Abstract
The metabolic syndrome refers to a constellation of signs including abdominal obesity, elevated serum triglycerides, low HDL-cholesterol, elevated blood pressure and insulin resistance. Today approximately one third of the adult population has the metabolic syndrome. While there is little doubt that the signs constituting the metabolic syndrome frequently cluster, much controversy exists over the definition, pathogenesis, or clinical utility. Here we present evidence from the field of comparative physiology that the metabolic syndrome is similar to the biological process that animals engage to store fat in preparation for periods of food shortage. We propose that the metabolic syndrome be changed to fat storage condition to more clearly align with its etiology. Obesity in humans is likely the consequences of both genetic predisposition (driven in part by thrifty genes) and environment. Recent studies suggest that the loss of the uricase gene may be one factor that predisposes humans to obesity today. Understanding the process animals engage to switch from a lean insulin-sensitive to an obese insulin-resistant state may provide novel insights into the cause of obesity and diabetes in humans, and unique opportunities for reversing their pathology.
Keywords: hibernation, insulin resistance, metabolic syndrome, obesity
In 1923 the Swedish physician, Eskil Kylin, noted that obese subjects not uncommonly have elevated glucose levels, elevated blood pressure and hyperuricemia.1 Subsequently others also observed a clustering of signs, including obesity, glucose intolerance, hypertriglyceridemia, and hyperuricemia or gout in subjects at risk for developing diabetes.2–7 In 1989 Reaven elaborated on these cluster of signs which he called syndrome X and suggested that insulin resistance was the underlying pathophysiological mechanism.8 Shortly thereafter the cluster became known as the metabolic syndrome, and was given various definitions, for which one of the most popular is the one provided by the Adult Treatment Panel (ATP) III of the National Cholesterol Education Program (NCEP-ATPIII). According to the NCEP-ATPIII definition, an individual must have three of the following five characteristics: 1) Abdominal Obesity determined by waist circumference (Men > 102 cm; Women > 88 cm); 2) Triglycerides ≥150 mg/dL; 3) Low HDL cholesterol levels (Men < 40 mg/dL; Women < 50 mg/dL); 4) Blood pressure≥130/≥85 mmHg; and 5) Fasting glucose ≥ 110 mg/dL.9 Based on this definition, approximately 34% of the US 10 and 27% of the southern European11 adult population have metabolic syndrome.
Controversy exists, however, on the use of the term, metabolic syndrome.12–13 While the frequent clustering of the signs are not denied, the concept that insulin resistance may be the underlying mechanism has also been challenged 14. Agreement on definitions and cut-off levels have been difficult.14 There are also other findings frequently associated with metabolic syndrome but which are not included in the definition, such as fatty liver, microalbuminuria, sleep problems, hyperuricemia, inflammation, loss of muscle and bone mass, kidney stones and evidence of oxidative stress and endothelial dysfunction. The predictive value of metabolic syndrome as a risk factor for cardiovascular disease also does not appear any better than its individual components. This has led to some societies to recommend abandonment of the term metabolic syndrome.14 Even Gerald Reaven, whose paper brought the concept to national attention in the 1990s, has questioned whether it retains any useful value 15. It has been stated that until a better understanding of the underlying cause of metabolic syndrome is provided, the entity will remain controversial.12
In this paper we extend previous arguments16 to propose that the metabolic syndrome has many similarities with the normal biological processes by which animals store fat in preparation for periods of food shortage. The primary difference is that humans with metabolic syndrome continue to accumulate fat. As such, we propose to rename the metabolic syndrome “fat storage syndrome” to better characterize its underlying physiological basis.
Accumulating Fat is a Normal Survival Process
A longstanding tenet in the medical literature is that the development of insulin resistance and other features of metabolic syndrome is a pathophysiological process, meaning that it is either caused by a disease or represents a dysregulation of normal physiological mechanisms 8, 12. However, we would argue that it instead represents a normal physiological process, as it is engaged throughout the animal kingdom as a survival mechanism to avert starvation.
One of the basic concepts of Darwinian evolutionary theory is survival of the fittest, in which it was proposed that those individuals who could best adapt to the changing environment would survive to successfully reproduce and thereby be the ones to pass their genes to subsequent generations.17 Evolution has been considered a natural experimental process to select animal design characteristics with a survival advantage and one of the most challenging aspects of life is to be able to survive during times of food shortage. While some animals have learned to do this by storing foods, others have developed intricate ways to store fat (triglycerides) in their tissues, especially the white adipose tissues, the liver, and the blood. In the setting of food shortage, survival may not truly favor those who are lean and fit, but rather those with the greatest fat stores, and hence a more appropriate term than “survival of the fittest” might be “survival of the fattest”. Indeed, an understanding of the particular adaptations of different species in nature may lead to novel interventions for the prevention and treatment of human diseases, such as metabolic syndrome. As discussed by Singer18 this approach underlies the principles of biomimicry, which is the science that studies nature's models and, inspired by these designs and processes, try to solve human health problems.
