Abstract
Background & Aims
Esophageal cancer is increasing in incidence in US, especially among patients with Barrett’s esophagus (BE). Statins might prevent this cancer. We performed a systematic review with meta-analysis of studies that evaluated the effect of statins on the risk of esophageal cancer.
Methods
We conducted a systematic search of Medline, Embase, and Web of Science through August 2012. Studies were included if they evaluated exposure to statins, reported the development of esophageal cancer, and reported relative risks or odds ratios (ORs), or provided data for their estimation. Summary OR estimates with 95% confidence intervals (CIs) were calculated using the random-effects model. The analysis included 13 studies (including a post-hoc analysis of 22 randomized controlled trials) reporting 9285 cases of esophageal cancer among 1,132,969 patients.
Results
Meta-analysis of the studies showed a significant (28%) reduction in incidence of esophageal cancer among patients who took statins (adjusted OR, 0.72; 95% CI, 0.60–0.86), though there was considerable heterogeneity among studies. In analyzing a subset of patients known to have BE (5 studies, 312 esophageal adenocarcinomas [EAC] developed in 2125 patients), statins were associated with a significant (41%) decrease in the risk of EAC, after adjusting for potential confounders (adjusted OR, 0.59; 95% CI, 0.45–0.78) with consistent results among all studies. The number needed to treat with statins to prevent 1 case of EAC in patients with BE was 389.
Conclusions
Meta-analysis of existing observational studies indicates that statins protect against esophageal cancer and reduce the risk of EAC in patients with BE.
Keywords: Esophageal cancer risk, cholesterol-lowering drugs, HMG CoA reductase inhibitors, chemoprevention
INTRODUCTION
Esophageal cancer (EC) is the 6th most common cancer in men worldwide with a dismal 5-year survival rate.1 While the incidence of esophageal squamous cell cancer (ESCC) is declining worldwide, the incidence of esophageal adenocarcinoma (EAC) has been rapidly rising, now forming majority of EC in the Western world. This increase may be partly attributable to the obesity epidemic.2, 3
Barrett’s esophagus (BE) is the most significant risk factor for development of EAC, and surveillance and early recognition of high grade dysplasia (HGD) and/or EAC may improve survival.4, 5 However, endoscopic surveillance of all patients with BE is expensive and not cost-effective.6 Hence, current strategies for improved management of EC are aimed at identifying patients at high risk for progression to EAC and identifying chemopreventive agents.
Statins, or 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, used for primary and secondary prevention of cardiovascular diseases, may decrease the risk of cancers.7–9 In vitro and animal studies have shown that in addition to cholesterol reduction, statins have anti-proliferative, pro-apoptotic, anti-angiogenic and immunomodulatory effects, which prevent cancer development and growth.10 This effect has also been shown in EC cell lines.11–14 Some recent observational studies have shown that statins may be associated with a lower risk of EC, particularly in patients with BE,15–17 whereas others have shown no beneficial effect.18, 19.
To better understand this issue, we performed a systematic review with meta-analysis of existing randomized controlled trials (RCTs) and observational studies that investigated the association between statins and risk of developing EC, in particular, the risk of development of EAC or progression of dysplasia in patients with BE.
METHODS
This systematic review was conducted following guidance provided by the Cochrane Handbook 20 and Kanwal and White 21 and is reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.22 The process followed a priori established protocol.
Data Sources and Search Strategy
A systematic literature search of Medline (1980 through August 31, 2012), Embase (1988 through August 31, 2012) and Web of Science (1993 through August 31, 2012) databases was conducted by two study investigators (S.S. and P.S.), independently, for all relevant articles on the effect of statin use on the risk of EC in all patients. Keywords used in the search included “HMG-CoA reductase inhibitor(s)”, “statin(s)”, “atorvastatin”, “fluvastatin”, “lovastatin”, “pravastatin”, “rosuvastatin,” or “simvastatin,” combined with “cancer” or “neoplasm(s)” or “Barrett’s”. The title and abstract of studies identified in the search were reviewed to exclude studies that did not answer the research question. The full text of each remaining article was read to determine whether it contained information on the topic of interest. We also manually searched review article bibliographies and abstracts of gastrointestinal/oncological society meetings for the years 2003–2012, and consulted with experts in the field. When incomplete information was available, attempts were made to contact the corresponding authors of the studies for additional information.
Study Selection
Studies considered in this meta-analysis were either randomized controlled trials (RCTs) or observational studies that met the following inclusion criteria: (1) evaluated and clearly defined exposure to statins, (2) reported EC incidence and (3) reported relative risks (RR) (for cohort studies) or odds ratio (OR) (for case-control studies) or provided data for their calculation. Inclusion was not otherwise restricted by study size, language or publication type. Articles were excluded from the analyses if there was insufficient data for determining an estimate of RR/OR and a 95% confidence interval (CI), though these were included in the systematic review and described qualitatively. When there were multiple publications from the same population, only data from the most recent comprehensive report were included.
Data Abstraction and Quality Assessment
Data were independently abstracted onto a standardized form by two authors (S.S. and A.G.S.). The following data were collected from each study: study design, time period of study/year of publication, country of the population studied, primary outcome reported type, dose and duration of statin use, information source for exposure measurement, total number of persons in each group (exposed v. not exposed), OR and 95% CIs with and without adjustment for potential confounders, and potential confounders used for adjustment. Data on the following confounding risk factors for EC were extracted from each study: age, sex, race, cigarette smoker, body mass index (BMI), presence of BE (and if present, length of BE segment and grade of dysplasia), other medication use (aspirin/non-steroidal anti-inflammatory drugs [NSAIDs]/proton pump inhibitors [PPIs]) and alcohol use was assessed. Conflicts in data abstraction were resolved by consensus, referring back to the original article.
