Abstract
IL-10 is important in the resistance response of BALB/c mice to experimental Pseudomonas aeruginosa corneal infection. However, the cellular mechanism(s) by which this anti-inflammatory cytokine is regulated remains unknown. Since the mammalian target of rapamycin (mTOR), regulates IL-10 in other disease models, the current study tested its role in bacterial keratitis. After infection, corneas of rapamycin vs. control-treated BALB/c mice showed worsened disease and real-time RT-PCR confirmed that mTOR mRNA levels were significantly decreased. Rapamycin treatment also increased clinical score, PMN infiltration (determined by MPO assay), and bacterial load, but diminished PMN bactericidal activity. Inhibition of mTOR also led to elevated mRNA and protein levels of IL-12p40, matrix metalloproteinase 9 (MMP-9), and iNOS, while mRNA and protein levels of IL-10, its regulator/effector STAT-3, and SOCS3 (a pro-inflammatory cytokine regulator) were decreased. Furthermore, mTOR inhibition reduced levels of pro-apoptotic caspase-3 and increased levels of Bcl-2 (anti-apoptotic), indicative of delayed apoptosis. mTOR inhibition also altered genes related to TLR signaling, including elevation of TLR4 and 5, and IL-1R1, with decreases in IRAK-1 and an inhibitor of NFκB, NFκBIL-1. Rapamycin treatment also increased levels of IFN-γ and Cebpb, a gene that regulates expression of preprotachykinin-A (PPT-A; the precursor of Substance P). Collectively, these data, as well as a rescue experiment using rIL-10 together with rapamycin, which decreased PMN in cornea, provide concrete evidence that mTOR regulates IL-10 in P. aeruginosa-induced bacterial keratitis and is critical to balancing pro- and anti-inflammatory events, resulting in better disease outcome.
Keywords: Rapamycin, mTOR, IL-10, mice, cornea
Introduction
Pseudomonas (P.) aeruginosa infection in resistant Th2-responder BALB/c mice elicits production of both pro- and anti-inflammatory cytokines. Among those that are anti-inflammatory, IL-10 has been show as critical in balancing cytokines such as IFN-γ and decreasing stromal damage, while promoting successful pathogen elimination (1, 2). Nevertheless, the cellular mechanisms regulating IL-10 in the P. aeruginosa-infected cornea of BALB/c mice have not yet been determined. In this regard, in other models, the mammalian target of rapamycin (mTOR) has been of interest as it is upstream of IL-10 (3–5) and a target of a diverse array of microbes, growth factors, hormones, and amino acids that elicit a host innate immune response.
mTOR itself is a serine-threonine kinase that also mediates cell growth and proliferation, ribosome biogenesis, and cytoskeletal organization (6,7). It is contained within two functional complexes: mTORC1 and mTORC2 and is active when complexed with Raptor, mLST8, and PRAS40 (forming mTORC1), or when complexed with Rictor, mLST8, and Sin1 (forming mTORC2) (6,7).
mTORC1 alone is sensitive to inhibition by the macrolide antibiotic rapamycin that blocks the formation of the complex described above. Clinically, rapamycin is used as an immunosuppressant in allogeneic transplantation. However, while used successfully in clinical practice for immunosuppression after kidney transplant (8), recent evidence suggests that it may have inflammatory side effects, including: fever, anemia, and glomerulonephritis (9). Moreover, inhibition of mTOR in mice can enhance LPS-induced shock that correlates with elevated levels of pro-inflammatory cytokines such as IL-12p40 (10).
Similarly, another study showed that inhibition of mTOR diminished IL-10 levels, while elevating IL-12p40 (and IL-23) in vitro, which was protective in vivo in experimental Listeria monocytogenes infection (5). In contrast, it has been shown that in corneal P. aeruginosa infection, IL-10 is required for resistance in the BALB/c mouse and that if macrophages (a source of the cytokine) are depleted, levels of IL-10 are decreased with concurrent elevation of IFN-γ, resulting in an overall worsening of disease (1). PMN, a source of IL-12p40 (11) are of prime importance in bacterial keratitis, and if dysregulated, can enhance stromal destruction and disease (11). Yet, the effects of rapamycin treatment on PMN remain untested and relevant to determine in bacterial keratitis.
In summary, this study provides evidence that IL-10 is regulated in the infected cornea by the mTOR pathway. This conclusion is based upon data showing that inhibition of mTOR by rapamycin decreased anti-inflammatory cytokines, particularly IL-10, upregulated several pro-inflammatory cytokines (IL-12p40, IL-23, and IFN-γ) and their regulators, and that administration of rIL-10 with rapamycin reversed increased PMN in cornea, one effect of mTOR inhibition.. Furthermore, rapamycin downregulated caspase-3 and upregulated Bcl-2, the latter prolonging inflammatory cell viability, possibly further contributing to stromal destruction and unresolved disease.
Materials and Methods
Mice
Female 8-week-old BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in accordance with the National Institutes of Health guidelines. The animals were treated humanely in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Bacterial culture and infection
P. aeruginosa cultures (strain 19660; purchased from the American Type Culture Collection Manassas, VA) were grown in peptone tryptic soy broth (PTSB) at 37°C on a 150 rpm rotary shaker for 18 h to an optical density reading between 1.3–1.8 at 540 nm. Stock cultures were centrifuged at 6000 × g for 10 min, washed once with sterile saline, recentrifuged, and resuspended to a final concentration of approximately 1 × 104 CFU/µL (1). Under a stereoscopic microscope (magnification=40X), a sterile 25 5/8 gauge needle was used to scar the left central cornea of ethyl ether-anesthetized BALB/c mice. After which a 5 µL suspension of P. aeruginosa was topically applied. To analyze phagocytic capacity, GFP-expressing P. aeruginosa was grown to a logarithmic phase in PTSB with kanamycin (50 µg/mL), washed twice with PBS, resuspended and diluted in PTSB (with kanamycin) and used as described below for phagocytosis assays.
Ocular response to bacterial infection
Corneal disease was graded using an established scale (12): 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; +3 = dense opacity, covering the entire anterior segment; and +4 = corneal perforation. After infection, a clinical score was recorded (days 1, 3, and 5) for each group of mice (n=5/group/ treatment/assay). Lastly, representative corneas were photographed using a slit lamp to document the effects of rapamycin vs. control treatment.
Rapamycin treatment
Rapamycin (LC Laboratories, Woburn, MA) was prepared to a concentration of 20 µg/µl in 100% ethanol and stored at −20°C. Before intraperitoneal injection, the rapamycin in ethanol was diluted in sterile PBS. To test the appropriate concentration of rapamycin, BALB/c mice (n=5/group/time/assay) were anesthetized with ether and intraperitoneally injected with 100 µL of rapamycin (1.5 or 3.0 mg/kg) (5) or sterile PBS (Mediatech, Manassas, VA) on the day before infection (d= −1) and each day through 5 days, post-infection (p.i.). Results indicated that the higher concentration of rapamycin significantly lowered IL-10 protein levels (comparative data not shown) and thus all subsequent experiments were done using the higher concentration of rapamycin prepared as described above. In a separate experiment BALB/c mice were injected with rapamycin as described above with or without injection of recombinant (r) IL-10 (R&D Systems, Minneapolis, MN). rIL-10 (1 µg/µl) was injected subconjunctivally the day before infection and a similar amount was injected intraperitoneally on the day of infection and at one day p.i. Corneas were harvested for MPO assay at 1 and 3 days p.i. as described below.
Quantification of corneal PMN
An MPO (myeloperoxidase) assay was used to quantitate the polymorphonuclear neutrophilic (PMN) leukocyte infiltrate in the corneas of rapamycin and PBS-treated BALB/c mice (n=5/group/time) at 1 and 3 days p.i. Corneas were harvested, homogenized in 1 mL of potassium phosphate buffer (50 mM, pH 6.0) containing 0.5% hexodecyltrimethylammonium bromide (Sigma-Aldrich) using glass micro-tissue grinders. The samples were freeze-thawed four times and centrifuged at 14,000 rpm for 10 min. To measure MPO, a 100 µL sample of supernatant was added to fresh 2.9 mL o-dianisidine dihydrochloride substrate buffer (16.7 mg/mL; Sigma-Aldrich) with 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was read every 30 seconds for 5 min on a Heλios αspectrophotometer (Thermo Fisher Scientific, Waltham, MA). Afterward, units of MPO/cornea were calculated: one unit of MPO activity is equivalent to approximately 2 × 105 PMN/µL (13).