There are numerous examples in the zoological literature of the importance of fat in survival of different animal species. Insect larvae store fat in their fat bodies which they use to support their life when in the pupal stage.19 Adult insects also rely on their fat stores to provide energy when food is not available. One striking example relates to the work of Weis-Fogh, who performed energy balance studies in African desert locusts.20 Weis-Fogh determined that the locusts use fat for more than 85% of their energy needs when they are in flight. He further demonstrated that locusts with 10% fat could last only 12 hours in flight, whereas those with 15% fat would last 20 hours. As such, he calculated that when the swarms of locusts would make the 600 mile trek from southern Morocco to Portugal, many individuals would perish during the flight from exhaustion of their fat stores.20
Some fish store fat for their survival. Lungfish live in freshwater lakes in Africa, but during the heat of the summer the water can evaporate and the lungfish must burrow into the mud to survive by burning stored fat until the waters return, which may be a year or more (termed estivation).21 Another example is the salmon, who stop eating when they migrate up fresh water rivers to spawn. During these migrations they can consume up to 60–80% of their fat, and if the fat stores become depleted, the fish will die.22–23
One of the best examples of storing fat as a means for survival is the freshwater Pacu fish (Piaractus mesopotamicus), which lives in the Amazon and Orinoco River. Every year these rivers flood during the tropical rains, and the river levels may rise 6 to 18 meters, flooding 70,000 to 300,000 square kilometers of rain forest. During this time of flooding the Pacu enters into the flooded jungle to feast on ripe fruit that has fallen into the river. The Pacu rapidly becomes fat, and its fat content can reach 28%. When the waters recede, the Pacu quits eating and lives off its fat stores for up to 6 months; during this time its fat content dwindles down to 10% or less.24–25
Yet another example is the water holding desert frog (Cyclorana platycephalus) that lives in the deserts of Australia where it prepares for droughts by storing fat in pads in its feet. Some frogs accumulate up to one-fifth of their weight in these food pads. When drought occurs, they burrow into the sand and use their fat stores, where they may survive for years without food. However, the amount of fat is critical for survival. Indeed, only 10% of frogs can survive a drought of 5 years duration, and it is those frogs who were able to store 20% or more of their overall weight as fat in their feet.26
Birds also exploit stored fat for their survival. As an example, Emperor Penguins double their weight in fat to help them survive without any food for two to four months during the Antarctic winter while they are nesting inland.27–28 Long-distance migrating birds, such as the godwit, also accumulate large amounts of fat prior to their migrations.29
Hibernating mammals, such as the 13-lined ground squirrel double their weight in the late summer in preparation for winter. The animals emerge lean from hibernation in spring, having burned these fat stores to survive the winter without eating.30 The gray whale increases its fat stores markedly prior to migrating to Baja, Mexico where it breeds. During the 6 months it travels to Baja and back it will fast and lose as much as 30 % of its weight.31–32
Classical Sites of Fat Storage
Some animals store fat in unusual places, such as the footpad in the water holding desert frog, the tail of the lizard, and the neck of the horse. However, for most animals, the storage of fat is similar to that observed in humans, and occurs primarily in the blood (as serum triglycerides), in the liver (steatosis), or in the fat tissues themselves (adipocytes). Birds in particular store large amounts of fat in the liver. For example, the hummingbird, which ingest a large amount of nectar during the day can develop such an extreme fatty liver that this organ is actually creamy white by the end of the day.33 However, because of their extremely high metabolism, hummingbirds may actually deplete their fat stores during the night, forcing them to enter torpor until the morning comes when they can feed on nectar again.34 Other animals, such as the Minke whale, can have such high serum triglycerides that the serum appears lipemic.35 Hibernating squirrels are known to increase their serum and liver triglyceride content during the summer and then use these lipid stores as their energy source during hibernation.36 Regardless, the fact that animals purposefully develop fatty liver, elevated triglycerides and visceral fat stores is reminiscent of features observed in patients with metabolic syndrome.
Insulin Resistance is a Feature of Fat Storage Syndrome
An important feature of fat storage is that animals also may become insulin resistant. For example, hibernating mammals, such as the marmot and squirrel, develop hyperinsulinemia and insulin resistance while they are accumulating fat in late summer.16, 37 Likewise, the European Garden Warbler increases its fat content in its liver, fat tissues and blood and becomes insulin resistant prior to migrating across the Sahara to subtropical Africa.38 The desert gerbil (Psammomys obesus), which is so predisposed to becoming fat that it is also called the fat sand rat, will become diabetic shortly after being placed on a high protein diet, also suggesting it may be prediabetic in the wild.39 Horses are also at risk for developing obesity and insulin resistance.40
Should We Reappraise the Metabolic Syndrome based on Comparative Physiology?
