Abstract
Excessive concentrations of oxidized phospholipids (OxPL), the products of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine (PAPC) oxidation have been detected in atherosclerosis, septic inflammation, and ALI; and have been shown to induce vascular barrier dysfunction. In contrast, oxidized PAPC (OxPAPC) at low concentrations exhibit potent barrier protective effects. The nature of such biphasic effects remains unclear. We tested the hypothesis that barrier-disruptive effects of high OxPAPC doses on endothelial cell (EC) monolayer are defined by fragmented products of PAPC oxidation (lyso-PC, POVPC, PGPC), while barrier enhancing effects are mediated by full length oxidated PAPC products and may be reproduced by single compounds contained in the OxPAPC such as PEIPC. All three fragmented OxPAPC products increased EC permeability in a dose-dependent manner, while PEIPC decreased it and reversed barrier disruptive effects of lyso-PC, POVPC and PGPC monitored by measurements of transendothelial electrical resistance. Immunofluorescence staining and western blot analysis showed that PGPC mimicked the cytoskeletal remodeling and tyrosine phosphorylation of adherens junction (AJ) protein VE-cadherin leading to EC barrier dysfunction induced by high OxPAPC concentrations. Barrier-disruptive effects of PGPC were abrogated by ROS inhibitor, N-acetyl cysteine, or Src kinase inhibitor, PP-2. The results of this study show that barrier disruptive effects of fragmented OxPAPC constituents (lyso-PC, POVPC, PGPC) are balanced by barrier enhancing effects of full length oxygenated products (PEIPC). These data strongly suggest that barrier disruptive effects of OxPAPC at higher concentrations are dictated by predominant effects of fragmented phospholipids such as PGPC, which promote ROS-dependent activation of Src kinase and VE-cadherin phosphorylation at Tyr658 and Tyr731 leading to disruption of endothelial cell AJs.
Keywords: endothelium, oxidized phospholipids, vascular permeability, cytoskeleton, VE-cadherin
Introduction
Elevated circulating levels of oxidized phospholipids (OxPL) have been found in a variety of pathological conditions accompanied by oxidative stress including atherosclerosis, autoimmune diseases, lung injury and sepsis (1). Lipid oxidation in acute respiratory distress syndrome (ARDS) is manifested by elevated concentrations of lipid peroxidation products, such as 4-hydroxy-2-nonenal and 8-isoprostane (2). Exhaled 8-isoprostane has been even used as biomarker and diagnostic tool for evaluation of oxidative stress in patients with COPD, lung injury and ARDS (3, 4). Generation of OxPL by oxidation of arachidonic acid-containing membrane phospholipids occurs upon inflammatory activation of various cell types including myeloid cells (neutrophils and monocytes), epithelium, fibroblasts, vascular endothelium. Inflammatory events lead to formation of hydroperoxides and accumulation of fragmented and full length products of cell membrane phospholipid oxidation such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-phosphatidylcholine (PGPC), lysophosphatidyl choline (lyso-PC), 1-palmitoyl-2-(5, 6-epoxyisoprostane E2)-sn-glycero-3-phsphocholine (PEIPC) and others (5, 6).
Increased levels of OxPL present in the injured lung significantly influence pulmonary endothelial cell (EC) functions including inflammatory responses and barrier regulation (7–10). However, in vitro and in vivo studies demonstrate opposite effects of high and low OxPL doses on vascular permeability and lung injury, which remain an intriguing question in vascular biology, and further understanding of this phenomenon is of great importance.
Previous reports demonstrate that 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidyl choline (PAPC) oxidized in vitro induced a pronounced barrier enhancing response in vascular endothelium when present in low concentrations (5–20 µg/ml) in either pulmonary or systemic circulation (11). In addition, these OxPAPC doses attenuated agonist-induced EC permeability and prevented pulmonary vascular leak caused by mechanical ventilation at high tidal volume (9, 11, 12). These effects were due to OxPAPC-induced stimulation of small GTPases Rac1 and Rap1, which promoted enhancement of peripheral actin cytoskeleton and EC junctions (11, 13). In addition, low OxPAPC doses stimulated membrane translocation of negative regulator of Rho signaling, p190RhoGAP, which suppressed Rho pathway of vascular permeability (14). In turn, higher OxPAPC doses exhibit opposite effects and cause rapid endothelial monolayer barrier disruption (11, 12). The nature of contrasting biological activities of high and low OxPAPC concentrations and dose-dependent regulation of vascular endothelial barrier properties remains unclear, however diversity of compounds generated during non-enzymatic oxidation of PAPC may be an important factor defining EC permeability response.
This study used purified compounds detected in OxPAPC to test the hypothesis that barrier disruptive effects and signaling mechanisms triggered in pulmonary endothelium by high OxPAPC concentrations are dictated by increasing concentrations of fragmented products of PAPC oxidation (POVPC, PGPC, lyso-PC), while barrier enhancing effects are driven by full length PAPC oxygenation products such as PEIPC. These studies suggest important implications of the pathology-specific OxPL composition in the modulation of vascular pathologies by oxidized phospholipids.
