Abstract
Background
Tandem mass spectrometry has been proposed as a method of diagnosing or predicting the development of common complex neonatal diseases. Our objective was to identify metabolites associated with common complications of prematurity.
Methods
We performed a retrospective analysis of medical data and metabolite measurements from routine neonatal screening on 689 preterm (<37 weeks of gestational age) neonates.
Results
We observed higher levels of phenylalanine in infants with respiratory distress syndrome (RDS; P=1.7×10−5), the only association that was significant after correction for multiple testing. We found suggestive significance (P<0.001) of higher essential amino acids in infants with patent ductus arteriosus (PDA). Functionality of these findings was explored in the ductus arteriosus (DA) isolated from term and preterm mouse pups. None of the amino acids had a direct vasodilatory effect on the isolated DA.
Conclusion
We found newborns with RDS had higher levels of phenylalanine that may be due to impaired phenylalanine hydroxylase activity. We also detected marginally higher levels of all measured essential amino acids in infants with PDA. We did not find dilation of the mouse ductus for these metabolites indicating that instead of potentially causing PDA they are likely serving as markers of catabolism.
Introduction
Comprehensive metabolic profiling at birth is a critical public health program nationally, as well as internationally, to detect rare congenital conditions that if identified early can be treated. If left untreated, these disorders can cause lifelong morbidities or death. In addition to state-mandated newborn screening, longitudinal metabolic profiling with high-throughput methods such as tandem mass spectrometry may prove critical for monitoring, diagnosing and treating conditions as they develop in the neonatal intensive care unit (NICU) (1–3). While there are several metabolites currently used for routine monitoring of overall health in the NICU, including glucose, blood gas values and electrolytes, the measurement of analytes with high-throughput methods such as tandem mass spectrometry used in newborn screening programs has not been implemented in the NICU setting for monitoring preterm infant health or potential risk for common complex diseases.
Preterm and/or sick neonates are known to have distinct metabolic profiles, often defined by amino acid and acylcarnitine measurements compared to their term and/or healthy counterparts (4, 5). While stress and immature liver and kidney function may explain some of the observed metabolic differences, there are likely many other factors contributing to an infant’s metabolism at birth including fetal and maternal influences, perinatal events, and genetic background. Gestational age and birth weight are known contributors to variation in metabolic profiles; however, few studies have examined specific conditions that often accompany low birth weight and early gestational age (3, 6–9). A few studies have applied nuclear magnetic resonance spectroscopy (NMR) analysis of urine to examine neonates with patent ductus arteriosus, intrauterine growth restriction, asphyxiation and in children with neuropathies (3, 6–9). This technique offers a promising approach for distinguishing patterns of metabolites altered in specific neonatal diseases and conditions.
State-wide neonatal screening is generally performed from dried blood spots collected 1–3 days after birth. Many metabolites are identified through expanded newborn screening using tandem mass spectrometry. To our knowledge no study has used these values to examine associations with complications commonly associated with prematurity. Our objective was to determine if metabolites from routine newborn screening associate with common diseases of prematurity and could therefore be potential biomarkers or therapeutic targets for critical illnesses in the neonatal intensive care unit. We examined metabolite measurements from routine newborn screening in 689 preterm (<37 weeks gestation) newborns to identify metabolite associations with common complications of prematurity, including patent ductus arteriosus (PDA), respiratory distress syndrome (RDS) and necrotizing enterocolitis (NEC). We followed-up metabolic findings with PDA using a previously established mouse model to test ductus arteriosus (DA) contractility. There are several different knockout mouse models that have PDA, each has respiratory distress, pulmonary congestion, and possible fatality if a large PDA cannot be treated or rescued. We tested the vasodilatory effects of candidate metabolites on the mouse DA to estimate the functionality of our metabolic associations.