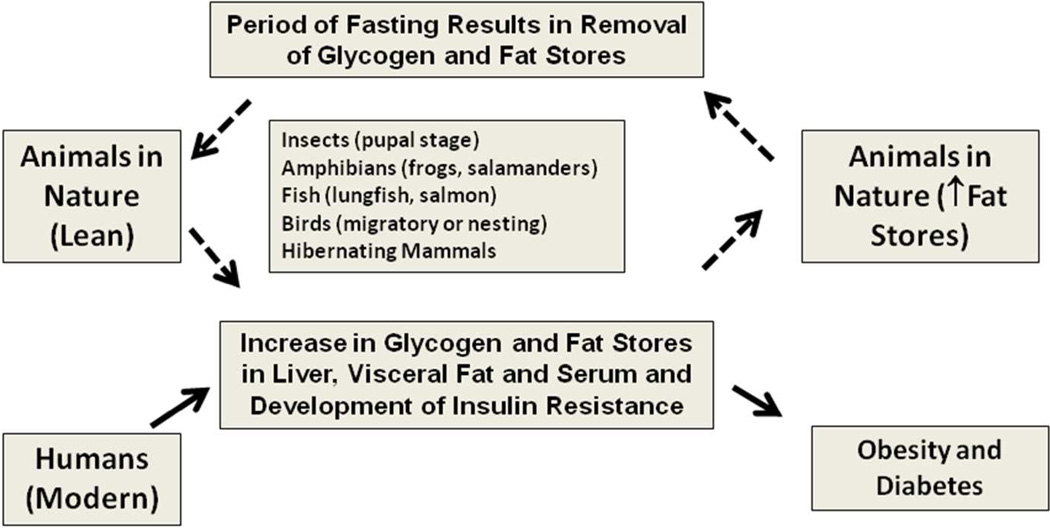
Most of the studies in the field of medicine focus on classical fields such as physiology, genetics, and molecular biology. Comparative physiology is often not at the top of the list. As such, the observation of a clustering of signs such as insulin resistance, hypertriglyceridemia, elevated blood pressure, abdominal obesity, and fatty liver may appear as a dysregulation of normal physiology. However, studies in a wide variety of animals support the concept that these signs may represent an adaptive form of fat storage (Figure 1). The fact that there is a continuum from a state of leanness to marked fat storage, as well as variability among and within species as to the degree with which fat is stored can also explain the difficulty in establishing strict definitions for metabolic syndrome or fat storage condition in humans. In some respects, the variations in manifestation of fat storage condition may not be dissimilar from systemic lupus erythematosus, in which diagnosis is made by the presence of four of eleven criteria; yet in the latter everyone agrees that the condition exists. By recognizing that fat storage condition is a normal biological process found in many animals to protect them from food shortage, we believe that we should focus not so much on the reality of this condition, or on its predictive components, but rather on the fundamental mechanisms that trigger the change back and forth between a lean, insulin sensitive animal to an obese, insulin resistant animal. One significant difference between humans and animals in the wild, of course, is that many animals become obese prior to a period of fasting, such as during hibernation, long distance migration, or nesting. According to this reasoning, our predisposition to obesity may simply be due to the continued availability of food.
Figure 1. The Metabolic Syndrome as a Disorder of Fat Storage.
Many animals develop features consistent with metabolic syndrome as part of the normal physiological processes involved in fat storage (shown as dotted lines). This suggests that metabolic syndrome may represent a form of fat storage. However, most animals undergo a period of fasting that brings the animal back to their regular weight, whereas many humans will progressively increase their fat stores until they become frankly obese or diabetic (solid lines).