Quantification of viable bacteria
Bacteria were quantitated at 1 and 3 days p.i. in individual infected corneas of BALB/c mice (n=5/group/time) after rapamycin or PBS treatment. Each cornea was homogenized in 1 mL sterile PBS containing 0.25% BSA; 100 µL of the corneal homogenate was serially diluted 1:10 in the same solution. Select dilutions were plated in triplicate on pseudomonas isolation agar plates (Becton-Dickinson, Franklin Lakes, NJ). Plates were incubated overnight at 37°C and the number of viable bacteria manually counted. Results are reported as 1 × 103 CFU/cornea ± SEM.
Quantification of PMN phagocytic and intracellular killing capacity
PMN from BALB/c mice were isolated (14) and processed essentially as described before (15). Briefly, mice received an i.p. injection of a 9% casein solution (1.0 mL) and a second similar injection 24 h later. PMN (90% of elicited cells) were lavaged from the peritoneal cavity 3 h after the second injection, washed, and isolated using a Percoll gradient. Viable cells (≥94.3%) were stained with Trypan blue and counted using a Cellometer Vision CBA Analysis System v. 2.1.0. (Nexcelon Bioscience LLC, Lawrence, MA) and resuspended in RPMI (with 10% FBS) at the desired concentration for in vitro stimulation. Cells were treated with rapamycin (0.01 µM and 1.0 µM) or sterile 1X PBS (negative and positive controls) for 18 h at 37°C. Cells were washed to remove the inhibitor, then resuspended in media. PMN viability after rapamycin treatment was decreased from ≥94.3% to between 87.5 to 90.4%.
For phagocytosis assays (16), 1 × 106 CFU/µL of GFP-labeled bacteria was added to each well (a ratio of 2.5:1, bacteria to PMN) of a twelve well plate, omitting bacterial addition to the negative control wells. Plates were centrifuged at 500 × g for 10 min and then incubated for 2 h at 37°C. Cells were washed twice to remove bacteria, then incubated with 0.04% Trypan blue to quench the fluorescence of surface-associated bacteria and to again measure PMN viability (≥87.5%). To analyze intracellular killing, PMN were incubated with P. aeruginosa, harvested, and treated as above. Then, after 1 h incubation at 37°C, 200 µg/mL gentamicin (16) was added for an additional 3 h to ensure killing of extracellular bacteria. Cells were washed twice with Hank’s Balanced Salt Solution (HBSS). Supernatant was removed and 0.5 mL of ice-cold water was added to lyse PMN. Samples were kept on ice for 10 min after which 0.5 mL 2X HBSS was added to a final volume of 1 mL. 250 µL of cell suspension was plated in triplicate on pseudomonas isolation agar (Becton-Dickinson) and incubated for 24 h at 37°C. Afterward, colonies were manually counted and recorded as the mean CFU ± SEM. The experiment was repeated twice.
Real-time RT-PCR
Mice were sacrificed; normal and infected corneas from rapamycin- and PBS-treated mice (n=5/group/time) were removed (1, 3, and 5 days p.i.). Individual corneas were briefly stored in RNA STAT-60™ (Tel-Test, Friendsville, TX) at −20°C before processing. Total corneal RNA was extracted with chloroform (200 µL/mL RNA STAT-60™; Sigma-Aldrich) and precipitated overnight with isopropanol (500 µL/mL RNA STAT-60™; Sigma-Aldrich). Samples were washed in cold 75% ethanol. To produce a cDNA template for the PCR reaction, 1 µg of each RNA sample was reverse transcribed using Moloney-murine leukemia virus (M-MLV). The 20 µL mixture contained 200 U M-MLV-reverse transcriptase, 10 U of RNase inhibitor, 500 ng oligo(dT) primers, 10 mM dNTPs, 100 mM DTT, and M-MLV reaction buffer (all from Invitrogen, Carlsbad, CA). cDNA products were diluted 1:25 with diethylpyrocarbonate-treated water and a 2 µL cDNA aliquot was used for real-time RT-PCR. mRNA levels of mTOR, STAT-3, IL-10, IL-12p40, IL-23, inducible nitric oxide synthase (iNOS), matrix metallopeptidase (MMP-9), suppressor of cytokine signaling 3 (SOCS3), caspase-3, B cell lymphoma (Bcl)-2, Cebpb, NFκB inhibitor-like 1 (NFκBIL-1), TLR4, TLR5, IFN-γ, IL-1 receptor (IL-1R1), and interleukin-1 receptor-associated kinase 1 (IRAK-1) were tested by real-time RT-PCR (MyiQ™ Single Color Real-Time PCR Detection System; Bio-Rad, Hercules, CA). Real-Time SYBR® Green/Fluorescein PCR Master Mix (Bio-Rad) was used for the PCR reaction with primer concentrations of 10 µM. After a pre-programmed hot start cycle (3 min at 95°C), the parameters used for PCR amplification were: 15 sec at 95°C and 60 sec at 60°C with the cycles repeated 45 times. The fold differences in gene expression were calculated after normalization to β-actin and are expressed as the relative mRNA concentration ± SEM. The primer pair sequences used for real-time RT-PCR are shown in Table I.
TABLE I.
Nucleotide sequences of mouse primers for real-time RT-PCR.
| Gene | GenBank No. | Primer Sequence (5’ - 3’) | Size (bp) |
|---|---|---|---|
| β-Actin | NM_007393.3 | F - GAT TAC TGC TCT GGC TCC TAG C | 147 |
| R - GAC TCA TCG TAC TCC TGC TTG C | |||
| Bcl-2 | NM_009741 | F - GGA CTT GAA GTG CCA TTG GT | 127 |
| NM_177410.2 | R - AGC CCC TCT GTG ACA GCT TA | ||
| caspase-3 | NM_009810.2 | F - TGG GCC TGA AAT ACC AAG TC | 147 |
| R - AAA TGA CCC CTT CAT CAC CA | |||
| Cebpb | NM_009883.3 | F - TGATGCAATCCGGATCAA | 63 |
| R - CACGTGTGTTGCGTCAGTC | |||
| IFN-γ | NM_008337.3 | F - GTT ACT GCC ACG GCA CAG TCA TTG | 125 |
| R - ACC ATC CTT TTG CCA GTT CCT CCA G | |||
| IL-1R1 | NM_001123382.1 | F - CTC TGC TTC TTG ACA ACG TGA GCT TC | 87 |
| NM_008362.2 | R - TAT AGT CCC CTC TGT GCT CTT CAG CC | ||
| IL-10 | NM_010548.2 | F - TGC TAA CCG ACT CCT TAA TGC AGG AC | 126 |
| R - CCT TGA TTT CTG GGC CAT GCT TCT C | |||
| IL-12p40 | NM_008352.2 | F - GGT CAC ACT GGA CCA AAG GGA CTA TG | 121 |
| R - ATT CTG CTG CCG TGC TTC CAA C | |||
| IL-23 | NM_031252.2 | F - AAT AAT GTG CCC CGT ATC CAG T | 142 |
| R - GCT CCC CTT TGA AGA TGT CAG | |||
| iNOS | NM_010927.3 | F - TCC TCA CTG GGA CAG CAC AGA ATG | 118 |
| R - GTG TCA TGC AAA ATC TCT CCA CTG CC | |||
| IRAK-1 | NM_008363.2 | F - CAGAACCACCACAGATCATCATC | 87 |
| R - GGCTATCCAAGACCCCTTCTTC | |||
| MMP-9 | NM_013599.2 | F - CTC TAC AGA GTC TTT GAG TCC GGC AG | 119 |
| R - TCA GGA ACT TCC AGT ACC AAC CGT C | |||
| mTOR | NM_020009.2 | F - ACC GGC ACA CAT TTG AAG AAG | 110 |
| R - CTC GTT GAG GAT CAG CAA GG | |||
| NFκBIL-1 | NM_010909.4 | F - CCCTGATGCTTACACGGACTT | 105 |
| R - CAGCCCAGAATCTGCCCAG | |||
| SOCS3 | NM_007707.3 | F - GGG GAC CAA GAA CCT ACG C | 131 |
| R - CTG GAG GCG GCA TGT AGT G | |||
| STAT-3 | NM_011486.4 | F - TGG CAC CTT GGA TTG AGA GTC | 117 |
| R - GCA GGA ATC GGC TAT ATT GCT | |||
| TLR4 | NM_021297.2 | F - CGC TTT CAC CTC TGC CTT CAC TAC AG | 109 |
| R - ACA CTA CCA CAA TAA CCT TCC GGC TC | |||
| TLR5 | NM_016928.2 | F - TGC TCA AAC ACC TGG ATG CTC ACT AC | 112 |
| R - ACA GCC GCC TGG ATG TTG GAG ATA TG |
ELISA
After mice were treated with rapamycin or sterile PBS, IL-10, IL-12p40, p-STAT-3, Cebpb and IFN-γ protein levels were determined in normal and infected mouse corneas (n=5/group/time, repeated once) using ELISA kits (R&D Systems; except Cebpb which was purchased through antibodies-online.com, Atlanta, GA). For these assays, tissue was prepared as described before (2). 50 µL (IL-12p40, IFN-γ) or 100 µL (IL-10, p-STAT-3, and Cebpb) aliquots of each supernatant were assayed in duplicate according to the manufacturer’s instructions (R&D Systems; Cebpb – antibodies-online.com). Sensitivities for the assays were: >4.0 pg/mL (IL-10), 0.6 – 2.7 pg/mL (IL-12p40), 33 pg/mL (Cebpb), >2.0 pg/mL (IFN-γ), and >4.48 pg/mL (p-STAT-3) (R&D Systems). Results are expressed as pg/mL (fg/mL only for IFN-γ) of protein ± SEM.