The methodologic quality of case-control and cohort was assessed by two authors independently (A.G.S. and P.S.) using the Newcastle-Ottawa scale.23 In this scale, observational studies were scored across three categories: selection (4 questions) and comparability (2 questions) of study groups, and ascertainment of the outcome of interest (3 questions), with all questions with a score of one, except for comparability of study groups, where separate points were awarded for controlling age and/or sex (maximum two points). Any discrepancies were addressed by a joint reevaluation of the original article.
Outcomes assessed
The primary analysis focused on assessing the risk of all EC (EAC + ESCC) among users of statins. In addition, we also performed a subgroup analysis of studies restricted to patients with BE, to assess if statin use modifies the risk of progression to EAC (and/or HGD).
Data synthesis and Analysis
We used the random-effects model described by DerSimonian and Laird to calculate summary OR and 95% CI.24 We assessed heterogeneity between study-specific estimates using 2 methods.21, 25 Firstly, the Cochran’s Q statistical test for heterogeneity, which tests the null hypothesis that all studies in a meta-analysis have the same underlying magnitude of effect, was measured. Because this test is underpowered to detect moderate degrees of heterogeneity,26 a p-value of <0.10 was considered suggestive of significant heterogeneity. Secondly, to estimate what proportion of total variation across studies was due to heterogeneity rather than chance, I2 statistic was calculated. In this, a value of <30%, 30%–60%, 60%–75% and >75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively.21, 27 Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to study characteristics (as described above). In this analysis also, a p-value for differences between subgroups of <0.10 was considered statistically significant (i.e., a value of p<0.10 suggested that stratifying based on that particular study characteristic partly explained the heterogeneity observed in the analysis). We explored potential causes of heterogeneity by stratifying for several methodological and clinical features of studies. This included study design (case-control v. cohort), method of determining exposure to statins (pharmacy prescription database v. patient interview v. electronic medical record [EMR]), study location (North America v. Europe), study setting (hospital-based v. population-based) and risk of bias in exposure and outcome assessment due to lack of sufficient information (abstract only v. fully published manuscript). The presence of publication bias was assessed quantitatively using Begg and Mazumdar adjusted rank correlation test (publication bias considered present if p≤0.10)28 and qualitatively, by visual inspection of funnel plots of the logarithm of ORs versus their standard errors.29 All P values were two tailed. For all tests (except for heterogeneity and publication bias), a probability level <0.05 was considered statistically significant.
Since outcomes were relatively rare, OR were considered approximations of relative risk (RR). Adjusted OR, where reported in studies, was used for analysis to account for confounding variables. We also calculated the number needed to treat with statins to prevent 1 case of EC in the general population, as well as in high-risk patients with BE, based on current estimates of incidence of EC.30–33
All calculations and graphs were performed using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ).
RESULTS
Search Results
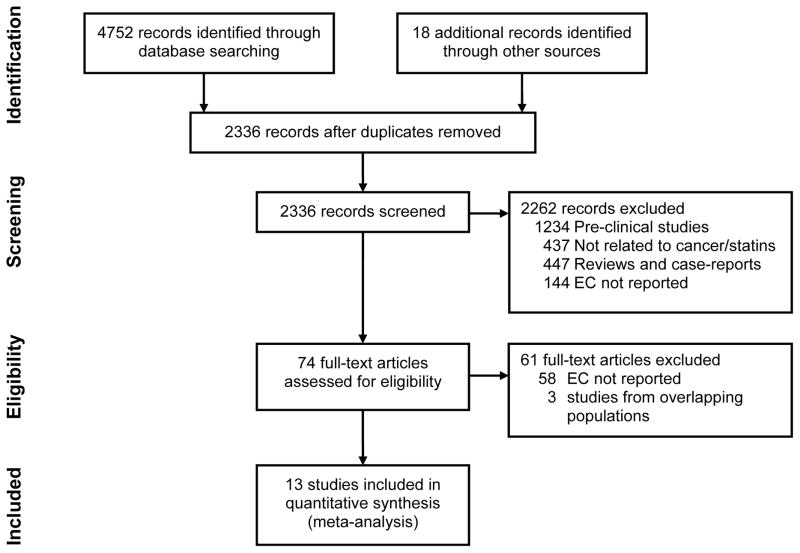
Of a total 2336 unique studies identified using our search criteria, 13 studies fulfilled our inclusion criteria and were included in the meta-analysis (7 case-control, 5 cohort, 1 post-hoc analysis of 22 RCTs),15–19, 24, 34–41 3 of which had been published only in the abstract form.34, 37, 39 Figure 1 summarizes the process of study identification, inclusion and exclusion. These cumulatively reported 9,285 cases of EC in 1,132,969 patients, primarily derived from a cohort of subjects seen in primary care clinics (with or without known esophageal or cardiovascular diseases). Five of these studies were performed in patients with known BE, and evaluated the risk of development of EAC (except 2 studies which combined risk of development of HGD and/or EAC or any dysplasia and/or EAC).15–18, 39 These cumulatively reported 312 cases of EAC in 2,125 patients with BE. Seven studies reported data from 3 population databases, hence only the most comprehensive reports from these were included, i.e., 1 study out of 2 from the QResearch database, UK,19, 42 2 out of 3 studies from the General Practice Research Database, UK,34, 36, 43 and 1 out of 2 studies from the Veterans’ Affairs national database, USA were included.15, 44
Figure 1.
Flowsheet summarizing study identification and selection.
Characteristics of Included Studies
The characteristics of these studies are shown in Table 1. The earliest study period began in 1983, and the latest ended in 2010. While studies in patients with BE reported development of EAC, studies on all cases of EC provided insufficient data on histological subtypes to study effects of statins on EAC and ESCC separately.