Relationship to the Thrifty Gene Hypothesis
It is also possible that humans may have acquired (or lost) genes in our past that may predispose us to obesity today. Over 50 year ago James Neel proposed the thrifty gene hypothesis, in which he suggested that the epidemic of obesity and diabetes today are due to the acquisition of genes that occurred during periods of famine in our past that would facilitate fat stores and insulin resistance.41 This hypothesis has been controversial, with some papers supporting 42–44 and others criticizing 45–47 the hypothesis. One of the problems is that until recently, no thrifty genes had been identified. However, there is now evidence that it was not the gain of a gene, but rather the loss of the gene, uricase, that may have functioned to stimulate the thrifty phenotype.48
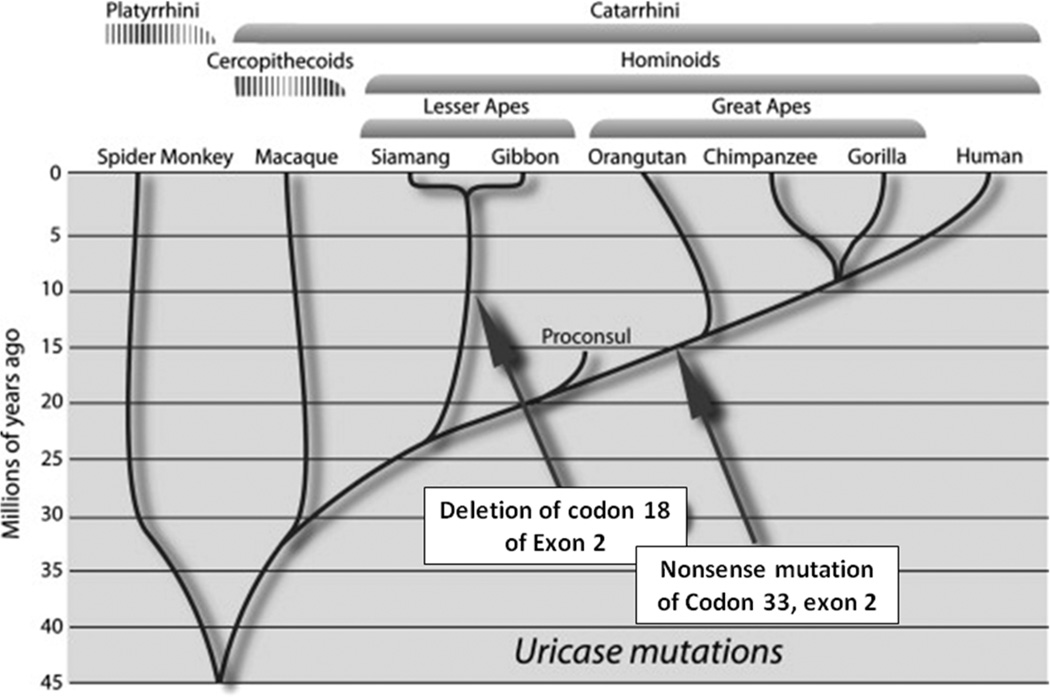
Uricase is a hepatic enzyme that degrades uric acid, eventually generating allantoin. It is present in all mammals except for the apes and humans. For the latter species, uricase was lost due to parallel mutations affecting the great apes and humans (occurring around 15 million years ago) and the lesser apes (occurring around 10 million years ago)(Figure 2).49–50 As such, humans and apes have higher serum uric acid levels than other mammals.
Figure 2. Parallel Mutations in Uricase occurred During the Evolution of Hominoids in the Miocene.
Adapted from Johnson RJ et al50 with permission of Elsevier.
The fact that there were parallel mutations of uricase in two lineages of apes suggest that there was an evolutionary benefit to the uricase mutation to apes in the mid Miocene. It is thus important to understand the paleoenvironment of this period. In the early Miocene (approximately 20 million years ago), all apes lived in Africa, where their diet consisted almost entirely of fruit. Then global cooling began, possibly initiated by volcanic activity in the Rift Valley, and leading to an expansion of the polar ice caps and a fall in the sea level. A land bridge that connected Africa with Eurasia appeared around 17 million years ago, and numerous species exited Africa, including giraffes, anteaters and apes. Shortly thereafter ape species can be found throughout Turkey and Europe.
Initially the apes in Europe and Turkey lived in tropical rain forests similar to those present in Africa. As global cooling continued, there was a loss of tropical rain forests in Europe with the replacement by deciduous trees. The climate became more seasonal, and the ape populations retreated to isolated colonies, where there is evidence for intermittent starvation (based on dental studies).51 By 8- 9 million years ago, all apes had become extinct in Europe. In contrast, the apes in Africa were able to continue with their regular diet of tropical fruit throughout the year, for while cooling also occurred here, it resulted primarily in a retraction of the tropical rain forest rather than their replacement as occurred in Europe.
While the extinction of apes in Europe may appear like a biological dead-end, there is increasing evidence from the fossil record that the ancestors to humans and modern apes came from a European ape that returned to Africa prior to the final extinctions. Both Kenyapithecus and Dryopithecus have been proposed as our candidate ancestors to have made the exodus “Back to Africa”.52–53 It was during this period that the uricase mutation occurred in our ancestors. Based on increasing evidence that the loss of uricase can increase fat stores and enhance the effects of fructose (see below), Johnson and Andrews hypothesized that the uricase mutation likely occurred in Europe as it would have provided a survival advantage under the conditions of seasonality and dwindling fruit availability.48, 54
The effect of the uricase mutation on fat storage and metabolic syndrome has only recently been appreciated. While initial studies focused on the potential benefit of uric acid as an antioxidant in the circulation, more recent studies show that an elevated serum uric acid likely has a direct role to increase fat stores and induce features of metabolic syndrome. Indeed, lowering serum uric acid has been found to reduce fatty liver, serum triglycerides, blood pressure and improve insulin resistance in a variety of animal models.55–57 Epidemiological studies show unequivocally that hyperuricemia is a consistent independent risk factor for obesity, fatty liver, insulin resistance and hypertension.58–61 Some genetic studies have linked polymorphisms in uric acid transport with hypertension, obesity and metabolic syndrome62–63 whereas others have not.64,65 Early clinical studies are also suggestive of a benefit of lowering serum uric acid on metabolic syndrome66–67, although larger, randomized studies are needed before any definitive conclusions can be made.
