columbia, MD) analysis of 2,6-DCIP (Sigma; St. Louis, MO) reduction at 600 nm for 20 minutes. Measurements were collected at 5 minute intervals and normalized to protein content.
Statistical Analysis
Data were analyzed using SYSTAT 13 (SYSTAT Software Inc., Chicago, IL). All data are presented as mean value ± standard error of the mean, with n equal to the number of experiments in each group. A repeated-measures analysis of variance was used for sequential time-based measurements for each test solution against its own baseline value. Group comparisons were made based on percent change in absorbance and average slopes. Probability values less than 0.05 were considered significant.
RESULTS
Succinate Dehydrogenase Activity
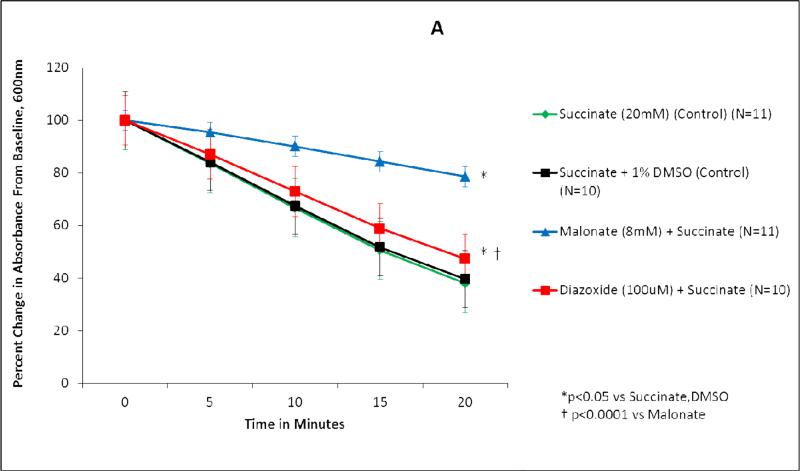
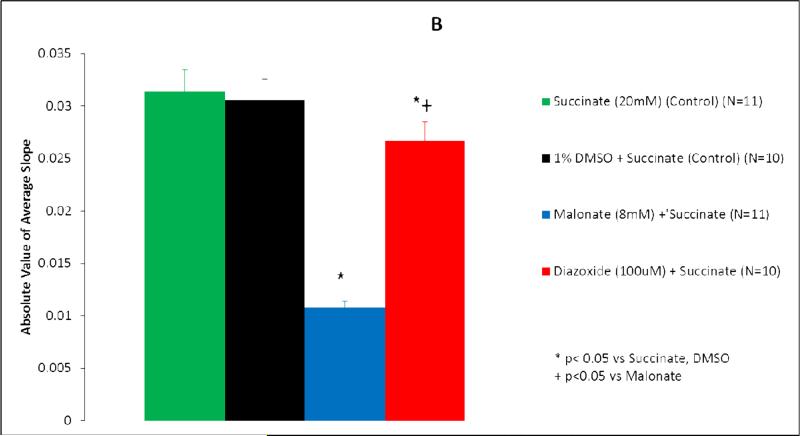
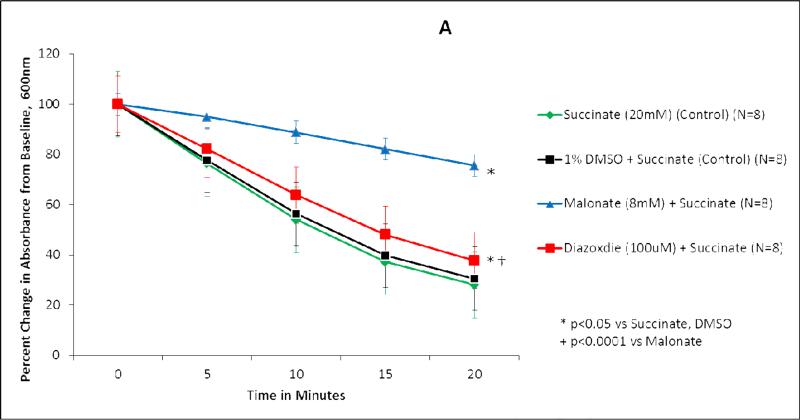
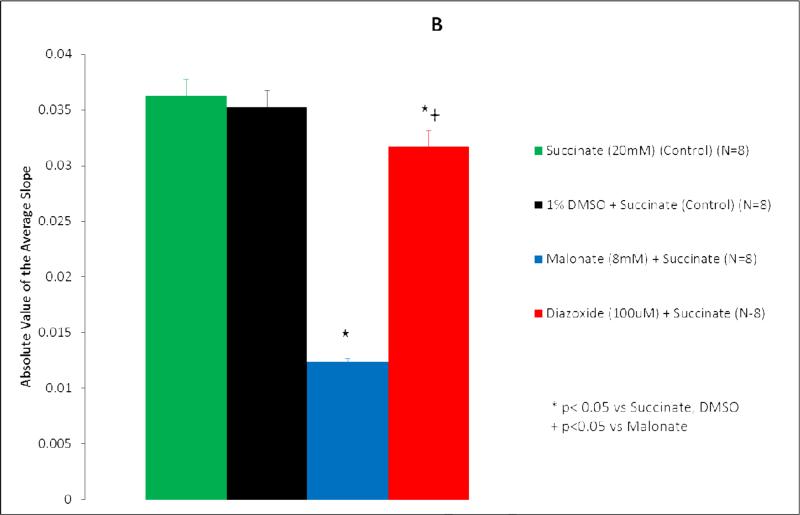
Succinate dehydrogenase activity is represented as both percent change in absorbance over time (Figure 1A, 2A) and as the absolute value of the average slope (Figure 1B, 2B). SDH activity is inversely proportional to absorbance.
