Abstract
Copper is essential for the function of the mitochondrial electron transport chain and several antioxidant proteins. However, in its free form copper can participate in Fenton-like reactions that produce reactive hydroxyl radicals. Aryl-hydrocarbon receptor (AhR) agonists, including the most potent polychlorinated biphenyl (PCB) congener, 3,3',4,4',5-pentachlorobiphenyl (PCB126), increase copper levels in rodent livers. This is accompanied by biochemical and toxic changes. To assess the involvement of copper in PCB toxicity, male Sprague Dawley rats were fed an AIN-93G diet with differing dietary copper levels: low (2 ppm), adequate (6 ppm), and high (10 ppm). After three weeks, rats from each group were given a single ip injection of corn oil (control), 1, or 5 μmol/kg body weight PCB126. Two weeks following injections, biochemical and morphological markers of hepatic toxicity, trace metal status, and hepatic gene expression of metalloproteins were evaluated. Increasing dietary copper was associated with elevated tissue levels of copper and ceruloplasmin. In the livers of PCB126-treated rats the hallmark signs of AhR activation were present, including increased cytochrome P-450 and lipid levels, and decreased glutathione. In addition a doubling of hepatic copper levels was seen and overall metals homeostasis was disturbed, resulting in decreased hepatic selenium, manganese, zinc and iron. Expression of key metalloproteins was either decreased (cytochrome c oxidase), unchanged (ceruloplasmin and CuZnSOD) or increased (tyrosinase, metallothionein 1 and 2) with exposure to PCB126. Increases in metallothionein may contribute/reflect the increased copper seen. Alterations in dietary copper did not amplify or abrogate the hepatic toxicity of PCB126. PCB126 toxicity, i.e. oxidative stress and steatosis, is clearly associated with disturbed metals homeostasis. Understanding the mechanisms of this disturbance may provide tools to prevent liver toxicity by other AhR agonists.
Introduction
Copper, an essential trace element with multiple biological roles, can be found in trace concentrations (3–10 μg/g wet weight) in vital organs of the human body, including the liver, brain, heart, and kidneys.1 Copper is necessary for cellular respiration and energy production, since the copper-containing cytochrome c oxidase is part of the electron transport chain that reduces oxygen. Copper also functions as a key component of the antioxidant enzyme copper zinc superoxide dismutase (CuZnSOD), which detoxifies reactive superoxide radicals. However, copper can also participate in superoxide catabolism as a pro-oxidant by catalyzing a Fenton-like reaction that generates reactive hydroxyl radicals.2 Therefore a fine control of tissue copper levels is of critical importance.
In previous studies, we observed that hepatic copper levels were significantly increased following exposure to 3,3',4,4',5-pentachlorobiphenyl (PCB126).3 PCB126, the most toxic PCB congener4,5, can assume a more co-planar conformation similar to that of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) due to the lack of chlorine atoms adjacent to the biphenyl bridge in the 2,2',6,6' positions. These congeners, known as dioxin-like PCBs, are potent aryl hydrocarbon receptor (AhR) agonists6 and induce cytochrome P-450 (CYP) 1A.7,8 Changes seen following exposure of rodents to potent AhR agonists, like TCDD and PCB126, besides the increase in hepatic copper8–10 include liver hypertrophy, increased metallothionein, 8-OHdG and reduced hepatic glutathione levels11,12, reduced hepatic selenium and reduced selenium-dependent glutathione peroxidase activity and mRNA levels.3,13,14 In addition, it is well shown that this class of compounds causes ROS and free radical related pathologies.
Little is known about the role copper plays in PCB126-induced toxicity, if any. In this study, we investigate dietary copper's involvement in PCB126-induced alterations to hepatic redox status and toxicity. In addition, gene expression of several proteins whose functions include intra- and extracellular metal trafficking (MT & Cp), pigment production (Tyr) and electron transport (CytOx) were investigated for potential explanation as to the cause of copper increase. We hypothesized that reducing the dietary intake of copper would result in a reduction of the amount of copper available for Fenton-like reactions and thereby decrease toxicity as measured by biochemical changes and morphology. On the other hand, high levels of dietary copper were expected to produce additive or synergistic effects with PCB126. To this end, male rats, placed on purified diets containing 2 (low), 6 (adequate), or 10 (high) ppm copper, were subsequently administered a single dose of 1 (low), or 5 (high) μmol/kg of PCB126, and tissue metal levels, biochemical and morphological changes were determined to evaluate toxicity and potentially explain the increased copper seen.
Methods and Materials
Chemicals
All chemicals were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO) unless otherwise stated. PCB126 (3,3′,4,4′,5-pentachlorobiphenyl) was prepared by an improved Suzuki-coupling method of 3,4,5-trichlorobromobenzene with 3,4-dichlorophenyl boronic acid utilizing a palladium-catalyzed cross-coupling reaction.15 The crude product was purified by aluminum oxide column and flash silica gel column chromatography and recrystallized from methanol. The final product purity was determined by GC/MS analysis to be > 99.8% and its identity confirmed by 13C NMR. Caution: PCBs and their metabolites should be handled as hazardous compounds in accordance with NIH guidelines.
Animals
This animal experiment was conducted with approval from the Institutional Animal Care and Use Committee of the University of Iowa. Male Sprague-Dawley rats, 4–5 weeks old, from Harlan Sprague-Dawley (Indianapolis, IN) weighing 75–100 grams were housed in individual wire cages in a controlled environment maintained at 22°C with a 12 h light-dark cycle and water ad libitum. Animals were randomly divided into three dietary groups, and were fed ad libitum an AIN-93G based diet (Supplemental Table 1) containing a low (2 ppm), adequate (6 ppm), or high (10 ppm) copper level (diets obtained from Harland Teklad, Madison, WI). These concentrations were chosen since the normal AIN-93G diet, commonly used diet for rats, contains 6 ppm copper and levels of 2 and 10 ppm copper represent only modest changes from this normal diet and not expected to produce toxic effects. Moreover, 1 ppm copper in the diet was shown not to change liver and kidney metallothionein compared to 6 ppm copper controls.16 Following three weeks of acclimatization, a period shown to achieve steady state copper levels17, animals were administered a single ip injection of vehicle (5 ml/kg body wt of stripped corn oil; Acros Chemical Company, Pittsburgh, PA), or vehicle with a 1 μmol/kg (326 μg/kg) or 5 μmol/kg (1.63 mg/kg) dose of PCB126. Two weeks following the injection, a time frame sufficient for liver pathology to manifest8, the now 9–10 week old rats were euthanized using carbon dioxide asphyxiation followed by cervical dislocation. Blood was collected by cardiac puncture. Livers and kidneys were excised, weighed, and further processed as described below.
Hepatic subcellular fractions preparation
Liver tissues were excised immediately following sacrifice, and homogenized in ice-cold 0.25 M sucrose solution, pH 7.4. The homogenate was centrifuged at 10,000g for 20 min. The resulting supernatant was then centrifuged at 100,000g for 1 h. This supernatant, containing the cytosolic fractions, was dispensed and aliquoted. The pellet containing the microsomal fraction was washed twice with cold sucrose solution and resuspended in that solution. Protein concentrations were determined by the method of Lowry et al..18 All samples were stored at −80°C until further use.
Measurement of CYP1A1 activity
CYP1A1 activity was determined in hepatic microsomal fractions by the method of Burke and Mayer19 with slight modifications, measuring the ethoxyresorufin deethylase (EROD) activity with ethoxyresorufin as the substrate. The resulting fluorescent resorufin product from the monooxygenase reaction was detected using a Perkin-Elmer LS 55 spectrofluorometer at excitation wavelength of 550 nm and emission wavelength of 585 nm.