One of the more important interactions of uric acid is with fructose. Fructose is distinct from glucose in that it raises intracellular uric acid, due to the rapid and unchecked phosphorylation of fructose with ATP depletion and consequent degradation of nucleotides. This reaction is driven by fructokinase C, and has a critical role in driving the metabolic phenotype in response to fructose.68 We have recently found that the intracellular uric acid has an important role in fat accumulation from fructose by causing both direct effects on mitochondria as well as increasing the expression and activity of fructokinase itself (MA Lanaspa et al, unpublished). Consistent with this finding, the inhibition of uricase amplifies the effects of fructose to induce insulin resistance, raise blood pressure and increase serum triglycerides in rats.69 Furthermore, we have recently been able to study the ancestral primate uricase and have shown that hepatocytes (HepG2 cells) exposed to fructose show a blunted triglyceride response (Lanaspa MA and Gaucher EA et al, unpublished). Consistent with the evidence that uric acid regulates fructokinase, a recent study showed that subjects with an elevated serum uric acid show a greater ATP depletion in response to fructose.70
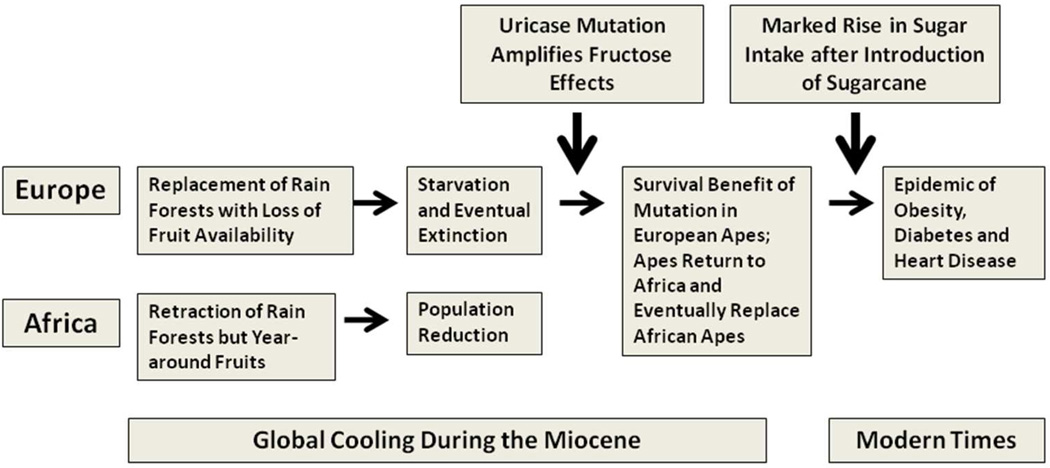
The uricase mutation that occurred in the Miocene amplified the biological effects of fructose from fruits to enhance fat storage, and was likely of great benefit to apes during this period of famine. However, with this type of diet, serum uric acid levels were only modestly higher than that of uricase-expressing primates. Indeed, in a study we conducted at the San Diego Zoo, the uricase-expressing primates have serum uric acid levels in the 1–2 mg/dl range whereas the apes lacking uricase have uric acid levels in the 3 mg/dl range.71 This latter level of uric acid is also similar to what is observed in tribes living on Paleolithic diets, such as the Yanomamö Indians.72 However, the introduction of sugarcane into the western diet led to a dramatic increase in fructose intake (Figure 3). The rise in sugar intake has closely paralleled the rise in serum uric acid levels, obesity and diabetes throughout the world.73–74 Sugary soft drinks, a major source of added sugars, predict the development of obesity, insulin resistance and diabetes 75–78. In addition to stimulating fatty liver, elevated triglycerides and insulin resistance, fructose also induces leptin resistance that blunts satiety, resulting in enhanced intake of foods with high fat content.79
Figure 3. The Thrifty Gene Hypothesis and Uricase.