Materials and Methods
Study Population
This is a retrospective analysis of data collected between 2001 and 2009 as part of a prospective cohort for studying the epidemiology and genetics of preterm birth (10, 11). Study samples were collected at the University of Iowa Children’s Hospital in Iowa City, IA after approval by the University of Iowa Institutional Review Board (IRB200506792). Signed informed consent was obtained from all families for enrollment. Gestational age and birth weight were obtained from the medical record. There were 689 preterm infants included for analysis. Infants were included in data analysis if they were born preterm (delivery at 23 weeks and 0 days through 36 weeks and 6 days gestation), had not received a blood transfusion at the time of sample collection, and had their sample collected between 24 and 72 hours after birth. Existing data, collected by medical chart review, was evaluated for 22 medical and demographic factors; completeness of the data varied across subjects. While exploratory analysis was performed for all examined variables and correction for multiple testing accounts for all tests performed we do not present data on the following variables as they did not show relevant associations and had a large percentage of missing data (shown in parentheses): length at birth (30.9%), occipital frontal circumference (29.0%), feeding method at time of newborn screen (20.8%), highest direct bilirubin (70.4%), hemoglobin at birth (52.1%), bronchopulmonary dysplasia (30.6%), intraventricular hemorrhage (37.9%), periventricular leukomalacia (21.6%), retinopathy of prematurity (30.2%) and sepsis (62.0%).
Diagnosis of Complications of Prematurity
All infants less than 28 weeks gestational age were examined by a pediatric cardiologist using echocardiography between day 5 and 7 of life, regardless of symptoms. Infants greater than or equal to 28 weeks gestation are examined by echocardiography between day 5 and 7 of life if a murmur suggestive of a PDA is present. Any preterm infant was examined using echocardiography if they had symptoms of congestive heart failure. PDA was diagnosed if flow was detected through the DA. No infant received prophylactic indomethacin (i.e., in the first two days of life). RDS was defined radiologically in combination with the requirement of supplemental oxygen for 2 h or more. Surfactant was not administered as prophylaxis, but if treatment was needed it occurred within the first hour after birth. Necrotizing enterocolitis (NEC) was described using the modified Bell staging criteria (12). A stage of 2a or greater was considered positive for necrotizing enterocolitis..
Tandem Mass Spectrometry Data
Data on analyte measurements were provided by the State of Iowa Hygienic Laboratory and linked to the clinical medical record data. Approval for use of the data provided by the State of Iowa Hygienic Laboratory was granted by the Iowa Department of Public Health. Newborn dried blood spot (DBS) specimens are collected, dried and handled according to the Clinical Laboratory Standards Institute (CLSI) guideline (13). Screening procedures in Iowa are based on previously established methodology (14–16). Briefly, a derivatization method is used in which butyl esters of acylcarnitines and amino acids are prepared from the extracts. Tandem mass spectrometry is performed with Waters Quattro Micro triple quadrupole tandem mass spectrometers (Milford, MA), equipped with an electrospray ionization source operated in the positive ion mode. Multiple reaction monitoring (MRM) mode is used to scan for specific mass ion intensities. Concentrations are obtained from the ratio of ion intensity (cps) at the mass that represents a specific analyte compared to its isotopically labeled internal standard and correcting for blood volume in a 1/8 inch DBS punch. Both internal and external spiked control specimens, a normal control specimen, and a blank are analyzed with each batch of specimens. The external spiked control specimens are obtained from Newborn Screening Quality Assurance Program at the Centers for Disease Control. Data was provided on 13 amino acids and 36 acylcarnitines (Table 1).
Table 1.
List of Analytes Examined.
| Amino Acids (umol/L) |
| Alanine (ALA) |
| Arginine (ARG) |
| Argininosuccinate (ASA)a |
| Citrulline (CIT) |
| Glutamate (GLU) |
| Glycine (GLY)b |
| Leucine (LEU) |
| Methionine (MET) |
| Ornithine (ORN)b |
| 5-oxoproline (5-OxoPro)b |
| Phenylalanine (PHE) |
| Tyrosine (TYR) |
| Valine (VAL) |
| Acylcarnitines (umol/L) |
| Carnitine free (C0) |
| Acetylcarnitine (C2) |
| Propionylcarnitine (C3) |
| Malonylcarnitine (C3-DC) |
| Butyrylcarnitine+Isobutyrylcarnitine (C4) |
| Methylmalonylcarnitine (C4-DC) |
| 3-Hydroxybutyrylcarnitine (C4-OH)b |
| Isovalerylcarnitine+Methylbutyrylcarnitine (C5) |
| Tiglylcarnitine (C5:1)a |
| Glutarylcarnitine (C5-DC) |
| 3-Hydroxyisovalerylcarnitine (C5-OH) |
| Hexanoylcarnitine (C6) |
| Methylglutarylcarnitine (C6-DC) |