Immunohistochemistry
After sacrificing mice, normal and 1 and 5 days p.i. eyes were enucleated from rapamycin- or PBS-treated animals (n=5/group/time), embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Torrance, CA) and snap frozen in liquid nitrogen. Sections (10 µm) were cut, fixed in acetone, and incubated in blocking agent as described before (1). Sections were simultaneously incubated with individual primary antibodies: rabbit anti-mouse caspase-3 (1:800; Cell Signaling, Danvers, MA) and hamster anti-mouse Bcl-2 (1:100; BD Biosciences, San Jose, CA) in blocking agent for 1 h. Then, sections were rinsed with phosphate buffer and incubated for another hour with Alexa Fluor 633 goat anti-rabbit secondary antibody for caspase-3 (1:1500; Invitrogen) and Alexa Fluor 546 goat anti-hamster for Bcl-2 (1:1500; Molecular Probes, Eugene, OR) in 0.01M Tris-HCl buffer. Sections were rinsed in the latter buffer and then incubated with nuclear acid stain (SYTOX® Green, 1:20,000; Lonza, Walkersville, MD) and rinsed again. Digital images were captured on a confocal laser-scanning microscope (TCS SP2, Leica Microsystems, Exton, PA). Negative controls for each group were processed similarly, with the exception that each primary antibody was replaced by the same host species IgG.
Nitrite concentration (Griess assay)
NO production was measured in uninfected corneas and 1 and 5 day p.i. after PBS vs. rapamycin-treatment (n=5/group/time) using Griess reagent (Sigma) which quantifies the stable end product nitrite. Corneal samples were homogenized in sterile PBS with micro-tissue grinders and 100 µL aliquots were incubated in 100 µL Griess reagent for 15 min at room temperature. Absorbance was measured at 570 nm and nitrite concentrations calculated using a sodium nitrite standard curve. The data is reported as the mean µM/cornea concentration of nitrite ± SEM.
Toll-like receptor PCR array
The corneas of rapamycin- vs. PBS-treated mice (n=5/group/time) were harvested at three days p.i., pooled, and processed for cDNA using a RT2 First Strand Kit (Qiagen Inc., Valencia, CA). mRNA levels of 84 genes were profiled using a Toll-Like Receptor RT2 Profiler™ PCR Array (Qiagen Inc.). Selected genes that had at least a two-fold or greater change were tested by real-time RT-PCR and ELISA assay.
Statistical analysis
The difference in clinical score between two groups was tested by the Mann-Whitney U test. For all other experiments, an unpaired, two-tailed, Student’s t test (for comparisons between two groups) was used. All data were considered significant at p ≤ 0.05. Each experiment was repeated at least once to ensure reproducibility and data from a representative experiment are shown. Quantitative data is expressed as the mean + the standard error of the mean (SEM), except for the clinical score data in which medians are shown.
Results
Disease response, mTOR inhibition, and PMN infiltration
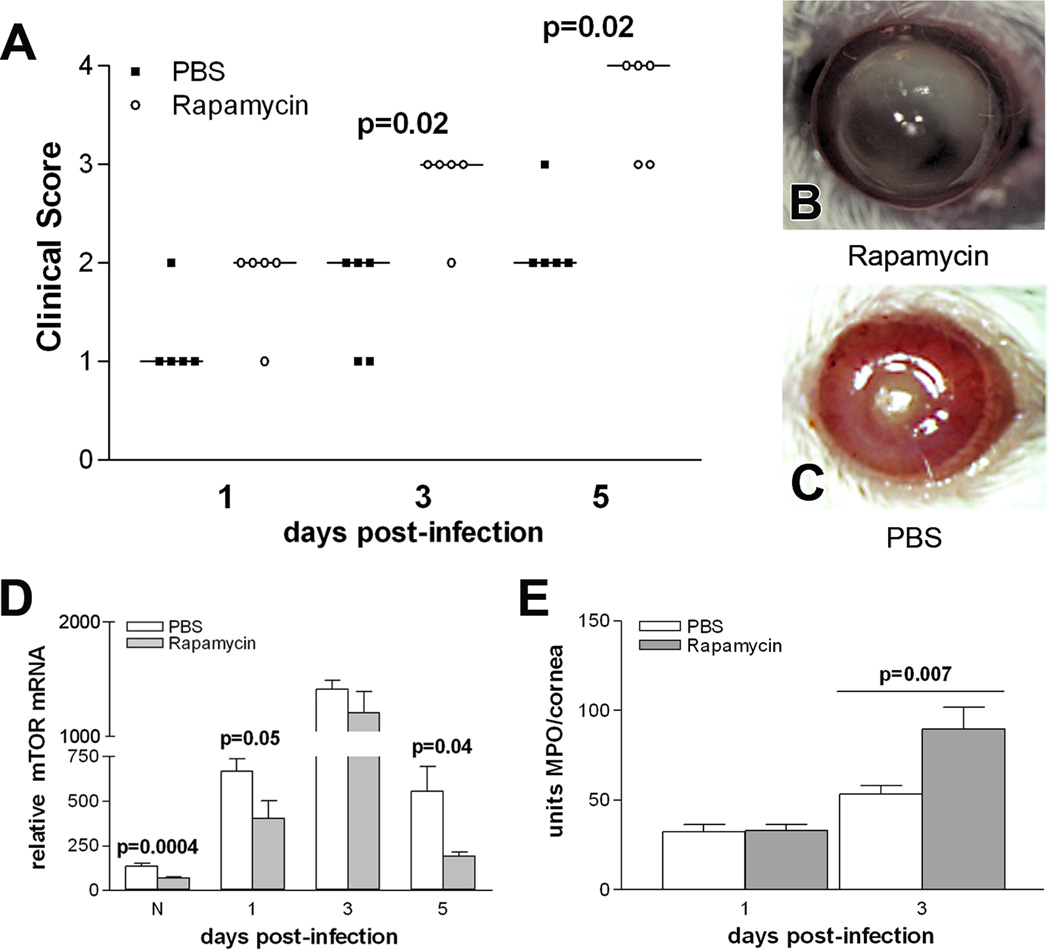
More severe disease (Fig. 1A) was seen after rapamycin treatment at 3 and 5 days p.i. (p=0.02 for both) when compared with PBS-treated controls; no significant difference was observed at 1 day p.i. Photographs taken with slit lamp of representative eyes from both groups at 5 days p.i. (Fig. 1B, C) confirmed that rapamycin treatment increased corneal opacity and resulted in corneal thinning (Fig. 1B), while the cornea of PBS-treated mice exhibited slight central opacity (Fig. 1C). mTOR mRNA levels also were tested using real-time RT-PCR (Fig. 1D). Rapamycin downregulated mTOR when compared to PBS treatment in the normal, uninfected cornea and at 1 and 5 days p.i. (p=0.0004, p=0.05, p=0.04). An MPO assay was used to quantitate PMN in the infected cornea of both groups (Fig. 1E) and revealed that after rapamycin vs. PBS treatment, MPO levels were significantly increased at 3 (p=0.007), but did not differ between groups at 1 day p.i.
Figure 1.
Effects of rapamycin treatment in vivo. Clinical scores (A) were significantly increased at 3 and 5 days p.i. in rapamycin vs. PBS treated mice. Photographs with a slit lamp confirmed that the cornea of rapamycin (B) vs. PBS (C) treated mice had increased opacity and corneal thinning. Rapamycin vs. PBS also significantly decreased mTOR mRNA levels (D) in normal uninfected cornea, and at 1 and 5 days p.i. MPO levels (E) were significantly elevated at 3 days p.i. in rapamycin vs. PBS treated mice, with no differences at 1 day p.i. Bars show median values; magnification = 7X.