Table 1.
Characteristics of included studies assessing the risk of esophageal cancer with statin use
| Study | Design | Location | Setting | Time period | Exposure assessment | Total subjects | EC cases | Variables adjusted for | Study quality (NOS)Δ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome/Exposure | Overall quality score (maximum 9) | |||||||||
| Studies on patients with BE | ||||||||||||
| Nguyen et al44 | C-C | USA | Population-based | 2000–2004 | Pharmacy | 812 | 116 | 1–3,6,10 | *** | ** | *** | 8 |
| Kastelstein et al16 | Cohort | Netherlands | Hospital-based | 2003–2010 | Patient interview | 570 | 38‡ | 1,2,6,8,9 | **** | ** | *** | 9 |
| Kantor et al18 | Cohort | USA | Hospital-based | 1983–2009 | Patient interview | 411 | 56 | 1,2,5,6 | *** | ** | *** | 8 |
| Beales et al17 | C-C | UK | Hospital-based | NR | Patient interview | 255 | 85 | 1,2,4–6,11,12 | **** | ** | *** | 9 |
| Altawil et al [abstr]39 | Cohort | USA | Hospital-based | 2004–2010 | EMR | 77 | 17Π | NR | *** | ** | *** | 8 |
| Studies on all patients with esophageal cancer | ||||||||||||
| Kaye et al36 | C-C | UK | Population-based | 1990–2002 | Pharmacy | 18,088 | 100 | NR | *** | * | ** | 6 |
| Vinogrodova et al19 | C-C | UK | Population-based | 1998–2008 | Pharmacy | 16,200 | 3,159 | 1,2,4,5,6,7,10 | *** | ** | *** | 8 |
| Bhutta et al [abstr]34 | C-C | UK | Population-based | 2000–2008 | Pharmacy | 21,475 | 4,242 | 1,2,4–7,11 | ** | ** | ** | 6 |
| Marelli et al38 | Cohort | USA | Population-based | 1990–2009 | EMR | 91,714 | 73 | 1–3,5,6 | **** | ** | *** | 9 |
| Friedman et al35 | Cohort | USA | Population-based | 1994–2003 | Pharmacy | 361,859 | 68 | NR | **** | - | *** | 7 |
| Khurana et al [abstr]37 | C-C | USA | Population-based | 1998–2004 | NR | 484,226 | 659 | 1,5,7,12 | * | * | ** | 4 |
| Lai et al | C-C | Asia | Population-based | 2000–2009 | NR | 2,745 | 549 | 1,2,6,12 | *** | * | * | 5 |
| CTT40 | Post-hoc analysis of RCTs | Europe, Australia North America | Hospital-based | 2012† | Variable | 134,537 | 123 | 1,2,4,5,10 | N/A | N/A | N/A | N/A |
-EAC or HGD (in patients with Barrett’s),
- all dysplasia or EAC,
- EAC + squamous cell Ca,
- year of publication;
-Study quality assessment of observational studies performed using the Newcastle-Ottawa scale (each asterisk represents if individual criterion within the subsection were fulfilled) [C-C = Case-control, DM = Diabetes mellitus, NSAIDs = Non-steroidal anti-inflammatory drugs, NR = Not reported, NOS = Newcastle Ottawa Score, RCT = Randomized Controlled Trials]; 1=age, 2=sex, 3=race, 4= BMI, 5=smoking, 6=NSAIDs/aspirin, 7=DM, 8=BE length, 9=BE histology, 10=other comorbidities, 11=other medications, 12=alcohol use
Quality of Included Studies
Seven studies were considered high-quality. Table 1 depicts the methodological quality of all studies. Most studies adjusted for the following confounders: age (10/13), sex (9/13), BMI (4/13), smoking (6/13), NSAID/aspirin use (8/13). In most studies, >90% of all patients were on PPIs. In all these studies, a temporal relation of development of EC to statins was established by excluding cases of EC developing prior to exposure to statins, though there was variable duration of minimum statin use before new incident EC cases were included for analysis (0 months–2 years, where reported).