Total Glutathione analysis
Total Glutathione levels were determined in liver tissues homogenized in 5% salicylic acid based on the methods of Griffith20 and Anderson21, using 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) as the substrate. The reaction catalyzed by glutathione reductase and NADPH results in the formation of 2-nitro-5-thiobenzoate (TNB) and a yellow color which can be measured at 412 nm. The absorbance change at 412 nm was followed in a Beckman DU-670 spectrophotometer for 5 min. The rate of yellow color accumulation is proportional to the amount of total glutathione in the sample. Total glutathione levels are expressed per mg hepatic protein. Oxidized glutathione was not measured due to additional oxidation of samples before analysis as a result reduced glutathione was not measured.
Measurement of Superoxide Dismutase (SOD) activities
Hepatic SOD activities were determined by the method of Spitz and Oberley22, measuring the reduction of nitroblue tetrazolium (NBT) colormetrically, with xanthine-xanthine oxidase as a source of superoxide anion radicals. Absorbance change at 560 nm was followed in a Beckman DU-670 spectrophotometer for 5 min. To determine manganese SOD (MnSOD) activity, the assay was performed in the presence of 5 mM cyanide, a level that inhibits copper zinc SOD (CuZnSOD) activity. CuZnSOD activity was subsequently determined by subtracting MnSOD activity from total SOD activity. One unit of SOD activity is defined as the amount of protein that yields 50% of maximal inhibition of NBT reduction by superoxide anion radicals.
Serum Ceruloplasmin measurement
Total Serum ceruloplasmin was determined using an ELISA kit purchased from ALPCO (Salem, NH). Briefly, ceruloplasmin present in the serum binds to an anti-ceruloplasmin antibody, which then forms complexes with an anti-ceruloplasmin antibody conjugated with horseradish peroxidase. The addition of a chromogenic substrate, 3,3',5,5'-tetramethylbenzidine, causes an absorbance change at 450 nm, which is used as a measure of the concentration of ceruloplasmin in the test sample. The quantity of bound enzyme correlates directly with the concentration of ceruloplasmin in the sample tested.
4-Hydroxynonenal (4-HNE) determination
Liver 4-HNE levels were measured using an ELISA kit from Cell Biolabs, Inc. (San Diego, CA). Briefly, homogenized liver tissue samples were incubated with an anti-HNE-His antibody, followed by incubation with a HRP-conjugated secondary antibody. Incubation with the provided substrate solution results in an absorbance change at 450 nm which was read in a Molecular Devices SpectraMax 340 96-well plate reader. The quantity of HNE-His adduct in the tissue homogenates was determined by comparing the recorded absorbance with that of a known HNE-BSA standard curve.
Hepatic metalloprotein gene expression analysis
Gene expression for select metalloproteins was determined by a two-step quantitative RT-PCR method using an Eppendorf Realplex Mastercycler. Total RNA was isolated from hepatic tissue using a Qiagen RNeasy Mini kit following the manufacturer's instructions. The RNA was reverse transcribed with random primers using a High Capacity cDNA Reverse Transcription Kit from Applied Biosystems. 1–10 ng cDNA was used with 300 nM primers and Power SYBR green PCR master mix from Applied Biosystems for the real time RT-PCR. Samples were run as duplicates and a mean value was used for further calculations. Six biological replicates were evaluated for each treatment group. Actin was used as the reference gene. Either the Pfaffl method or a standard curve method was used to determine relative gene expression. All primers, excluding tyrosinase, have been published before (sequences are given in Supplemental Table 2) and were produced by IDT (Coralville, IA)23–26.
Trace elements determination
Metal concentrations in liver, kidney and blood were quantitatively determined with an elemental mass spectrometer, the Inductively Coupled Plasma – Mass Spectrometry (ICP-MS). ICP-MS was selected because of its low detection limits and multi-element capacity27. Tissues were pretreated with HNO3 acid digestion prior to ICP-MS measurement, and metal concentrations in the treated tissues were determined in an Agilent 7500ce ICP-MS equipped with a CETAC ASX520 auto sampler.
Histology
Sections of liver were fixed in formalin and glutaraldehyde for light microscopic and transmission electron microscopic examinations, respectively. Formalin-fixed tissues were routinely processed, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin and eosin. Glutaraldehyde-fixed tissues were stained with osmium tetroxide for lipid evaluation, embedded in epoxy resin, sectioned at 50–100 nm and stained with uranyl acetate and lead citrate. Frozen sections of selected liver samples were sectioned at 8 μm and stained with oil red O for lipid evaluation (ORO). ORO liver sections were imaged at 400× magnification (DP72, Olympus) and analyzed using ImageJ software. The percent staining was calculated by dividing the stained area by the total parenchymal area. Two images were analyzed per tissue section.
Statistics
The effects of PCB126 and dietary copper on various response endpoints were studied using ANOVA analysis via procedure PROC GLM in the statistical analysis package SAS (version 9.2). Dunnett's test was used to compare PCB126 with the corn oil vehicle control and the normal with the high and low dietary copper levels. This comparison was conducted separately for PCB126 treatment and dietary copper levels (one-way ANOVA) and also jointly (two-way ANOVA) (Table 1). In two-way ANOVA, the interaction term was removed if it was not significant at level 0.05. When applying Dunnett's test to the PCB126 treatment, the effect of dietary copper was controlled by using lsmeans statement in PROC GLM. The same was done when applying Dunnett's test to dietary copper levels.
Table 1.
Two-way ANOVA analysis of the effects of dietary copper, PCB126, and interaction
| Dietary Copper | PCB126 | Interaction Effect | |
|---|---|---|---|
| Growth Rate (%) | - | ↓<0.0001 | - |
| Feed Consumption | - | ↓<0.0001 | - |
| Rel. Liver Weight (%) | ↓0.0227 | ↑<0.0001 | - |
| Rel. Kidney Weight (%) | - | - | - |
| Liver EROD Activity | - | ↑<0.0001 | - |
| Liver MnSOD Activity | - | ↓0.0037 | - |
| Liver CuZnSOD Activity | - | - | - |
| Liver Total SOD Activity | - | - | - |
| Liver Glutathione | ↓0.0005 | ↓0.0026 | - |
| Serum Cp | ↑<0.0001 | - | 0.0019 |
| Liver 4-HNE | - | ↓0.0145 | - |
| Liver Cp Expression | ↑0.0197 | - | - |
| Liver Tyr Expression | - | ↑0.0066 | - |
| Liver CytOxI Expression | - | ↓<0.0001 | - |
| Liver CytOxIV Expression | - | ↓<0.0001 | 0.0002 |
| Liver MT1 Expression | ↑0.0001 | ↑<0.0001 | - |
| Liver MT2 Expression | ↑0.0072 | ↑<0.0001 | - |
| Liver Copper | ↑<0.0001 | ↑<0.0001 | 0.0001 |
| Liver Selenium | ↑0.0011 | ↓<0.0001 | 0.0020 |
| Liver Iron | - | ↓0.0002 | - |
| Liver Zinc | ↑<0.0001 | ↓<0.0001 | - |
| Liver Manganese | ↑0.0006 | ↓<0.0001 | - |
| Kidney Copper | ↑<0.0001 | ↑0.0005 | - |
| Kidney Selenium | ↑<0.0001 | ↑<0.0001 | - |
| Kidney Iron | - | - | - |
| Kidney Zinc | ↑0.0017 | - | - |
| Kidney Manganese | ↑0.0081 | ↑0.0111 | - |
| Blood Copper | ↑<0.0001 | ↑0.0281 | 0.0013 |
| Blood Selenium | ↑0.0026 | - | - |
| Blood Iron | ↑0.0130 | - | - |
| Blood Zinc | - | ↓0.0019 | - |
| Blood Manganese | ↓0.0001 | - | - |
Only p-values <0.05 are reported
Results
Effects on growth, feed consumption, and organ weights
Weight gain and feed consumption are early indicators of general toxicity of a treatment. Weight gain (Supplemental Figure 1a) and feed consumption (Supplemental Figure 1b) were not significantly influenced by the different copper diets, although rats on the low (2 ppm) copper diet had a slightly higher growth rate than those on normal or high copper diets, even with PCB126-treatment. Overall rats treated with PCB126 gained weight at a lower rate as the corn oil control rats (Table 1), but for the individual groups this reduced growth was significant only with the high (5 μmol/kg) dose of PCB126 (Supplemental Figure 1a). Animals that were treated with PCB126 also overall consumed slightly less feed (Table 1), which was significant for the individual group at the high PCB-dose with all diets and lower (1 μmol/kg) PCB dose in the low and high copper diet group.