Materials and Methods
Cell culture and reagents
Human pulmonary artery endothelial cells (HPAECs) were obtained from Lonza (Allendale, NJ) and used at passages 5–8. All experiments were performed in EGM growth medium (Lonza) containing 2% fetal bovine serum unless otherwise specified. Texas Red-conjugated phalloidin and Alexa Fluor 488-labeled secondary antibodies were purchased form Molecular Probes (Eugene, OR). Antibodies to phospho-VE-cadherin were purchased from Invitrogen (Carlsbad, CA), and VE-cadherin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Diphospho-MLC and β-tubulin antibodies, rabbit anti-human VEGFR2 antibody, HRP-linked anti-mouse and anti-rabbit IgG were obtained from Cell Signaling (Beverly, MA). PP2 inhibitor (4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) was purchased from Calbiochem (La Jolla, CA). Unless otherwise specified, all biochemical reagents were obtained from Sigma (St. Louis, MO). 1-Palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidyl choline (PAPC), 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphatidylcholine (POVPC), 1-palmitoyl-2-glutaroyl-sn-glycero-phosphatidylcholine (PGPC) and lysophosphatidyl choline (lyso-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Oxidation of PAPC was performed by exposure of dry lipid to air as previously described (15). The extent of oxidation was measured by positive ion electrospray mass spectrometry (ESI-MS) as previously described (15). 1-Palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phsphocholine (PEIPC) was synthesized as previously described (16). After completion of oxidation, the phospholipids were stored at −70°C dissolved in chloroform and were used within 2 weeks of MS analysis.
Measurement of transendothelial electrical resistance
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance (TER) across confluent endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as previously described (11, 12).
Western Blot
Protein extracts were subjected to SDS-polyacrylamide gel electrophoresis, transferred to PVDF membrane, and probed with antibodies of interest, as previously described (17).
Surface protein biotinylation
Cells were treated with 20 µg/ml PGPC or 2 µg/ml PEIPC for 30 min, washed with PBS at 37°C and incubated for 10 min with 5 mM Sulfo-NHS-SS-Biotin (Pierce Biotechnology, Rockford, IL) at RT. Subsequently, cells were washed two times with ice-cold PBS with 100mM glycine, lysed for 30 min on ice in 1% Triton-100 PBS and cell lysate centrifuged at 10,000 × g for 10 min at 4°C. Equal amounts of cell lysates were incubated with 60 µL of Streptavidin-agarose (Pierce Biotechnology, Rockford, IL) for 1 hr at 4°C. Beads were washed three times with ice-cold PBS and boiled in LDS sample buffer with 5% 2-mercaptoethanol. Samples were centrifuged for 1 min at 1,000 × g and supernatants were subjected to western blot analysis with VE-cadherin antibody.
Immunofluorescence microscopy
HPAECs grown to 95–100% confluence on gelatinized coverslips were stimulated with oxidized phospholipids followed by immunofluorescence staining as previously described (11). Texas Red conjugated Phalloidin (Molecular Probes) was used to visualize filamentous actin. Analysis of immunofluorescence staining was performed using an Olympus IX71 microscope with × 60 objective lens (Tokyo, Japan).
Statistical analysis
The results are expressed as means ± SD of 3 to 5 independent experiments. Stimulated samples were compared with the controls using the unpaired Student t-test. For multiple-group comparisons, a 1-way variance analysis and post hoc multiple comparisons tests were used. P < 0.05 was considered statistically significant.
Results
Dose dependent effects of fragmented and full length products of PAPC oxidation on endothelial permeability
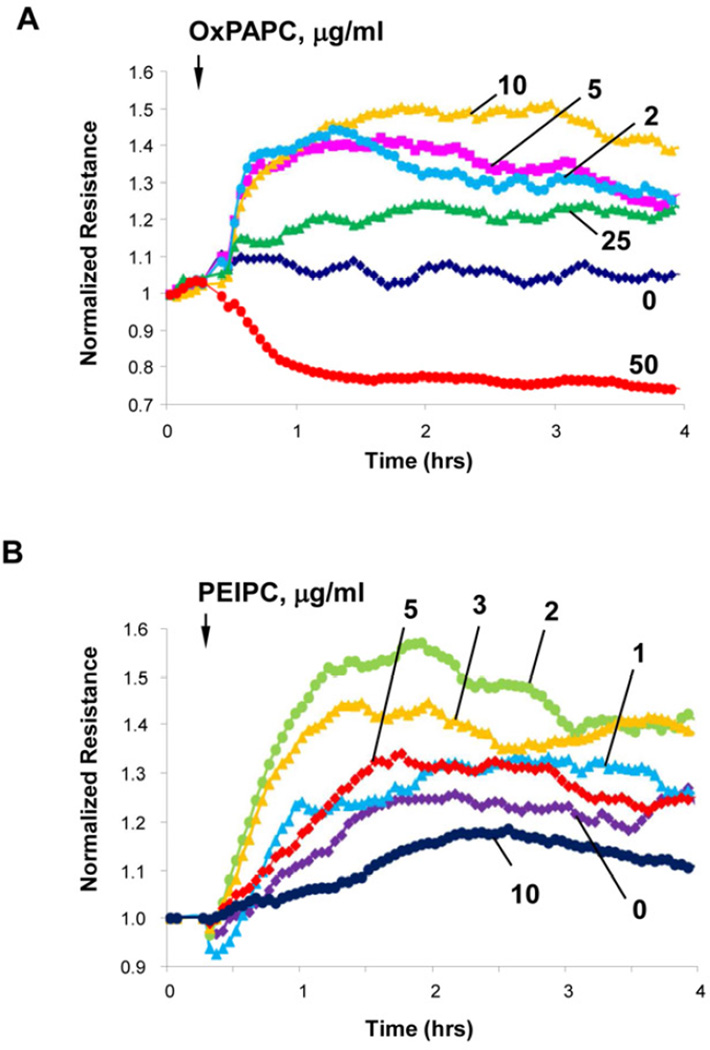
Stimulation of EC monolayers with high or low OxPAPC doses induces a differential permeability response with barrier enhancement observed at low OxPAPC concentration (2–25 µg/ml) and barrier disruption observed at higher concentrations (Figure 1A). The disparate effects of OxPAPC at different doses on TER were also associated with increases in cortical actin cytoskeleton and dramatic enhancement of continuous line of VE-cadherin positive adherens junctions in EC stimulated with low OxPAPC dose (10 µg/ml) previously reported by our group (18). These effects were in sharp contrast with disappearance of VE-cadherin from cell junctions and formation of intercellular gaps observed in EC stimulated with high OxPAPC dose (100 µg/ml) (18). To test whether such biphasic effect is due to complex composition of OxPAPC preparation, we studied effects of single purified OxPL molecules present in the OxPAPC: PEIPC, POVPC, PGPC, and lyso-PC (6). PEIPC causes a dose-dependent and sustained increase in TER reflecting EC monolayer barrier enhancement which reached maximal levels at 1–3 µg/ml PEIPC (the estimated PEIPC content in the barrier protective OxPAPC doses) and declined at higher PEIPC concentrations (Figure 1B). In contrast, fragmented products of PAPC oxidation, PGPC, lyso-PC and POVPC, caused monophasic dose dependent TER decline reflecting increased EC permeability, which was observed at the 5–100 µg/ml concentration range (Figure 2ABC).