Risk of Esophageal Cancer – All Studies
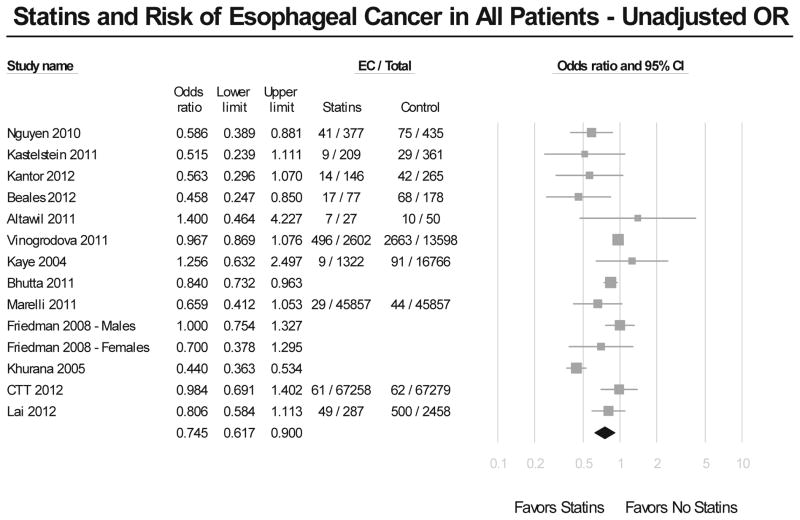
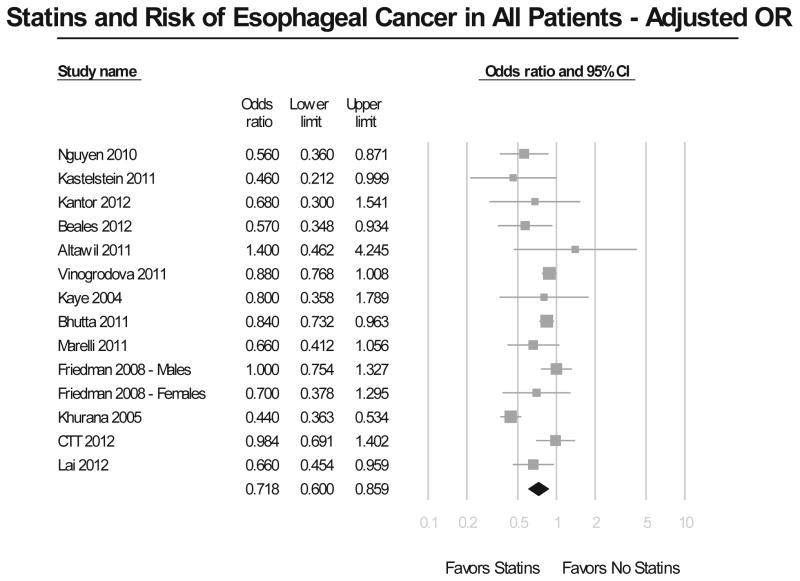
Meta-analysis of all studies assessing the risk of EC revealed that the use of statins was associated with a significant 26% reduction in EC incidence (Figure 2A). The results showed considerable heterogeneity across all studies, with a Cochran’s Q test p<0.01, and the corresponding I2=80%. There was no evidence of publication bias, both quantitatively (Begg and Mazumdar’s p=0.46), and qualitatively, on visual inspection of the funnel plot. This risk reduction in EC incidence with statin exposure persisted after adjusting for potential confounders (adjusted OR, 0.72; 95% CI, 0.60–0.86) (Figure 2B), even though the results till showed substantial heterogeneity (Cochran’s Q test p<0.01, I2=74%).
Figure 2.
Figure 2A. Summary of unadjusted odds ratios assessing the risk of esophageal cancer with statin exposure in all included studies.
Figure 2B. Summary of adjusted odds ratios assessing the risk of esophageal cancer with statin exposure in all included studies.
On restricting analysis to only 7 high-quality observational studies (Newcastle-Ottawa score ≥8),15–19, 38, 39 use of statins continued to show a significant protective effect against development of EC (adjusted OR, 0.70; 95% CI, 0.56–0.88). This analysis revealed only moderate heterogeneity (Cochran’s Q p=0.13, I2=40%).
Sensitivity Analysis
Given significant heterogeneity in meta-analysis of all included, we performed sensitivity analysis to better understand the source of heterogeneity (Table 2). The method of documenting statin exposure (pharmacy record, EMR or patient interviews) showed a non-significant trend towards explaining heterogeneity in studies. There was no significant difference in the subgroups based on study design, study location, setting or publication type.
Table 2.
Subgroup analysis of all studies
| Subgroups | No. of studies | Unadjusted OR | 95% CI | Adjusted OR | 95% CI | Heterogeneity between groups (p)* | |
|---|---|---|---|---|---|---|---|
| EC type | EAC + ESCC | 8 | 0.80 | 0.65–1.00 | 0.75 | 0.61–0.93 | 0.18 |
| EAC (patients with BE) | 5 | 0.57 | 0.44–0.75 | 0.59 | 0.45–0.78 | ||
| Study design | Case-control | 7 | 0.71 | 0.54–0.94 | 0.66 | 0.51–0.85 | 0.19 |
| Cohort | 5 | 0.76 | 0.60–1.00 | 0.79 | 0.62–1.02 | ||
| RCT (post-hoc analysis) | 1 | 0.98 | 0.69–1.40 | NR | NR | ||
| Publication type | Full text | 10 | 0.80 | 0.68–0.94 | 0.78 | 0.68–0.89 | 0.73 |
| Abstracts | 3 | NR | NR | 0.70 | 0.39–1.24 | ||
| Study location | USA | 6 | 0.67 | 0.48–0.92 | 0.68 | 0.48–0.95 | 0.30 |
| Europe | 5 | 0.85 | 0.70–1.03 | 0.82 | 0.72–0.93 | ||
| Study setting | Population-based | 8 | 0.76 | 0.61–0.95 | 0.71 | 0.57–0.88 | 0.82 |
| Hospital-based | 5 | 0.70 | 0.47–1.03 | 0.74 | 0.53–1.05 | ||
| Method of ascertainment to exposure | Pharmacy | 5 | 0.88 | 0.77–1.01 | 0.85 | 0.77–0.94 | 0.10 |
| EMR | 2 | 0.81 | 0.42–1.56 | 0.81 | 0.42–1.55 | ||
| Interview | 3 | 0.51 | 0.35–0.75 | 0.56 | 0.39–0.82 | ||
- for adjusted OR; EAC – Esophageal adenocarcinoma, ESCC – Esophageal squamous cell carcinoma, N/A – Not applicable, NR – Not reported
To assess whether any one study had a dominant effect on the summary OR, each study was excluded and its effect on the main summary estimate was evaluated. No study markedly affected the summary estimate or p-value for heterogeneity among the other summary estimates. Sufficient data were not available to perform stratified analyses based on age, gender, histologic type of EC or statin dose.