The relative liver weight (liver weight in percent of final body weight), was significantly and dose-dependently increased by PCB126 (Figure 1a). Absolute liver weights were also significantly increased by the PCB126 treatment (data not shown). In addition, statistical analysis revealed an overall significant effect of copper diets on relative liver weight (Table 1). In contrast, relative kidney weights were not significantly affected by dietary copper levels or PCB126 (data not shown).
Figure 1.
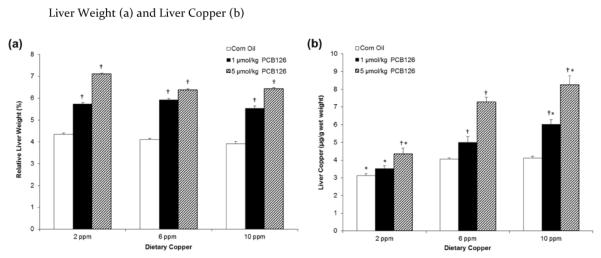
PCB126 significantly increased liver weight (a) and hepatic copper (b) in a dose-dependent manner. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
Effects on liver, kidney, and blood copper
Liver copper was significantly and dose-dependently affected by both dietary copper and PCB126 and also exhibited an interaction effect (Table 1). Low (2 ppm) dietary copper significantly diminished hepatic copper levels while high dietary copper increased them which was significant in all PCB126-treatment groups (Figure 1b). In addition, a dose-dependent increase in hepatic copper in rats treated with PCB126 was seen. Significance was seen for both doses with the adequate and high copper diet but, only the high PCB126 dose in the low copper diet group (Figure 1b).
In the kidneys the copper levels, determined as μg/g tissue wet weight, were overall slightly higher than in the liver. The trends due to copper diet and PCB126 treatment mirrored those in the liver (Table 2). Low and high dietary copper significantly increased and decreased kidney copper levels, respectively, and the high (5 μmol/kg) dose of PCB126 significantly increased kidney copper levels compared to corn oil-treated controls. However, in low dose PCB126 treated rats levels were not significant compared to controls. Blood copper was also significantly affected by both dietary copper and PCB126 with an interaction effect (Table 1). The trends were the same as in the kidneys and liver within normal and high copper diet group (Table 2). Interestingly, blood copper was dose-dependently diminished by PCB126 in rats receiving low dietary copper.
Table 2.
Kidney (μg/g) and blood copper (μg/L) under each experiment condition and significance of various comparisons (adjusted using Dunnett's test)
| Kidney Copper (μg/g tissue wet weight) | Blood Copper (μg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment | Dietary Copper Level | Dietary Copper Level | ||||||
|
|
||||||||
| Low (2 ppm) | Adequate (6 ppm) | High (10 ppm) | Overall | Low (2 ppm) | Adequate (6 ppm) | High (10 ppm) | Overall | |
| Corn Oil | 4.34 ± 0.09 (-,*) | 6.44 ± 0.36 (-,-) | 7.28 ± 0.42 (-,-) | - | 490 ± 51 (-,*) | 793 ± 22 (-,-) | 890 ± 34 (-,-) | - |
| 1 μmol/kg PCB126 | 4.25 ± 0.12 (-,*) | 6.70 ± 0.49 (-,-) | 7.83 ± 0.44 (-,-) | - | 420 ± 61 (-,*) | 955 ± 58 (-,-) | 1003 ± 41 (-,-) | - |
| 5 μmol/kg PCB126 | 4.46 ± 0.13 (-,*) | 8.00 ± 0.86 (-,-) | 9.24 ± 0.48 (†,-) | † | 342 ± 64 (-,*) | 1007 ± 53 (†,-) | 1167 ± 56 (†,-) | † |
|
| ||||||||
| Overall | * | - | * | * | - | * | ||
Results are expressed as mean ± SEM. Each group contained 4–6 animals. One-way ANOVA was used to examine the difference between each PCB126 level and the corn oil treatment, "†" in the parentheses indicates a significant difference due to PCB treatment. Similarly, a "*" in the parentheses indicates significant differences between low or supplemental and the adequate dietary copper level. Significance for each factor based on two-way ANOVA is indicated in the bottom margin for copper diet (*) and in the right margin for PCB treatment (†). The level for significance is 0.05 and n=6 for each group.
Effects of copper diets and PCB126 on liver cytochrome P450 1A1 activity
Hepatic CYP1A1, as determined by measuring EROD activity, was significantly induced by treatment with PCB126, more so with the lower (1 μmol/kg) dose than with the higher (5 μmol/kg) dose of PCB126 (Figure 2). Two-way ANOVA did not indicate an effect of dietary copper levels on EROD activity; however, rats receiving less (2 ppm) copper in the diet had a significantly lower baseline level of CYP1A1 activity than the corresponding corn oil control with normal or high dietary copper levels (Figure 2).
Figure 2.
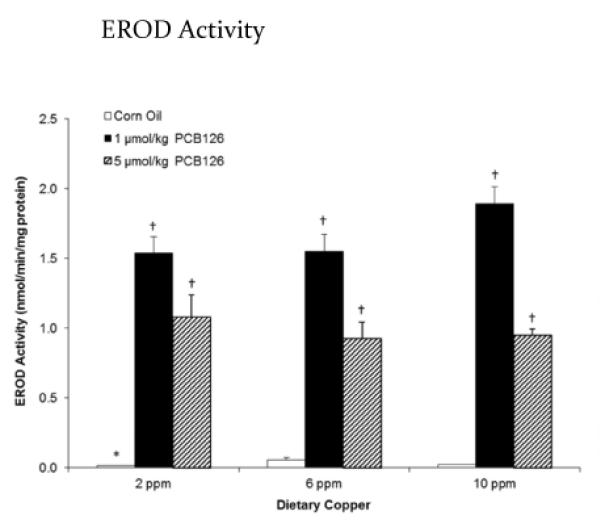
PCB126 significantly induced EROD activity. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
Effects of copper diets and PCB126 on liver SOD activities
Different dietary copper levels might be expected to have an influence on CuZnSOD activity. Neither the dietary copper level nor treatment with PCB126 alone or both in combination had an influence on hepatic CuZnSOD activity or total SOD activity (Table 3). MnSOD activity was diminished by PCB126. This effect was significant in the high dietary copper group administered 5 μmol/kg PCB126 and overall at both PCB doses.
Table 3.