| Octanoylcarnitine (C8) |
| Octenoylcarnitine (C8:1) |
| Suberylcarnitine (C8-DC)b |
| Decanoylcarnitine (C10) |
| Decenoylcarnitine (C10:1) |
| Decadienoylcarnitine (C10:2)b |
| Dihydrosebacylcarnitine (C10-DC)b |
| Dodecanoylcarnitine (C12) |
| Dodecenoylcarnitine (C12:1) |
| Tetradecanoylcarnitine (C14) |
| Tetradecenoylcarnitine (C14:1) |
| Tetradecadienoylcarnitine (C14:2) |
| 3-Hydroxytetradecanoylcarnitine (C14-OH)a |
| Palmitoylcarnitine (C16) |
| Palmitoleylcarnitine (C16:1) |
| 3-Hydroxypalmitoleylcarnitine (C16:1-OH)a |
| 3-Hydroxypalmitoylcarnitine (C16-OH)a |
| Stearoylcarnitine (C18) |
| Oleoylcarnitine (C18:1) |
| 3-Hydroxyoleoylcarnitine (C18:1-OH)a |
| Linoleoylcarnitine (C18:2) |
| 3-Hydroxylinoleoylcarnitine (C18:2-OH)b |
| 3-Hydroxystearoylcarnitine (C18-OH)a |
results are not presented for these metabolites that had low variability (standard deviation is ≤0.01umol/L)
metabolites added or dropped from the tandem mass spectrometry panel within collection period
Statistical Analysis
Statistical modeling techniques were applied to identify maternal factors and conditions that may significantly influence neonatal metabolic profiles. All metabolites were transformed with the Box Cox transformation to normalize each distribution. Multiple statistical modeling methods were compared and few differences were identified; therefore, analysis of variance (ANOVA) was performed adjusting for year of sample collection, assay lot changes, gestational age and birth weight. The significance of our results were not greatly affected when including only gestational age, only birth weight or both covariates in the model. Standardized residuals were examined for outliers and measurements that were < −3.5 or > 3.5 were removed. Analysis included 924 ANOVA models (22 clinical variables × 42 analyte and ratio measurements) and a Bonferonni significance threshold of P<5×10−5 was used to correct for multiple testing. All analyses were performed with Stata, version 12.0 (Stata Corporation, College Station, TX). There were 18 infants identified as having an abnormal MS/MS test result on the first screen after birth, of whom 17 had a normal repeat screen and thus were interpreted as false positive results; one infant with a positive screen was diagnosed with medium chain acylcarnitine-CoA dehydrogenase deficiency (MCADD). None of the significantly associated factors increased the false positive rate for the expanded newborn screening panels.
Functional Studies in the Mouse
Adult female CD1 mice (Charles River, Raleigh, NC) were bred to produce timed pregnancies. Dams were anesthetized by intraperitoneal injection of 0.4 ml of 2.5% avertin (2,2,2-tribromoethanol in tert-amyl alcohol; Sigma-Aldrich, St. Louis, MO), followed by isoflurane inhalation (Baxter, Deerfield, IL) to facilitate fetal anesthesia. Pregnant dams were euthanized by cervical dislocation and offspring were delivered by uterine incision. Experiments were conducted in accordance with National Institutes of Health animal care standards and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University. L-valine, L-methionine, L-phenylalanine, and L-leucine (Sigma-Aldrich, St. Louis, MO) were prepared as concentrated stock solutions in aqueous buffer. Final solvent concentration was limited to 0.01% and was not expected to alter ex vivo study results (17).
Response of the isolated ductus arteriosus was examined by pressurized vessel myography, as previously described (17). Briefly, anesthetized fetal mouse pups were delivered at term (day 19) and preterm (day 17) gestation by C-section and immediately placed in ice-cold modified Krebs buffer which had been gassed with a 5% CO2/95% nitrogen mix (deoxy Krebs) to maintain stable pH and mimic in utero O2 conditions. The ductus arteriosus, along with a portion of the transverse aorta, and the pulmonary arteries were then dissected free while submerged under cold deoxy Krebs, to maintain ductus relaxation. The ductus was then mounted on glass cannulae (150–170 μm) in a custom-made chamber designed for pressure myography (Instrumentation and Model Facility, University of Vermont) and secured with single-stranded nylon sutures derived from a larger braid. The chamber was then placed on an inverted microscope where continuous measurements of the internal diameter and distending pressure were made (100X) (IonOptix, Milton, MA). Pressure was applied to the proximal inlet of the secured vessel using an elevated column of Krebs buffer and measured using a calibrated manometer (Living Systems Instrumentation, Burlington, VT). The ductus was superfused (6 ml/min) with Krebs solution at 36.5–37.5° C. After an equilibration period of approximately 40 minutes at 37 ° C and 5 mmHg of pressure, a stepwise elevation of pressure was applied, up to a working pressure of 20 mm Hg. The isolated ductus was subsequently challenged with two consecutive doses of 50 mM KCl to verify contractility. Poorly responsive vessels were discarded.