The uricase mutation occurred during global cooling in the mid Miocene and has been postulated to have provided a survival advantage to European apes who were undergoing periodic starvation due to the loss of fruit availability in the cooler seasonal months. Specifically, the mutation amplified the effects of fructose in fruits to increase fat stores. Later the introduction of sugar led to a major increase in fructose availability and likely has a role in the epidemic rise in obesity and diabetes.54
These recent studies help explain various controversies related to the thrifty gene hypothesis. Because the uricase mutation increases the susceptibility to fructose-induced metabolic syndrome, it helps to explain why the mutation is present in everyone, yet obesity is not. Hence, obesity is a consequence of both environmental factors (such as fructose intake) coupled with an underlying genetic mutation (such as mutation in uricase). The argument that the thrifty gene hypothesis is unlikely to have occurred because humans have only been present for a million years or less is also not valid, for our studies suggest that the mutation occurred much earlier (during the Miocene). Indeed, we have also postulated that the loss of vitamin C due to a mutation in L-gulono-gamma-lactone oxidase nearly 50 million years ago may have represented another genetic change that would enhance the thrifty phenotype.50
Conclusion
In conclusion, metabolic syndrome in humans can be considered a type of fat storage condition. Increasing evidence suggests that metabolic syndrome today represents an interaction between genetic changes that we acquired to protect us during food shortage, coupled with changes in our environment. One of the genetic changes appears to be the loss of the uricase gene in the Miocene.
Acknowledgements
NIH RO-1 HL-68607 and NIH grants RC4 DK090859 provided support for this paper. This paper represents a publication of the Colorado Obesity Research Initiative.
Footnotes
Disclaimer/Conflict of Interest.
Dr Johnson is listed as an inventor on patent applications with the University of Florida related to lowering uric acid as a means for preventing or treating the metabolic syndrome. Dr Johnson and Dr Lanaspa are listed as inventors on patent applications from the University of Colorado on blocking fructose metabolism in the treatment of metabolic syndrome in response to carbohydrates. Finally, Dr. Johnson has a lay book, The Fat Switch (2012, Mercola.com) that discusses the role of fructose in metabolic syndrome in more detail. All other investigators have no conflicts.
References
- 1.Kylin E. [Studies of the hypertension-hyperglycemia-hyperuricemia syndrome] Studien uber das Hypertonie-Hyperglykamie-hyperurikamiesyndrome. Zentralblatt fur innere Medizin. 1923;44:105–127. [Google Scholar]
- 2.Herman JB, Medalie JH, Goldbourt U. Diabetes, prediabetes and uricaemia. Diabetologia. 1976;12:47–52. doi: 10.1007/BF01221964. [DOI] [PubMed] [Google Scholar]
- 3.Haller H. [Epidemiology and associated risk factors of hyperlipoproteinemia] Z Gesamte Inn Med. 1977;32:124–128. [PubMed] [Google Scholar]
- 4.Phillips GB. Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:1729–1733. doi: 10.1073/pnas.74.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer P. [Diagnosis of primary hyperlipoproteinemias] Z Gesamte Inn Med. 1977;32:128. contd. [PubMed] [Google Scholar]
- 6.Phillips GB. Sex hormones, risk factors and cardiovascular disease. The American journal of medicine. 1978;65:7–11. doi: 10.1016/0002-9343(78)90685-x. [DOI] [PubMed] [Google Scholar]
- 7.Herman JB, Goldbourt U. Uric acid and diabetes: observations in a population study. Lancet. 1982;2:240–243. doi: 10.1016/s0140-6736(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition. 1997;13:65. doi: 10.1016/s0899-9007(96)00380-2. discussion 4, 6. [DOI] [PubMed] [Google Scholar]
- 9.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 10.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 11.Gavrila D, Salmeron D, Egea-Caparros JM, et al. Prevalence of metabolic syndrome in Murcia Region, a southern European Mediterranean area with low cardiovascular risk and high obesity. BMC public health. 2011;11:562. doi: 10.1186/1471-2458-11-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brietzke SA. Controversy in diagnosis and management of the metabolic syndrome. Med Clin North Am. 2007;91:1041–1061. doi: 10.1016/j.mcna.2007.06.005. vii-viii. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran D, Reddy KS. The metabolic syndrome: looking beyond the debates. Clin Pharmacol Ther. 2011;90:19–21. doi: 10.1038/clpt.2011.116. [DOI] [PubMed] [Google Scholar]
- 14.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 15.Reaven GM. The metabolic syndrome: time to get off the merry-go-round? Journal of internal medicine. 2011;269:127–136. doi: 10.1111/j.1365-2796.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin SL. Mammalian hibernation: a naturally reversible model for insulin resistance in man? Diab Vasc Dis Res. 2008;5:76–81. doi: 10.3132/dvdr.2008.013. [DOI] [PubMed] [Google Scholar]