Figure 1. Dose-dependent effects of OxPAPC and PEIPC on transendothelial electrical resistance.
Human pulmonary artery endothelial cells (HPAECs) were seeded in polycarbonate wells with gold microelectrodes. After 24 hr of culture, HPAEC were stimulated with various concentrations of: A - OxPAPC (2, 5, 10, 25 and 50 µg/ml); or B - PEIPC (1, 2, 3, 5 and 10 µg/ml), and measurements of transendothelial electrical resistance (TER) were monitored over 4 hrs using an electrical cell-substrate impedance sensing system (ECIS). Results are representative of five independent experiments.
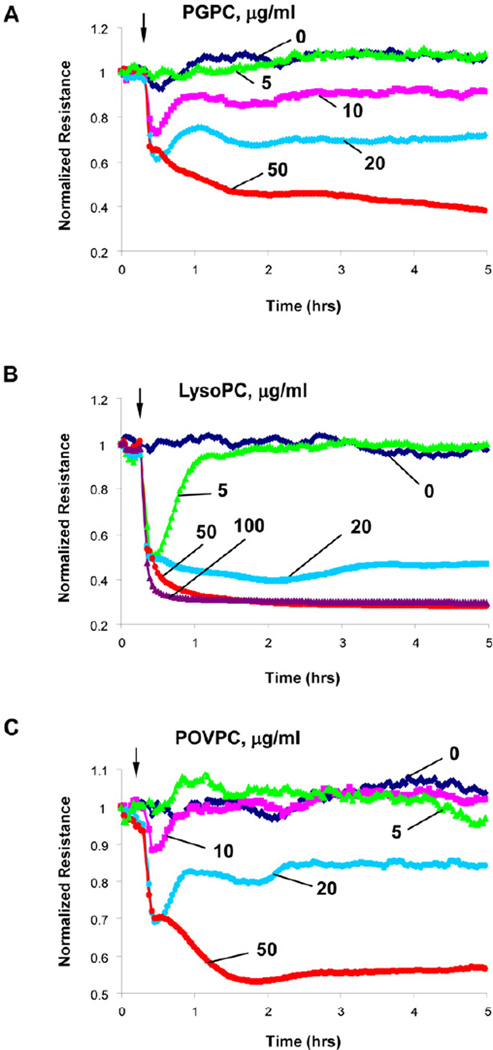
Figure 2. Dose-dependent effects of PGPC, lyso-PC and POVPC on transendothelial electrical resistance.
HPAECs seeded in polycarbonate wells with gold microelectrodes were stimulated with various concentrations of: A – PGPC; B – lyso-PC; and B – POVPC, and measurements of transendothelial electrical resistance (TER) were monitored over 5 hrs using an electrical cell-substrate impedance sensing system (ECIS). Results are representative of five independent experiments.
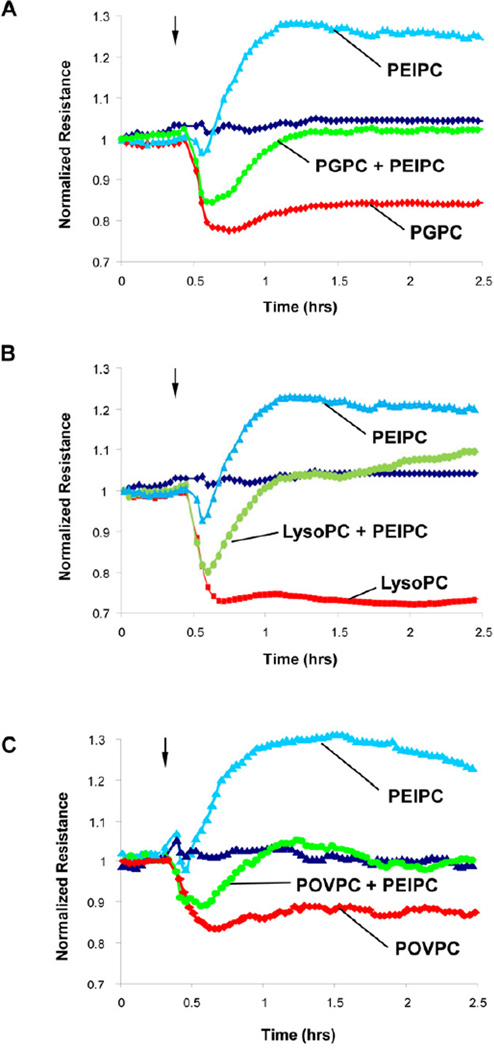
EC barrier dysfunction caused by fragmented phospholipids can be rescued by PEIPC
Co-treatment of EC monolayers with combination of PGPC, lyso-PC or POVPC (20 µg/ml) and PEIPC (2 µg/ml) reversed TER decline observed in ECs treated with each fragmented phospholipid alone (Figure 3ABC). These data demonstrate antagonistic effects of fragmented and full length products of PAPC oxidation when added in combination. Results in Figures 2 and 3 demonstrate similar dose-dependent effects of three fragmented products of PAPC oxidation. Preliminary immunofluorescence analysis also showed similar patterns of VE-cadherin dissolution from cell junction areas upon EC treatment with lyso-PC, POVPC or PGPC (data not shown). Since published studies did not reveal significant functional differences between these compounds in other cell systems, we focused on PGPC as representative member of this group. The effects of EC treatment with PEIPC and PGPC on intracellular localization of VE-cadherin, a key protein of adherens junctions involved in regulation of endothelial barrier function, were tested in the following studies.
