Abstract
The strength and duration of extracellular dopamine concentrations are regulated by the presynaptic dopamine transporter (DAT) and dopamine D2 autoreceptors (D2autoRs). There is a functional interaction between these two proteins. Activation of D2autoRs increases DAT trafficking to the surface whereas disruption of this interaction compromises activities of both proteins and alters dopaminergic transmission. Previously we reported that DAT expression and activity are subject to modulation by protein kinase Cβ (PKCβ). Here, we further demonstrate that PKCβ is integral for the interaction between DAT and D2autoR. Inhibition or absence of PKCβ abolished the communication between DAT and D2autoR. In mouse striatal synaptosomes and transfected N2A cells, the D2autoR-stimulated membrane insertion of DAT was abolished by PKCβ inhibition. Moreover, D2autoR-stimulated DAT trafficking is mediated by a PKCβ-ERK (extracellular signal-regulated kinase) signaling cascade where PKCβ is upstream of ERK. The increased surface DAT expression upon D2autoR activation resulted from enhanced DAT recycling as opposed to reduced internalization. Further, PKCβ promoted accelerated DAT recycling. Our study demonstrates that PKCβ critically regulates D2autoR-activated DAT trafficking and dopaminergic signaling. PKCβ is a potential drug target for correcting abnormal extracellular dopamine levels in diseases such as drug addiction and schizophrenia.
Keywords: dopamine transporter, dopamine D2 autoreceptor, Protein kinase Cβ, trafficking, extracellular signal regulated protein kinase
Introduction
Extracellular dopamine (DA) levels are regulated by presynaptic DA transporter (DAT) and DA D2 autoreceptor (D2autoR). Reuptake of DA through DAT into neurons is the primary mechanism for removing extracellular DA and terminating DA action, whereas D2autoR provides an inhibitory feedback on DA exocytosis, DA synthesis, and dopaminergic neuron firing (Jones et al. 1999, L’Hirondel et al. 1998, Cubeddu & Hoffmann 1982, Pothos et al. 1998, Haubrich & Pflueger 1982). There is an anatomical and a functional interaction between DAT and D2autoR. DAT and D2autoR co-localize in striatal presynaptic terminals (Hersch et al. 1997), and demonstrate a physical coupling in heterologous cells (Lee et al. 2007). D2autoR agonists increase surface DAT expression and activity (Eriksen et al. 2010, Bolan et al. 2007). Coexpression of D2autoR facilitates the expression of surface DAT (Lee et al. 2007), whereas disruption of the DAT and D2autoR interaction compromises the activity of both proteins (Dickinson et al. 1999, L’Hirondel et al. 1998, Lee et al. 2007, Jones et al. 1999). More significantly, the interaction between DAT and D2autoR has clinical relevance. A reduced coupling of striatal DAT and D2autoR has been reported in postmortem brains of schizophrenics (Lee et al. 2009). Moreover, a DAT polymorphism in humans with attention deficit/hyperactive disorder (ADHD) disrupts the normal interaction between DAT and D2autoR, leading to an anomalous DA efflux (Bowton et al. 2010). These data suggest an important physiological role of DAT and D2autoR interaction in regulation of dopaminergic transmission.
DAT undergoes trafficking upon exposure to DAT substrates or DA D2 receptor agonists. Although DAT substrate-elicited DAT trafficking and its regulation by second-messenger systems have been studied extensively [see review (Chen et al. 2010)], knowledge of the molecular mechanisms underlying D2autoR-stimulated DAT trafficking is limited. A potential candidate to govern the interaction between DAT and D2autoR is protein kinase C (PKC), a common modulator for DAT and D2R trafficking and function (Giambalvo & Wagner 1994, Giambalvo 2004, Namkung & Sibley 2004). Recently, we reported that a specific PKC isoform, PKCβ, promotes rapid DAT trafficking to the surface in N2A neuroblastoma cells upon short-term exposure to DAT substrate amphetamine (Chen et al. 2009, Furman et al. 2009a) whereas long-term PKC activation leads to DAT internalization (Chen et al. 2010, Schmitt & Reith 2010). Further, there are reports that PKC can regulate D2autoR function. PKC activation reduces the inhibitory effect of D2autoR on electrical stimulation-evoked DA exocytosis (Cubeddu et al. 1989) and reduces D2autoR-stimulated immobilization of calcium (Liu et al. 1992, Morris et al. 2007). D2autoR-stimulated DAT trafficking in heterologous cells is dependent on activation of ERK (extracellular signal-regulated kinase), a downstream signaling molecule (Bolan et al. 2007, Lee et al. 2007). Here we make a new observation that, in addition to ERK, PKCβ is required for D2autoR-elicited trafficking of intracellular DAT to the membrane surface. Moreover, PKCβ is upstream of ERK. This study reveals an important contribution of PKCβ in maintaining homeostasis of the presynaptic dopaminergic transmission.
Materials and Methods
Dopamine receptor nomenclature
Both DA D2 receptor (D2R) and D3 receptor (D3R) are expressed in the dopaminergic neurons (Diaz et al. 2000, Sesack et al. 1994), thus we use D2autoR as a general term for presynaptic D2-like autoreceptors for experiments conducted in animal tissues. Although DA D3 receptors exist in dopamine neurons, evidence is strong that the dopamine autoreceptor in mice is the D2 subtype (Mercuri et al. 1997). D2R has two splice variants: short and long (D2S and D2L, respectively). Evidence suggests that the short variant D2S functions as a presynaptic autoreceptor (Wang et al. 2000, Usiello et al. 2000). The short splice variant D2S was transfected into N2A cells. We determined by quantitative PCR that N2A cells do not express D2R or D3R receptors.
Cell culture and transfection
N2A neuroblastoma cell lines were grown in Opti-MEM I media (Life Technology) supplemented with 10% fetal growth serum and 1% penicillin/streptomycin. Cells were transiently transfected with human D2S with FLAG tag (FLAG-hD2S, a gift from Dr. David Sibley) and human DAT with HA tag (HA-hDAT, a gift from Dr. Jonathan Javitch) using the calcium phosphate method. The FLAG tag was fused at the extracellular N terminus of D2S. The HA tag was inserted in the second extracellular loop of DAT as described previously (Furman et al. 2009b).
