Abstract
Myelin-associated inhibitor/NgR1 signaling has important roles in modulation of synaptic plasticity, with demonstrated effects on cognitive function. We have previously demonstrated that NgR1 and its ligands are upregulated in the hippocampus of aged rats with impaired spatial learning and memory, but it is unknown whether increased expression of these proteins indicates a potential increase in pathway signaling because NgR1 requires co-receptors for signal transduction through RhoA. Two co-receptor complexes have been identified to date, comprised of NgR1 and LINGO-1, and either p75 or TROY. In this study, we assessed the expression of LINGO-1, p75 and TROY, and the downstream effector RhoA in mature adult (12 months) and aged (26 months) male Fischer 344/Brown Norway hybrid rats classified as cognitively impaired or cognitively intact by Morris water maze testing. The hippocampal distribution of NgR1 and its co-receptors was assessed to determine whether receptor/co-receptor interaction, and therefore signaling through this pathway, is possible. Protein expression of LINGO-1, p75, TROY, and RhoA was significantly elevated in cognitively impaired, but not intact, aged rats compared to mature adults, and expression levels correlated significantly with water maze performance. Co-localization of NgR1 with LINGO-1, p75 and TROY was observed in hippocampal neurons of aged, cognitively impaired rats. Further, expression profiles of NgR1 pathway components were demonstrated to classify rats as cognitively intact or cognitively impaired with high accuracy. Together, this suggests that hippocampal induction of this pathway is a conserved phenomenon in cognitive decline that may impair learning and memory by suppressing neuronal plasticity.
Keywords: aging, cognitive decline, Nogo receptor, Morris water maze, Fischer 344/Brown Norway hybrid rat
Introduction
Hippocampus-associated spatial learning and memory is one of the most profoundly impaired cognitive domains in aged adults (Schaie, 1996). Although progress has been made in identifying proteins, processes, and electrophysiological correlates of learning and memory dysregulated in the aged hippocampus, the mechanisms underlying age-related deficits of spatial learning and memory remain elusive. We have demonstrated that hippocampal expression of myelin-associated inhibitors (MAIs) of neuronal plasticity, including MAG, Nogo-A and OMgp and their common neuronal receptor, NgR1, is upregulated specifically in aged cognitively impaired, but not age-matched cognitively normal, rats (VanGuilder et al., 2011b; VanGuilder et al., 2012). The potential role of the MAI/NgR1 pathway in age-related cognitive decline is of particular interest due to recent demonstrations that MAI/NgR1 signaling can impair learning and memory (Lenzlinger et al., 2005; Walmsley & Mir, 2007; Karlen et al., 2009; Gillani et al., 2010). This is one of the few pathways identified to date that is altered specifically with cognitive decline, rather than as a broad effect of aging. Further, MAI/NgR1 signaling may be related to multiple hippocampal phenomena associated with cognitive decline, such as decreased expression of glutamate receptors and calcium/calmodulin-dependent protein kinase 2α expression (Shi et al., 2007; Peng et al., 2011; VanGuilder et al., 2011b), and reduced numbers of high-efficiency synapses (Geinisman et al., 1986; Nicholson et al., 2004; Zagrebelsky et al., 2005; Lee et al., 2008).
MAI/NgR1 signaling requires NgR1 co-receptors for signal transduction (Fournier et al., 2001). Two heterotrimeric NgR1 receptor complexes have been identified: one comprised of NgR1, LINGO-1 and p75 (Wang et al., 2002a; Wong et al., 2002; Yamashita et al., 2002; Mi et al., 2004); the other comprised of NgR1, LINGO-1 and TROY (Park et al., 2005; Shao et al., 2005; Wills et al., 2012). Signaling through these NgR1 co-receptor complexes activates RhoA (Niederost et al., 2002; Yamashita et al., 2002; Shao et al., 2005), leading to inhibitory modulation of neuronal plasticity (Yamashita et al., 2002; McGee et al., 2005; Lee et al., 2008; Delekate et al., 2011; Murakoshi et al., 2011). Like other components of the MAI/NgR1 pathway, NgR1 co-receptors are implicated in regulation of activity-dependent synaptic plasticity and cognitive function. Antagonism of TROY and LINGO-1 increases neuronal structural plasticity (Park et al., 2005; Shao et al., 2005; Ji et al., 2006), while NgR1-TROY signaling inhibits synaptogenesis (Wills et al., 2012). Genetic manipulation studies demonstrate that p75 expression is inversely related to hippocampal spine density, Schaffer collateral long-term potentiation (LTP), and spatial learning and memory ability (Barrett et al., 2010; Zagrebelsky et al., 2010). It is our hypothesis that increased hippocampal signaling through the NgR1 pathway in subjects with cognitive decline impairs behavioral correlates of spatial learning and memory. To better understand the potential role of MAI/NgR1 signaling in age-related spatial learning and memory deficits, we examined the hippocampal expression of LINGO-1, p75, TROY and RhoA, the co-localization of co-receptors with NgR1, and the relationship of MAI/NgR1 signaling machinery to spatial learning and memory impairment in a naturally-occurring rat model of human cognitive decline.