Drug studies, testing 4–5 vessels per compound, were conducted in a continuously recycling circuit with a total volume of 20 ml. After equilibration, the Krebs buffer was aerated with 12% O2 to mimic newborn oxygen conditions, which resulted in submaximal constriction of the vessel (pre-constriction). Activity of the DA after each dose was monitored for 20 minutes or until stable. After the complete series of cumulative doses (10−9 M to 10−3 M) the vessel was exposed to the nitric oxide donor sodium nitroprusside (SNP, 10−5 M) to document the capacity for vasodilation. Subsequently, exposure to 50 mM KCl was used to determine viability of the preparation at the conclusion of the experiment. Ductus preparations were responsive to vasoconstrictive stimuli, including KCl and increased oxygen tension. Exposure to the nitric oxide donor SNP at the completion of each dose-response experiment overcame oxygen-induced pre-constriction and produced relaxation to baseline levels of resting tone in all experiments. Vessels returned to their original diameter after return to the start-up deoxygenated conditions. The strong response to KCl, oxygen, and SNP suggest that a significant contractile or vasodilatory effect of the study compounds would be detected using this approach.
Results
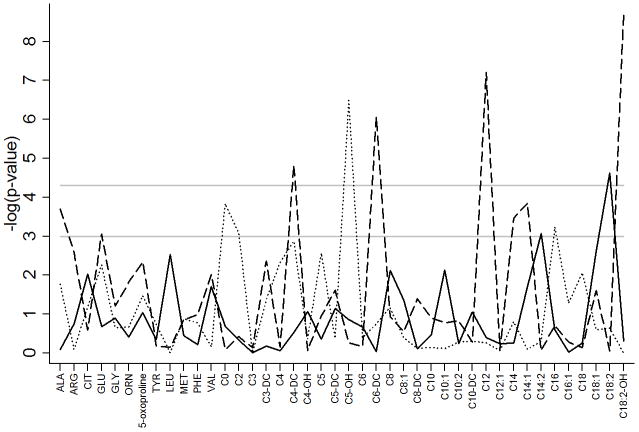
The metabolites examined are presented in Table 1 and demographic characteristics of the cohort are described in Table 2. Associations with demographic and clinical factors were examined for each metabolite after adjustment for gestational age, birth weight, year of sample collection and change in the assay during the study period. Increased concentrations of free carnitine (C0) and multiple acylcarnitines (C2, C4-DC, C14 and C18:2) were observed in males compared to females (Table 3). Several other acylcarnitines were either higher (C6-DC–P=8.5×10−7, C12 – P=6.2×10−8 and C18:2-OH – P=2.0×10−9) or lower (C4-DC – P=1.5×10−5) in the summer months of birth (Figure 1). Additionally, C5-OH (P=3.2×10−7) was lower in twin compared to singleton births and C18:2 (P=2.4×10−5) increased with increasing age at time of sample collection (Figure 1).
Table 2.
Demographic characteristics
| Variable | Mean±SD/Number (Percent) | # Obs. | % Missing |
|---|---|---|---|
| Gestational age (weeks) | 31.3±3.3 | 688 | 0.1 |
| Birth weight (grams) | 1,767±719 | 689 | 0.0 |
| Infant gender (Male) | 380 (55.2%) | 689 | 0.0 |
| Infant race (Caucasian) | 592 (87.8) | 674 | 2.2 |
| Twin/multiple | 130 (18.9%) | 689 | 0.0 |
| Season of birth | 689 | 0.0% | |
| Winter (Dec–Feb) | 197 (28.6%) | ||
| Spring (Mar–May) | 141 (20.5%) | ||
| Summer (Jun–Aug) | 164 (23.8%) | ||
| Fall (Sep–Nov) | 187 (27.1%) | ||
| Age (hours) at time of sample collection | 29.3±6.9 | 689 | 0.0 |
| APGAR score at 1 minute | 6.2±2.1 | 681 | 1.2 |
| APGAR score at 5 minutes | 7.9±1.3 | 682 | 1.0 |
| Highest total bilirubin (mg/dL) | 9.5±3.1 | 646 | 6.2 |
| Respiratory distress syndrome (RDS) | 364 (60.6%) | 601 | 12.8 |
| Patent ductus arteriosus (PDA) | 133 (19.9%) | 669 | 2.9 |
| Necrotizing enterocolitis (NEC) | 12 (2.0%) | 611 | 11.3 |
| Congenital anomaly | 50 (7.3%) | 689 | 0.0 |
The number of observations represents the total number of individuals with non-missing data for a given variable.
