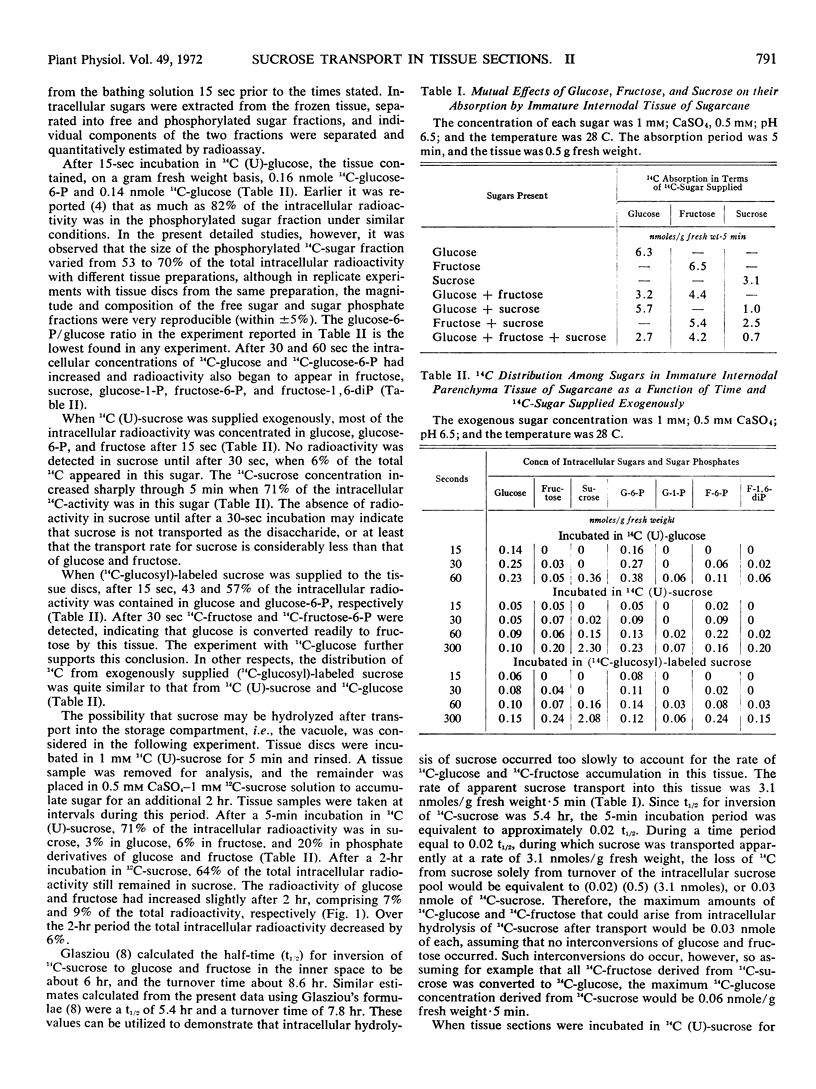
Abstract
The mechanism by which sucrose is transported into the inner spaces of immature internodal parenchyma tissue of sugarcane (Saccharum officinarum L. var. H 49-5) was studied in short term experiments (15 to 300 seconds). Transport of sucrose, glucose, and fructose was each characterized by a Vmax of 1.3 μmoles/gram fresh weight·2 hours, and each of these three sugars mutually and competitively inhibited transport of the other two. When 14C-glucose was supplied exogenously, 14C-glucose 6-phosphate and 14C-glucose were the first labeled compounds to appear in the tissue; no 14C-sucrose was detected until after 60-second incubation. After 15-second incubation in 14C-sucrose, all intracellular radioactivity was in glucose, fructose, glucose 6-phosphate, and fructose 6-phosphate; trace amounts of 14C-sucrose were found after 30 seconds and after 5 minutes, 71% of the intracellular radioactivity was in sucrose. Although it was possible that sucrose was transported intact into the inner space and then immediately hydrolyzed, it was shown that the rate of hydrolysis under these conditions was too low to account for the rate of hexose accumulation. Pretreatment of the tissue with rabbit anti-invertase antiserum eliminated sucrose transport, but had no effect on glucose transport. Since the antibodies did not penetrate the plasmalemma, it was concluded that sucrose was hydrolyzed by an invertase in the free space prior to transport. The glucose and fructose moieties, or their phosphorylated derivatives, were then transported into the inner space and sucrose was resynthesized. No evidence for the involvement of sucrose phosphate in transport was found in these experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedetti G., D'Agnolo G., Pocchiari F. Anion-exchange chromatography of glycolysis intermediates. J Chromatogr. 1970 May 20;49(1):53–56. doi: 10.1016/s0021-9673(00)93610-0. [DOI] [PubMed] [Google Scholar]
- Bowen J. E. Sugar transport in immature internodal tissue of sugarcane: I. Mechanism and kinetics of accumulation. Plant Physiol. 1972 Jan;49(1):82–86. doi: 10.1104/pp.49.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- Glasziou K. T. Accumulation & transformation of sugars in stalks of sugar cane. Origin of glucose & fructose in the inner space. Plant Physiol. 1961 Mar;36(2):175–179. doi: 10.1104/pp.36.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasziou K. T. Accumulation and Transformation of Sugars in Sugar Cane Stalks. Plant Physiol. 1960 Nov;35(6):895–901. doi: 10.1104/pp.35.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Glasziou K. T. Direct Evidence for Translocation of Sucrose in Sugarcane Leaves and Stems. Plant Physiol. 1964 Mar;39(2):180–184. doi: 10.1104/pp.39.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. Sugar accumulation by sugar-cane storage tissue: the role of sucrose phosphate. Biochem J. 1964 Dec;93(3):521–526. doi: 10.1042/bj0930521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- PUTMAN E. W., HASSID W. Z. Sugar transformation in leaves of Canna indica. I. Synthesis and inversion of sucrose. J Biol Chem. 1954 Apr;207(2):885–902. [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]