Materials and Methods
Animals and tissues
The housing and cognitive assessment of animals (Table 1) from which study samples were derived have been described elsewhere (VanGuilder et al., 2011a; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). Briefly, mature adult (12 months) and aged (26 months) Fischer 344 × Brown Norway (F1) hybrid male rats were acquired from the National Institute on Aging rodent colony at Harlan Industries (Indianapolis, IN) and housed in the specific pathogen-free Barrier Facility at the OUHSC Reynolds Oklahoma Center on Aging. The OUHSC animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all animal procedures were approved by the OUHSC Institutional Animal Care and Use Committee in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council’s Guide for the Care and Use of Laboratory Animals. Rats were singly housed in laminar-flow cages with food (Purina Mills, Richmond, IN) and water freely available, and the Barrier Facility maintained constant temperature/humidity levels and a 12-hour light/dark cycle. For two weeks prior to behavioral testing, rats were handled by laboratory personnel during the light phase to minimize stress during Morris water maze assessment of hippocampal learning and memory (described below). To avoid potential confounding effects of behavioral testing on expression of hippocampal transcripts and proteins, rats (cohort 1, Table 1) were sacrificed by decapitation one week following the conclusion of testing. Alternatively, rats (cohort 2, Table 1) were anesthetized with ketamine/xylazine and transcardially perfused with 6U/mL heparin sodium salt in phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS (pH 7.4) for immunohistochemical assessment. No animals meeting exclusionary criteria (e.g., pituitary tumors, cortical atrophy, peripheral tumors) were included in these studies.
Table 1.
Animal and sample information
| Cohort | Rat strain, sex | Rat age | Group size | Sample type | Analysesa,b | Partially described in |
|---|---|---|---|---|---|---|
| 1 | Fischer 344 × Brown Norway (F1), male | Adult: 12mos. | Adult: n=7 | Hippocampal subregion dissections (CA1, CA3, DG) | IB (RH)c qPCR (LH) |
VanGuilder et al., 2011 VanGuilder et al., 2012 VanGuilder Starkey et al., 2012 |
| Aged: 26mos. | Aged intact: n=7 | |||||
| Aged impaired: n=10 | ||||||
| 2 | Fischer 344 × Brown Norway (F1), male | Aged: 26mos. | Aged impaired: n=3 | Perfusion-fixed sagittal hemispheres | IHC |
VanGuilder et al., 2011 VanGuilder et al., 2012 |
IB: immunoblotting; qPCR: quantitative real-time polymerase chain reaction; IHC: immunohistochemistry
RH: right hemisphere; LH: left hemisphere
Inclusion of protein samples passing quality control assessments yielded the following sample sizes for immunoblot analysis: CA1: adult n=7, aged intact n=7, aged impaired n=9; CA3: adult n=7, aged intact n=6, aged impaired n=10; DG: adult n=5, aged intact n=7, aged impaired n=10.
Morris water maze testing
Our methods for assessment of spatial learning and memory by Morris water maze testing has been described extensively elsewhere (Freeman et al., 2009; VanGuilder et al., 2011a; VanGuilder et al., 2011b; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). The water maze consisted of a round tank (1.7m in diameter, 0.6m high) filled with water made opaque by water-based white food coloring, with a retractable escape platform submerged 2cm below the surface, and surrounded by a dark curtain with fixed-position visual reference cues. Maze activity was recorded by a center-mounted digital camera mounted above the maze tank, and performance was analyzed using automated tracking software (Noldus Ethovision XT, Wageningen, Netherlands). Maze testing was conducted in four two-day blocks, with each block consisting of five acquisition trials (60 seconds each, 2–3 trials/day) with the escape platform fixed in place and one probe trial with the platform retracted to an inaccessible depth. After the last probe trial, visual performance was evaluated in four consecutive swim trials with the escape platform visible to ensure that visual deficits did not hinder maze performance. All testing (i.e., acquisition and probe trials and visible-platform testing) was performed during the light phase.