Risk of Esophageal Adenocarcinoma - Barrett’s Esophagus
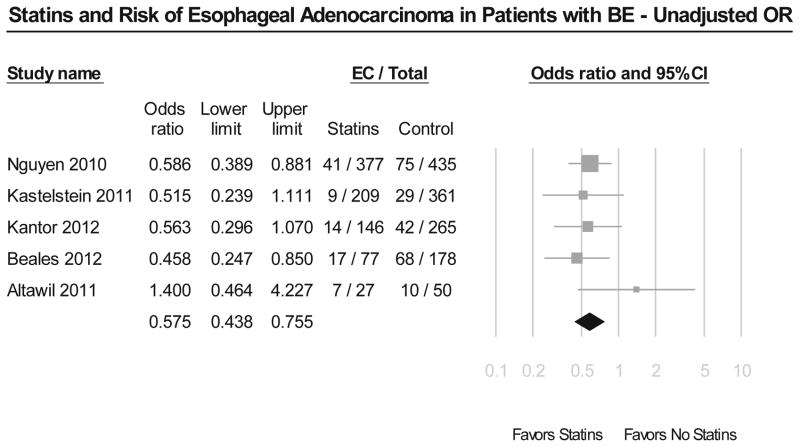
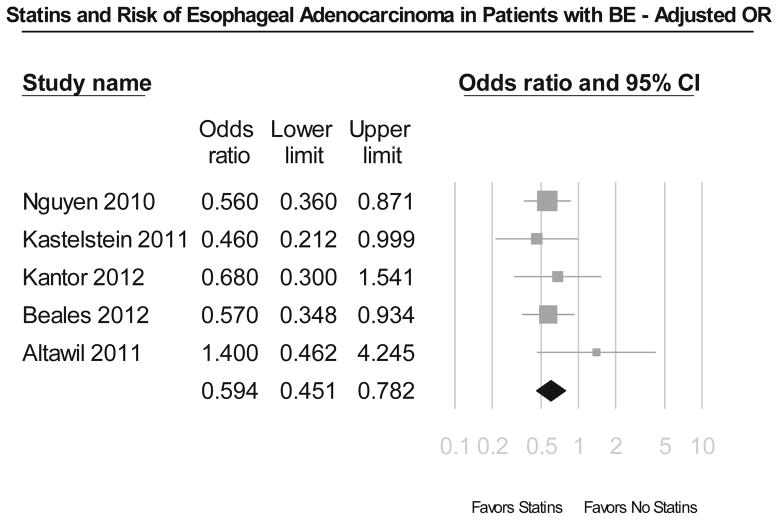
Meta-analysis of 5 studies in patients with BE revealed that use of statins was associated with a statistically significant 43% reduction in development of EAC and/or HGD (unadjusted OR, 0.57; 95% CI, 0.44–0.75) (Figure 3A). These results were very consistent across all included studies, with Cochran’s Q test p value 0.54, and the corresponding I2 statistic being 0%. There was no evidence of publication bias on visual inspection of the funnel plot. This remained after adjustment for potential confounders, including use of NSAIDs/aspirin and length and dysplasia status of BE (adjusted OR, 0.59; 95% CI, 0.45–0.78) (Figure 3B).
Figure 3.
Figure 3A. Summary of unadjusted odds ratios assessing the risk of esophageal adenocarcinoma and/or high grade dysplasia with statin exposure in patients with Barrett’s esophagus.
Figure 3B. Summary of adjusted odds ratios assessing the risk of esophageal adenocarcinoma and/or high grade dysplasia with statin exposure in patients with Barrett’s esophagus.
Subgroup Analysis
On pooled analysis of 2 studies, a greater protective effect against development of EAC with longer duration of statin use (>5 years) was noted (adjusted OR, 0.44; 95% CI, 0.24–0.78), as compared to shorter duration of statin use (<5 years) (adjusted OR, 0.55; 95% CI, 0.28–1.09), though this difference was not statistically significant.16, 17 Meta-analysis of the 2 studies that assessed the combined effect of statin and NSAID/aspirin use on the development of EAC in BE, demonstrated a marked 72% reduction in EAC incidence, which was higher than the use of either class of medications (adjusted OR, 0.28; 95% CI, 0.14–0.56).16, 17
Number Needed to Treat
Due to significant heterogeneity between studies, a single summary estimate for number needed to treat with statins to prevent one case of EC could not inferred. However, on restricting analysis to studies only on patients with BE, we could estimate the number needed to prevent one case of EAC. In a population-based study of patients with BE, the baseline histology at presentation showed no dysplasia in 73%, low-grade dysplasia in 13%, HGD in 3% and EAC in 9%.45 Patients with BE have reported rates of EAC of 6.3, 3.3, 16.9 and 65.8 per 1,000 patient years in all patients with BE, patients with non-dysplastic BE, BE with low-grade dysplasia and BE with high-grade dysplasia, respectively.31–33 In these groups, the number needed to treat with statins to prevent one case of EAC per year, would be 389, 741, 146 and 39, respectively, considering the 41% risk reduction with statin use after adjusting for potential confounders.