Confocal microscopy of DAT trafficking in N2A cells
Cells were seeded on coverslips 12 hrs after transfection. Coverslips were coated with 0.5 mg/ml poly-D-lysine. Forty-eight hours after the transfection, cells were incubated with vehicle (Veh), a specific PKCβ inhibitor LY379196 (LY, 100 nM, 30 min) or the ERK inhibitor PD98059 (PD, 10 μM, 15 min) at 37°C before treatment with the D2R/D3R agonist quinpirole (Quin, 1 μM) or Veh for 30 min. The reaction was stopped by washing cells with cold PBS, and blocked with 4% normal goat serum in PBS for 1 hr on ice. To label surface DAT protein, cells were incubated with a mouse anti-HA antibody (Covance, Gaithersberg, MD) followed by secondary goat anti-mouse Alexa Fluor 594 at 4°C. Then cells were fixed with 4% paraformaldehyde in PBS for 15 min on ice and permeabilized by 0.1% Triton X-100 in PBS for 10 min at room temperature for the subsequent cytosolic DAT labeling. Cells were incubated with a rabbit anti-HA antibody (Covance) followed by secondary goat anti-rabbit Alexa Fluor 647. Cells were mounted onto glass slides with ProLong Gold anti-fade reagent (Life Technology) for imaging.
Images of cells displaying fluorescent signals were acquired on an Olympus FluoView 500 confocal microscope with a 60x by 1.35N.A. oil objective. Images were obtained by taking a series of images every 0.2 μm through the cell and combing the images into a composite stack. Sequential scans were taken to prevent the overlap of laser signal. For the laser configuration, Alexa Fluor 594 was excited by a 543nm laser and passed through a BA560-600nm bandpass filter whereas Alexa Fluor 647 was excited by a 633nm laser and passed through a BA660IF bandpass filter. Z-slices of cells were quantified using Image J software (NIH). The background signal from neighboring untransfected cells was subtracted from the total fluorescence for all quantified signals to determine the specific intensity of fluorescence. The surface DAT content was calculated as the ratio of the specific intensity of surface labeling to that of the intracellular labeling of DAT.
D2S-activated DAT recycling and internalization in N2A cells
N2A cells transfected with HA-DAT and FLAG-D2S were treated with vehicle (V) or quinpirole (Q, 100 nM) in the presence or absence of LY379196 (LY, 100 nM). DAT recycling and internalization were determined according to the published protocol (Loder & Melikian 2003, Boudanova et al. 2008b). To determine whether quinpirole or LY379196 had an effect on DAT recycling, cells were biotinylated (1 mg/kg) at 4°C for 60 min. Then cells were warmed up to room temperature and treated with V+V, V+Q, LY+V and LY+Q in the presence of sulfo-NHS-SS-biotin (1.5 mg/ml) for 30 min. Biotinylation was rapidly quenched with 100 mM glycine for 15 min at 4°C. Cells were lysed in RIPA buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate and 1% Triton X-100) containing protease inhibitors. Biotinylated and non-biotinylated proteins from equal amounts of cell lysates were separated by strepavidin pulldown. Samples were analyzed by SDS-PAGE and immunoblotted by DAT antibody (MAB369). The biotinylated DAT was normalized by the DAT from the non-biotinylated fraction. The data were calculated as the percent of the vehicle treatment.
To determine whether DAT internalization was affected by quinpirole or LY379196 treatment, cells were biotinylated with 2 mg/ml sulfo-NHS-SS-biotin. Following biotinylation, one set of cells were washed with PBS/Ca/Mg and kept on ice to determine the total initial surface DAT and the stripping efficiency. To initiate endocytosis, the other set of cells were warmed up to room temperature and treated with V+V, V+Q, LY+V and LY+Q for 30 min. The endocytic reaction was stopped by replacing the reaction solution with the cold NT buffer (150 mM NaCl, 1 mM EDTA, 20 mM Tris, pH 8.6). The remaining surface biotin was stripped twice by the reducing agent Tris(2-carboxyethyl)phosphine (TCEP) in NT buffer for 20 min at 4°C. The strip efficiency was determined in each experiment and was more than 95%. After stripping, cells were washed with cold PBS and lysed in RIPA buffer. The internalized proteins were pulled down by strepavidin beads. The biotinylated DAT was normalized by the DAT from the non-biotinylated fraction. The data were calculated as the percent of the vehicle treatment.
Animals
Generation of PKCβ wild type (PKCβ+/+) and knockout mice (PKCβ−/−) was described previously (Leitges et al. 1996). PKCβ+/+ and PKCβ−/− mouse breeders had been backcrossed with C57BL/6J mice ten times. Experimental PKCβ+/+ and PKCβ−/− mice were age- (2–3 months old) and gender-matched. Mice had free access to the standard Purinarodent chow and water and were maintained in a temperature-and humidity-controlled environment on a 12-h dark/light cyclewith lights on at 7:00 AM. Animal use and procedures were in accordance with the National Institutes of Health guidelines, and approved by the Institutional Animal Care and Use Committee at University of Michigan.
Quinpirole-stimulated DAT trafficking in mouse striatal synaptosomes
Preparation of mouse striatal synaptosomes and the procedure for surface striatal DAT biotinylation were previously described (Chen et al. 2009). The striatal tissues were dissected by a means of brain matrix, and included both the ventral and the dorsal striatum. To study the time course of quinpirole-induced DAT trafficking, mouse synaptosomes were treated for 1, 5, 15, 30 or 60 min at 37°C with quinpirole (10 μM) or vehicle in Kreb-Ringer’s buffer (KRB) containing 24.9 mM NaHCO3, pH 7.4, 1.2 mM KH2PO4, 146.2 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 10 mM glucose, 0.05 mM ascorbic acid,50 μM pargyline. To study the effect of PKCβ inhibition on quinpirole-induced DAT trafficking, synaptosomes were pretreated with a specific PKCβ inhibitor LY379196 (100 nM) or KRB for 1 hour before the quinpirole treatment (15 min). Then samples were biotinylated by membrane non-permeable sulfo-NHS-SS-biotin. Surface DAT expression was calculated as the ratio of the biotinylated DAT to the total DAT. Data were expressed as the relative surface DAT content upon quinpirole treatment vs. vehicle treatment in the same time frame of drug exposure for comparison of genotype difference in DAT trafficking.
Quinpirole-stimulated DA uptake in mouse striatal synaptosomes
Striatal synaptosomes were pre-incubated with KRB or 10 μM quinpirole for 10 min at 37°C. DA uptake was measured for 1 min at 37°C in KRB containing 10 nM [3H]DA (specific activity 23.5 Ci/mmol; Perkin Elmer) and 500 nM unlabeled DA. Non-specific [3H]DA uptake was determined in the presence of 30 μM cocaine. The DA uptake assay was conducted as described (Chen et al. 2009). Data were expressed as percent of the vehicle treatment, and analyzed by paired-Student’s t-test for each genotype.
To ensure that potential compensatory changes in PKCβ−/− mice were not confounding the results, the effect of the PKCβ inhibitor LY379196 on [3H]DA uptake was determined. Synaptosomes were incubated with LY379196 (100 nM) for 1 hr, PD98059 (10 μM) for 15 min or KRB at 37°C before treatment with quinpirole (10 μM) or vehicle for 5 min prior to measurement of [3H]DA uptake. DA uptake was performed for 5 min at 37°C in the presence of 500 nM unlabeled DA and 10 nM [3H]DA.