Table 3.
Metabolite Associations with Gender
| Female (N=309) | Male (N=380) | P-value | |
|---|---|---|---|
| C0 | 22.7±9.8 | 26.5±10.6 | 3.2×10−10 |
| C2 | 24.3±10.7 | 28.3±11.5 | 1.4×10−8 |
| C4-DC | 0.10±0.05 | 0.12±0.06 | 6.4×10−6 |
| C14 | 0.21±0.08 | 0.24±0.09 | 1.6×10−6 |
| C18:2 | 0.23±0.17 | 0.26±0.17 | 3.5×10−5 |
Means and SD in uM units.
ANOVA model adjusted for major assay change, year of sample collection and adjustment for birth weight and gestational age.
Figure 1.
Metabolite associations with infant demographics. Associations are represented by the negative logarithm of the p-value with the solid line representing age at time of sample collection, the dashed line representing season of birth and the dotted line representing twin or multiple gestations. The first horizontal gray solid line at −log(p)=3 represents a p-value of ≤0.001, the second horizontal gray solid line at −log(p)=4.3 represents Bonferroni corrected significance threshold of p≤5×10−5.
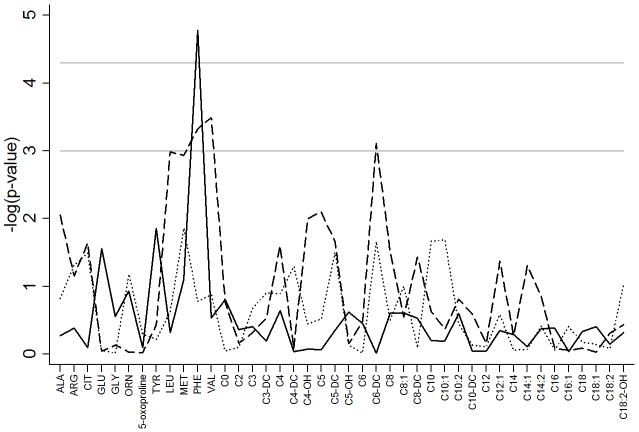
Figure 2 represents metabolite associations with complications of prematurity including respiratory distress syndrome (RDS), patent ductus arteriosus (PDA)and necrotizing enterocolitis (NEC). Phenylalanine was significantly (P=1.7×10−5) increased in infants with RDS compared to controls and this was the only complication of prematurity to have an association that remained significant after correction for multiple testing (Table 4). Additional adjustment for prenatal steroids did not alter the significance of this result (P=2.1×10−5). This association remained when examining infants less than 32 weeks gestation (P=0.03).
Figure 2.
Metabolite associations with complications of prematurity. Associations are represented by the negative logarithm of the p-value with the solid line representing RDS, the dashed line representing PDA and the dotted line representing NEC. The first horizontal gray solid line at −log(p)=3 represents a p-value of ≤0.001, the second horizontal gray solid line at −log(p)=4.3 represents Bonferroni corrected significance threshold of p≤5×10−5.
Table 4.
Metabolite Associations with Complications of Prematurity
| RDS | No (N=237) | Yes (N=364) | Model 1 | Model 2 | Model 3 |
| P-valuea | P-valueb | P-valuec | |||
| PHE | 61.6±18.7 | 72.6±29.9 | 1.7×10−5 | 4.1×10−4 | 0.03 |
| PDA | No (N=536) | Yes (N=133) | Model 1 | Model 2 | Model 3 |
| P-valuea | P-valueb | P-valuec | |||
| LEU | 127.0±91.9 | 159.1±55.7 | 1.0×10−3 | 2.7×10−3 | 0.01 |
| MET | 25.8±20.5 | 33.1±17.9 | 1.2×10−3 | 0.02 | 0.02 |
| PHE | 66.7±30.4 | 77.0±20.8 | 4.7×10−4 | 1.2×10−3 | 9.3×10−4 |
| VAL | 93.6±53.7 | 123.2±48.8 | 3.2×10−4 | 1.0×10−3 | 8.3×10−3 |
| C6-DC | 0.03±0.04 | 0.05±0.09 | 7.8×10−4 | 6.1×10−3 | 0.02 |
Means and SD in uM units.