DISCUSSION
Identification of potential agents for chemoprevention in EC is highly desirable. Aspirin, NSAIDs and PPIs may have some potential chemopreventive effects in patients with BE but they are not without significant side effects.46, 47 In this comprehensive meta-analysis of all existing studies in more than 1.13 million patients with 9,285 cases of EC, we found that use of statins is associated with a 28% reduction in the risk of EC, after adjusting for confounding variables, though there was substantial methodological heterogeneity in studies. This effect is more pronounced and consistent in patients with BE, in which statin use is associated with a 41% reduction in risk of neoplastic progression, after adjusting for potential confounders, including use of NSAIDs/aspirin and baseline BE segment length and dysplasia grade. This chemopreventive effect of statins in patients with BE is comparable to the 32% reduction in the risk of EAC seen in patients with BE with aspirin use.46
The strengths of this analysis include the comprehensive and systematic literature search, consistency of association between statins and EAC in patients with BE between studies with different designs, its ability to partially evaluate the potential influence of measured confounders on the summary estimates and its evaluation of duration effect. The likelihood of important selection or publication bias in our meta-analysis is small. During the identification and selection process, we did not exclude any article because of methodological characteristics and when sufficient information was not available, we attempted to obtain unpublished results from the corresponding authors. Our results are consistent with a previous small systematic review48 Alexandre et al included only 2 studies in patients with BE and 3 studies in general population cohorts. Their estimates for risk were 0.53 (95% CI, 0.36–0.78), and 0.86 (95% CI, 0.78–0.94); respectively. 48. Likewise, in another meta-analysis by Kuoppala et al, only 1 study on the effect of statins on esophageal cancer was included.8 In our analysis, we added numerous additional studies, including data from randomized trials, to bring the evidence base up-to-date, and provide a more robust pooled estimate on the effect of statins on EC risk. With the larger number of studies, we were able to perform multiple subgroup analyses, evaluate heterogeneity and the presence of publication bias.
Antineoplastic Effects of Statins
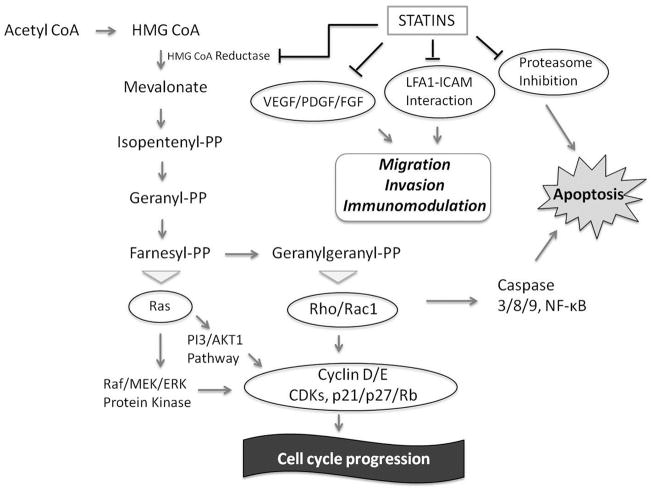
In vitro and animal studies have shown that statins exert antineoplastic effects through both HMG-CoA reductase-dependent and HMG-CoA reductase-independent pathways (Figure 4). The primary mechanism of action of statins on cholesterol reduction is by competitive inhibition of HMG-CoA reductase. This prevents post-translational prenylation of the Ras/Rho superfamily, which are important mediators of cell growth, differentiation and survival.10
Figure 4. Mechanism of anticancer effects of statins.
In vitro and animal studies have shown that statins exert antineoplastic effects through both HMG-CoA reductase-dependent and HMG-CoA reductase-independent pathways. The primary mechanism of action of statins on cholesterol reduction is by competitive inhibition of HMG-CoA reductase, the rate limiting step in cholesterol biosynthesis, blocking the conversion of HMG-CoA into mevalonate. Consequently, statins can inhibit several of the downstream products of the mevalonate pathway including the generation of isoprenoids. This prevents post-translational prenylation of small signaling G-proteins of the Ras/Rho/Rac superfamily, which are important mediators of cell growth, differentiation and survival.10 They also exert pro-apoptotic effects through regulation of Rho and RAF-mitogen activated protein kinase 1-extracellular regulated kinase (MEK-ERK) pathway through a HMG-CoA reductase dependent mechanism, by activating caspases and decreasing Bcl-2.52, 53 Statins inhibit the activation of the proteosome pathway, limiting the breakdown of cyclin-dependent kinase inhibitors p21 and p27, thus allowing these molecules to exert their growth-inhibitory effects.54 In addition, they also exert an anti-inflammatory and immunomodulatory effects, modifying the cell adhesion cascade through both HMG-CoA reductase-dependent and HMG-CoA reductase-independent effects.10
This anti-neoplastic effect of statins has also been demonstrated in human-derived EC cell lines through inhibition of Ras farnesylation, ERK and Akt signaling pathways as well as by inhibiting COX-2, which are expressed in both EAC and ESCC.11, 12, 14 Using three different EAC cell lines, Ogunwobi and colleagues have shown that statins inhibit cell proliferation and induce apoptosis in a concentration dependent manner, additive to inhibition of COX-2 with either NS-398 or celecoxib. Statins up-regulated the proapoptotic proteins Bad and Bax in these EAC cell lines.11 These studies suggest that statins may offer exciting potential as chemopreventive agents in EC, especially, BE.49 Unfortunately, there are no animal models that effectively mimic human disease in terms of risk factors, molecular pathogenesis and response to interventions.50
Dose and Duration of Statin Use
A meta-analysis of effects of different doses of statins was not possible due to limited information form individual studies. Data from the QResearch database as well as the General Practice Research Database, as well as from a cohort of patients with BE demonstrate that higher dose of statins (simvastatin ≥40mg or equivalent) may be more beneficial for both EAC and ESCC, than low dose statin (simvastatin <40mg per day).17, 42, 43 In the individual patient data analysis of 5 RCTs conducted by the CTT collaboration, there was no significant difference in incidence of EC with ‘more’ or ‘less’ statin use (EC incidence, ‘more’ v. ‘less’: 20/19829 v. 21/19783).40
In our analysis, we found that prolonged statin use conferred a greater protective effect of statins in patients with BE. While Nguyen et al15 reported that cumulative filled statin prescription for >12 months has a reduced risk of EAC compared to those with ≤ 12 month or those with no statin prescription, Beales et al17 reported that use of statins for more than 5 years was associated with a significantly lower incidence of EAC, as compared to 6 months-5 years of use [adjusted OR (95% CI) for statin use >5 yrs v. 2–5 yrs v. 6 months–2 yrs: 0.41 (0.15–0.0.85) v. 0.58 (0.27–1.43) v. 0.77 (0.29–1.87)]. Kastelstein et al and Friedman et al, however, did not find a significant difference in the effect of statins when used for more than 5 years or less.16, 35 Using time-varying analysis in the large QResearch database, Cox et al demonstrated that the reduction in risk of EC was apparent one to three years after starting statins and persisted during the first five years of treatment, and in women the risk returned to normal within the first year after stopping treatment and in men one to three years after stopping treatment.42
Differences in Observational Studies and Clinical Trials
The chemopreventive effect of statins was seen primarily in observational studies which accounted for a large majority of the included EC cases (9,162 cases, 98.7%). RCTs included in the study did not demonstrate any significant chemopreventive effect of statins, though these accounted for a small minority of the included EC cases (123 cases, 1.3%). Importantly, the clinical trials included in the meta-analysis represented post-hoc analysis of 22 RCTs performed on the effect of statins on cardiovascular mortality. By design, the patients enrolled in these RCTs were at low risk for development of EC. Also, given the small number of cases of EC, the studies were not adequately powered to detect a significant difference in the placebo and statin group with regard to development of EC. Moreover, since the occurrence of EC was not the primary objective of these trials so patients were not routinely screened for development of EC; this might have affected the detection rate of EC. The follow-up duration in these RCTs was short. These factors may explain why current clinical trials of statins for prevention of cardiovascular mortality do not demonstrate a chemopreventive effect of statins against EC.