Percoll-purification of striatal synaptosomes
To dissect the presynaptic D2autoR signaling from the postsynaptic D2R/D3R signaling upon agonist quinpirole stimulation, striatal synaptosomes were purified on a percoll gradient as described (Dunkley et al. 1988) to eliminate the majority of the postsynaptic components. Briefly, crude striatal synaptosomes pooled from striata of 4 wildtype mice were suspended in KRB and loaded on a percoll gradient consisting of 23%, 15%, 10% and 3% percoll made in 0.32M sucrose containing 1 mM EDTA. The gradient was centrifuged, and layers 3 and 4 were combined, washed and resuspended in KRB as purified striatal synaptosomes for determination of PKCβ and ERK phosphorylation.
Phosphorylation of PKCβ and ERK in percoll-purified mouse synaptosomes
Phosphorylation of PKCβI and PKCβII were used as a measurement of PKCβ activation. Percoll-purified striatal synaptosomes from PKCβ+/+ mice were resuspended in KRB and pretreated with or without the ERK inhibitor PD98059 (10 μM) for 15 min at 37°C before addition of quinpirole (10 μM) or KRB for 5 min. The reaction was stopped with cold KRB, and samples were immediately centrifuged. The pellets were lysed in RIPA buffer containing phosphatase and protease cocktail inhibitors (PhosStop and Complete Mini, respectively, Roche, Indianapolis, IN). PKCβ activation was determined by detecting phosphorylated PKCβI and PKCβII (pPKCβI and pPKCβII) at threonine 642 and threonine 641, respectively, using phosphospecific antibodies. The total content of PKCβI and PKCβII (tPKCβI and tPKCβII, respectively) was determined using rabbit anti-PKCβI or -PKCβII antibody. PKCβ I or PKCβII activity was expressed as a ratio of pPKCβI to tPKCβI or pPKCβII to tPKCβII.
To determine ERK activity, percoll-purified striatal synaptosomes from both PKCβ+/+ and PKCβ−/− mice were pretreated with or without 100 nM LY379196 for 1 hour at 37°C before treatment with KRB, phorbol 12-myristate 13-acetate (PMA, 1 μM), or quinpirole (10 μM) for 5 min. The levels of phospho-ERK (pERK) and total ERK (tERK) were detected using rabbit anti-pERK and mouse anti-ERK antibodies, respectively. ERK activity was expressed as a ratio of pERK to tERK.
Drugs and reagents
Amphetamine, dopamine and quinpirole were purchased from Sigma Aldrich (St. Louis, MO). PD98059 and phorbol 12-myristate 13-acetate were from Calbiochem (San Diego, CA). Sulfo-NHS-SS-biotin, strepavidin and Tris(2-carboxyethyl)phosphine (TCEP) were from Proteochem Inc. (Denver, CO). LY379196 was a generous gift from Eli Lilly Company (Indianapolis, IN). The DAT antibody MAB369 was from Millipore (Billerica, CA), antibodies for ERK and phosphorylated ERK were from Cell Signaling Technology (Danvers, MA), antibodies for PKCβ and phosphorylated PKCβ were from Santa Cruz Biotechnology (Santa Cruz, CA). All the other chemicals were from Sigma Aldrich (Saint Louis, MO) unless otherwise indicated. Goat anti-mouse Alexa Fluor 594 and goat anti-rabbit Alexa Fluor 647 were from Life Technology (Grant Island, NY)
Statistical analyses
Results are presented as means ± standard error of the means (SEM). Comparisons between two groups were made by paired two-tailed Student’s t-test. Comparisons among multiple groups were made by one- or two-way ANOVA with post hoc Bonferroni test using Systat (Chicago, IL). Statistical significance was set at P < 0.05.
Results
Inhibition of PKCβ and ERK disrupts D2S-activated DAT trafficking in N2A cells
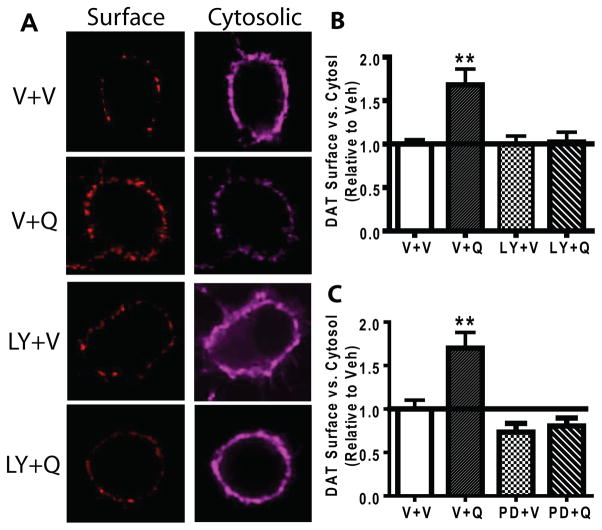
We initially examined whether D2S-activated DAT trafficking is PKCβ and ERK dependent in HA-DAT-FLAG-D2S-N2A cells using a highly specific PKCβ inhibitor LY379196 (Jirousek et al. 1996). Bolan et al. (2007) demonstrated that quinpirole enhanced trafficking of DAT to the surface in heterologous N2A cells. Further, we previously showed that PKCβ was instrumental in promoting rapid substrate-induced trafficking of DAT to the surface in N2A cells (Furman et al. 2009a). We utilized confocal microscopy to determine that PKCβ critically modulated D2S-stimulated DAT trafficking in N2A cells. Representative images for staining of surface and cytosolic DAT upon quinpirole treatment in the presence or absence of LY379196 are shown (Fig. 1A). A one-way ANOVA indicated a significant main effect of quinpirole and LY379196 on trafficking of DAT, F(3,85)=7.833, p<0.01(N=14–42). Compared to vehicle, quinpirole (1 μM, 30 min) increased the surface content of DAT (Fig. 1B, p<0.05). The PKCβ inhibitor, LY379196, alone did not increase surface DAT, but blocked quinpirole-induced DAT trafficking to the surface (Fig. 1B). Next, we examined whether inhibition of ERK would affect DAT trafficking in a similar manner as did inhibition of PKCβ. Similarly to LY379196, the ERK inhibitor PD98059 (10 μM) blocked quinpirole-stimulated DAT trafficking to the surface without significantly affecting the basal DAT surface content (Fig. 1C).
Fig. 1.