For acquisition trials, the escape platform location was constant throughout behavioral testing, while start positions were pseudo-randomized. Rats’ path length to the escape platform was measured during acquisition trials, with decreasing path length indicating improved maze performance. Probe trials (30 seconds each) were performed after each five-trial acquisition block, and the average distance to the escape platform location (i.e., mean proximity) was measured. Upon conclusion of testing, a composite “mean proximity to platform location” measure was calculated for each animal, reflecting performance across four probe trials. Using this metric, lower values indicate better performance (i.e., less distance from the target area), and higher values indicate worse performance. Based on mean proximity values, aged rats performing within the range of adults (i.e., probe trial performance within one standard deviation below the best performing adult rat and one standard deviation above the worst performing adult rat) were classified as cognitively intact. Aged rats with probe trial performance inferior to the adult group (i.e., more than one standard deviation above the worst performing adult) were classified as cognitively impaired. This stratification approach yielded the following group sizes in cohort 1: adult n=7; aged cognitively intact n=7; aged cognitively impaired n=10 (Table 1). Data regarding all cohort 2 animals are presented in Supplemental Table 1. Differences in group probe trial performance were verified by one-way analysis of variance (ANOVA) with Student Newman Keuls (SNK) post hoc testing. Retrospective repeated-measures ANOVA analysis of acquisition data was performed across acquisition blocks to confirm that all groups learned the maze task. Mean swim speed throughout testing was also monitored to ensure that maze performance was not confounded by motor deficits.
Dissection of hippocampal CA1, CA3 and DG subregions
These samples have been described and partially characterized in previous reports (VanGuilder et al., 2011a; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). Briefly, hippocampi from cohort 1 (Table 1) were rapidly excised and Cornu Ammonis area 1 (CA1), Cornu Ammonis area 3 (CA3) and dentate gyrus (DG) subregions were individually collected by dissecting the dorsomedial hippocampus perpendicular to the longitudinal axis, and then along the hippocampal fissure (Newton et al., 2005; VanGuilder et al., 2011a; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). Accuracy of subregion dissections was assessed by qPCR analysis of transcripts previously demonstrated to be enriched in CA1 [nephroblastoma overexpressed/Nov (Zhao et al., 2001; Lein et al., 2004) and Wolfram syndrome 1/Wfs1 (Takeda et al., 2001; Fanselow & Dong, 2010)], CA3 [neuronatin/Nnat (Greene et al., 2009; Oyang et al., 2011) and sparc-osteonectin, cwcv and kazal like domains proteoglycan/Spock1 (Bonnet et al., 1996; Lein et al., 2004)], or DG [desmoplakin/Dsp (Lein et al., 2004; Hagihara et al., 2009) and T-cell lymphoma invasion and metastasis 1/Tiam1 (Greene et al., 2009)]. qPCR assessments of dissection precision were performed as described below using TaqMan Gene Expression Assays (Table 2). Only adult rats were included in this analysis to avoid the potential confound of age-related alterations in gene expression or distribution.
Table 2.
qPCR primers/probe information
| Target | Full name | TaqMan Gene Expression Assay |
Assay design | Amplicon length |
Purpose |
|---|---|---|---|---|---|
| Lingo-1 | Leucine rich repeat and Ig domain containing 1 | Rn03993618_s1 | intra-exon | 109 | Assessment of NgR1 pathway components |
| p75 | Nerve growth factor receptor | Rn00561634_m1 | exon-spanning | 60 | Assessment of NgR1 pathway components |
| TROY | Tumor necrosis factor receptor superfamily, member 19 | Rn01534699_m1 | exon-spanning | 60 | Assessment of NgR1 pathway components |
| RhoA | Ras homolog family member A | Rn04219609_m1 | exon-spanning | 100 | Assessment of NgR1 pathway components |
| Nov | Nephroblastoma overexpressed | Rn00578390_m1 | exon-spanning | 61 | Validation of subregion dissections (CA1 enriched) |
| Wfs1 | Wolfram syndrome 1 homolog | Rn00582735_m1 | exon-spanning | 95 | Validation of subregion dissections (CA1 enriched) |
| Nnat | Neuronatin | Rn00822063_m1 | exon-spanning | 66 | Validation of subregion dissections (CA3 enriched) |