Figure 1.
Diazoxide inhibits succinate dehydrogenase (SDH) activity in wild type mice mitochondria. (A) Isolated mitochondria were exposed to test solutions for 20 minutes. SDH activity is represented as percent change in absorbance over time. Green line, succinate (20 mM) (control) (n=11); black line, succinate + DMSO (control) (n=10); blue line, malonate (8 mM) + succinate (n=11); red line, diazoxide (100 uM) + succinate (n=10). *p<0.05 vs succinate, DMSO; †p<0.0001 vs malonate. (B) SDH activity represented as the average slope. Green bar, succinate (20 mM) (control) (n=11); black bar, 1% DMSO + succinate (control) (n=10); blue bar, malonate (8 mM) + succinate (n=11); red bar, diazoxide (100 uM) + succinate (n=10). *p<0.05 vs succinate, DMSO; †p<0.05 vs malonate.
Figure 2.
Diazoxide inhibits succinate dehydrogenase (SDH) activity in sulfonylurea receptor subunit 1 knockout mice mitochondria. (A) Isolated mitochondria were exposed to test solutions for 20 minutes. SDH activity is represented as percent change in absorbance over time. Green line, succinate (20 mM) (control) (n=8); black line, 1% DMSO + succinate (control) (n=8); blue line, malonate (8 mM) + succinate (n=8); red line, diazoxide (100 uM) + succinate (n=8). *p<0.05 vs succinate, DMSO; †p<0.0001 vs malonate. (B) SDH activity is represented as average slope. Green bar, succinate (20 mM) (control) (n=8); black bar, 1% DMSO + succinate (control) (n=8); blue bar, malonate (8 mM) + succinate (n=8); red bar, diazoxide (100 uM) + succinate (n=8). *p<0.05 vs succinate, DMSO; †p<0.05 vs malonate.
Succinate, the substrate for succinate dehydrogenase, served as the control for wild type and SUR1(-/-) mitochondrial experiments (Figures 1-2). Succinate demonstrated the greatest SDH activity (Figures 1,2). Mitochondria exposed to succinate + DMSO (vehicle) resulted in SDH activity similar to succinate alone in both wild type (p=0.611) and SUR1(-/-) mice (p=0.994) (Figures 1,2).
In wild type mice mitochondria, the addition of malonate to succinate resulted in significant inhibition of SDH activity (p<0.0001 vs Succinate) (Figure 1). The addition of 100 μM DZX to succinate also resulted in significant inhibition of SDH activity (p=0.002 vs Succinate), to a smaller degree than malonate (p<0.0001 vs malonate).
In SUR1 (-/-) mice mitochondria, the addition of malonate to succinate also significantly inhibited SDH activity (p<0.0001 vs Succinate) (Figure 2). The addition of 100 μM DZX to succinate inhibited SDH activity (p<0.0001 vs Succinate) to a smaller degree than malonate (p<0.0001 vs malonate).
When the average slopes of absorbance were compared, the presence of both malonate and DZX inhibited SDH activity in wild type and SUR1 (-/-) mice (p<0.05 vs succinate) (Figures 1B, 2B). DZX inhibited SDH activity to a smaller degree than malonate in both groups (p<0.05 vs malonate).