PKCβ and ERK inhibitor blocked quinpirole-stimulated DAT trafficking to the surface in N2A cells. N2A cells were transiently transfected with HA-DAT and FLAG-D2S. Cells were pretreated with LY379196 (LY, 100 nM, 30 min), PD98059 (PD, 10 μM, 15 min) or vehicle (V) prior to quinpirole (Q, 1 μM, 30 min) or V treatment. Following the treatment, live cells were labeled for surface DAT, then fixed and permeabilized for intracellular DAT labeling with different fluorophores. The surface DAT content was calculated as the ratio of the fluorescent intensity of surface labeling to that of the intracellular labeling. (A) Representative confocal images of surface and cytosolic DAT treated with quinpirole (Q) or vehicle (V) in the presence or absence of LY379196 (LY). (B) Quinpirole stimulated DAT trafficking to the surface (p<0.05, N=19) whereas LY379196 blocked quinpirole-induced DAT trafficking (n=15). (C) The ERK inhibitor blocked quinpirole-stimulated DAT trafficking to the surface. PD98059 alone did not have an effect on basal DAT trafficking (N=14). Error bars represent SEM. *p<0.05 vs. the vehicle treatment.
PKCβ promotes D2S-stimulated DAT recycling in N2A cells
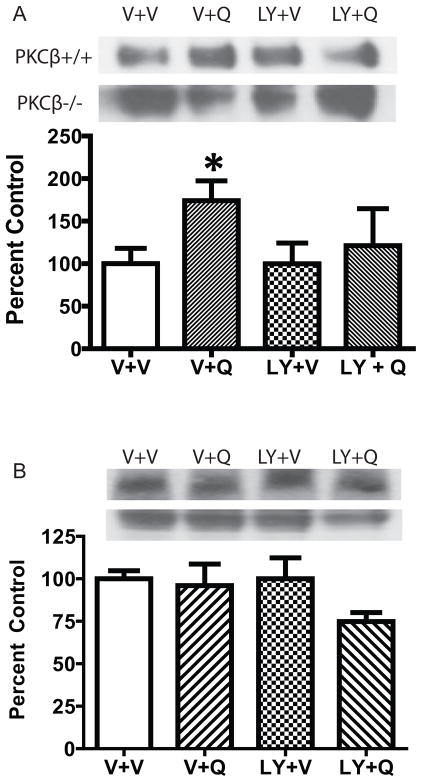
The increased surface DAT expression upon D2S stimulation could result from an increase in the insertion of DAT into the membrane or a reduction in the internalization of DAT. We interrogated this by examining the effect of quinpirole on both recycling and internalization of DAT in N2A cells co-expressing DAT and D2S. As shown in Fig. 2A, quinpirole (100 nM) significantly increased the recycling rate of DAT over 30 min compared to vehicle (two-tailed paired t-test, t = 2.788, df = 5, p<0.05). Incubation with LY379196 alone did not alter the recycling rate of DAT, but blocked the effect of quinpirole. On the contrary, there was no effect of either quinpirole or LY379196 on the internalization rate of DAT when co-expressed with D2S (Figure 2B, N=5). These data suggest that the increased surface DAT expression by quinpirole indeed resulted from accelerated DAT trafficking to the membrane instead of decreased DAT internalization.
Fig. 2.
Quinpirole stimulated DAT recycling without an effect on DAT internalization. N2A cells transiently transfected with HA-DAT and FLAG-D2S were treated with vehicle (V), quinpirole (100 nM, 30 min), LY379196 (100 nM), or LY+Q. (A) Representative blots and summary of the DAT recycling (N=6). Quinpirole accelerated the DAT recycling rate compared to the vehicle treatment (*p<0.05). LY379196 itself did not have an effect on DAT recycling, but blocked quinpirole-induced increased DAT recycling. (B) Representative blots and summary of DAT internalization (N=5). The DAT internalization rate did not differ between quinpirole and vehicle treatment and LY379196 did not affect DAT internalization either. The data from the LY+Q group did not differ from V+V or LY+V. Error bars represent SEM.
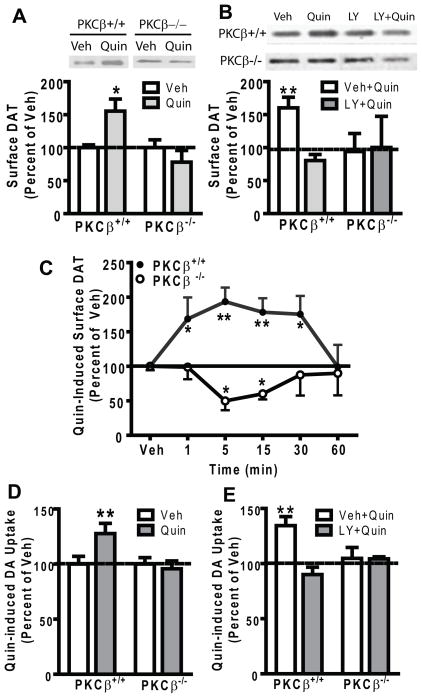
Inhibition of PKCβ abolishes quinpirole-induced increases in surface DAT content in mouse striatal synaptosomes
In DAT-D2S-N2A cells, we demonstrated that PKCβ and ERK were involved in D2S-stimulated DAT trafficking. However, we wished to establish this in a more physiological system and to verify our results without reliance on a small molecule PKC inhibitor. Therefore we continued our interrogation into the role of PKCβ in D2autoR-stimulated DAT trafficking by using mice with a deletion of PKCβ. To investigate whether PKCβ regulates D2autoR-modulated DAT trafficking in striatal synaptosomes, surface DAT content in PKCβ+/+ and PKCβ−/− mice was determined by biotinylation in the presence or absence of quinpirole. Treatment with quinpirole (10 μM) significantly increased surface DAT expression in PKCβ+/+ mice (two-tailed paired t-test, t=5.476, df = 3, p<0.05,), but not in PKCβ−/− mice (Fig. 3A). To exclude the possibility that neuronal adaptations in the absence of PKCβ might have complicated the response to quinpirole in PKCβ−/− mice, synaptosomes from PKCβ+/+ mice were treated with the PKCβ inhibitor LY379196. Mimicking PKCβ−/− mice, pretreatment with LY379196 (100 nM) significantly prevented the quinpirole-induced increase in striatal surface DAT content in PKCβ+/+ mice (two-tailed paired t-test, t=4.480, df=3, p<0.05), and had no effect on PKCβ−/− mice (Fig. 3B). These data are consistent with our findings in N2A cells (Fig. 1).
Fig. 3.