ANOVA model adjusted for major assay change, year of sample collection and adjustment for birth weight and gestational age.
ANOVA model adjusted for major assay change, year of sample collection and adjustment for birth weight and gestational age and excluding infants on TPN, twins and infants with congenital anomalies.
ANOVA model adjusted for major assay change, year of sample collection, birth weight and gestational age in infants <=32 weeks gestation.
While not significant after correction for multiple testing it is notable that multiple essential amino acids were of suggestive significance (P≤1.0×10−3). Specifically LEU, MET, PHE and VAL were higher in infants with PDA (Figure 2 and Table 4) compared to those without PDA. C6-DC was higher in infants with PDA (P=7.8×10−4) and in infants born in the summer. Including both covariates in the same model with C6-DC (and adjusting for birth weight, gestational age, year of collection and change in the assay during the study period) did not decrease the significance of the association between C6-DC and PDA (P=5.6×10−3). While 15.0% of the infants with PDA received total parenteral nutrition (TPN) before the newborn screen, excluding these individuals (N=67) from the analysis did not alter the significance of the results (Table 4). Results also remained significant when examining only infants <32 weeks gestation. We further followed associations with essential amino acids and PDA with functional studies in the mouse. The vascular response to L-valine, L-methionine, L-phenylalanine and L-leucine was examined in the isolated term or preterm ductus arteriosus. Exposure to cumulative doses of each compound failed to produce a significant change in ductal tone (Figure 3).
Figure 3.
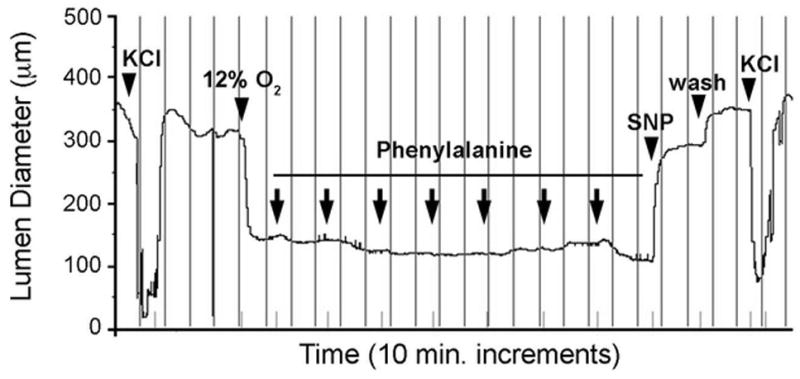
Response of the isolated murine ductus arteriosus to phenylalanine. Representative tracing of a term gestation mouse ductus that was mounted in a pressure myography chamber under deoxygenated conditions. Submaximal constriction of the ductus lumen diameter by oxygen was not overcome by exposure to increasing concentrations of phenylalanine (10−9 M to 10−3 M). In contrast, exposure to the NO donor SNP (10−5 M) produced marked dilation and return to resting baseline dimensions. Terminal exposure to 50mM KCl confirmed responsiveness of the preparation at completion of the study. Vertical lines represent 10 minute intervals. Arrowheads indicate serial 10-fold increases in phenylalanine concentration in the perfusion bath.
Discussion
Recently, measuring the metabolic profile in urine has been proposed as a method for monitoring progression of diseases that often develop in newborns born preterm (4). We identified specific complications of prematurity associated with essential amino acid levels. Specifically, newborns with respiratory distress syndrome had higher levels of phenylalanine (PHE) compared to controls. Higher PHE concentrations have also been observed in adult patients with respiratory distress (23, 24). PHE could also be increased due to impaired phenylalanine hydroxylase activity. It has been hypothesized that oxidative stress due to inflammation can deplete 5,6,7,8-tetrahydrobiopterin, a cofactor for this enzyme (25).
While not significant after correction for multiple testing we detected marginally higher levels of all measured essential amino acids (total leucine, methionine, phenylalanine and valine) in infants with patent ductus arteriosus (PDA). Branched-chain amino acids (BCAA) which include leucine, isoleucine, and valine are generated through catabolism and extra-hepatic tissues, including cardiac muscle (26). We did not find dilation of the mouse ductus for these metabolites indicating that instead of potentially causing PDA they are likely serving as markers of catabolism which is heightened in neonates where the patent ductus is failing to close. This result is intriguing as recent reports have suggested that persistent patency of the ductus arteriosus at 3–4 days of life can be predicted by using the first urine passed by preterm neonates (9). While the study does not reveal the specific metabolites associated with the prediction it asserts not only that using a metabolomics approach can predict PDA but also that it can distinguish the responders from the non-responders to ibuprofen therapy (9). As our dried blood spot samples are taken at 1–3 days of life, right before the identification of persistent patency of the ductus, we may be detecting the distinguishing metabolic patterns that are also observed in urine samples.