CuZnSOD, MnSOD, and Total SOD activity (U/mg protein) under each experiment condition and significance of various comparisons (adjusted using Dunnett's test)
| CuZnSOD Activity (U/mg protein) | MnSOD Activity (U/mg protein) | Total SOD Activity (U/mg protein) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Treatment | Dietary Copper Level | Dietary Copper Level | Dietary Copper Level | |||||||||
|
| ||||||||||||
| Low (2 ppm) | Adequate (6 ppm) | High (10 ppm) | Overall | Low (2 ppm) | Adequate (6 ppm) | High (10 ppm) | Overall | Low (2 ppm) | Adequate (6 ppm) | High (10 ppm) | Overall | |
| Corn Oil | 256 ± 36 (-,-) | 223 ± 25 (-,-) | 224 ± 40 (-,-) | - | 83.1 ± 3.9 (-,-) | 91.4 ± 5.5 (-,-) | 98.6 ± 9.2 (-,-) | - | 339 ± 37 (-,-) | 314 ± 27 (-,-) | 323 ± 36 (-,-) | - |
| 1 μmol/kg PCB126 | 240 ± 26 (-,-) | 270 ± 32 (-,-) | 284 ± 61 (-,-) | - | 68.6 ± 9.9 (-,-) | 74.5 ± 7.6 (-,-) | 82.6 ± 6.5 (-,-) | † | 308 ± 29 (-,-) | 344 ± 32 (-,-) | 367 ± 60 (-,-) | - |
| 5 μmol/kg PCB126 | 225 ± 27 (-,-) | 296 ± 40 (-,-) | 283 ± 33 (-,-) | - | 67.6 ± 8.3 (-,-) | 81.8 ± 8.1 (-,-) | 61.7 ± 5.5 (*,-) | † | 293 ± 26 (-,-) | 378 ± 47 (-,-) | 344 ± 32 (-,-) | - |
|
| ||||||||||||
| Overall | - | - | - | - | - | - | - | - | - | |||
Results are expressed as mean ± SEM. Each group contained 4–6 animals. One-way AN OVA was used to examine the difference between each PCB126 level and the corn oil treatment, "†" in the parentheses indicates a significant difference due to PCB treatment. Similarly, a "*" in the parentheses indicates significant differences between low or supplemental and the adequate dietary copper level. Significance for each factor based on two-way ANOVA is indicated in the bottom margin for copper diet (*) and in the right margin for PCB treatment (†). The level for significance is 0.05.
Effects on total hepatic glutathione
Treatment with PCB126 caused a reduction in total GSH in the livers of rats (Figure 3). Although this effect seems pronounced and dose dependent with normal and high copper diets, significance was seen only with the high dose of PCB126 in the normal copper group when compared with the corresponding corn oil control. Two-way ANOVA confirmed that overall both factors, PCB126 and dietary copper, diminished total hepatic glutathione levels (Table 1).
Figure 3.
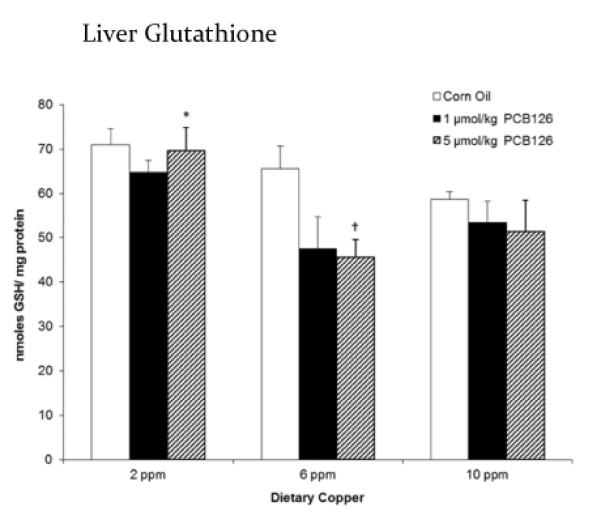
PCB126 and dietary copper significantly decreased total liver glutathione suggesting increased oxidative stress. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
Effects on Liver 4-HNE
Increased cellular levels of 4-HNE, a product of lipid peroxidation, are an indicator of oxidative stress. Different copper levels in the diet did not influence the hepatic 4-HNE either alone or in PCB-treated animals (Table 1; Supplemental Figure 2). Overall, PCB126 reduced 4-HNE, but at the level of individual data point a significant reduction was only seen with the high dose of PCB126 on low copper diet.
Effects on serum ceruloplasmin and hepatic ceruloplasmin gene expression
Ceruloplasmin is the major copper-carrying protein in the blood and synthesized in the liver. Serum ceruloplasmin was significantly diminished in all animals receiving the low (2 ppm) copper diet (Figure 4a). In those on normal (6 ppm) or high (10 ppm) dietary copper feed, PCB126 caused an increase in serum ceruloplasmin. In addition, two-way ANOVA revealed an interaction effect of dietary copper and PCB126 on serum ceruloplasmin levels (Table 1). Its gene expression resembled serum ceruloplasmin levels in that the low (2 ppm) copper diet diminished ceruloplasmin mRNA overall significantly, although at the individual data point levels this was only significant with the corn oil and low PCB treatment (Table 1; Figure 4b).
Figure 4.
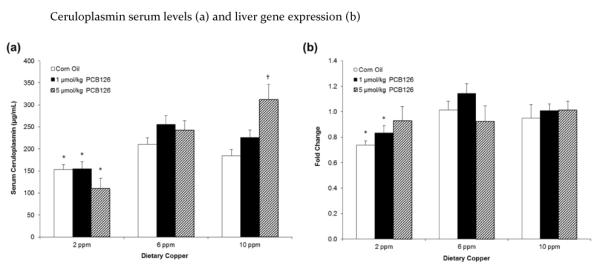
Dietary copper and not PCB126 caused a significant increase in the serum levels (a) and hepatic expression (b) of ceruloplasmin. Fold change is calculated relative to 6 ppm dietary copper and corn oil treatment. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
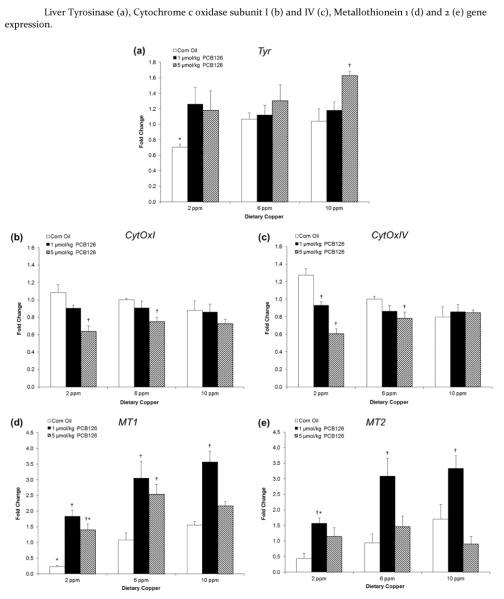
Effects on hepatic tyrosinase, cytochrome c oxidase and metallothionein gene expression
The copper-containing enzyme tyrosinase is the rate-limiting enzyme in the synthesis of melanin and also involved in the oxidation of other endogenous phenols. PCB126 treatment, particularly the high dose, caused an increase in tyrosinase expression in the high (10 ppm) dietary groups which reached significance in the high copper diet group and overall (Table 1; Figure 5a).
Figure 5.
Tyrosinase expression (a) was induced upon PCB126 treatment and in a near dose-dependent manner whereas dietary copper had little effect. PCB126 treatment significantly decreased cytochrome c oxidase subunit I (b) and subunit IV (c) gene expression. PCB126 treatment and dietary copper increased the expression of both MT1 and MT2. Fold change is calculated relative to 6ppm dietary copper and corn oil treatment. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
Cytochrome c oxidase, complex IV in the electron transport chain, uses copper and has genetic information in both the nucleus (subunit IV) and the mitochondrion (subunit I). PCB126 treatment significantly decreased gene expression in the low (2 ppm) and adequate (6 ppm) dietary groups and had an overall effect on both subunits (Table 1; Figures 5b and c). In addition, two-way ANOVA revealed an interactive effect of copper diet and PCB126 treatment on the gene expression of cytochrome c oxidase subunit IV, the nuclear subunit.