On the other hand, the chemopreventive effect of statins seen in observational studies may also represent an over-estimate its true effect. Observational studies lack the experimental random allocation of the intervention necessary to test exposure-outcome hypotheses optimally. Despite adjusting for numerous covariates, it is not possible to eliminate the potential of residual confounding. It is possible that the observed decreased risk of EC seen in statin users may relate to a healthy user bias.51 Healthier patients taking statins may be more likely to be prescribed preventive medications and/or be more compliant with preventive health measures, as compared to patients not taking statins. The latter poorly compliant patients may have other unhealthy lifestyle practices predisposing them to EC. However, given the strength and consistency of association especially in patients with BE, along with experimental and in vitro data that suggests biological plausibility of the beneficial effects of statins in preventing EAC, suggests that this is unlikely to be primary driver of results. Observational studies are inherently not able to definitely establish when the intervention may exert its influence, the minimum effective dose/duration and frequency of medication intake is required to achieve a benefit. Although we found a potential duration benefit, differing exposure definitions between studies make recommendations regarding dosing regimens problematic.
Limitations
The included studies did not provide data based on histological subset of EC. It is well known that EAC and ESCC have differing risk factors, and different pathogenesis. Data from current studies are limited to infer a protective association between statin use and development of ESCC. In a study from UK using the General Practice Research Database, Bhutta et al showed an inverse association between statin use and development of ESCC (OR, 0.41; 95% CI, 0.21–0.80).43 Additionally, all studies did not adjust for the same confounders. They generally failed to account for one or more of the following risk factors for EC: presence of BE including length of segment and presence of baseline dysplasia, concomitant use of NSAIDs/aspirin or PPIs, obesity and smoking status. However, using the adjusted data for any use of statins had little effect on the summary OR, suggesting that any difference attributable to using different confounders for adjustment is likely small. Another potential limitation that particularly applies to case-control studies evaluating EC is recall bias. However, in most studies pharmacy drug prescriptions information was used, and hence, the effects of this are likely minimal.
Implications for Practice
In patients with multiple risk factors for EAC (e.g., obese men with known long-segment, non-dysplastic BE or BE with low-grade dysplasia), statins may have a chemo-protective effect on dysplasia progression and development of EAC. Additionally, given significant potential benefit of statins in reducing risk of development of EAC in patients with BE with HGD, future prospective studies may assess whether statin use in these patients after therapeutic endoscopic intervention may prevent recurrence.
Conclusions
In summary, meta-analysis of all studies suggest that statin use is associated with a reduced risk of EC, with greater and most consistent benefit on the risk of EAC in patients with known BE. Longer duration of statin use, in combination with aspirin/NSAIDs may provide greater protective effect. Given the high mortality rates after a diagnosis of EC, these results support chemoprevention trials evaluating statins in populations at high risk of developing EAC.
Acknowledgments
We sincerely thank Dr. Andrew Hart for sharing additional information from their original study for the purposes of this meta-analysis.