Bacterial load, PMN phagocytosis and intracellular killing
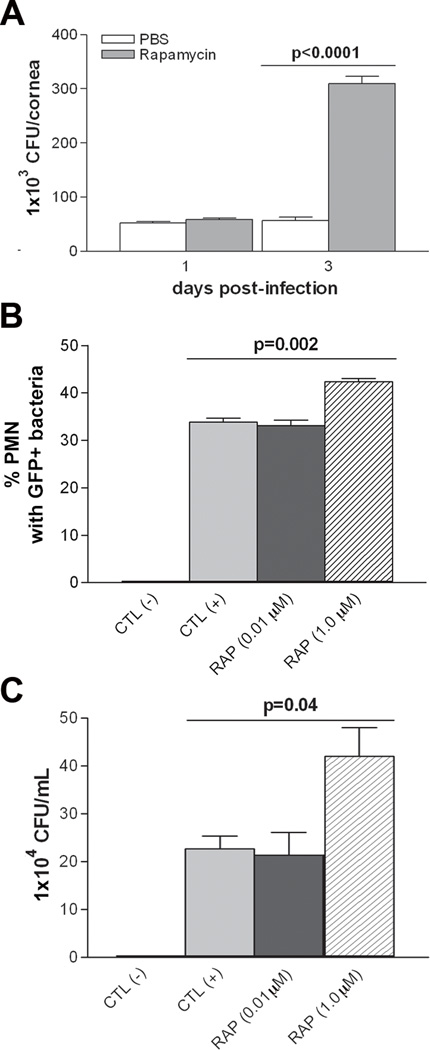
Viable bacterial load in the cornea of rapamycin- vs. PBS-treated mice was significantly higher at 3 days p.i. (Fig. 2A; p<0.0001); no significant difference was observed at 1 day p.i. An in vitro PMN phagocytosis assay (Fig. 2B) compared PBS with no bacteria (negative control), plus GFP-labeled bacteria (positive control), and GFP-labeled bacteria with rapamycin at either 0.01 or 1.0 µM concentration. Rapamycin at 1.0 µM concentration significantly increased the % of PMN containing GFP-labeled bacteria (p=0.002) over the positive control. However, no differences were detected between the positive control and the lower concentration of rapamycin. The ability of PMN to kill bacteria was similarly assessed amongst the groups and was significantly compromised (Fig. 2C; p=0.04) compared to the positive control, only at a concentration of 1.0 µM rapamycin.
Figure 2.
Rapamycin effects on PMN phagocytosis and intracellular killing. (A) Bacterial load was significantly elevated at 3 days p.i. in rapamycin vs. PBS treated mice, with no differences at 1 day p.i. (B) Rapamycin treatment (1.0 µM) significantly increased PMN phagocytosis when comparing the percent of cells containing GFP+ bacteria with the positive control. No effect was seen at the lower concentration (0.01µM). (C) Plate counts assessed bacterial killing and only 1.0 µM rapamycin showed a significant increase in bacterial load (decreased killing) compared with the positive control group.
Real-time RT-PCR of rapamycin effects on STAT-3, IL-10, IL-12p40 and IL-23 mRNA levels
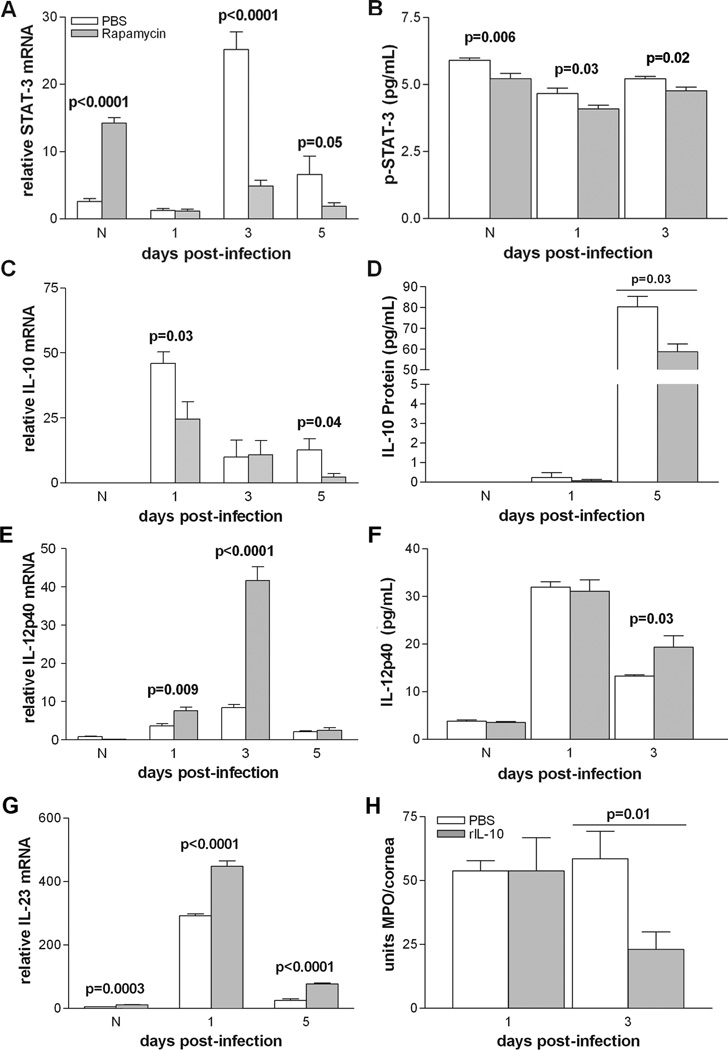
The balance between pro- and anti-inflammatory cytokines is critical to disease resistance in the infected cornea of BALB/c mice (1, 2). Thus, real-time RT-PCR was used to characterize the role of mTOR in their regulation. Figure 3 (A, C, E, G) shows corneal mRNA levels of STAT-3, IL-10, IL-12p40, and IL-23 at various times after rapamycin or PBS treatment. Rapamycin treatment resulted in a significant increase in STAT-3 mRNA expression (Fig. 3A) in the normal cornea and a decrease at 3 and 5 days after infection (p<0.0001, p<0.0001, p=0.05) when compared to PBS controls. No difference was detected at 1 day p.i. between the two groups. IL-10 mRNA expression (Fig. 3C) was significantly decreased at 1 and 5 days after infection (p=0.03 and p=0.04) following rapamycin treatment, with no difference between groups in the normal cornea or at 3 days p.i. In contrast, IL-12p40 (Fig. 3E) mRNA was significantly increased after rapamycin treatment at 1 and 3 days p.i. (p=0.009 and p<0.0001), with no difference between groups in the normal cornea or at 5 days p.i. mRNA levels of IL-23 (Fig. 3G) also were increased significantly in normal cornea, and at 1 and 5 days after infection (p=0.0003, p<0.0001 for both times p.i.).
Figure 3.
(A–H). Effects of rapamycin treatment on STAT-3, IL-10 and IL-12 expression levels in cornea and rIL-10 rescue experiment. After rapamycin treatment: STAT-3 mRNA (A) levels were downregulated at 3 and 5 days p.i., but increased in the normal uninfected cornea and unchanged at 1 day p.i.; mRNA levels of IL-10 (C) were decreased at 1 and 5 days p.i., undetected in the normal uninfected eye and no different between groups at 3 days p.i.; IL-12p40 (E) mRNA levels were increased at 1 and 3 days p.i., but did not differ between groups at 5 days p.i. or in the normal cornea. IL-23 mRNA (G) was increased after rapamycin treatment in normal cornea, and at 1 and 5 days p.i. For protein, p-STAT-3 (B) was downregulated after rapamycin treatment in normal and at 1 and 3 days p.i. IL-10 protein levels (D) were not detected in either group in normal cornea, did not differ at 1 day p.i. between groups, but were decreased after rapamycin treatment at 5 days p.i. IL-12 p40 protein (F) was increased at 3 days p.i., but did not differ between groups in the normal cornea or at 1 day p.i. Injection of rIL-10 (H) together with rapamycin vs. PBS treatment, decreased PMN (MPO assay) at 3 but not 1 day p.i.
Rapamycin treatment leads to a pro-inflammatory environment, but rIL-10 reverses rapamycin elevation of MPO
To selectively confirm the real-time RT-PCR data, ELISA assays were performed to determine the protein levels of p-STAT-3, IL-10, and IL-12p40 (Fig. 3B, D, F). Rapamycin treatment significantly decreased levels of p-STAT-3 (Fig. 3B), the activated form of STAT-3, in the normal cornea and at 1 and 5 days p.i. (p=0.006, p=0.03, p=0.02). In the normal cornea, IL-10 protein levels were below detectability for both groups (Fig. 3D) and did not differ between groups at 1 day p.i. However, at 5 days p.i., rapamycin decreased IL-10 levels compared to control treatment (p=0.03). In contrast, IL-12p40 (Fig. 3F) was increased in the rapamycin-treated group at 3 days p.i. (p=0.03) with no differences seen between groups in the normal cornea or 1 day after infection. Lastly, when rIL-10 was injected with rapamycin vs. rapamycin treatment alone (Fig. 3H), MPO activity was decreased, reversing the effects of rapamycin at 3 days p.i. (p=0.01), but not at one day p.i.