DISCUSSION
Current knowledge supports that the KATP channel is nature's own defense against myocardial ischemia. Pharmacological openers of KATP channels have been found to be cardioprotective in multiple animal models (1-5). Diazoxide, a KATP channel opener, has been found to limit myocyte volume and contractility derangements in a model of myocardial stunning (6-10). The exact mechanism of this protection is unknown.
Proposed hypotheses of DZX's mechanism of action implicate a sarcolemmal KATP channel, a mitochondrial KATP channel, or KATP channel-independent effects of channel openers themselves (25). We have eliminated the possibility of a sKATP location by demonstrating that DZX does not induce K+ current via sKATP channels, and that DZX cardioprotection requires KATP channel subunit SUR1 (not present in ventricular sKATP channels composed of Kir6.2 and SUR2A subunits) (14). We have also documented that the KATP channel subunit SUR1 is required for DZX cardioprotection implicating KATP channel involvement (14). The present study was conducted to investigate the inhibition of SDH as a mechanism of action of DZX independent of KATP channel subunit SUR1.
Similar to previous studies, the present study demonstrated that DZX inhibited SDH activity in wild type mice mitochondria. DZX also inhibited SDH activity in SUR1 (-/-) mice suggesting SDH and SUR1 have independent roles. Given that SUR1 has been identified in ventricular tissue but not a part of a sKATP channel, the present study could support DZX action at a mitochondrial location involving SDH with downstream involvement of a channel with SUR1 subunits (12-13). In addition, the present study could support that SDH forms a portion of the mKATP channel complex separate from SUR1.
Malonate, a competitive inhibitor of SDH, was associated with the greatest inhibition of SDH (79%) in wild type mice. Diazoxide reduced SDH activity by 47%. Similar results were found by other investigators using lower doses of succinate and DZX (16-17).
SDH inhibition was somewhat less in SUR1 (-/-) mice (38%) compared to wild type mice (47%). No other experiments have explored SDH inhibition in genetically altered mice.
Until the genotype of the mitochondrial KATP channel is established, the precise mechanism of action of diazoxide remains elusive and its investigation requires indirect methods. The present study suggests that diazoxide inhibits SDH even in animals lacking the SUR1 KATP channel subunit. This is consistent with independent involvement of SDH and a mitochondrial KATP channel in diazoxide's mechanism of action and that SUR1 may be an upstream modulator of a mitochondrial KATP channel.
Diazoxide provides cardioprotection in multiple animal models and in the setting of cardiac surgery (26). Further translational studies will involve intact heart and large animal models of various forms of myocardial ischemia with potential future human studies. Prior to these experiments it is vital to define the precise mechanism of action of diazoxide.
Limitations
Mitochondrial SDH activity was represented as change in absorbance over time and as the average slope of absorbance. The interpretation of the average slope is limited by deviation of the data from true linear changes.
Succinate dehydrogenase enzyme expression level was assumed to be equivalent in each mitochondrial pellet analyzed as protein concentration was normalized and pellets were randomly assigned to treatment groups.
Both male and female mice were included in this study and the effect of sex on SDH activity and implications for the findings described in this study are unknown.
Acknowledgments
Financial Support: This study was supported by American Heart Association Grant in Aid 09GRNT202045 (JSL), Thoracic Surgery Foundation for Research and Education Nina Starr Braunwald Career Award (JSL), NIH RO 1HL098182-01A1 (JSL), and NIH5T32 HL007776 (MMA).
TABLE OF ABBREVIATIONS
- DZX
diazoxide
- KATP
adenosine triphosphate sensitive potassium channel
- Kir
potassium inward rectifying subunit
- SUR
sulfonylurea receptor subunit
- sKATP
sarcolemmal KATP
- mKATP
mitochondrial KATP
- SDH
succinate dehydrogenase
- 3-NPA
3-nitropropionic acid
- 5-HD
5-hydroxydecanoate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 98th Annual Clinical Congress, Chicago, IL, October 2012.