Metallothionein is a cysteine rich intracellular protein that binds a wide range of metals from essential metals like Cu, Zn and Se to the potentially toxic metals Cd, Hg and As. Metallothionein isoforms 1 and 2 were significantly increased by both dietary copper and PCB126 exposure (Table 1; Figure 5d and e). Induction reached a peak at 1 μmol/kg PCB126 dose similar to the induction of CYP1A1 that was seen in the EROD activity.
Effects on liver, kidney, and blood selenium
Liver selenium was significantly affected overall (2-way ANOVA) by both dietary copper (increasing) and PCB126 (decreasing), with an interaction effect for the two factors (Table 1). In addition, PCB126 caused a significant decrease in hepatic selenium even at the low dose. In contrast to the liver, kidney selenium levels were increased by PCB126 at the high (5 μmol/kg) dose (Supplemental Table 3a). Two-way ANOVA showed that blood selenium was inversely affected by dietary copper.
Effects on liver, kidney, and blood iron
Liver and kidney iron levels were not influenced by dietary copper levels, but blood iron was significantly increased overall by increasing amounts of copper in the diet (Supplemental Table 3b). Liver iron was significantly diminished by PCB126 in the adequate (6 ppm) and high (10 ppm) dietary copper groups. Blood iron was not influenced by PCB126 overall. No significant effects of PCB126 on kidney iron levels were observed.
Effects on liver, kidney, and blood zinc
Two-way ANOVA showed that PCB126 caused a significant decrease in liver zinc levels, while high dietary copper increased zinc significantly (Table 1; Supplemental Table 3c). Kidney zinc was significantly increased overall by dietary copper but not PCB126. In contrast, blood zinc was significantly decreased overall by PCB126, but not affected by dietary copper.
Effects on liver, kidney, and blood manganese
PCB126 also caused an overall significant decrease in liver manganese (Table 1; Supplemental Table 3d). However, there was an overall increase by dietary copper with no interaction effect of the two factors (Table 1). Two-way ANOVA revealed a significant overall increase in kidney manganese due to dietary copper and PCB126. Blood manganese was not affected by PCB126, but was significantly decreased with increasing dietary copper (Table 1).
Histology
The histologic examination revealed that PCB126 treated livers had hepatocellular enlargement due to cytoplasmic lipid accumulation with a mild increase in cytoplasmic density. Additionally periportal hepatocytes had cytoplasmic clearing consistent with a hydropic change. There was a PCB dose-response in severity of these hepatocyte alterations. Apoptotic hepatocytes were identified in rats treated with the high dose of PCB126 in the adequate (6 ppm) and high (10 ppm) dietary copper groups although only 1–2 rats were affected in each group. No histologic changes associated with dietary copper levels were noticed.
Evaluation and quantification of ORO staining showed an increase in liver lipid in PCB-treated rats (Figure 6). In the control groups, significantly lower lipid content was observed in the low (2 ppm) copper diet group.
Figure 6.
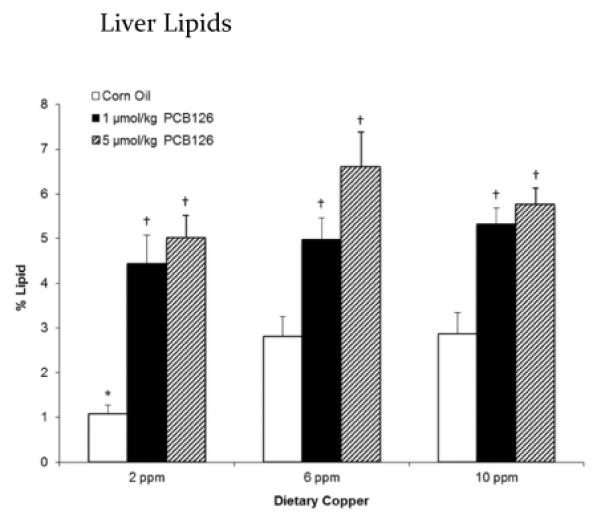
The percent of lipids in the liver increased with exposure to PCB126 with little effect from dietary copper. Error bars represent SEM. Each group contained 4–6 animals. * p < 0.05 as compared to adequate (6 ppm) dietary copper. † p < 0.05 as compared to Corn Oil vehicle control.
Liver sections were examined by transmission electron microscopy to determine whether ultrastructural differences due to PCB treatment or different dietary copper levels could be detected (Supplemental Figures 3a, b and c). The observed ultrastructural changes were consistent with PCB administration including increased cytoplasmic lipid and areas that appeared to have increased smooth endoplasmic reticulum although no quantification of sER was conducted. There was no evidence of lipid within the smooth endoplasmic reticulum. There were no significant ultrastructure differences between dietary copper groups administered the same PCB treatment.
Discussion
Copper's ability to act as both an essential antioxidant and a prooxidant makes it an intriguing target in toxicity. Copper absorbed in the body is mostly bound to a transporter protein, ceruloplasmin, or sequestered in cells bound to chaperones. In the unbound state, copper can react with hydrogen peroxide and generate highly reactive hydroxyl radicals in a Fenton-like reaction. These reactive oxygen species (ROS) can bind to lipids, proteins, and DNA, and cause covalent modifications.28 Acute copper toxicity can result in various pathologies, while chronic copper toxicity can cause severe hepatic and neurologic damage.29,30 Because of the small therapeutic range, copper metabolism and excretion is tightly controlled and regulated.31 Although toxicity as a result of direct disruption of copper homeostasis has been well studied, little is known about the effects of xenobiotics on this sensitive balance. PCBs, a family of persistent environmental pollutants, can cause ROS generation and disrupt copper homeostasis.32,33 Consistent with previous findings, we observed that the 5 μmol/kg dose PCB126 significantly slowed the growth of rats, an effect that can partially be explained by the lower feed consumption observed (Supplemental Figure 1). Liver weights were significantly increased by PCB126, particularly by the well tolerated lower dose of PCB126, reflecting liver hypertrophy (Figure 1a). The high or low dietary copper diets did not ameliorate or enhance the hypertrophy generally seen by this dioxin-like PCB.
We observed a significant induction of EROD (CYP1A1) activity following PCB126 exposure, confirming its potent binding and activation of the AhR. The higher dose of the toxicant PCB126 did not further increase the induction response of CYP1A1. We have observed this non-linear trend both in vitro and in vivo. In an earlier study using chick embryo hepatocytes, we found that dioxin – like PCBs caused potent induction of total cytochrome P-450 content and EROD activities with increasing concentrations until a maximum was reached. Further concentration increases resulted in marked reduction of total cytochrome P-450 and EROD activity.34 We have also observed this effect in the rat as well.3,35–37 Other groups have seen similar trends with PCB related compounds both in cells38–40 and in animals.41–43 Decrease in EROD activity seen with higher doses of PCB126 maybe the result of inhibition or inactivation of CYP1A1 by PCB126. Several papers have been published showing this trend in both marine animals and vertebrates.44–46
Strong induction of cytochrome P450 without the presence of a substrate is known to increase oxidative stress in vitro, if that effect takes place in vivo it may be involved in the cancer promoting activity of such compounds. Surprisingly, we did not observe an increase in the lipid peroxidation product 4-HNE and although total GSH was somewhat lowered in PCB-treated animals this was only significant for the 6 ppm copper-high PCB126 group. Although not shown by the parameters tested, countless other studies have shown the connection between ROS and dioxin-like PCBs.