Real-time RT-PCR testing downstream effectors of IL-10
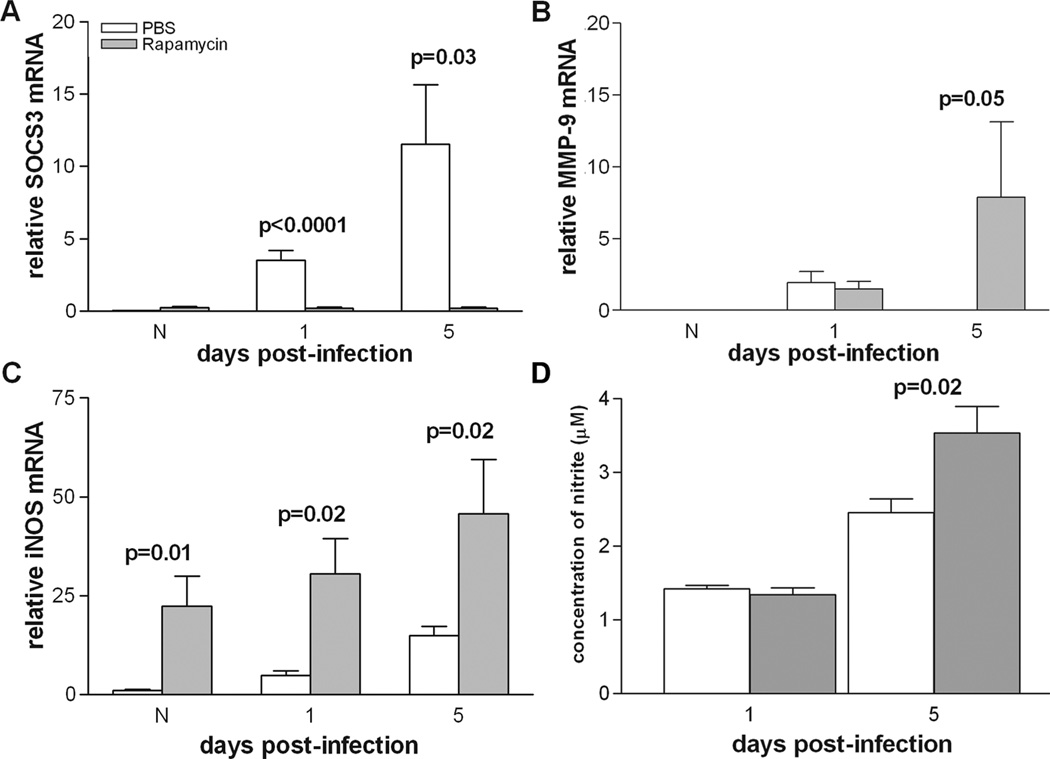
To explore the impact of IL-10 downregulation, real-time RT-PCR was used to test several downstream effectors of IL-10: SOCS3, MMP-9, and iNOS (Fig. 4 A–C). Treatment with rapamycin significantly decreased mRNA levels of the pro-inflammatory cytokine regulator SOCS3 (Fig. 4A) at both 1 and 5 days p.i. (p<0.0001 and p=0.03) compared to PBS controls, with no difference between groups in the normal cornea. In contrast, rapamycin treatment significantly increased levels of MMP-9 at 5 days p.i. (Fig. 4B; p=0.05) with no differences between groups in the normal cornea or at 1 day p.i. Furthermore, the nitric oxide (NO) catalyzing enzyme iNOS was significantly upregulated after rapamycin vs. PBS treatment (Fig. 4C) in the normal cornea and at both 1 and 5 days after infection (p=0.01, p=0.02, p=0.02).
Figure 4.
Response of IL-10 effectors to rapamycin treatment. mRNA levels for SOCS3 (A) were significantly decreased at 1 and 5 days p.i. after rapamycin vs. PBS treatment. mRNA levels of MMP-9 (B) were upregulated at 5 days p.i., while iNOS mRNA levels (C) were significantly increased in normal cornea and at 1 and 5 days p.i. Nitrite levels (D) were significantly increased in the rapamycin-treated group only at 5 days p.i.
Griess analysis of nitrite production
Levels of NO were tested using a Griess assay that measures nitrite concentration (Fig. 4D). At 1 day p.i., there was no significant difference in corneal nitrite levels between PBS- and rapamycin-treated mice. However, by 5 days p.i., nitrite levels were significantly increased in the rapamycin-treated group (p=0.02).
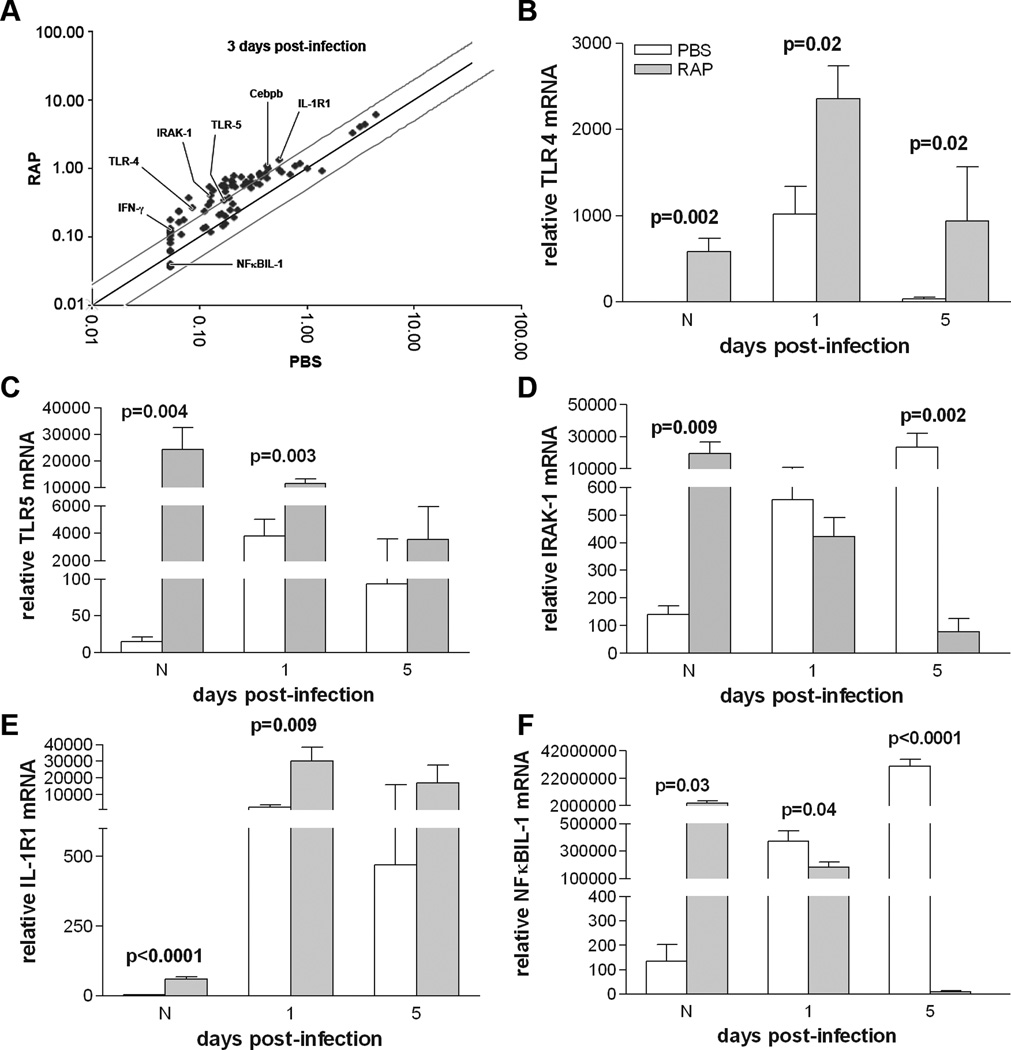
RT2 Profiler PCR array and real-time RT-PCR of TLR-related genes
TLR-related genes were profiled in the cornea at 3 days p.i. using a PCR array. Several genes with greater than 2-fold change in expression were selected for further analysis using real-time RT-PCR (labeled in Fig. 5A and shown in Table II): TLR4, TLR5, IRAK-1, NFκBIL-1, IL-1R1, IFN-γ, and Cebpb. Rapamycin treatment significantly increased mRNA expression levels of TLR4 in the normal cornea and at 1 and 5 day p.i. (Fig. 5B; p=0.002, p=0.02, p=0.02). TLR5 mRNA levels also were increased in the normal cornea and at 1 day p.i. (Fig. 5C; p=0.004, p=0.003) with no significant change at 5 days p.i. IRAK-1, whose signaling leads to the translocation of NFκB from the cytoplasm to the nucleus was significantly upregulated with rapamycin treatment in the normal cornea (Fig. 5D, p=0.009), but by 5 days p.i., levels were downregulated (Fig. 5D; p=0.002). IL-1R1 was significantly increased by rapamycin treatment in the normal cornea and at 1 day p.i. (Fig. 5E; p<0.0001 and p=0.009). No significant difference was observed at 5 days p.i. Finally, an inhibitor of NFκB translocation to the nucleus, NFκBIL-1, was significantly downregulated with rapamycin treatment at 1 and 5 days p.i. but upregulated in the normal cornea (Fig. 5F; p=0.04, p<0.0001, p=0.03).