Figure 3. Effects of PEIPC cotreatment with PGPC, lyso-PC and POVPC on transendothelial electrical resistance.
HPAECs seeded in polycarbonate wells with gold microelectrodes were stimulated with PEIPC (2 µg/ml) alone or were co-stimulated with PEIPC and: A – PGPC (20 µg/ml); B – lyso-PC (20 µg/ml); and B – POVPC (20 µg/ml), and measurements of transendothelial electrical resistance (TER) were monitored over 5 hrs using an electrical cell-substrate impedance sensing system (ECIS). Results are representative of three independent experiments.
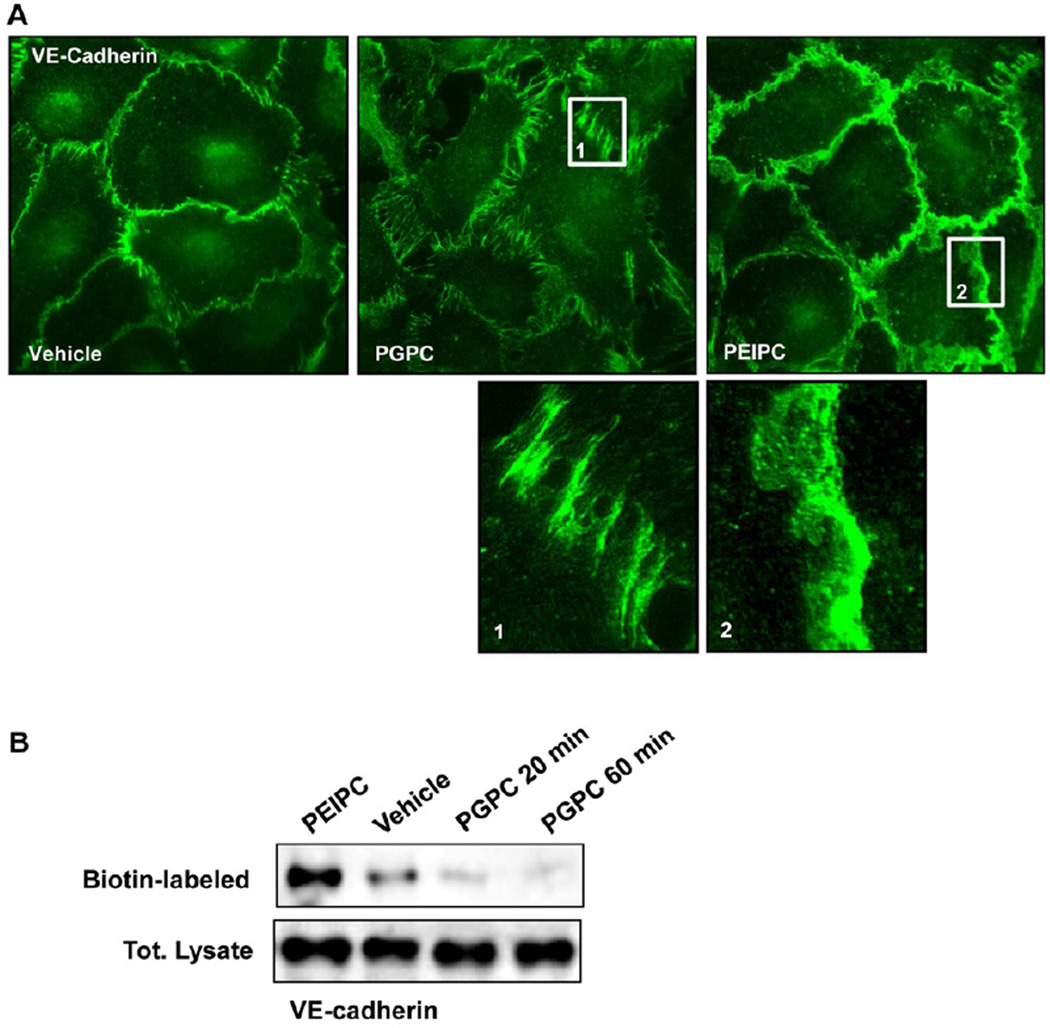
Effects of PEIPC and PGPC on VE-cadherin localization and surface expression
Immunofluorecence staining of adherens junction protein VE-cadherin in HPAEC monolayers treated with PEIPC and PGPC showed distinct patterns of AJ remodeling. We observed increased VE-cadherin positive areas at the cell-cell junctions of PEIPC-treated cells depicting enhanced AJ. In contrast, PGPC induced significant reduction of VE-cadherin immunoreactivity at cell-cell junctions (Figure 4A). Enlarged images demonstrate a discontinuous pattern of VE-cadherin distribution at cell junction areas of PGPC-treated cells and large continuous area of VE-cadherin immunoreactivity at cell-cell junctions of PEIPC treated cells (Figure 4A, insets 1, 2).