PKCβ inhibition abolishes quinpirole-induced increase in striatal surface DAT expression and DA uptake in mice. (A) Striatal synaptosomes from the PKCβ+/+ and PKCβ−/− mice were incubated for 15 min with vehicle (Veh) or quinpirole (Quin, 10 μM) before biotinylation of surface DAT. Quinpirole increased surface DAT content in PKCβ+/+ mice but not in PKCβ−/− mice. Representative blots for surface DAT biotinylation under various conditions are shown. Surface DAT was calculated as a ratio of surface biotinylated DAT to the total DAT from the lysate. Data were expressed as percent of the Veh treatment in each genotype. Veh values for ratios of biotinylated/total DAT for PKCβ+/+ and PKCβ−/− mice were 1.1 ± 0.1 and 0.7 ± 0.2, respectively. (B) Pretreatment with the PKCβ inhibitor LY379196 (LY, 100 nM, 1 hr) blocked the quinpirole-induced increase in surface DAT content in PKCβ+/+ mice compared to the Veh treatment and had no effect on surface DAT in PKCβ−/− mice. Representative blots for surface DAT biotinylation under various conditions are shown. Data were calculated as percent of the Veh+Veh treatment. (C) Quinpirole induced a significant time-dependent DAT trafficking in PKCβ+/+ mice, There was a significant increase in surface DAT expression upon quinpirole treatment at 1, 5, 15 and 30 min compared to the Veh treatment. By contrast, quinpirole did not increase surface DAT expression at any time point in PKCβ−/− mice (N=13, 6, 6, 9, 3 and 5 for Veh, 1, 5, 15, 30 and 60 min, respectively). *p<0.05, **p<0.01 vs. Sal, post hoc Bonferroni test. (D) Quinpirole (Quin) increased DA uptake in striatal synaptosomes from PKCβ+/+ mice (N=6, Veh vs. Quin, paired t-test, **p<0.01) but not from PKCβ−/− mice. Data were expressed as percent of Veh treatment in each genotype. Veh values for [3H]DA uptake in PKCβ−/− mice were 94% of that in PKCβ+/+ mice, N=6. (E) The PKCβ inhibitor LY379196 (LY, 100 nM) inhibited quinpirole-stimulated DA uptake in striatal synaptosomes from PKCβ+/+ mice (**p<0.01). Data were calculated as percent of Veh+Veh treatment. Error bars represent SEM.
To further ascertain that PKCβ−/− mice indeed had a blunted response to quinpirole stimulation in surface DAT expression, a time course of quinpirole treatment on DAT trafficking was conducted. A two-way ANOVA revealed a significant main effect of genotype on the time course of quinpirole-induced DAT trafficking, F(1, 73) = 38.73, p<0.001 (Fig. 3C) F(5,45)=5.265, p<0.01, N=6–15. In post-hoc analyses, compared with the vehicle treatment, surface DAT expression in PKCβ+/+ mice was significantly increased at 1, 5, 15 and 30 min of quinpirole, but not at 60 min. By contrast, quinpirole did not increase the surface DAT content in PKCβ−/− mice at any time point. Rather, quinpirole significantly reduced the surface DAT after 5 and 10 min treatment.
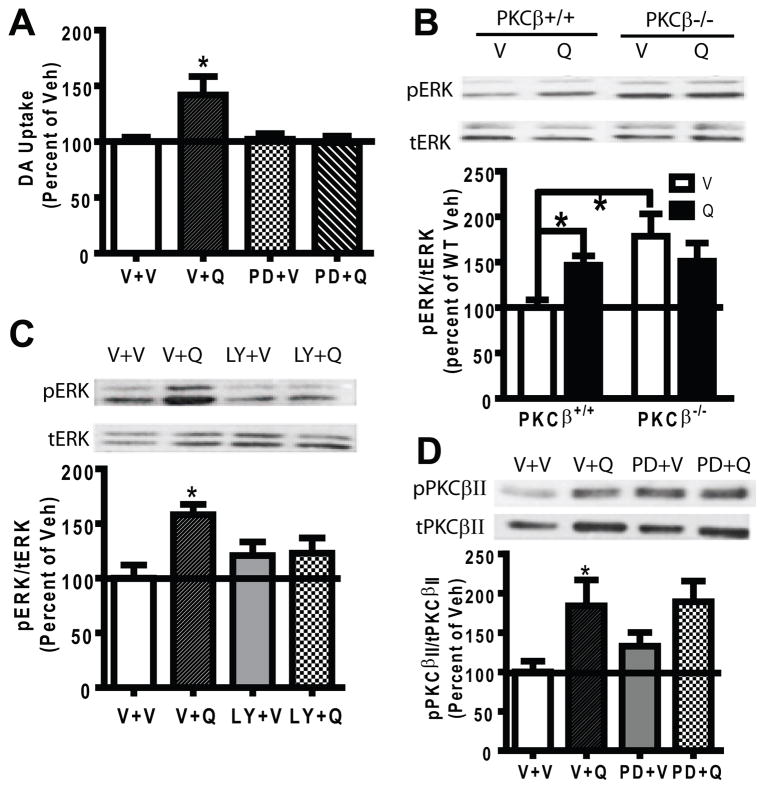
To verify that quinpirole-stimulated insertion of DAT into surface membranes had functional significance, DA uptake was measured in striatal synaptosomes from PKCβ+/+ and PKCβ−/− mice. Quinpirole pretreatment (10 μM) significantly increased DA uptake in PKCβ+/+ mice (Fig. 3D, two-tailed paired t-test, t = 4.438, df = 6, p<0.005), but not in PKCβ−/− mice. Moreover, LY379196 significantly blocked the quinpirole-induced increase in DA uptake in PKCβ+/+ mice (Fig. 3E, two-tailed paired t-test, t=5.005, df = 3, p < 0.05), and had no effect in PKCβ−/− mice (Fig. 3E), suggesting that the absence of quinpirole-stimulated DAT trafficking in PKCβ−/− mice indeed resulted from the absence of PKCβ. These data demonstrate that quinpirole-stimulated DAT trafficking to the surface paralleled the increased DAT activity.