A neonate’s metabolism continues to evolve after birth and is influenced by a host of factors including maternal metabolism, nutrition, toxicological insults, perinatal events and genetic background. Consistent with previous reports we identified strong associations between the majority of measured metabolites and gestational age and birth weight (4, 5, 18). Specifically, amino acids increased and medium- and long-chain acylcarnitines decreased with decreasing gestational age and birth weight. Likely explanations for these differences are immature liver, kidney, and adrenal function. Additionally, we found significantly lower acylcarnitine levels in females compared to males. Several studies have described that in adults total carnitine is lower in females compared to males (19–21). A possible explanation is estradiol levels, which have been shown to have an inverse relationship with plasma carnitine concentrations in rats (22). Our associations with the complications of prematurity were independent of potential confounders including gestational age, birth weight and gender.
Our study was retrospective in nature, and therefore we are not able to adequately capture or account for all of potential confounding influences. Additionally, we received only a snapshot of a select group of metabolites at one time point after birth. To better assess the metabolites we have identified as potential biomarkers for complications of prematurity we would need longitudinal samples collected throughout the course of the condition. This was a retrospective examination of data and several of the variables included in our analysis had a large amount of missing data points and therefore those results would need to be interpreted with caution. One limitation of our experimental model is that the mouse DA closes more rapidly after birth than the human DA; however, in relative terms the closure rate is similar. Additionally, while prematurely born mice cannot survive outside the womb, studies on the preterm fetal DA can be performed in vitro, to determine the response to vasoactive stimuli. While human DA tissues would be ideal they have limited availability and viability therefore the mouse model of DA can serve as a surrogate for functional assessment of human data. Our data shows that neonatal essential amino acids, as measured through routine newborn screening, are elevated in certain complications that accompany preterm birth including respiratory distress syndrome and patent ductus arteriosus. These metabolites are potential biomarkers for the presence and severity of several complications of prematurity.
Acknowledgments
We would also like to express our gratitude to the coordinating medical and research staff at the University of Iowa Hospitals and Clinics in Iowa City, IA; including a special thanks to research coordinators Laura Knosp and Susan Berends. We would like to express our thanks to the Congenital and Inherited Disorders Advisory Committee, particularly Kim Piper for her enthusiastic support and management. We thank Sara Copeland at the Health Resources Services Administration for her guidance and support on this project. We would also like to thank Franklin Delin and Dariush Shirazi from the State Hygienic Laboratory for their assistance in the acquisition of the newborn screening data. We would also like to thank Susie McConnell, Nancy Davin and Erin Brothers-Smith for administrative support.
Financial Support: March of Dimes (1-FY05-126 and 6-FY08-260), National Institute of Health (K99 HD-065786, R01 HD-52953, R01 HD-57192, and R01 HL-109199) and the Children’s Miracle Network through the University of Iowa (Grant #2224). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
References
- 1.Atzori L, Antonucci R, Barberini L, Griffin JL, Fanos V. Metabolomics: a new tool for the neonatologist. J Matern Fetal Neonatal Med. 2009;22 (Suppl 3):50–3. doi: 10.1080/14767050903181500. [DOI] [PubMed] [Google Scholar]
- 2.Antonucci R, Atzori L, Barberini L, Fanos V. Metabolomics: the “new clinical chemistry” for personalized neonatal medicine. Minerva Pediatr. 2010;62:145–8. [PubMed] [Google Scholar]
- 3.Fanos V, Barberini L, Antonucci R, Atzori L. Metabolomics in neonatology and pediatrics. Clin Biochem. 2011;44:452–4. doi: 10.1016/j.clinbiochem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Atzori L, Antonucci R, Barberini L, et al. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front Biosci (Elite Ed) 2011;3:1005–12. doi: 10.2741/e306. [DOI] [PubMed] [Google Scholar]