Figure 5.
TLR RT2 Profiler PCR array and real-time RT-PCR comparing rapamycin to PBS treatment. A scatter plot of several genes from PCR array that exhibited at least 2 fold change at 3 days p.i. are shown (A). These also were tested by RT-PCR: mRNA levels for TLR4 (B) were elevated in the normal uninfected cornea and at 1 and 5 days p.i; TLR5 mRNA levels (C) were upregulated in normal and at 1 day, but not 5 days p.i. IRAK-1 levels (D) were elevated in normal, unchanged at 1 and decreased at 5 days p.i. For IL-1R1 (E), levels were increased in the normal and at 1 day p.i., but were not significantly different at 5 days p.i. For NFκBIL-1 (F), levels were increased in normal, but decreased at 1 and 5 days p.i.
TABLE II.
Selected TLRs from the RT2 Profiler PCR array
| Gene | Fold up- or down-regulation |
|---|---|
| Cebpb | 3.09 |
| IFN-γ | 3.00 |
| IL-1R1 | 4.07 |
| IRAK-1 | 3.31 |
| NFκBIL-1 | 2.57 |
| TLR-4 | 3.12 |
| TLR-5 | 2.63 |
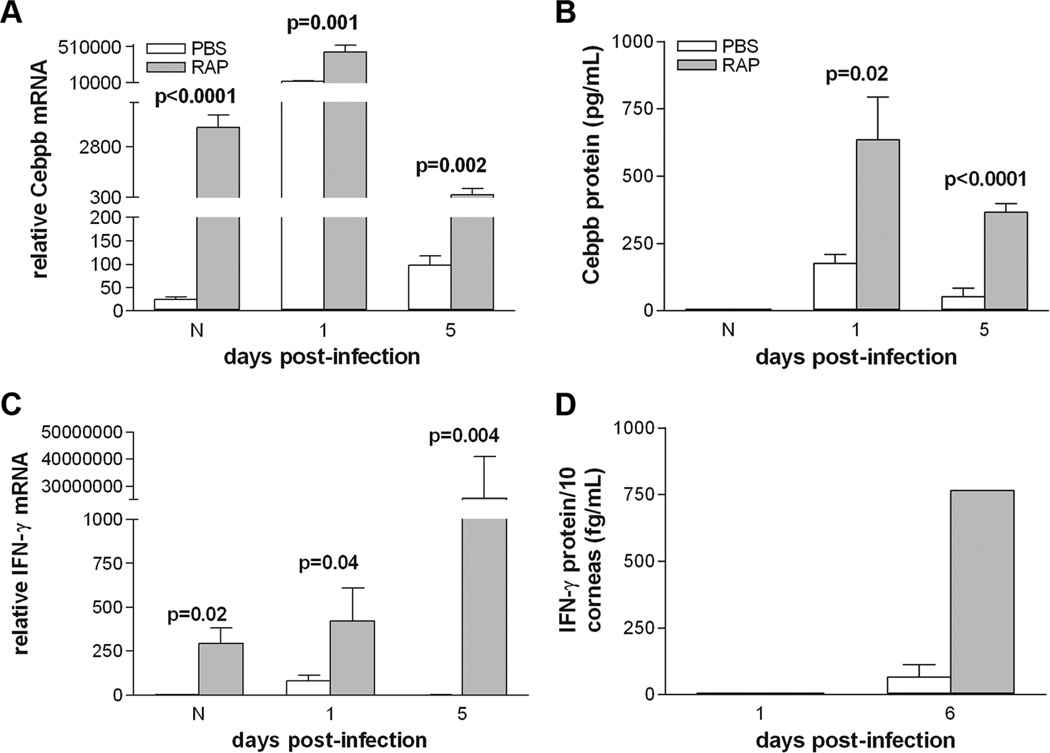
Rapamycin increases pro-inflammatory genes Cebpb and IFN-γ
Rapamycin treatment significantly increased mRNA levels of pro-inflammatory Cebpb in the normal cornea and at 1 and 5 days after infection (Fig. 6A; p<0.0001, p=0.001, p=0.002). Likewise, IFN-γ was upregulated in the rapamycin-treated group in the normal cornea and at 1 and 5 days p.i. (Fig. 6C; p=0.02, p=0.04, p=0.004). Rapamycin inhibition of mTOR also resulted in an increase in protein levels of Cebpb at 1 and 5 days p.i. (Fig. 6B, p=0.02, p<0.0001). It also resulted in increased IFN-γ expression at 6 days p.i., with no difference between groups at 1 day p.i. (Fig. 6D)
Figure 6.
(A–D). Effect of rapamycin vs. PBS treatment on pro-inflammatory cytokines. Rapamycin treatment significantly increased Cebpb mRNA (A) and IFN-γ (C) in the normal cornea and at 1 and 5 days p.i. Cebpb protein levels (B) were increased at 1 and 5 days p.i., but did not differ between groups in the normal cornea. IFN-γ protein (D) was significantly increased at 6 days p.i., but was undetectable at 1 day p.i. in either group.
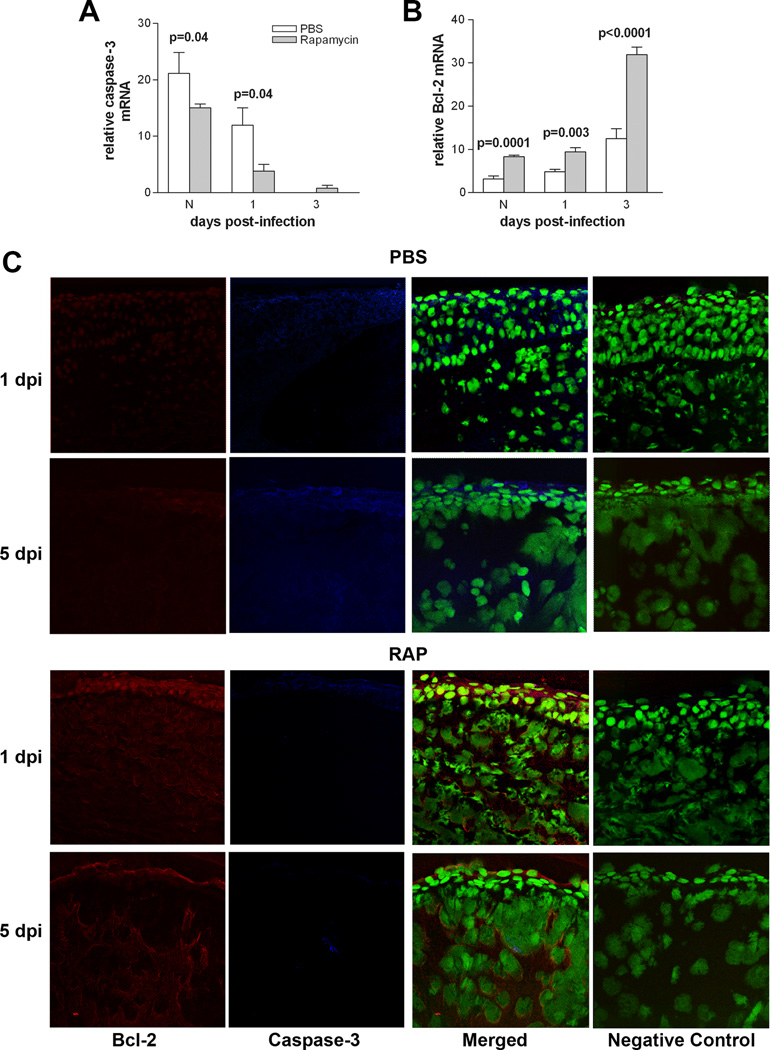
Rapamycin increases pro vs anti-apoptotic genes
To test the effects of rapamycin treatment on apoptosis, caspase-3 and Bcl-2 were assayed by real-time RT-PCR and immunostaining. Caspase-3 (Fig. 7A) was significantly decreased in the normal cornea of rapamycin- vs. PBS-treated group and at 1 day p.i. (p=0.04 for both). However, no significant differences between groups was seen at day 3 p.i. Bcl-2 was significantly increased in the normal cornea and at 1 and 3 days p.i. (Fig. 7B; p=0.0001, p=0.003, and p<0.0001).