Past studies have shown that dioxin-like compounds, including PCB126, caused significant increases in hepatic copper levels.8,11 Consistent with those observations, we observed a significant dose-dependent increase in hepatic copper levels in all rats exposed to PCB126. Liver copper was also increased by high dietary copper levels. As a result, the 10 ppm copper diet treated with 5 μmol/kg PCB126 had an increase of liver copper levels of about 100% compared to the no treatment controls on adequate copper (Figure 1b). Despite these considerable differences in the liver copper levels due to diet and PCB126, there were no effects on hepatic total or CuZnSOD activity (Table 2). These results support the assumption that the dietary copper levels used in this study did not reduce or increase liver copper to a physiologically relevant level.47
To investigate the cause of increased hepatic copper, the levels of copper-containing enzymes along with metallothionein were investigated as a cause of copper sequestration. Interestingly, with exception of the high copper diet – high PCB126 group, PCB126 treatment did not significantly increase ceruloplasmin in the serum nor were any changes in its gene expression in the liver observed, even though levels of copper were increased in both tissues (Figure 4). The increase seen in the high copper diet – high PCB126 group may be the result of increased production of the longer-lived holoceruloplasmin from the apoceruloplasmin but more studies are needed to confirm this.48–50 We hypothesized that other copper-containing proteins or another mechanism was involved in the PCB-induced increase in copper. Tyrosinase, also known as monophenol monooxygenase, is a copper-containing protein that has been shown to be induced by exposure to TCDD in melanocytes through activation of the Ah receptor.51 Its expression was not affected by dietary copper, but PCB126 treatment slightly increased tyrosinase gene expression in the liver, particularly in the high copper diet group (Figure 5a). This study provides the first evidence of hepatic tyrosinase expression and its modulation by PCB126. This small increase may contribute to the overall increase in hepatic copper that is seen with PCB exposure and may change the biotransformation pathway of endogenous and exogenous compounds.
Cytochrome C Oxidase, or Complex IV in the electron transport chain, is made up of 13 different subunits, 10 genes from the nucleus and 3 genes from the mitochondrion, and uses two copper atoms and two heme groups.52 In this study we found a PCB126-related decrease in mRNA for both subunit I, from mitochondrion, and subunit IV, from the nucleus, but no significant effect of the copper diets (Figures 5b and c). Complex IV functions in the final step in the electron transport chain to reduce O2 to H2O.53 Disruption of the electron transport chain may result in the generation of reactive superoxide anions54 and subsequent increase in antioxidant enzymes.55 In addition, other AhR agonists have been reported to disrupt the electron transport chain and diminish ATP levels in the liver.56,57 A decrease in ATP levels may result in decreases in hepatic copper export mediated by copper-transporting ATPases (in humans ATP7A and ATP7B)58,59, but further studies are needed to test this hypothesis.
Hepatic metallothionein 1 and 2 gene expression was significantly increased with both dietary copper and PCB126 (Table 1; Figure 5d and e). This is consistent with previous findings using similar planar dioxin-like compounds.8,11 Metallothionein has a greater binding affinity for copper than for zinc which potentially explains the decrease of zinc in the liver. In addition, metallothionein is able to bind up to 12 atoms of copper60 so even the modest increase that is seen with PCB126 (3-fold) could explain the increases in hepatic copper. Although the protein levels of metallothionein will need to be investigated to confirm this hypothesis along with the levels of other copper chaperones.
Consistent with our previous observations3,8, PCB126 caused a significant decrease in liver selenium, zinc, manganese and iron levels, the opposite of the effect on copper (Figure 1b). It has been suggested that copper is preferentially absorbed into the liver over other trace metals during periods of stress.61 Selenium was decreased in the liver even at the lower dose and was increased in the blood and kidneys. These observations suggest that PCB126 caused an increase in selenium excretion from the liver into the blood and increased uptake into the kidneys, possibly by conjugation to electrophilic compounds.62 Selenium is incorporated into several antioxidant enzymes and inactivation of those enzymes, due to lack of selenium, would result in an increased susceptibility to oxidative stress.63,64 Zinc was also decreased in the liver, zinc is an essential element in CuZnSOD, but this enzyme activity was not reduced in the liver, indicating that Zn levels did not fall below the required minimum. Manganese, however, a crucial component of MnSOD, is the most important mitochondrial antioxidant defense system and an enzyme that is implicated in cancer and neurodegenerative diseases. MnSOD activity was significantly reduced by PCB126 exposure. Assuming that manganese availability is the limiting factor for MnSOD activity (this issue is explored in detail in a separate report65), this toxic effect of PCB126 on an important antioxidant enzyme is mediated through a disturbance of metal homeostasis and not through direct effects on the enzyme or its gene regulation. Iron was also decreased in the liver by PCB126. Disruption of heme synthesis, specifically inhibition of uroporphyrinogen decarboxylase, has been linked to TCDD and may explain the changes in iron status but further studies need to be done.40
As expected, large cytoplasmic lipid vacuoles were visible in the livers of PCB126-treated rats (Supplemental Figure 3b&c). We observed a potential increase in the lipid synthesizing smooth endoplasmic reticulum (SER), but no lipid accumulation was observed in either smooth or rough endoplasmic reticulum (Supplemental Figure 3b&c). Oil-Red-O quantification confirmed an increase in lipid accumulation with PCB126 administration over all dietary groups. In a separate study, we have confirmed the increases in hepatic lipid levels in the livers of rats treated with PCB126.37 This suggests a potential increase in lipid synthesis and/or disruption of lipid metabolism (Figure 6). Alterations to hepatic lipid profiles has been shown to be linked to complexed copper66, although the mechanism of this change is unknown. Interestingly, dietary copper only lowered the liver lipids in the low copper diet and did not show an effect in the high copper diet animals. This is in contrast to the increased lipid accumulation seen in Wilson's Disease, a genetic disorder that causes the buildup of copper in the liver.67 Although the increases in hepatic copper seen in Wilson's Disease are orders of magnitude higher. This may suggest that a higher copper diet than the one that we used in this study is needed to see a more pronounced copper related effect. However, steatosis observed in PCB126-treated rats is also observed in the early stages of Wilson's Disease and is believed to be the result of oxidative damage caused by excess copper and Fenton chemistry. Steatosis may also be related to copper accumulation itself, independent of oxidative stress. Thus the disruption of copper homeostasis by PCB126 may be a/the major mechanism of steatosis of this and possibly other strong AhR agonists.
The involvement of copper in PCB126-induced toxicity is not well understood. Since AhR agonists cause increases in hepatic copper levels, this study sought to determine mechanisms of PCB126-induced liver toxicity related to copper levels. PCB126 did increase copper levels in the liver as expected but this increase was not fully explained by increases in copper containing enzymes. However the increases seen in hepatic metallothionein gene expression may explain the increased copper. In addition, the moderately increased availability of copper through the diet did not enhance or diminish the toxicity of PCB126. It is unclear whether increased hepatic copper directly causes toxicity or is a byproduct of toxicity and how this is related to the disturbed homeostasis in other essential minerals. Further studies will be needed to fully elucidate the involvement of copper and other metals in PCB126 toxicity.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Gregor Luthe for the synthesis of PCB126, the Radiation and Free Radical Research Core Facility (P30 CA 086862), and members of laboratory for help with the animal studies.
Funding
Support for this research was supported by NIH (ES 013661, ES 05605). I.K.L and W.D.K gratefully acknowledge support from the Iowa Superfund Research Program (P42 ES013661) Training Core.