- 5.Oladipo OO, Weindel AL, Saunders AN, Dietzen DJ. Impact of premature birth and critical illness on neonatal range of plasma amino acid concentrations determined by LC-MS/MS. Mol Genet Metab. 2011;104:476–9. doi: 10.1016/j.ymgme.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Chu CY, Xiao X, Zhou XG, et al. Metabolomic and bioinformatic analyses in asphyxiated neonates. Clin Biochem. 2006;39:203–9. doi: 10.1016/j.clinbiochem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Favretto D, Cosmi E, Ragazzi E, et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem. 2012;402:1109–21. doi: 10.1007/s00216-011-5540-z. [DOI] [PubMed] [Google Scholar]
- 8.Dessi A, Atzori L, Noto A, et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): urine reveals markers of metabolic syndrome. J Matern Fetal Neonatal Med. 2011;24 (Suppl 2):35–9. doi: 10.3109/14767058.2011.605868. [DOI] [PubMed] [Google Scholar]
- 9.Fanos V, Antonucci R, Barberini L, Noto A, Atzori L. Clinical application of metabolomics in neonatology. J Matern Fetal Neonatal Med. 2012 doi: 10.3109/14767058.2012.663198. [DOI] [PubMed] [Google Scholar]
- 10.Steffen KM, Cooper ME, Shi M, et al. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27:672–80. doi: 10.1038/sj.jp.7211806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehn NL, Cooper ME, Orr K, et al. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res. 2007;62:630–5. doi: 10.1203/PDR.0b013e3181567bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. Newborn screening for preterm, low birth weight, and sick newborns; approved guideline. CLSI Document I/LA31-A. 2004;29:1–29. [Google Scholar]
- 14.Turgeon C, Magera MJ, Allard P, et al. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin Chem. 2008;54:657–64. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- 15.Chace DH, Lim T, Hansen CR, De Jesus VR, Hannon WH. Improved MS/MS analysis of succinylacetone extracted from dried blood spots when combined with amino acids and acylcarnitine butyl esters. Clin Chim Acta. 2009;407:6–9. doi: 10.1016/j.cca.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Chace DH, DiPerna JC, Mitchell BL, Sgroi B, Hofman LF, Naylor EW. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin Chem. 2001;47:1166–82. [PubMed] [Google Scholar]
- 17.Reese J, Waleh N, Poole SD, Brown N, Roman C, Clyman RI. Chronic in utero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosus contractile pathways and prevents postnatal closure. Pediatr Res. 2009;66:155–61. doi: 10.1203/PDR.0b013e3181aa07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illsinger S, Schmidt KH, Lucke T, Vaske B, Bohnhorst B, Das AM. Plasma and urine amino acid pattern in preterm infants on enteral nutrition: impact of gestational age. Amino Acids. 2009 doi: 10.1007/s00726-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 19.Borum PR. Plasma carnitine compartment and red blood cell carnitine compartment of healthy adults. Am J Clin Nutr. 1987;46:437–41. doi: 10.1093/ajcn/46.3.437. [DOI] [PubMed] [Google Scholar]
- 20.Harper P, Wadstrom C, Cederblad G. Carnitine measurements in liver, muscle tissue, and blood in normal subjects. Clin Chem. 1993;39:592–9. [PubMed] [Google Scholar]
- 21.Reuter SE, Evans AM, Chace DH, Fornasini G. Determination of the reference range of endogenous plasma carnitines in healthy adults. Ann Clin Biochem. 2008;45:585–92. doi: 10.1258/acb.2008.008045. [DOI] [PubMed] [Google Scholar]
- 22.Borum PR. Regulation of the Carnitine Deficiency Syndromes. In: Frenkel RA, McGarry JD, editors. Carnitine biosynthesis, metabolism and functions. Academic Press; New York: 1980. pp. 115–126. [Google Scholar]
- 23.Siegel JH. Cardiorespiratory manifestations of metabolic failure in sepsis and the multiple organ failure syndrome. Surg Clin North Am. 1983;63:379–99. doi: 10.1016/s0039-6109(16)42987-7. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CD, Wu WG, Wang JH, Chen SM, Chiang BN. Proton nuclear magnetic resonance studies of plasma to determine metabolic status of patients with adult respiratory distress syndrome. Clin Chem. 1989;35:667–70. [PubMed] [Google Scholar]
- 25.Ploder M, Neurauter G, Spittler A, Schroecksnadel K, Roth E, Fuchs D. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids. 2008;35:303–7. doi: 10.1007/s00726-007-0625-x. [DOI] [PubMed] [Google Scholar]
- 26.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]