Figure 7.
Effect of rapamycin treatment on apoptosis. Rapamycin vs. PBS treatment: (A) significantly downregulated caspase-3 mRNA in the normal cornea and at 1 day p.i. with no differences between groups at 3 days p.i.; (B) Bcl-2 mRNA was upregulated in normal cornea and at 1 and 3 days p.i. (C) Confocal images show a decrease in caspase-3 (blue) and increase in Bcl-2 (red) at 1 and 5 days p.i. in the cornea of rapamycin vs. PBS treated mice. Controls in which the primary antibody was replaced with same host species IgG were negative. SYTOX® Green (green) was used for nuclear labeling. Magnification=208X.
Qualitative differences in immunostaining for caspase-3 (blue) and Bcl-2 (red) were detected between rapamycin- vs. PBS-treated groups (Fig. 7C) at 1 and 5 days p.i. Bcl-2 staining was markedly more intense at 1 and 5 days p.i. in the rapamycin-treated group while caspase-3 was barely detectable when compared with PBS controls. In the PBS group, staining for caspase-3 was increased at 1 and 5 days p.i., while Bcl-2 staining was markedly reduced. Representative same host species IgG negative controls are shown for the rapamycin- and PBS-treated groups.
Discussion
Previous study has shown that in P. aeruginosa induced keratitis, macrophages restrict bacterial growth, limit PMN influx, and upregulate IL-10, which is critical to balancing/downregulating pro-inflammatory cytokines such as IFN-γ, and that these contribute to the resistance response of BALB/c mice (1). The present work expands upon these findings by examining the relationship between IL-10 and its regulation by mTOR in the infected BALB/c cornea.
mTOR is an upstream regulator of immunoregulatory cytokines (e.g., IL-10) in other disease paradigms and is implicated in both growth and inflammatory pathways (3, 5). Previous work also has shown that mTOR plays a role in regulating the IL-10/IL-12 axis in LPS stimulated macrophages and controls the innate immune response (4).
The present work has specifically demonstrated the adverse effects of rapamycin treatment after P. aeruginosa infection in the cornea. The data provide evidence that decreased IL-10 and increased IL-12p40 and IL-23 levels are the foundation for these effects. Although pro-inflammatory cytokines such as IL-12p40 are essential to amplify the innate immune response, without anti-inflammatory cytokines such as IL-10 counterbalancing these effects, the inflammatory response is unchecked (4), as seen in the study reported herein. In fact, a rescue experiment, the injection of rIL-10 together with rapamycin vs. rapamycin treatment alone revealed decreased PMN number in the cornea. These data provide concrete evidence of at least one mechanism by which IL-10 participates to balance inflammatory responses in the cornea.
Inhibition of mTOR gene activation is a common technique used to characterize mTOR’s function. Rapamycin is an often used inhibitor that acts at this level (3–7), and has been shown to downregulate mRNA levels of mTOR in other systems (18), supportive of our findings in the keratitis model. Rapamycin is used clinically for immunosuppression after kidney transplant and inflammatory conditions are not routinely seen (8). In addition, at the concentration used for the studies reported herein, others (19) have shown that rapamycin levels in blood had therapeutic immunosuppressive effects in animal models of graft versus host disease. Nonetheless, Säemann, et al. (9) reviewed the role of mTOR inhibition by rapamycin in transplant immunity and found that, despite preliminary benefits to prevent rejection, side effects accompanied its use, including: rash, fever, pneumonitis, glomerulonephritis, and anemia. Another study showed that administration of rapamycin increased the lethality of endotoxin (LPS)-mediated shock in mice (10) and resistance to antimicrobial therapy (20).
These effects suggest that inhibition of mTOR has profound impact on elements of the inflammatory response, including infiltrating cells such as PMN. In this regard, we have shown that rapamycin increased PMN number in the infected cornea; this implies that bacterial clearance should be enhanced, however, viable bacterial plate counts were increased when compared with controls. Thus, the phagocytic and bactericidal capacity of these cells to engulf and kill P. aeruginosa was examined. The ability of mouse PMN to phagocytize bacteria was enhanced at the higher concentration (1.0 µM) of rapamycin used, however, the higher concentration of rapamycin decreased intracellular killing capacity. These data are somewhat consistent with other work that examined the effects of rapamycin (concentration of 10–20 nM) on human PMN function (in cirrhosis patients), and determined that inhibition of mTOR decreased PMN bactericidal activity (21), but not uptake. Differences between their and our data could reflect our usage of murine vs. human cells and that we used a higher concentration of rapamycin.
Furthermore, our data show that rapamycin treatment led to upregulated deleterious downstream effectors of IL-10 (e.g., MMP-9, iNOS), and downregulated cytokines that participate in control of the inflammatory response (e.g., SOCS3, STAT-3). The data also are in agreement with past studies in that others have shown that SOCS3 is a mediator of LPS-stimulated IL-10 release in response to infection (22). In addition, SOCS3-deficient mice are vulnerable to enhanced expression of Th1 inflammatory cytokines, promoting infiltration of inflammatory cells (e.g., PMN, macrophages, T-cells) (23).
Previously, our laboratory demonstrated that rMMP-9-treated BALB/c mice exhibited worsened disease based on increased clinical disease score, MPO, Langerhans cell number, and protein levels of pro-inflammatory cytokines (24). MMP-9 is among the most widely studied MMP in the cornea, as it preferentially degrades basement-membrane components such as type IV collagen. Here, loss of mTOR signaling led to an increase in MMP-9 mRNA. Furthermore, PMN are a source of matrix metalloproteinases (24) and since rapamycin increased the number of these cells in the cornea after infection, it is likely that they contribute to elevated levels of MMP-9. Ultimately, this increase fosters destruction of the corneal stromal cytoarchitecture and decreased ability for tissue restoration (24).
Berlato, et al. (22) observed a correlation between decreased inducible NO synthase (iNOS) levels and cells that were treated with IL-10. This is consistent with our data, as we have shown that inhibition of mTOR elevates iNOS and nitrite levels. In previous reports, our laboratory has shown that iNOS is constitutively expressed in the BALB/c cornea and is required for bacterial killing (25). However, overproduction of iNOS-derived NO (through upregulation of IFN-γ) is associated with susceptibility to P. aeruginosa infection (26) and these past data are consistent with current studies.
Herein, we establish that rapamycin affects numerous elements of the TLR pathway that are instrumental in the response to P. aeruginosa infection. Others have characterized TLR4 and TLR5 as regulators of P. aeruginosa-induced keratitis in macrophages (27). In our laboratory, we have demonstrated that TLR4 is constitutively produced and activated in the BALB/c cornea after infection (28) and is required for resistance against P. aeruginosa. Its signaling cascade includes IRAK-1 and leads to the translocation of NFκB from the cellular cytoplasm to the nucleus, causing the transcription of pro-inflammatory genes such as IFN-γ and IL-1β (whose receptor, IL-1R1, is also upregulated by rapamycin). Presently, we have shown that rapamycin-treated mice have increased levels of TLR4, TLR5, and IRAK-1 mRNA, indicating increased stimulation of the pro-inflammatory pathway. Moreover, we also demonstrated that an inhibitor of NFκB translocation, NFκBIL-1, is downregulated in response to rapamycin treatment.
Cebpb increases expression of the ppt-A gene that gives rise to Substance P (SP) (29), a pro-inflammatory neuropeptide that worsens bacterial keratitis in BALB/c mice (2). In fact, preliminary data suggest that rapamycin and SP elicit a similar pattern of disease response in the cornea of P. aeruginosa-infected BALB/c mice, with a similar outcome (30). Here we have demonstrated that Cebpb is significantly upregulated in rapamycin-treated mice (mRNA, protein), however, SP regulation by Cebpb in the cornea has been untested and may require further examination.