- 17.Darwin C. First edition. London: John Murray; 1859. On The Origin of Species by Means of Natural Selection, or The Preservation of Favoured Races in the Struggle for Life. [PMC free article] [PubMed] [Google Scholar]
- 18.Singer MA. Vampire bat, shrew, and bear: comparative physiology and chronic renal failure. American journal of physiology Regul Integr Comp Physiol. 2002;282:R1583–R1592. doi: 10.1152/ajpregu.00711.2001. [DOI] [PubMed] [Google Scholar]
- 19.Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis-Fogh T. Fat Combustion and Metabolic Rate of Flying Locusts (Schistocerca gregaria Forskal. Philosoph Transs Royal Soc London. 1952;237:1–36. [Google Scholar]
- 21.Fishman AP, Galante RJ, Winokur AI. Estivation in the African Lungfish. Proc Am Philos Soc. 1992;136:61–72. [Google Scholar]
- 22.Jonsson N, Jonsson B, Hansen LP. Changes in Proximate Composition and Estimates of Energetic Costs During Upstream Migration and Spawning in Atlantic Salmon Salmo salar. J Animal Ecol. 1997;66:425–436. [Google Scholar]
- 23.McVeigh BR, Healey MC, Wolfe F. Energy expenditures during spawning by chum salmon Oncorhynchus keta (Walbaum) in British Columbia. J Fish Biol. 2007;71:1696–1713. [Google Scholar]
- 24.Kubitzki K, Ziburski A. Seed Dispersal in Flood Plain Forests of Amazonia. Biotropica. 1994;26:30–43. [Google Scholar]
- 25.Junk WJ. Temporary fat storage, an adaptation of some fish species to the waterlevel fluctuations and related environmental changes of the Amazon river. Amazoniana. 1985;9:315–351. [Google Scholar]
- 26.Van Beurden EK. Energy Metabolism of Dormant Australian Water-holding Frogs (Cyclorana platycephalus) Copeia. 1980;4:787–799. [Google Scholar]
- 27.Robin JP, Boucontet L, Chillet P, Groscolas R. Behavioral changes in fasting emperor penguins: evidence for a "refeeding signal" linked to a metabolic shift. The American journal of physiology Regul Integ Comp Physiol. 1998;274:R746–R753. doi: 10.1152/ajpregu.1998.274.3.R746. [DOI] [PubMed] [Google Scholar]
- 28.Challet E, le Maho Y, Robin JP, Malan A, Cherel Y. Involvement of corticosterone in the fasting-induced rise in protein utilization and locomotor activity. Pharmacology, biochemistry, and behavior. 1995;50:405–412. doi: 10.1016/0091-3057(94)00287-s. [DOI] [PubMed] [Google Scholar]
- 29.Klaasen M. Metabolic constraints on longterm migration in birds. J Exp Biol. 1996;199:57–64. doi: 10.1242/jeb.199.1.57. [DOI] [PubMed] [Google Scholar]
- 30.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 31.Millar JS, Hickling GJ. Fasting Endurance and the Evolution of Mammalian Body Size Functional Ecology. 1990;4:5–12. [Google Scholar]
- 32.Mrosovsky N, Sherry DF. Animal anorexias. Science (New York, NY. 1980;207:837–842. doi: 10.1126/science.6928327. [DOI] [PubMed] [Google Scholar]
- 33.Hartman FA, Brownell KA. Liver lipids in hummingbirds. The Condor. 1959;61:270–277. [Google Scholar]
- 34.Hargrove JL. Adipose energy stores, physical work, and the metabolic syndrome: lessons from hummingbirds. Nutr J. 2005;4:36. doi: 10.1186/1475-2891-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tryland M, Brun E. Serum chemistry of the minke whale from the northeastern Atlantic. J Wildl Dis. 2001;37:332–341. doi: 10.7589/0090-3558-37.2.332. [DOI] [PubMed] [Google Scholar]
- 36.Otis JP, Sahoo D, Drover VA, Yen CL, Carey HV. Cholesterol and lipoprotein dynamics in a hibernating mammal. PLoS ONE. 2011;6:e29111. doi: 10.1371/journal.pone.0029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florant GL, Lawrence AK, Williams K, Bauman WA. Seasonal changes in pancreatic B-cell function in euthermic yellow-bellied marmots. The American journal of physiology Regul Integ Comp Physiol. 1985;249:R159–R165. doi: 10.1152/ajpregu.1985.249.2.R159. [DOI] [PubMed] [Google Scholar]
- 38.Bairlein F. How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften. 2002;89:1–10. doi: 10.1007/s00114-001-0279-6. [DOI] [PubMed] [Google Scholar]
- 39.Shafrir E, Ziv E, Kalman R. Nutritionally induced diabetes in desert rodents as models of type 2 diabetes: Acomys cahirinus (spiny mice) and Psammomys obesus (desert gerbil) ILAR J. 2006;47:212–224. doi: 10.1093/ilar.47.3.212. [DOI] [PubMed] [Google Scholar]
- 40.Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J Vet Intern Med. 2010;24:467–475. doi: 10.1111/j.1939-1676.2010.0503.x. [DOI] [PubMed] [Google Scholar]
- 41.Neel JV. Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"? American journal of human genetics. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JC. The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol Rev Camb Philos Soc. 2006;81:183–205. doi: 10.1017/S1464793105006974. [DOI] [PubMed] [Google Scholar]
- 43.Eknoyan G. A history of obesity, or how what was good became ugly and then bad. Adv Chronic Kidney Dis. 2006;13:421–427. doi: 10.1053/j.ackd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Prentice AM, Hennig BJ, Fulford AJ. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int J Obes (Lond) 2008;32:1607–1610. doi: 10.1038/ijo.2008.147. [DOI] [PubMed] [Google Scholar]