Quinpirole activates presynaptic PKCβ in purified mouse striatal synaptosomes
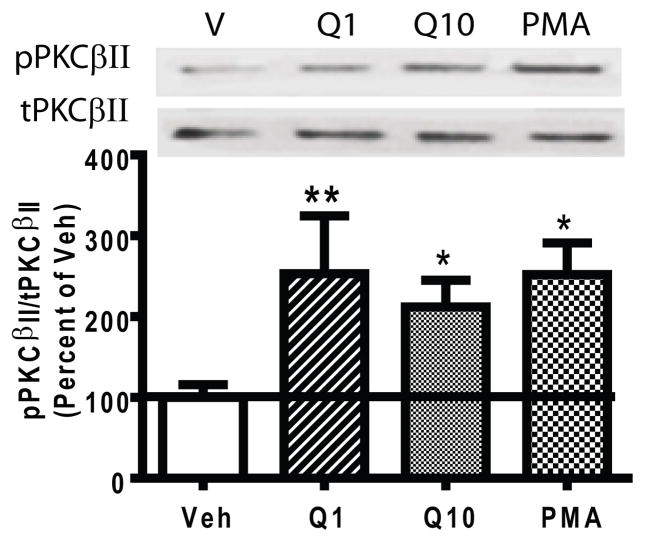
If D2autoR-stimulated DAT trafficking is PKCβ dependent, then activation of the D2autoRs should activate PKCβ. We assessed this by examining the effect of quinpirole on phosphorylation of the individual PKCβ isozymes at threonine 642 (in PKCβI) and threonine 641 (in PKCβII). Autophosphorylation of these carboxyl terminal threonines is required for active PKCβ (Edwards et al. 1999). Since PKCβ exists presynaptically in dopaminergic terminals (O’Malley et al. 2010) and postsynaptically (Yoshihara et al. 1991) in the striatum of animals, quinpirole-stimulated PKCβ activity was assayed in percoll-purified striatal synaptosomes. The percoll purification procedure eliminates the majority of postsynaptic membranes (Dunkley et al. 1988), and enriches synaptosomal signaling. Purified synaptosomes from PKCβ+/+ mice were treated with vehicle, quinpirole (1 μM or 10 μM), or the PKC activator PMA (1 μM). A one-way ANOVA indicated a significant drug effect on phosphorylation of PKCβII, F(3,11)=6.46, p<0.05. Both 1 μM and 10 μM quinpirole stimulated PKCβII activity by increasing phosphorylation of PKCβII compared to vehicle treatment (N=4, Fig. 4, p<0.05). However, neither dose of quinpirole enhanced phosphorylation of PKCβI (the values for pPKCβI/tPKCβI relative to the Veh treatment were 1.03 ± 0.09, 0.99 ± 0.76, and 1.55 ± 0.19 for quinpirole 1 and 10 μM and PMA, respectively). PMA significantly increased both pPKCβI and pPKCβII compared to the vehicle treatment (post hoc Bonferroni, p<0.05). These data suggest a specific role of PKCβII in D2autoR signaling.
Fig. 4.
Quinpirole stimulated PKCβII activity. Percoll-purified striatal synaptosomes prepared from PKCβ+/+ mice were incubated with vehicle (V), quinpirole (Q, 1 or 10 μM, 5 min) or PMA (1 μM, 1 min) as a positive control. The activity of PKCβII was determined by the level of phosphorylated PKCβII (pPKCβII) vs. total PKCβII (tPKCβII). Both 1 and 10 μM quinpirole increased PKCβII activity. Error bars represent SEM.
The PKCβ-ERK signaling cascade modulates D2autoR-activated DAT trafficking in mouse striatal synaptosomes
Our experiments in heterologous N2A cells demonstrated a role for both PKCβ and ERK in D2S-stimulation of DAT trafficking. Analogous to our studies in heterologous N2A cells, we further explored the effect of ERK in D2autoR modulation of DAT trafficking in the PKCβ−/− mice and queried whether or not PKCβ is a downstream or upstream signaling molecule of ERK. We first verified that ERK was required for D2S-stimulated DAT trafficking in PKCβ+/+ mice and blocked the effect of quinpirole to increase striatal DA uptake in PKCβ+/+ mice (Fig. 5A, N=6). Moreover, quinpirole increased phosphorylation of ERK in synaptosomes from PKCβ+/+ mice (Fig. 5B, lanes 1 and 2, p<0.05, N=5). We then examined whether PKCβ was upstream or downstream of ERK. If PKCβ were downstream of ERK, then deletion or inhibition of PKCβ should have no effect on the activation of ERK by quinpirole. Quinpirole-induced ERK phosphorylation (pERK) was examined in percoll-purified striatal synaptosomes from PKCβ−/− mice (N=5). A two-way ANOVA showed a significant effect of genotype on ERK activation, F(1,12) = 10.21, p<0.01. Quinpirole increased pERK in PKCβ+/+ mice but not in PKCβ−/− mice (Fig. 5B), suggesting that PKCβ is upstream of ERK following D2autoR activation. However, PKCβ−/− mice exhibited a significant increase in the basal pERK compared to PKCβ+/+ mice (Fig. 5B, p<0.05). The inability of quinpirole to further stimulate ERK activity in PKCβ−/− mice could have resulted from the absence of PKCβ or from a ceiling effect due to adaptation.
Fig. 5.
ERK activation by quinpirole required PKCβ. (A) A 15-min pretreatment with PD98059 (PD, 10 μM) blocked quinpirole (Q, 1 μM, 5 min)-stimulated DA uptake in striatal synaptosomes from PKCβ+/+ mice compared to vehicle (V) treatment. (B) Percoll-purified striatal synaptosomes from PKCβ+/+ and PKCβ−/− mice were treated with V or 10 μM quinpirole (Q) for 5 min (N=5). The upper panel shows representative blots of pERK (phosphorylated ERK) and tERK (total ERK) from a representative PKCβ+/+ and PKCβ−/− mouse. Data were converted as a ratio of pERK to tERK as a measurement of ERK activity, and calculated as a percent of the V treatment in PKCβ+/+ mice. Quinpirole significantly increased the pERK level in PKCβ+/+ but not in PKCβ−/− mice. The basal pERK level was significant higher in PKCβ−/− mice as compared to that from PKCβ+/+ mice (p<0.05). (C) Percoll-purified striatal synaptosomes from PKCβ+/+ mice were pretreated with the PKCβ inhibitor LY379196 (LY, 100 nM) or vehicle (V) for 1 hr, and the level of pERK in response to quinpirole (Q, 1 μM, 5 min) was determined (N=8). Representative blots of pERK and tERK were shown. LY379196 did not change the basal pERK, and blocked quinpirole-stimulated pERK. (D) Percoll-purified striatal synaptosomes were pretreated with the ERK inhibitor PD98059 (PD, 10 μM, 15 min) before vehicle or quinpirole treatment (Q, 1 μM, 5 min). The activity of PKCβII was determined by pPKCβII (phosphorylated PKCβII) vs. tPKCβII (total PKCβII) (N=8). Representative blots of pPKCβII and tPKCβII blots are shown in the upper panel. PD98059 itself did not change the basal pPKCβII level and did not block quinpirole-stimulated pPKCβII. Error bars represent SEM.
To further analyze the effect of PKCβ on quinpirole-stimulated ERK activity, striatal synaptosomes from PKCβ+/+ mice were pretreated with the PKCβ inhibitor LY379196 before quinpirole treatment. A one-way ANOVA revealed a significant effect of drug on ERK activity, F(3,31)=3.957, p<0.05 (Fig. 5C, lanes 3 and 4, N=8). LY379196 itself did not increase pERK, suggesting that the increased basal pERK in PKCβ−/− mice was due to adaptation. These data indicate that PKCβ is an upstream signaling molecule of ERK in quinpirole stimulation of D2autoR.
To further clarify that ERK is downstream of PKCβ in D2autoR signaling, pPKCβII was determined from percoll-purified striatal synaptosomes from PKCβ+/+ mice treated with quinpirole in the presence or absence of the ERK inhibitor PD98059. A one-way ANOVA revealed a significant effect of drug, F(3,31)=3.886, p<0.05 (N=8). Quinpirole-stimulated phosphorylation of PKCβII was unchanged in the presence of PD98059 (Fig. 5D), verifying that ERK is downstream of PKCβII in quinpirole-induced D2autoR signaling.