ABBREVIATIONS
- PCB
polychlorobiphenyl
- CuZnSOD
copper zinc superoxide dismutase
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- AhR
aryl-hydrocarbon receptor
- CYP1A1
cytochrome P450 1A1
- EROD
ethoxyresorufin deethylase
- DTNB
5,5′-dithio-bis-(2-nitrobenzoic acid)
- NBT
nitroblue tetrazolium
- MnSOD
manganese superoxide dismutase
- 4-HNE
4-hydroxynonenal
- RT-PCR
reverse transcription polymerase chain reaction
- ICP-MS
inductively coupled plasma – mass spectrometry
- ORO
oil red o
- Cp
ceruloplasmin
- Tyr
tyrosinase
- CytOxI
cytochrome c oxidase subunit I
- CytOxIV
cytochrome c oxidase subunit IV
- MT1
metallothionein isoform 1
- MT2
metallothionein isoform 2
- sER
smooth endoplasmic reticulum
- ROS
reactive oxygen species
- MnSOD
manganese superoxide dismutase
Footnotes
Supporting Information Available. Dietary components, primer sequences, organ metal status (Iron, Selenium, Zinc, Manganese), growth and feed consumption, liver lipid oxidation, electron micrographs of liver sections are provided. This information is available free of charge via the Internet at http://pubs.acs.org/.
Author Contribution
These studies form a portion of the dissertation research of I.K.L and W.D.K. who contributed equally to this study. Each should be considered “First Author”.
The authors declare no competing financial interest.
REFERENCES
- (1).Nordberg G. Handbook on the toxicology of metals. 3rd ed. Academic Press; Amsterdam; Boston: 2007. [Google Scholar]
- (2).Brewer GJ. The risks of free copper in the body and the development of useful anticopper drugs. Curr Opin Clin Nutr Metab Care. 2008;11:727–732. doi: 10.1097/MCO.0b013e328314b678. [DOI] [PubMed] [Google Scholar]
- (3).Lai IK, Chai Y, Simmons D, Watson WH, Tan R, Haschek WM, Wang K, Wang B, Ludewig G, Robertson LW. Dietary selenium as a modulator of PCB 126-induced hepatotoxicity in male Sprague-Dawley rats. Toxicological Sciences. 2011;124:202–214. doi: 10.1093/toxsci/kfr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- (5).Yoshizawa K, Heatherly A, Malarkey DE, Walker NJ, Nyska A. A critical comparison of murine pathology and epidemiological data of TCDD, PCB126, and PeCDF. Toxicol Pathol. 2007;35:865–879. doi: 10.1080/01926230701618516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- (7).Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- (8).Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: Effects on hepatic oxidative stress, glutathione and metals status. Environ Int. 2010;36:918–923. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Elsenhans B, Forth W, Richter E. Increased copper concentrations in rat tissues after acute intoxication with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Archives of toxicology. 1991;65:429–432. doi: 10.1007/BF02284268. [DOI] [PubMed] [Google Scholar]
- (10).Wahba ZZ, al-Bayati ZA, Stohs SJ. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the hepatic distribution of iron, copper, zinc, and magnesium in rats. Journal of biochemical toxicology. 1988;3:121–129. doi: 10.1002/jbt.2570030206. [DOI] [PubMed] [Google Scholar]
- (11).Nishimura N, Miyabara Y, Suzuki JS, Sato M, Aoki Y, Satoh M, Yonemoto J, Tohyama C. Induction of metallothionein in the livers of female Sprague-Dawley rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Life Sci. 2001;69:1291–1303. doi: 10.1016/s0024-3205(01)01212-7. [DOI] [PubMed] [Google Scholar]
- (12).Stohs SJ. Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Free Radic Biol Med. 1990;9:79–90. doi: 10.1016/0891-5849(90)90052-k. [DOI] [PubMed] [Google Scholar]
- (13).Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol. 1985;34:3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- (14).Twaroski TP, O'Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochem Pharmacol. 2001;62:273–281. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- (15).Luthe GM, Schut BG, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs): Synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2006 doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- (16).Dunn M, Blalock T, Cousins R. Metallothionein. Proc. Soc. Exp. Biol. Med. 1987;185:107–119. doi: 10.3181/00379727-185-42525a. [DOI] [PubMed] [Google Scholar]
- (17).Reeves P, DeMars L. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. The Journal of nutritional biochemistry. 2006;17:635–642. doi: 10.1016/j.jnutbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- (18).Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- (19).Burke MD, Mayer RT. Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos. 1974;2:583–588. [PubMed] [Google Scholar]
- (20).Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- (21).Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- (22).Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- (23).Banni M, Messaoudi I, Said L, El Heni J, Kerkeni A, Said K. Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Archives of environmental contamination and toxicology. 2010;59:513–519. doi: 10.1007/s00244-010-9494-5. [DOI] [PubMed] [Google Scholar]
- (24).Sullivan CJ, Teal TH, Luttrell IP, Tran KB, Peters MA, Wessells H. Microarray analysis reveals novel gene expression changes associated with erectile dysfunction in diabetic rats. Physiological genomics. 2005;23:192–205. doi: 10.1152/physiolgenomics.00112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ripamonti M, Vigano A, Moriggi M, Milano G, von Segesser LK, Samaja M, Gelfi C. Cytochrome c oxidase expression in chronic and intermittent hypoxia rat gastrocnemius muscle quantitated by CE. Electrophoresis. 2006;27:3897–3903. doi: 10.1002/elps.200600104. [DOI] [PubMed] [Google Scholar]
- (26).Wang B, Robertson LW, Wang K, Ludewig G. Species difference in the regulation of cytochrome P450 2S1: a lack of induction in rats by the aryl hydrocarbon receptor agonist PCB126. Xenobiotica. 2011;41:1031–1043. doi: 10.3109/00498254.2011.603763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. Biofactors. 2010;36:125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- (29).Butterworth RF. Metal toxicity, liver disease and neurodegeneration. Neurotox Res. 2010;18:100–105. doi: 10.1007/s12640-010-9185-z. [DOI] [PubMed] [Google Scholar]
- (30).Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26:268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- (31).Stern BR, Solioz M, Krewski D, Aggett P, Aw TC, Baker S, Crump K, Dourson M, Haber L, Hertzberg R, Keen C, Meek B, Rudenko L, Schoeny R, Slob W, Starr T. Copper and human health: biochemistry, genetics, and strategies for modeling dose-response relationships. J Toxicol Environ Health B Crit Rev. 2007;10:157–222. doi: 10.1080/10937400600755911. [DOI] [PubMed] [Google Scholar]
- (32).Hennig B, Hammock BD, Slim R, Toborek M, Saraswathi V, Robertson LW. PCB-induced oxidative stress in endothelial cells: modulation by nutrients. Int J Hyg Environ Health. 2002;205:95–102. doi: 10.1078/1438-4639-00134. [DOI] [PubMed] [Google Scholar]
- (33).Ohchi H, Kusuhara T, Katayama T, Ohara K, Kato N. Effects of dietary xenobiotics on the metabolism of copper, alpha-tocopherol and cholesterol in rats. J Nutr Sci Vitaminol (Tokyo) 1987;33:281–288. doi: 10.3177/jnsv.33.281. [DOI] [PubMed] [Google Scholar]