REFERENCES
- 1.Garlid KD, Paucek P, Yarov-Yarovoy B, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive potassium channels: possible mechanism for cardioprotection. Circulation Research. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 2.Grover GH, Garlid KD. ATP- sensitive potassium channels: a review of their cardioprotective pharmacology. J Molecul Cellul Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 3.Lim KHH, Javadov SA, Das M, et al. The effects of ischaemic preconditioning, diazoxide and 5-hydoxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousou AJ, Ericsson M, Federman M, et al. Opening of mitochondrial KATP channels enhances cardioprotection through the modulation of mitochondrial matrix volume, calcium, accumulation, and respiration. Am J Physiol Heart Circ Physiol. 2004;287:H1967–H1976. doi: 10.1152/ajpheart.00338.2004. [DOI] [PubMed] [Google Scholar]
- 5.Costa ADT, Quinlan CL, Andrukhiv A, et al. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–H415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani S, Prasad SM, Sellitto AD, et al. Myocyte volume and function in response to osmotic stress: Observations in the presence of an adenosine triphosphate-sensitive potassium channel opener. Circulation. 2005;112:219–223. doi: 10.1161/CIRCULATIONAHA.104.523746. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani S, Al-Dadah AS, Block JB, et al. Hyperkalemic cardioplegia-induced myocyte swelling and contractile dysfunction: prevention by diazoxide. Ann Thorac Surg. 2006;81:154–159. doi: 10.1016/j.athoracsur.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 8.Prasad SM, Al-Dadah AS, Byrd GD, et al. Role of the sarcolemmal adenosine triphosphate sensitive potassium channel in hyperkalemic cardioplegia-induced myocyte swelling and reduced contractility. Ann Thorac Surg. 2006;81:148–153. doi: 10.1016/j.athoracsur.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Al-Dadah AS, Voeller RK, Schuessler RB, et al. Maintenance of myocyte volume homeostasis during stress by diazoxide is cardioprotective. Ann Thorac Surg. 2007;84:857–863. doi: 10.1016/j.athoracsur.2007.04.103. [DOI] [PubMed] [Google Scholar]
- 10.Maffit SK, Sellitto AD, Al-Dadah AS, et al. Diazoxide maintains human myocyte volume homeostasis during stress. J Am Heart Assoc. 2012;1:1–8. 6. doi: 10.1161/JAHA.112.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shyng SL, Nichols CG. Octametric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flagg TP, Kurata HT, Masia R, et al. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circulation Research. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. Antisense oligodeoxynucleotides of sulfonylurea receptors inhibit ATP-sensitive K+ channels in cultured neonatal rat ventricular cells. Pflugers Arch. 1999;437:400–408. doi: 10.1007/s004240050794. [DOI] [PubMed] [Google Scholar]
- 14.Sellitto AD, Maffit SK, Al-Dadah AS, et al. Diazoxide maintenance of myocyte volume and contractility during stress: evidence for a non-sarcolemmal KATP channel location. J Thorac Cardiovasc Surg. 2010;140:1153–1159. doi: 10.1016/j.jtcvs.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafer G, Portenhauser R, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide. Action on succinate dehydrogenase and TCA-Cycle oxidations. Biochem Pharmacol. 1971;20:1271–1280. doi: 10.1016/0006-2952(71)90358-3. [DOI] [PubMed] [Google Scholar]
- 16.Hanley PJ, Mickel M, Loffler M, et al. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzeja PP, Bast P, Ozcan C, et al. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;254:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- 18.Ockaili RA, Bhargave P, Kukreja RC. Chemical preconditioning with 3-nitropropionic acid in hearts: role of mitochondrial KATP channel. Am J Physiol Heart Circ Physiol. 2001;280:H2406–H2411. doi: 10.1152/ajpheart.2001.280.5.H2406. [DOI] [PubMed] [Google Scholar]
- 19.Busija DW, Katakam P, Rajapakse NC, et al. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Research Bulletin. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochimica et Biophysica Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohllk N, Thomas PM, Huan E, Cote GJ. A human succinate-ubiquinone oxidoreductase CII-3 subunit gene ending in a polymorphic dinucleotide repeat is located within the sulfonylurea receptor (SUR) gene. Molecul Genet Metabolism. 1998;65:187–190. doi: 10.1006/mgme.1998.2752. [DOI] [PubMed] [Google Scholar]
- 22.Ardehali H, Chen Z, Ko Y, et al. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. PNAS. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide to Care and Use of Laboratory Animals. 8th Edition Institutes for Laboratory Animal Research, Division on Earth and Life Studies; National Research Council, National Institutes of Health; 2010. [Google Scholar]
- 24.Shiota C, Larsson O, Shelton KD, et al. Sulfonylurea receptor type 1 knockout mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biolog Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 25.Foster DB, Rucker J, Garlid AO, et al. Mitochondrial ROMK channel is a molecular component of MitoKATP. Circulation Research. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deja MA, Malinowski M, Golba KS, et al. Diazoxide protects myocardial mitochondria, metabolism, and function during cardiac surgery: a double-blind randomized feasibility study of diazoxide-supplemented cardioplegia. J Thorac Cardiovasc Surg. 2009;137:997–1004. doi: 10.1016/j.jtcvs.2008.08.068. [DOI] [PubMed] [Google Scholar]