Discussion
The present study reveals a significant new role of PKCβ in regulation of D2autoR-activated DAT trafficking in heterologous cultured cells and mouse striatal synaptosomes. We have demonstrated that PKCβ promotes D2autoR-activated insertion of DAT into the membrane by accelerating DAT recycling. A specific PKCβ → ERK signaling cascade is critical in D2autoR modulation of DAT trafficking.
The novel trafficking role of PKCβ in D2autoR-activated DAT translocation
PKC regulation of dynamic trafficking of neurotransmitter transporters is well documented (Chen et al. 2010, Schmitt & Reith 2010), but the importance of the specific PKC isoforms, notably PKCβ, in trafficking processes is newly emerging. PKCβ promotes trafficking of insulin-stimulated glucose transporter 4 and synaptic vesicles to membranes in adipocytes (Walaas et al. 1997, Wright et al. 2003) and primary cultured neurons (Lu et al. 1998, Yang et al. 2002), respectively. In this study we contribute a new example of PKCβ-dependent trafficking by delineating the role of PKCβ in promoting translocation of DAT to surface membranes upon D2autoR/D2S activation by accelerating the recycling of DAT. We found that quinpirole transiently decreased surface DAT in striatal synaptosomes from PKCβ−/− mice (Fig. 3C). The reduction in surface DAT may have occurred because normal recycling of DAT is dysregulated in the absence of PKCβ (Chen et al. 2009). The requirement of PKCβ for quinpirole-stimulated recycling of DAT to the surface could lead to a reduction in surface DAT when PKCβ is deleted. However, inhibition of PKCβ by LY379196 did not reduce surface DAT below control levels in the heterologous N2A cells (Fig. 1) whether cells were incubated with quinpirole for 30 min as shown in Fig. 1 or even times as short as 5 min (data not shown). Therefore it is possible that the transient decrease in DAT in response to quinpirole could be unique to PKCβ−/− mice.
The PKCβ-ERK signaling cascade modulates DAT trafficking
D2S-modulated DAT trafficking is sensitive to pertussis toxin and thus mediated by Gαi/o (Bolan et al. 2007). Our study suggests the additional requirement of PKCβII stimulation upon activation of D2autoR. Quinpirole stimulation of PKCβII activity promotes DAT trafficking while inhibition of PKCβ abolishes DAT trafficking mediated by D2autoR. PKCβI and PKCβII are isozymes encoded by a single gene locus which differ only in their C-terminus 50–52 amino acids (Ono et al. 1987). In vivo, they can behave as unique isoenzymes with distinct properties. Although both PKCβI and PKCβII couple to DAT (Johnson et al. 2005b, Hadlock et al. 2011), the PKCβII isozyme enhanced AMPH-stimulated reverse transport of DAT (Johnson et al. 2005b). In purified striatal synaptosomes from PKCβ+/+ mice, the selective activation of PKCβII but not PKCβI by quinpirole may be due to differing cellular localization of the proteins. For instance, activated PKCβII translocates to the membrane and interacts specifically with actin and the actin cytoskeleton (Blobe et al. 1996), which facilitates vesicle trafficking and exocytosis. Substrate-induced DAT trafficking to the surface is an exocytotic event that is regulated by soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins (Furman et al. 2009a). A specific PKCβII interaction with the cytoskeleton element may facilitate the trafficking and membrane insertion of DAT through activation of D2autoR. PKCβII activation by quinpirole is likely due to activation of PLCβ by the Gβγ subunit of Gαi/o (Liu et al. 2003). Cocaine has been shown to facilitate PKC maturation to prime PKC for response to the second messenger system (Xue et al. 2012).
Both D2autoR and DAT are subject to PKC regulation and contain identified PKC phosphorylation sites (Morris et al. 2007, Foster et al. 2002). Therefore, a PKCβ-dependent phosphorylation of DAT, D2autoR or both may underlie the association between DAT and D2autoR and facilitate DAT recycling. In fact, G protein-coupled receptor kinases (GRKs) regulate recycling of phosphorylated but not unphosphorylated D2autoR in HEK293 cells (Cho et al. 2010), supporting the notion that phosphorylation of D2S by protein kinases may result in altered trafficking of DAT. It warrants further investigator as to whether or not the increased surface DAT expression by quinpirole is due to co-trafficking of DAT and D2autoR. Nonetheless, our study not only adds a new member to the PKC-ERK signaling family, but also delineates PKCβII as the specific PKC form involved in the signaling.
D2autoR-induced DAT trafficking is distinct from DAT substrate-induced DAT trafficking
Although DAT trafficking induced by either D2autoR or DAT substrate involves PKCβ, the characteristics are distinct. D2autoR-stimulated DAT trafficking is relatively long-lived (≥15 min in striatal synaptosomes and 30 min in N2A-hDAT-D2S cells) as demonstrated in the current study compared to that stimulated by DAT substrate amphetamine (≤15 min) that we reported previously (Chen et al. 2009, Furman et al. 2009a, Johnson et al. 2005a). DAT substrate-induced DAT trafficking does not require D2autoR (Furman et al. 2009a). Interestingly, the processes may also be related to different conformational states of DAT. The increased surface DAT stimulated by amphetamine parallels increased amphetamine-induced DA efflux through DAT suggesting that it may be in a predominantly inward-facing conformation (Furman et al. 2009a). In contrast, the increased surface DAT stimulated by quinpirole parallels increased DA uptake (Fig. 3) which is reflected by an outward-facing conformation. Since quinpirole is a ligand for both D2R and D3R, the presynaptic D3R could also function as an autoreceptor (Zapata et al. 2001). Further investigation on the regulatory machinery of D3R-DAT trafficking will determine whether PKCβ is a common signaling pathway for presynaptic D2S and D3R regulation of DAT trafficking.
Conclusion
We show here that PKCβ is an important modulator of D2autoR-activated DAT trafficking in both mouse striatal synaptosomes and cultured cells. PKCβ facilitates D2autoR-stimulated DAT trafficking by increasing the recycling rate of DAT without an effect on DAT internalization. The signaling cascade of PKCβ-ERK critically modulates D2autoR-activated DAT trafficking. This study provides a new avenue for intervening in the interaction between DAT and D2autoR and controlling synaptic DA levels. This is of clinical significance for diseases such as schizophrenia, ADHD and drug addiction which may involve a dysfunctional dopamine autoreceptor function.