- (34).Rodman LE, Shedlofsky SI, Swim AT, Robertson LW. Effects of Polychlorinated Biphenyls on Cytochrome P450 Induction in the Chick Embryo Hepatocyte Culture. Archives of Biochemistry and Biophysics. 1989;275:252–262. doi: 10.1016/0003-9861(89)90371-8. [DOI] [PubMed] [Google Scholar]
- (35).Shedlofsky SI, Hoglen NC, Rodman LE, Honchel R, Robinson FR, Swim AT, McClain CJ, Robertson LW. 3,3',4,4',-tetrabromobiphenyl Sensitizes Rats to the Hepatotoxic Effects of Endotoxin by a Mechanism that Involves more than Tumor Necrosis Factor. Hepatology. 1991;14:1201–1208. [PubMed] [Google Scholar]
- (36).Robertson LW, Parkinson A, Campbell MA, Safe S. Polybrominated Biphenyls as Aryl Hydrocarbon Hydroxylase Inducers: Structure-Activity Correlations. Chem Biol Interact. 1982;42:53–66. doi: 10.1016/0009-2797(82)90141-7. [DOI] [PubMed] [Google Scholar]
- (37).Lai I, Dhakal K, Gapdupudi G, Miao L, Ludewig G, Robertson LW, Olivier A. N-acetylcystiene (NAC) diminishes the severity of PCB126-induced fatty liver in male rodents. Toxicology. 2012;302:25–33. doi: 10.1016/j.tox.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sawyer T, Safe S. PCB Isomers and Congeners: Induction ofAryl Hydrocarbon Hydroxylase and Ethoxyresorufin O-deethylase Enzyme Activities in Rat Hepatoma Cells. Toxicol Lett. 1982;13:87–94. doi: 10.1016/0378-4274(82)90142-4. [DOI] [PubMed] [Google Scholar]
- (39).Lambrecht RW, Sinclair PR, Bement WJ, Sinclair JF. Uroporphyrin Accumulation in Cultured Chick Embryo Hepatocytes: Comparison of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and 3,4,3',4'-Tetrachlorobiphenyl. Toxicol Appl Pharmacol. 1988;96:507–516. doi: 10.1016/0041-008x(88)90010-5. [DOI] [PubMed] [Google Scholar]
- (40).Sinclair PR, Bement WJ, Bonkovsky HL, Sinclair JF. Inhibition of uroporphyrinogen decarboxylase by halogenated biphenyls in chick hepatocyte cultures. Biochem J. 1984;222 doi: 10.1042/bj2220737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Schmoldt A, Benthe HF, Fruhling R. Induction of Rat Liver Enzymes by PCBs in Dependence on the Dose and Chlorine Content. Archives of toxicology. 1974;32:69–81. doi: 10.1007/BF00316228. [DOI] [PubMed] [Google Scholar]
- (42).Sawyer T, Jones D, Rosanoff K, Mason G, Piskorska-Pliszczynska J, Safe S. The Biologic and Toxic Effects of 2,3,7,8-tetrachlorodibenzo-p-dixoin in Chickens. Toxicology. 1986;39:197–206. doi: 10.1016/0300-483x(86)90136-8. [DOI] [PubMed] [Google Scholar]
- (43).Hamilton JW, Denison MS, Bloom SE. Development of basal and induced aryl hydrocarbon (benzo[a]pyrene) hydroxylase activity in the chicken embryo in ovo. Proc. Natl. Acad. Sci. 1983;80:3372–3376. doi: 10.1073/pnas.80.11.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Schlezinger J, White R, Stegeman J. Oxidative Inactivation of Cytochrome P-450 1A (CYP1A) Stimulated by 3,3',4,4'-Tetrachlorobiphenyl: Production of Reactive Oxygen by Veterbrate CYP1As. Mol. Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- (45).Hahn M, Woodward B, Stegeman J, Kennedy S. Rapid Assessment of Induced Cytochrome P4501A Protein and Catalytic Activity in Fish Hepatoma Cells Grown in Multiwell Plates: Response to TCDD, TCDF, and two Planar PCBs. Environ. Toxicol. Chem. 1996;15:582–591. [Google Scholar]
- (46).Hahn M, Lamb T, Schultz M, Smolowitz R, Stegeman J. Cytochrome P4501A induction and inhibition by 3,3',4,4'-tetrachlorobiphenyl in an Ah receptor-containing fish hepatoma cell line (PLHC-1) Aquat. Toxicol. 1993;26:185–208. [Google Scholar]
- (47).Harris ED. Copper as a cofactor and regulator of copper,zinc superoxide dismutase. J Nutr. 1992;122:636–640. doi: 10.1093/jn/122.suppl_3.636. [DOI] [PubMed] [Google Scholar]
- (48).Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, de Jong GJ, Gitlin JD. Mechanisms of Copper Incorporation into Human Ceruloplasmin. Journal of Biological Chemistry. 2002;277:46632–46638. doi: 10.1074/jbc.M206246200. [DOI] [PubMed] [Google Scholar]
- (49).Holtzman NA, Gaumnitz BM. Studies on the Rate of Release and Turnover of Ceruloplasmin and Apoceruloplasmin in Rat Plasma. J Biol Chem. 1970;245:2354–2358. [PubMed] [Google Scholar]
- (50).Gitlin JD, Schroeder JJ, Lee-Ambrose LM, Cousins RJ. Mechanisms of ceruloplasmin biosynthesis in normal and copper-deficient rats. Biochem J. 1992;282:835–839. doi: 10.1042/bj2820835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Luecke S, Backlund M, Jux B, Esser C, Krutmann J, Rannug A. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment cell & melanoma research. 2010;23:828–833. doi: 10.1111/j.1755-148X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- (52).Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- (53).Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421–429. doi: 10.1002/iub.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H(2)O(2) signaling. Antioxid Redox Signal. 2011;14:459–468. doi: 10.1089/ars.2010.3363. [DOI] [PubMed] [Google Scholar]
- (55).Di Giovanni S, Mirabella M, Papacci M, Odoardi F, Silvestri G, Servidei S. Apoptosis and ROS detoxification enzymes correlate with cytochrome c oxidase deficiency in mitochondrial encephalomyopathies. Molecular and cellular neurosciences. 2001;17:696–705. doi: 10.1006/mcne.2001.0970. [DOI] [PubMed] [Google Scholar]
- (56).Forgacs AL, Burgoon LD, Lynn SG, LaPres JJ, Zacharewski T. Effects of TCDD on the expression of nuclear encoded mitochondrial genes. Toxicol Appl Pharmacol. 2010;246:58–65. doi: 10.1016/j.taap.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Senft AP, Dalton TP, Nebert DW, Genter MB, Hutchinson RJ, Shertzer HG. Dioxin increases reactive oxygen production in mouse liver mitochondria. Toxicol Appl Pharmacol. 2002;178:15–21. doi: 10.1006/taap.2001.9314. [DOI] [PubMed] [Google Scholar]
- (58).Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88:826S–829S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Linz R, Lutsenko S. Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. Journal of bioenergetics and biomembranes. 2007;39:403–407. doi: 10.1007/s10863-007-9101-2. [DOI] [PubMed] [Google Scholar]
- (60).Li Y, Weser U. Circular Dichroism, Luminescence, and Electonic Absorption of Copper Binding Sites in Metallothionein and Its Chemically Synthesized alpha and beta Domains. Inorg. Chem. 1992;32:5526–5533. [Google Scholar]
- (61).Aggett PJ. Physiology and metabolism of essential trace elements: an outline. Clin Endocrinol Metab. 1985;14:513–543. doi: 10.1016/s0300-595x(85)80005-0. [DOI] [PubMed] [Google Scholar]
- (62).Muttenthaler M, Alewood PF. Selenopeptide chemistry. J Pept Sci. 2008;14:1223–1239. doi: 10.1002/psc.1075. [DOI] [PubMed] [Google Scholar]
- (63).Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- (64).Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- (65).Wang B, Simmons D, Klaren W, Olivier A, Wang K, Robertson LW, Ludewig G. (Submitted) Complex regulation of MnSOD: Effects of dietary manganese and PCB126 in the rat [Google Scholar]
- (66).Kennedy DC, Lyn RK, Pezacki JP. Cellular lipid metabolism is influenced by the coordination environment of copper. J Am Chem Soc. 2009;131:2444–2445. doi: 10.1021/ja809451w. [DOI] [PubMed] [Google Scholar]
- (67).Lalioti V, Sandoval I, Cassio D, Duclos-Vallee JC. Molecular pathology of Wilson's disease: a brief. J Hepatol. 2010;53:1151–1153. doi: 10.1016/j.jhep.2010.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.