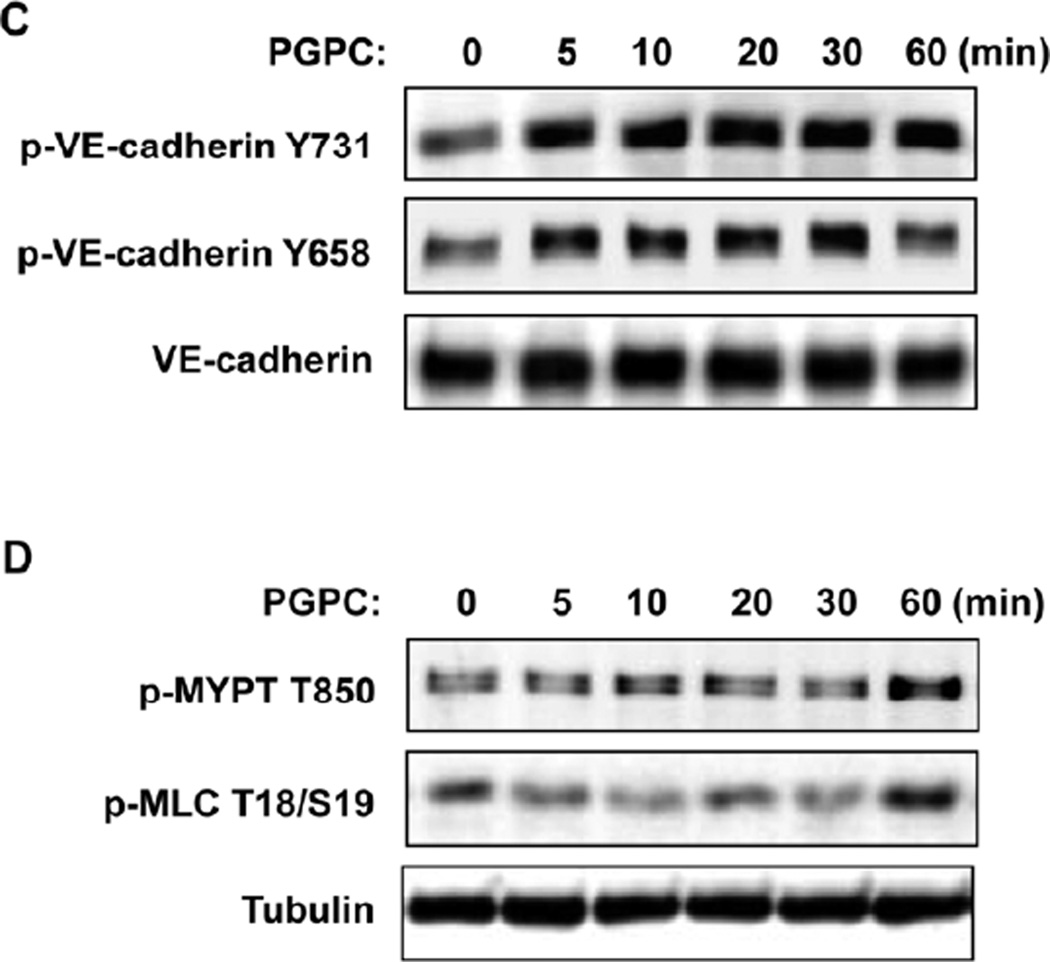
Figure 4. Effects of PGPC and PEIPC on intracellular distribution and phosphorylation of VE-cadherin and downstream Rho targets.
A – Endothelial monolayers grown on glass coverslips were stimulated with PGPC (30 µg/ml, 30 min) or PEIPC (2 µg/ml, 30 min) followed by immunofluorescence staining for VE-cadherin. Insets depict higher magnification areas of VE-cadherin immunoreactivity at cell-cell junctions of PGPC and PEIPC treated endothelial monolayers. Shown are representative results of three independent experiments. B - HPAECs were stimulated with PEIPC or PGPC for indicated periods of time, washed, and cell surface proteins were labeled with Sulfo-NHS-SS-Biotin as described in Methods. Cells were lysed, and biotinylated proteins precipitated with streptavidin-agarose. Presence of biotinylated VE-cadherin was evaluated by Western blot analysis. C - EC were stimulated with PGPC (30 µg/ml) for indicated periods of time, and VE-cadherin phosphorylation at Tyr731 and Tyr658 was examined by western blot. Staining with VE-cadherin antibody was used as normalization control. D - Time course of myosin light chain phosphatase (MYPT) and myosin light chain (MLC) phosphorylation induced by PGPC was examined by western blot with corresponding phospho-specific antibodies. µ-Tubulin antibody was used as normalization control.
PGPC-induced disappearance of VE-cadherin from AJ was further verified by a surface protein biotinylation assay as described in Methods section. After in situ biotinylation of cell surface proteins in control and PEIPC or PGPC stimulated cells, the level of biotinylated VE-cadherin was assessed by Western blot. PGPC caused disappearance of VE-cadherin from cell surface, while VE-cadherin surface expression was increased in PEIPC-treated endothelium (Figure 4B). These findings are consistent with differential effects of PEIPC and PGPC on EC morphological changes and monolayer barrier function.
Effects of PGPC on VE-cadherin phosphorylation and activation of Rho pathway
VE-cadherin is a key protein regulating endothelial cell-cell interactions and EC barrier function. Tyrosine phosphorylation at Tyr658 and Tyr731 impairs VE-cadherin activity towards formation of AJ (19). We next examined tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 in cells treated with PGPC. EC treatment with 20 µg/ml PGPC caused rapid and sustained VE-cadherin phosphorylation at both sites (Figure 4C).
Endothelial monolayer gap formation often results from the activation of myosin light chain (MLC) phosphorylation which triggers actomyosin contraction and disruption of cell-cell links (20). RhoA GTPase signaling plays a pivotal role in the induction of MLC phosphorylation in pulmonary endothelium via phosphorylation and inactivation of myosin phosphatase (MYPT) by Rho associated kinase (21–23). Western blot analysis of site-specific phosphorylation of MLC and Rho-kinase mediated phosphorylation of MYPT showed that basal MLC and MYPT phosphorylation levels observed in untreated cells were not affected for up to 30 min post PGPC treatment and slightly increased at 60 min (Figure 4D).