Lastly, tight regulation of the balance between apoptosis and cell survival, as well as, the timing of apoptotic events, is critical to immune defense (12, 31). Others have shown that persistence of apoptotic cells can worsen disease outcome (12, 31). In the susceptible C57BL/6 mouse, we have shown that PMN have delayed apoptosis that contributes to inflammation-induced tissue damage (12). In the same study, administration of SP to resistant BALB/c mice delayed apoptosis, leading to a similar outcome of worsened disease. Another study reported that in a mouse model of LPS-induced acute lung injury, delay of apoptosis also prolonged the inflammatory response (32). Similarly, treatment with rapamycin as we have reported, delayed apoptosis, which again is consistent with past studies and with the poor outcome of enhanced disease after infection.
In summary, inhibition of mTOR by rapamycin treatment, increased disease in the cornea of resistant BALB/c mice through downregulation of the anti-inflammatory cytokine, IL-10, and up regulation of pro-inflammatory IL-12p40 and IL-23. Furthermore, the absence of IL-10 led to dysregulation of several downstream effectors: MMP-9, SOCS3, STAT-3, and iNOS and elements of the TLR pathway: TLR4, TLR5, IRAK-1, IL-1R1, NFκBIL-1, Cebpb, and IFN-γ.Moreover, we have demonstrated that loss of mTOR signaling generates an anti-apoptotic corneal environment in which damaged and dying cells could contribute to further tissue destruction. Rapamycin treatment also increased PMN number, but reduced their capacity for intracellular killing. Lastly, rIL-10 together with rapamycin injection was capable of reducing PMN number, strengthening the tenet held herein that IL-10 is critical to disease resolution. Collectively, these data provide evidence that loss of mTOR signaling elicits a dysregulated pro-inflammatory response in the infected BALB/c mouse cornea that exacerbates P. aeruginosa induced keratitis.
Acknowledgments
Support: This work was supported by grants R01 EY002986, EY016058, and P30 EY004068 (to LDH) from the National Eye Institute, National Institutes of Health.
Special Abbreviations
- Bcl-2
B cell lymphoma-2
- Cebpb
CCAAT/enhancer binding protein, beta
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- MMP-9
matrix metallopeptidase-9
- MPO
myeloperoxidase
- mTOR
mammalian target of rapamycin
- PMN
polymorphonuclear cells (neutrophils)
- p.i.
post-infection
- SOCS3
suppressor of cytokine signaling 3
References
- 1.McClellan SA, Huang X, Barrett RP, van Rooijen N, Hazlett LD. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 2003;170:5219–5227. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 2.McClellan SA, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to Pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators down-regulated. Invest. Ophthalmol. Vis. Sci. 2008;49:1502–1511. doi: 10.1167/iovs.07-1369. [DOI] [PubMed] [Google Scholar]
- 3.Baker AK, Wang R, Mackman N, Luyendyk JP. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-α expression in macrophages by reducing IL-10 expression. Mol. Immunol. 2009;46:2249–2255. doi: 10.1016/j.molimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheekatla SS, Aggarwal A, Naik S. mTOR signaling pathway regulates the IL-12/IL-10 axis in Leishmania donovani infection. Med. Microbiol. Immunol. 2012;201(1):37–46. doi: 10.1007/s00430-011-0202-5. [DOI] [PubMed] [Google Scholar]
- 5.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M, Müller M, Säemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Guan K. Expanding mTOR signaling. Cell. Research. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 8.Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: A systematic review and meta-analysis of randomized trials. Transplantation. 2006;81(9):1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 9.Säemann MD, Haidinger M, Hecking M, Hörl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: Implications for transplant immunity. Am. J. Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A, Janssen K, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur. J. Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett LD, Rudner XL, McClellan SA, Barrett RP, Lighvani S. Role of IL-12 and IFN-γ in Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci. 2002;43(2):419–424. [PubMed] [Google Scholar]
- 12.Zhou Z, Barrett RP, McClellan SA, Zhang Y, Szliter EA, van Rooijen N, Hazlett LD. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci. 2008;49:4458–4467. doi: 10.1167/iovs.08-1906. [DOI] [PubMed] [Google Scholar]
- 13.Hazlett LD, Moon MM, Strejc M, Berk RS. Evidence for N-acetyl-mannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest. Ophthalmol. Vis. Sci. 1987;28:1978–1985. [PubMed] [Google Scholar]
- 14.Williams RN, Patterson CA, Eakins KE. Quantification of ocular inflammation: Evaluation of polymorphonuclear leukocyte inflammation by measuring myeloperoxidase activity. Curr. Eye. Res. 1983;2:465–470. doi: 10.3109/02713688208996350. [DOI] [PubMed] [Google Scholar]
- 15.Luo YaMDE. Current Protocols in Immunology. John Wiley and Sons: New York; 1997. Isolation of mouse neutrophils; pp. 3.20.21–23.20.26. [Google Scholar]
- 16.Szliter EA, Lighvani S, Barrett RP, Hazlett LD. Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J. Immunol. 2007;178:1105–1114. doi: 10.4049/jimmunol.178.2.1105. [DOI] [PubMed] [Google Scholar]
- 17.Hazlett LD, Masinick-McClellan SA, Barrett RP. Complement defects in aged mice compromise phagocytosis of Pseudomonas aeruginosa . Curr. Eye. Res. 1999;19(1):26–32. doi: 10.1076/ceyr.19.1.26.5337. [DOI] [PubMed] [Google Scholar]
- 18.Yu SY, Liu L, Li PU, Li J. Rapamycin inhibits the mTOR/p70S6K pathway and attenuates cardiac fibrosis in adriamycin-induced dilated cardiomyopathy. Thorac Cardiovasc Surg. 2012 doi: 10.1055/s-0032-1311548. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner SM, Sellin L, Vonend O, Schenker P, Buchner NJ, Flecken M, Viebahn R, Rump LC. Pneumonitis associated with sirolimus: Clinical characteristics, risk factors and outcome - A single-centre experience and review of the literature. Nephrol. Dial. Transplant. 2007;22:3631–3637. doi: 10.1093/ndt/gfm420. [DOI] [PubMed] [Google Scholar]
- 21.Rolas L, Makhezer N, Hadjoudj S, El-Benna J, Djerdjouri B, Elkrief L, Moreau R, Périanin A. Inhibition of mammalian target of rapamycin aggravates the respiratory burst defect of neutrophils from decompensated cirrhotic patients. Hepatology. 2012 doi: 10.1002/hep.26109. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 (SOCS3) as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J. Immunol. 2002;168:6404–6411. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, Yeh WI, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH, 3rd, Park K, Harrington LE, Raman C, Benveniste EN. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc. Natl. Acad. Sci. USA. 2012;109(13):5004–5009. doi: 10.1073/pnas.1117218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClellan SA, Huang X, Barrett RP, Lighvani S, Zhang Y, Richiert D, Hazlett LD. Matrix metalloproteinase-9 amplifies the immune response to Pseudomonas aeruginosa corneal infection. Invest. Ophthalmol. Vis. Sci. 2006;47(1):256–264. doi: 10.1167/iovs.05-1050. [DOI] [PubMed] [Google Scholar]
- 25.Hazlett LD, McClellan S, Goshgarian C, Huang X, Thakur A, Barrett R. The role of nitric oxide in resistance to P. aeruginosa ocular infection. Ocul. Immunol. Inflamm. 2005;13(4):279–288. doi: 10.1080/09273940590951016. [DOI] [PubMed] [Google Scholar]
- 26.McClellan SA, Lighvani S, Hazlett LD. IFN-γ: Regulation of nitric oxide in the P. aeruginosa-infected cornea. Ocul. Immunol. Inflamm. 2006;14:21–28. doi: 10.1080/09273940500545650. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J. Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Du W, McClellan SA, Barrett RP, Hazlett LD. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 2006;47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 29.Kovács KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux J. C/EBPβ couples dopamine signaling to substance P precursor gene expression in striatal neurons. J. Neurochem. 2006;98:1390–1399. doi: 10.1111/j.1471-4159.2006.03957.x. [DOI] [PubMed] [Google Scholar]
- 30.Foldenauer ME, McClellan SA, Hazlett LD. The Association for Research in Vision and Ophthalmology. Fort Lauderdale, FL: Broward County/Greater Fort Lauderdale Convention Center; 2012. May 06, “mTOR Inhibition: Similarity to Treatment with Substance P in Pseudomonas Infection.”. Poster Presentation. [Google Scholar]
- 31.Ren Y, Savill J. Apoptosis: The importance of being eaten. Cell. Death. Differ. 1998;5(7):563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 32.Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1- deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J. Immunol. 2002;169:6401–6407. doi: 10.4049/jimmunol.169.11.6401. [DOI] [PubMed] [Google Scholar]