Acknowledgments
This work was supported by NIH grants DA011697 (MEG), DA025954 and DA030890 (RC) and funding from University of Michigan Substance Abuse Research Center (RC). R.F. was supported by NIDA training grant T32-DA007281. We would like to thank Dr. Robert W. Gereau IV (Washington University, MO) for contributing PKCβ knockout mice on C57BL/6 background, Dr. Jonathan Javitch (Columbia University, NY) for the HA-DAT construct and Dr. David Sibley (NIH, MD) for the FLAG-D2S construct.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- D2autoR
dopamine D2-like autoreceptor
- D2S
dopamine D2 receptor short variant
- PKCβ
protein kinase Cβ
- ERK
extracellular signal-regulated kinase
Footnotes
The authors declare no conflict of interests.
Authorship credit
RC and MEG designed the experiments; RC, CPD, RF and SLS performed the experiments and collected the data; RC and MEG analyzed the data and wrote the manuscript; ML donated the PKCβ knockout mice on SV129 background and critically reviewed the manuscript.
References
- Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008b;39:211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30:6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Gnegy ME. Dopamine transporter trafficking: rapid response on demand. Future Neurol. 2010;5:123. doi: 10.2217/fnl.09.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RWt, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, Kim KM. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol Endocrinol. 2010;24:574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS. Operational characteristics of the inhibitory feedback mechanism for regulation of dopamine release via presynaptic receptors. J Pharmacol Exp Ther. 1982;223:497–501. [PubMed] [Google Scholar]
- Cubeddu LX, Lovenberg TW, Hoffman IS, Talmaciu RK. Phorbol esters and D2-dopamine receptors. J Pharmacol Exp Ther. 1989;251:687–693. [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, et al. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JA. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- Edwards AS, Faux MC, Scott JD, Newton AC. Carboxyl-terminal phosphorylation regulates the function and subcellular localization of protein kinase C betaII. J Biol Chem. 1999;274:6461–6468. doi: 10.1074/jbc.274.10.6461. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Jorgensen TN, Gether U. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Vaughan RA. Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J Biol Chem. 2002;277:25178–25186. doi: 10.1074/jbc.M200294200. [DOI] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci. 2009a;29:3328–3336. doi: 10.1523/JNEUROSCI.5386-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman CA, Lo CB, Stokes S, Esteban JA, Gnegy ME. Rab 11 regulates constitutive dopamine transporter trafficking and function in N2A neuroblastoma cells. Neurosci Lett. 2009b;463:78–81. doi: 10.1016/j.neulet.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambalvo CT. Mechanisms underlying the effects of amphetamine on particulate PKC activity. Synapse. 2004;51:128–139. doi: 10.1002/syn.10289. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT, Wagner RL. Activation of D1 and D2 dopamine receptors inhibits protein kinase C activity in striatal synaptoneurosomes. J Neurochem. 1994;63:169–176. doi: 10.1046/j.1471-4159.1994.63010169.x. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Nelson CC, Baucum AJ, 2nd, Hanson GR, Fleckenstein AE. Ex vivo identification of protein-protein interactions involving the dopamine transporter. J Neurosci Methods. 2011;196:303–307. doi: 10.1016/j.jneumeth.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich DR, Pflueger AB. The autoreceptor control of dopamine synthesis. An in vitro and in vivo comparison of dopamine agonists. Mol Pharmacol. 1982;21:114–120. [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- Jirousek MR, Gillig JR, Gonzalez CM, et al. (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem. 1996;39:2664–2671. doi: 10.1021/jm950588y. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005a;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005b;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- L’Hirondel M, Cheramy A, Godeheu G, Artaud F, Saiardi A, Borrelli E, Glowinski J. Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res. 1998;792:253–262. doi: 10.1016/s0006-8993(98)00146-2. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Liu F. Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse. 2009;63:710–712. doi: 10.1002/syn.20648. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. Embo J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Liu G, Ghahremani MH, Banihashemi B, Albert PR. Diacylglycerol and ceramide formation induced by dopamine D2S receptors via Gbeta gamma -subunits in Balb/c-3T3 cells. Am J Physiol Cell Physiol. 2003;284:C640–648. doi: 10.1152/ajpcell.00190.2002. [DOI] [PubMed] [Google Scholar]
- Liu YF, Civelli O, Grandy DK, Albert PR. Differential sensitivity of the short and long human dopamine D2 receptor subtypes to protein kinase C. J Neurochem. 1992;59:2311–2317. doi: 10.1111/j.1471-4159.1992.tb10125.x. [DOI] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yang H, Lenox RH, Raizada MK. Regulation of angiotensin II-induced neuromodulation by MARCKS in brain neurons. J Cell Biol. 1998;142:217–227. doi: 10.1083/jcb.142.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Morris SJ, Van H, II, Daigle M, Robillard L, Sajedi N, Albert PR. Differential desensitization of dopamine D2 receptor isoforms by protein kinase C: the importance of receptor phosphorylation and pseudosubstrate sites. Eur J Pharmacol. 2007;577:44–53. doi: 10.1016/j.ejphar.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem. 2004;279:49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- O’Malley HA, Park Y, Isom LL, Gnegy ME. PKCbeta co-localizes with the dopamine transporter in mesencephalic neurons. Neurosci Lett. 2010;480:40–43. doi: 10.1016/j.neulet.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Kikkawa U, Ogita K, et al. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236:1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Przedborski S, Davila V, Schmitz Y, Sulzer D. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Walaas O, Horn RS, Walaas SI. The protein kinase C pseudosubstrate peptide (PKC19-36) inhibits insulin-stimulated protein kinase activity and insulin-mediated translocation of the glucose transporter glut 4 in streptolysin-O permeabilized adipocytes. FEBS Lett. 1997;413:152–156. doi: 10.1016/s0014-5793(97)00898-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC, Fick CA, Olesen JB, Craig BW. Evidence for the involvement of a phospholipase C--protein kinase C signaling pathway in insulin stimulated glucose transport in skeletal muscle. Life Sci. 2003;73:61–71. doi: 10.1016/s0024-3205(03)00256-x. [DOI] [PubMed] [Google Scholar]
- Xue B, Guo ML, Jin DZ, Mao LM, Wang JQ. Cocaine facilitates PKC maturation by upregulating its phosphorylation at the activation loop in rat striatal neurons in vivo. Brain Res. 2012;1435:146–153. doi: 10.1016/j.brainres.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang X, Sumners C, Raizada MK. Obligatory role of protein kinase Cbeta and MARCKS in vesicular trafficking in living neurons. Hypertension. 2002;39:567–572. doi: 10.1161/hy0202.103052. [DOI] [PubMed] [Google Scholar]
- Yoshihara C, Saito N, Taniyama K, Tanaka C. Differential localization of four subspecies of protein kinase C in the rat striatum and substantia nigra. J Neurosci. 1991;11:690–700. doi: 10.1523/JNEUROSCI.11-03-00690.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Witkin JM, Shippenberg TS. Selective D3 receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology. 2001;41:351–359. doi: 10.1016/s0028-3908(01)00069-7. [DOI] [PubMed] [Google Scholar]