Role of ROS and Src in adherens junction disassembly and EC permeability caused by PGPC
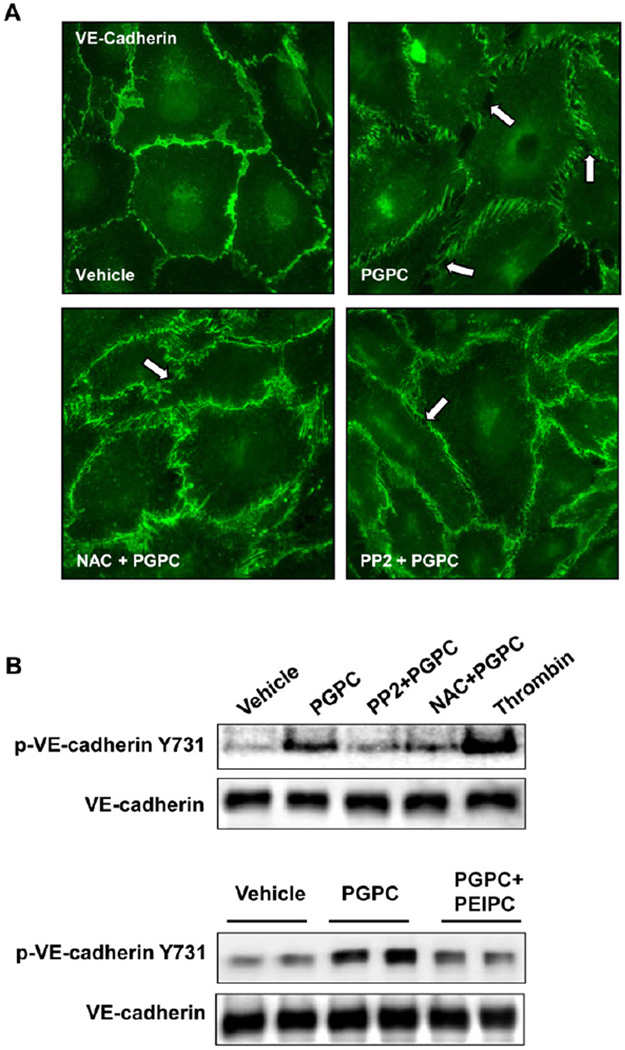
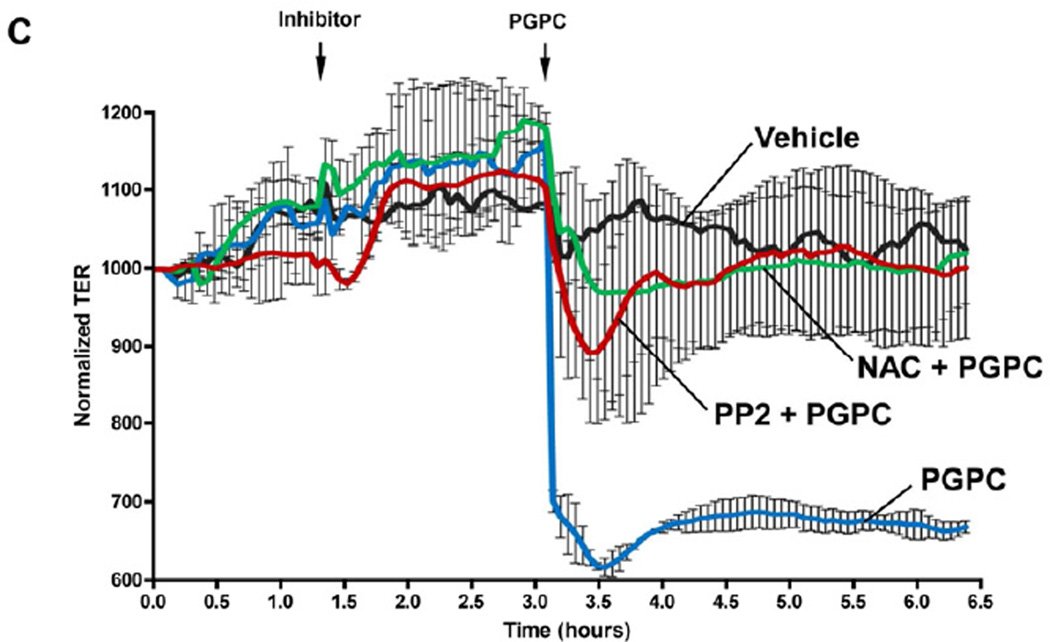
Previous study of barrier disruptive mechanisms triggered by high doses of OxPAPC showed activation of ROS signaling, which induced activation of Src and Src-dependent phosphorylation of VE-cadherin and led to disassembly of endothelial AJ (18). We further tested whether these effects of high OxPAPC doses can be reproduced by PGPC treatment. HPAEC pretreatment with Src kinase inhibitor PP2 (1µM) or antioxidant agent, N-acetyl cysteine (NAC, 1mM), prevented PGPC-induced disruption of continuous VE-cadherin-positive adherens junctions (Figure 5A) and abolished PGPC-induced VE-cadherin phosphorylation at Tyr731 (Figure 5B). Interestingly, co-treatment with PEIPC attenuated PGPC-induced VE-cadherin phosphorylation at Y731, which is consistent with attenuation of PGPC-induced TER decline by PEIPC shown in Figure 2. Preincubation with 1µM PP2 and 1mM NAC markedly attenuated a drop in transendothelial resistance caused by 20 µg/ml of PGPC (Figure 5C). These results suggest that ROS signaling and Src kinase activation play a key role in EC barrier dysfunction and AJ remodeling caused by PGPC. In addition, these data suggest that potential inhibitory effects of barrier protective OxPAPC compounds (or total OxPAPC at low doses) on adherens junction destabilization induced by fragmented phospholipids may involve inhibition of ROS signaling. Further studies are warranted to further test this mechanism.