- 45.Benyshek DC, Watson JT. Exploring the thrifty genotype's food-shortage assumptions: a cross-cultural comparison of ethnographic accounts of food security among foraging and agricultural societies. American journal of physical anthropology. 2006;131:120–126. doi: 10.1002/ajpa.20334. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarthy MV, Booth FW. Eating, exercise, and "thrifty" genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 47.Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the 'drifty gene' hypothesis. Int J Obes (Lond) 2008;32:1611–1617. doi: 10.1038/ijo.2008.161. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RJ, Andrews P, Benner SA, Oliver W. Theodore e. Woodward award: The evolution of obesity: insights from the mid- miocene. Trans Am Clin Climatol Assoc. 2010;121:295–305. discussion -8. [PMC free article] [PubMed] [Google Scholar]
- 49.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RJ, Gaucher EA, Sautin YY, Henderson GN, Angerhofer AJ, Benner SA. The planetary biology of ascorbate and uric acid and their relationship with the epidemic of obesity and cardiovascular disease. Medical hypotheses. 2008;71:22–31. doi: 10.1016/j.mehy.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skinner MF, Dupras TL, Moya-Sola S. Periodicity of linear enamel hypoplasia among Miocene Dryopithecus from Spain. J Paleopathol. 1995:195–222. [Google Scholar]
- 52.Andrews P, Kelley J. Middle Miocene dispersals of apes. Folia primatologica; international journal of primatology. 2007;78:328–343. doi: 10.1159/000105148. [DOI] [PubMed] [Google Scholar]
- 53.Begun DR. Middle Miocene hominoid origins. Science (New York, NY. 2000;287:2375. [PubMed] [Google Scholar]
- 54.Johnson RJ, Andrews P. Fructose, Uricase, and the Back-to-Africa Hypothesis. Evol Anthropol. 2010;19:250–257. [Google Scholar]
- 55.Xu CF, Yu CH, Xu L, Sa XY, Li YM. Hypouricemic therapy: a novel potential therapeutic option for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2010;52:1865–1866. doi: 10.1002/hep.23798. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 57.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grayson PC, Kim SY, Lavalley M, Choi HK. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010 doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 60.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes and Metabolism. 2009;35:447–451. doi: 10.1016/j.diabet.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Shafiu M, Johnson RJ, Turner ST, et al. Urate transporter gene SLC22A12 polymorphisms associated with obesity and metabolic syndrome in Caucasians with Hypertension. Kidney Blood Press Res. 2012 doi: 10.1159/000337370. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parsa A, Brown E, Weir MR, et al. Genotype-based changes in serum uric acid affect blood pressure. Kidney international. 2012;81:502–507. doi: 10.1038/ki.2011.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caulfield MJ, Munroe PB, O'Neill D, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q, Kottgen A, Dehghan A, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feig DI, Soletsky B, Johnson RJ. Effect of Allopurinol on the Blood Pressure of Adolescents with Newly Diagnosed Essential Hypertension. JAMA. 2008;300:922–930. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogino K, Kato M, Furuse Y, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3:73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 68.Ishimoto T, Lanaspa M, Le M, et al. Opposing Effects of Fructokinase C and A Isoforms on Fructose-Induced Metabolic Syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1119908109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanaspa M, Tapia E, Soto V, Sautin Y, Sanchez-Lozada LG. Uric acid and Fructose: Potential Biological Mechanisms. Sem Nephrol. 2011 doi: 10.1016/j.semnephrol.2011.08.006. in press. [DOI] [PubMed] [Google Scholar]
- 70.Abdelmalek MF, Lazo M, Horska A, et al. Higher Dietary Fructose Is Associated with Impaired Hepatic ATP Homeostasis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology (Baltimore, Md. 2012 doi: 10.1002/hep.25741. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Johnson RJ, Sautin YY, Oliver WJ, et al. Lessons from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in western society? Journal of comparative physiology B. 2009;179:67–76. doi: 10.1007/s00360-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. The American journal of clinical nutrition. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 75.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiology & behavior. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 79.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. American journal of physiology Regul Integr Comp Physiol. 2008;295:R1370–R1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]