Footnotes
Conflict of Interest: None
Author Contributions: S.S. and P.G.I. were involved in study concept and design; S.S., A.G.S. and P.S. were involved in acquisition of data; S.S., M.H.M. and P.G.I. were involved statistical analysis and interpretation of data; S.S. and P.G.I. were involved in drafting of the manuscript; A.G.S., P.S., and M.H.M. were involved critical revision of the manuscript for important intellectual content
Disclosures: Supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (RC4DK090413) and the American College of Gastroenterology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P, Negri E, La Vecchia C. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122:1118–29. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer. 2009;101:855–9. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–56. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 5.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 6.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–86. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24:4808–17. doi: 10.1200/JCO.2006.06.3560. [DOI] [PubMed] [Google Scholar]
- 8.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. European journal of cancer. 2008;44:2122–32. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins Are Associated With a Reduced Risk of Hepatocellular Cancer: A Systematic Review and Meta-analysis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.10.005. S0016-5085(12)01496-5 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 11.Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. American Journal of Gastroenterology. 2008;103:825–37. doi: 10.1111/j.1572-0241.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- 12.Konturek PC, Burnat G, Hahn EG. Inhibition of Barret’s adenocarcinoma cell growth by simvastatin: involvement of COX-2 and apoptosis-related proteins. J Physiol Pharmacol. 2007;58 (Suppl 3):141–8. [PubMed] [Google Scholar]
- 13.Sadaria MR, Reppert AE, Yu JA, Meng X, Fullerton DA, Reece TB, Weyant MJ. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg. 2011;142:1152–60. doi: 10.1016/j.jtcvs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Ye F, Zhang GH, Guan BX, Xu XC. Suppression of esophageal cancer cell growth using curcumin, (−)-epigallocatechin-3-gallate and lovastatin. World J Gastroenterol. 2012;18:126–35. doi: 10.3748/wjg.v18.i2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260–6. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology. 2011;141:2000–8. doi: 10.1053/j.gastro.2011.08.036. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 17.Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett’s oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2012 doi: 10.1097/MEG.0b013e3283543f01. [DOI] [PubMed] [Google Scholar]
- 18.Kantor ED, Onstad L, Blount PL, Reid BJ, Vaughan TL. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2012;21:456–61. doi: 10.1158/1055-9965.EPI-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinogradova Y, Coupland C, Hippisley-Cox J. Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer. 2011;11:409. doi: 10.1186/1471-2407-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPTGSe. Cochrane Handbook for Systematic Reviews of Interventions [updated March 2011] The Cochrane Collaboration; 2011. [Google Scholar]
- 21.Kanwal F, White D. “Systematic Reviews and Meta-analyses” in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–6. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Wells GASB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012 [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet. 1991;338:1127–30. doi: 10.1016/0140-6736(91)91975-z. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 29.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 30.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 31.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235–44. doi: 10.1016/j.cgh.2009.10.010. quiz e32. [DOI] [PubMed] [Google Scholar]
- 32.Wani S, Puli SR, Shaheen NJ, Westhoff B, Slehria S, Bansal A, Rastogi A, Sayana H, Sharma P. Esophageal adenocarcinoma in Barrett’s esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. American Journal of Gastroenterology. 2009;104:502–13. doi: 10.1038/ajg.2008.31. [DOI] [PubMed] [Google Scholar]
- 33.Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–6. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 34.Bhutta HY, Clark A, Holt S, Lewis MP, Hart A. Oesophageal Cancer - An Aetiological Investigation Into the Potential Protective Effect of Statins in the UK General Practice Research Database (GPRD) Gastroenterology. 2011;140:S166–S166. [Google Scholar]
- 35.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 36.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635–7. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khurana V, Chalasani R, Caldito G, Fort C. Statins reduce the incidence of esophageal cancer: a study of half a million US veterans. Gastroenterology. 2005;128:A93–A93. [Google Scholar]
- 38.Marelli C, Gunnarsson C, Ross S, Haas S, Stroup DF, Cload P, Clopton P, DeMaria AN. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol. 2011;58:530–7. doi: 10.1016/j.jacc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Altawil J, Irwin B, Jinjuvadia R, Antaki F. Statins Might Not Prevent Progression to Dysplasia and Cancer in Barrett’s Esophagus. American Journal of Gastroenterology. 2011;106:S32–S32. [Google Scholar]
- 40.Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, Bhala N, Holland L, Peto R, Keech A, Collins R, Simes J, Baigent C. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai SW, Liao KF, Lai HC, Muo CH, Sung FC. Atorvastatin correlates with decreased risk of esophageal cancer: a population-based case-control study from Taiwan. Libyan J Med. 2012:7. doi: 10.3402/ljm.v7i0.18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhutta HY, Alexandre L, Clark A, Holt S, Lewis MP, Hart A. Mo1542 Do Statins Prevent the Histological Subtypes of Esophageal Cancer? Prospective Data From the UK General Practice Research Database (GPRD) Gastroenterology. 2012;142:S-624. [Google Scholar]
- 44.Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:1299–304. doi: 10.1016/j.cgh.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu TT, Wang KK, Frederickson M, Geno DM, Locke GR, Prasad GA. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. American Journal of Gastroenterology. 2011;106:1447–55. doi: 10.1038/ajg.2011.130. quiz 1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao LM, Vaughan TL, Corley DA, Cook MB, Casson AG, Kamangar F, Abnet CC, Risch HA, Giffen C, Freedman ND, Chow WH, Sadeghi S, Pandeya N, Whiteman DC, Murray LJ, Bernstein L, Gammon MD, Wu AH. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–452. e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 48.Alexandre L, Clark AB, Cheong E, Lewis MP, Hart AR. Systematic review: potential preventive effects of statins against oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2012 doi: 10.1111/j.1365-2036.2012.05194.x. [DOI] [PubMed] [Google Scholar]
- 49.Fang D, Das KM, Cao W, Malhotra U, Triadafilopoulos G, Najarian RM, Hardie LJ, Lightdale CJ, Beales IL, Felix VN, Schneider PM, Bellizzi AM. Barrett’s esophagus: progression to adenocarcinoma and markers. Ann N Y Acad Sci. 2011;1232:210–29. doi: 10.1111/j.1749-6632.2011.06053.x. [DOI] [PubMed] [Google Scholar]
- 50.Hawk ET, Viner JL. Statins in esophageal cancer cell lines: promising lead? American Journal of Gastroenterology. 2008;103:838–41. doi: 10.1111/j.1572-0241.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 51.Patrick AR, Shrank WH, Glynn RJ, Solomon DH, Dormuth CR, Avorn J, Cadarette SM, Mogun H, Brookhart MA. The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Health. 2011;14:513–20. doi: 10.1016/j.jval.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, Denner L. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer research. 1998;58:76–83. [PubMed] [Google Scholar]
- 53.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer research. 2004;64:6461–8. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 54.Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7797–802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]