Figure 5. Effects of SRC and ROS inhibitors on remodeling of VE-cadherin-positive adherens junctions, VE-cadherin phosphorylation and EC permeability induced by PGPC.
HPAECs were pretreated with vehicle, PP2 (1µM), or NAC (1mM) for 30 min followed by stimulation with 30 µg/ml PGPC. A – Immunofluorescence staining for VE-cadherin was performed after 15 min of PGPC stimulation. Arrows depict areas of intercellular gaps. Shown are representative results of three independent experiments. B – The levels of VE-cadherin phosphorylated at Tyr731 and Y658 were detected by Western blot analysis and normalized to the total VE-cadherin content. Lower panels depict effect of co-treatment with PEIPC (2 µg/ml) on PGPC-induced VE-cadherin phosphorylation at Y731. C – TER measurements were monitored over 6.5 hrs. Time points of inhibitor and PGPC addition are indicated by vertical arrows. Shown are pooled data from three independent experiments expressed as mean ± SD.
Discussion
The complex role of OxPLs in modulation of inflammation and vascular dysfunction observed in various pathologies including atherosclerosis, sepsis and ARDS is well-recognized (7–10, 24, 25). These OxPL features are due to the complexity of OxPL-activated signaling mechanisms predominant in a given particular pathologic setting (26). In many cases, OxPLs exhibit pathological effects when accumulated chronically at high concentrations, for example at sites of atherosclerosis in the range of 10–100 µM/kg (15, 25), or when added exogenously at exceedingly high concentrations in experimental models (7).
Analysis of dose-dependent OxPAPC effects on EC permeability shows EC barrier enhancement by low OxPAPC concentrations and pronounced barrier disruption by high concentrations. Because OxPAPC represent a spectrum of products at different stages of oxidation (27, 28), it is possible that the overall EC barrier response to various doses of OxPAPC is dictated by effects of specific OxPL components prevailing at certain OxPL concentrations. This possibility was further tested in the present study.
Our data show rapid, unidirectional and dose dependent permeability increase in ECs treated with three groups of fragmented phospholipids, PGPC, POVPC and Lyso-PC. In contrast, full length oxygenated PAPC product, PEIPC, caused significant EC barrier enhancement at low concentrations. Of note, elevation of PEIPC concentrations over 5 ug/ml resulted in gradual loss of its barrier enhancing effect, and at 25 µg/ml PEIPC caused an EC permeability increase (data not shown). Characterization of signaling events leading to the weakening of EC barrier function at high PEIPC concentrations requires further investigation but was beyond the scope of this study. Furthermore, our results suggest that barrier disruptive effects of lyso-PC, POVPC or PGPC can be attenuated by addition of PEIPC or barrier-protective concentrations of OxPAPC.
Rapid barrier disruptive effects of high OxPAPC concentrations have been linked to production of reactive oxygen species and oxidative stress, leading to activation of protein tyrosine kinase activities including Src kinase (18). Src-mediated phosphorylation of VE-cadherin induced by high OxPAPC concentrations caused VE-cadherin internalization and disassembly of adherens junctions, leading to disruption of EC monolayer integrity and barrier dysfunction. The present study demonstrates that this critical mechanism of EC barrier dysfunction by high OxPAPC doses can be equally induced by single fragmented phospholipid, PGPC. The resulting phosphorylation of VE-cadherin at Tyr658 and Tyr731 is sufficient to prevent the binding of its partners, p120-and β-catenin, respectively, to the cytoplasmic tail of VE-cadherin, which leads to the inhibition of cell barrier function (29, 30). The reported activation of ROS production by fragmented phospholipids further supports this mechanism (31). In addition, the rapid EC permeability increase caused by PGPC was not associated with early activation of the Rho pathway and MLC phosphorylation dependent mechanisms. Previous report by our group demonstrated that, although the activation of Rho and MLC phosphorylation is not involved in the rapid barrier disruptive response, it however plays a role in the sustained permeability response induced by high OxPAPC doses (32). It is tempting to speculate that this mechanism can be also triggered by PGPC, and further studies are required to test this possibility. In addition, since barrier disruptive effects of fragmented phospholipids were attenuated by PEIPC, it is possible that PEIPC antagonizes pathologic signaling induced by PGPC. This interesting question also awaits further investigation.
The question regarding putative receptors selectively activated by fragmented and full length oxidized phospholipids, although not addressed in this study remains an interesting subject for future investigations. Existing data including our own studies suggest various classes of receptors that may become activated or transactivated (17, 32, 33) by OxPLs. Some receptors such as Toll-like receptor 4 and intracellular receptor PPARα bind both, full length and fragmented phospholipids (6, 34, 35 , 36), while others such as scavenger receptor CD36, platelet activating factor receptor or G-protein-coupled receptor GPR4 bind more selectively fragmented phospholipids (37–39). Reported data suggest OxPAPC-induced engagement of prostaglandin receptors EP2 and DP (40), which however did not mediate the barrier enhancing effects by OxPAPC (K. Birukov, unpublished data).
In conclusion, this study demonstrates opposite effects of isolated fragmented and full length oxygenated OxPAPC compounds on EC barrier function. The combination of single OxPL compounds mirrored the biphasic effects of OxPAPC on EC permeability. These data suggest the mechanism of dose-dependent EC barrier regulation by OxPAPC. Specifically, barrier enhancing effects of low OxPAPC concentrations are mediated by full length oxygenated PAPC products, such as PEIPC, whose effects prevail over fragmented phospholipid products at these conditions. In turn, at higher OxPAPC concentrations, protective effects of full length oxygenated compounds such as PEIPC diminish and become dominated by barrier disruptive effects of increasing doses of fragmented phospholipids such as PGPC, POVPC and lyso-PC. These data also emphasize the importance of OxPL composition in its biological effects towards vascular endothelium and shed the light on apparent controversy between reported pro-inflammatory effects of endogenous lipid peroxidation products containing large proportion of fragmented OxPLs or terminal lipid peroxidation products (i.e. MDA, 4-HNE), and protective effects of exogenously added OxPAPC containing minimal content of fragmented, and maximal content of full length oxygenated PAPC compounds.
Acknowledgments
Supported by HL087823, HL076259 (KGB); HL107920 (AAB) and HL064731 (LK, JAB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare. All authors have read the journal's policy on disclosure of potential conflicts of interest.
References
- 1.Bochkov VN, Oskolkova OV, Birukov KG, et al. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12(8):1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JM. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med. 1996;24(2):241–246. doi: 10.1097/00003246-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Montuschi P, Collins JV, Ciabattoni G, et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with copd and healthy smokers. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1175–1177. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ards. Chest. 1998;114(6):1653–1659. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- 5.Jerlich A, Schaur RJ, Pitt AR, Spickett CM. The formation of phosphatidylcholine oxidation products by stimulated phagocytes. Free Radic Res. 2003;37(6):645–653. doi: 10.1080/1071576031000091720. [DOI] [PubMed] [Google Scholar]
- 6.Subbanagounder G, Wong JW, Lee H, et al. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem. 2002;277(9):7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 7.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Z, Li J, Yang L, et al. Inhibition of lps- and cpg DNA-induced tnf-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L808–L816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- 9.Nonas S, Birukova AA, Fu P, et al. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12(1):R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonas SA, Miller I, Kawkitinarong K, et al. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173(10):1130–1138. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birukov KG, Bochkov VN, Birukova AA, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via cdc42 and rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 12.Birukova AA, Fu P, Chatchavalvanich S, et al. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L924–L935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 13.Birukova AA, Zebda N, Fu P, et al. Association between adherens junctions and tight junctions via rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226(8):2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birukova AA, Zebda N, Cokic I, et al. P190rhogap mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res. 2011;317(6):859–872. doi: 10.1016/j.yexcr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson AD, Leitinger N, Navab M, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272(21):13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 16.Jung ME, Berliner JA, Koroniak L, et al. Improved synthesis of the epoxy isoprostane phospholipid peipc and its reactivity with amines. Organic letters. 2008;10(19):4207–4209. doi: 10.1021/ol8014804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singleton PA, Chatchavalvanich S, Fu P, et al. Akt-mediated transactivation of the s1p1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res. 2009;104(8):978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starosta V, Wu T, Zimman A, et al. Differential regulation of endothelial cell permeability by high and low doses of oxpapc. Am J Respir Cell Mol Biol. 2012;46(3):331–341. doi: 10.1165/rcmb.2011-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and ve-cadherin in the control of vascular permeability. J Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 20.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 21.Birukova AA, Smurova K, Birukov KG, et al. Role of rho gtpases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67(1):64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Essler M, Amano M, Kruse HJ, et al. Thrombin inactivates myosin light chain phosphatase via rho and its target rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 23.van Nieuw Amerongen GP, van Delft S, Vermeer MA, et al. Activation of rhoa by thrombin in endothelial hyperpermeability: Role of rho kinase and protein tyrosine kinases. Circ Res. 2000;87(4):335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 24.Bochkov VN, Kadl A, Huber J, et al. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419(6902):77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 25.Subbanagounder G, Leitinger N, Schwenke DC, et al. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20(10):2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 26.Birukov KG. Oxidized lipids: The two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8(3):223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 27.Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81(10):613–626. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 28.Furnkranz A, Leitinger N. Regulation of inflammatory responses by oxidized phospholipids: Structure-function relationships. Curr Pharm Des. 2004;10(8):915–921. doi: 10.2174/1381612043452929. [DOI] [PubMed] [Google Scholar]
- 29.Alcaide P, Newton G, Auerbach S, et al. P120-catenin regulates leukocyte transmigration through an effect on ve-cadherin phosphorylation. Blood. 2008;112(7):2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of ve-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280(36):31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 31.Zmijewski JW, Landar A, Watanabe N, et al. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33(Pt 6):1385–1389. doi: 10.1042/BST20051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birukova AA, Lee S, Starosta V, et al. A role for vegfr2 activation in endothelial responses caused by barrier disruptive oxpapc concentrations. PLoS One. 2012;7(1):e30957. doi: 10.1371/journal.pone.0030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimman A, Mouillesseaux KP, Le T, et al. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2007;27(2):332–338. doi: 10.1161/01.ATV.0000252842.57585.df. [DOI] [PubMed] [Google Scholar]
- 34.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (tlr) signaling is restricted to tlr2 and tlr4: Roles for cd14, lps-binding protein, and md2 as targets for specificity of inhibition. J Biol Chem. 2008;283(36):24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oskolkova OV, Afonyushkin T, Preinerstorfer B, et al. Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J Immunol. 2010;185(12):7706–7712. doi: 10.4049/jimmunol.0903594. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Shi W, Tontonoz P, et al. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87(6):516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 37.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor cd36. J Biol Chem. 2002;277(41):38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 38.Qiao J, Huang F, Naikawadi RP, et al. Lysophosphatidylcholine impairs endothelial barrier function through the g protein-coupled receptor gpr4. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L91–L101. doi: 10.1152/ajplung.00508.2005. [DOI] [PubMed] [Google Scholar]
- 39.Pegorier S, Stengel D, Durand H, et al. Oxidized phospholipid: Povpc binds to platelet-activating-factor receptor on human macrophages. Implications in atherosclerosis. Atherosclerosis. 2006;188(2):433–443. doi: 10.1016/j.atherosclerosis.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Li R, Mouillesseaux KP, Montoya D, et al. Identification of prostaglandin e2 receptor subtype 2 as a receptor activated by oxpapc. Circ Res. 2006;98(5):642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]