| Spock1 | Sparc/osteonectin, cwcv and kazal-like domains proteoglycan (testican) 1 | Rn01441970_m1 | exon-spanning | 148 | Validation of subregion dissections (CA3 enriched) |
| Dsp | Desmoplakin | Rn01434648_m1 | exon-spanning | 65 | Validation of subregion dissections (DG enriched) |
| Tiam1 | T-cell lymphoma invasion and metastasis 1 | Rn01477317_m1 | exon-spanning | 61 | Validation of subregion dissections (DG enriched) |
| Actb | Actin, beta | Rn00667869_m1 | exon-spanning | 91 | Endogenous control |
RNA isolation and qPCR
Subregion dissections were homogenized in TRI-Reagent using a bead mill. RNA was extracted by incubation with 10% 1-bromo-3-chloropropane and standard phase separation, and was precipitated in isopropanol overnight at −20°C. RNA was purified using the RNeasy Mini kit (Qiagen), eluted in molecular grade H2O, and assessed for quality and quantity by microfluidic chip (2100 Expert Bioanalyzer NanoChip, Agilent Technologies, Santa Clara, CA) and spectrometry (NanoDrop ND1000, Thermo Scientific, Wilmington, DE), respectively. To verify the accuracy of hippocampal subregion dissections while avoiding potential age-related confounds in mRNA expression, transcripts enriched in CA1, CA3 and DG (see above and Table 2) were quantified by qPCR in samples derived from cohort 1 adult animals only (n=7). Transcripts for LINGO-1, p75, TROY and RhoA were quantified in all RNA samples from cohort 1 rats (Table 1; adult: n=7, aged intact: n=7, aged impaired n=10) by qPCR using our previously described methods (Bixler et al., 2011; VanGuilder et al., 2011a; VanGuilder Starkey et al., 2012). cDNA was synthesized using the High-capacity cDNA Reverse Transcription kit (Applied Biosciences, Life Technologies, Carlsbad, CA). qPCR was performed with a 7900HT Sequence Detection System (Applied Biosystems) using TaqMan Assay-On-Demand gene-specific primer/probe assays (Applied Biosystems) with β-actin as the endogenous control (Table 2). Data were analyzed using the 2−ΔΔCt method with automatic thresholding (SDS 2.2.2 software, Applied Biosystems), and statistically evaluated by one-way ANOVA with SNK post hoc testing.
Protein extraction and quantitation
Detergent-soluble protein was isolated by homogenizing subregion dissections in a Tween20-based protein lysis buffer [1% Tween20, 100mM NaCl, 20mM HEPES, 1mM EDTA, 1mM dithiothreitol, 1mM Na3VO4, and protease inhibitors (Complete Mini EDTA-free protease inhibitor cocktail tablets, Roche Applied Science, Indianapolis, IN)] using a bead mill (Retsch TissueLyzer II, Qiagen, Valencia, CA). Homogenates were incubated for 15 minutes at 4°C with gentle rocking, and insoluble material was removed by centrifugation (12,500 × g, 15 minutes, 4°C. The supernatant, containing soluble protein, was collected and protein concentrations were determined by bicinchoninic acid quantitation (Pierce, Rockford, IL). As described previously (VanGuilder et al., 2010; VanGuilder et al., 2011a; VanGuilder et al., 2011b; VanGuilder et al., 2012), for each hippocampal subregion (CA1, CA3, DG) assessed, a gel loaded with equal protein (15µg) from all 24 cohort 1 animals samples (adult: n=7, aged intact: n=7, aged impaired n=10) was fixed in 15% ethanol/1% citric acid (pH 2.3), stained with Deep Purple total protein stain (GE Healthcare, Piscataway, NJ) and quantitated using semi-automated digital densitometry (ImageQuant TL, GE Healthcare) to ensure equal protein content and sample integrity of samples used for immunoblot analyses. Samples with signs of degradation or irregular banding patterns were excluded from analysis of protein expression. This quality control assessment yielded the following sample sizes for protein quantitation: adult (CA1: n=7; CA3: n=7; DG: n=5), aged intact (CA1: n=7; CA3: n=6; DG: n=7) and aged impaired (CA1: n=9; CA3: n=10; DG: n=10).
Protein-level expression of LINGO-1, p75, TROY and RhoA was quantified in CA1, CA3 and DG dissections using standard immunoblotting methods with sample identities masked to investigators. Protein samples were denatured by incubation for 5 minutes at 95°C in LDS sample buffer (Invitrogen, Carlsbad, CA) with 50mM dithiothreitol. 15µg of each prepared protein sample was denatured at 95°C prior to SDS-PAGE separation using Criterion Tris-HCl precast 4–20% acrylamide gradient gels (BioRad, Hercules, CA). Separated proteins were transferred to hydrophobic polyvinylidene difluoride membranes (HyBond-P, GE Healthcare), blocked with 3% bovine serum albumin in PBS containing 1% Tween-20, and incubated with primary antibodies (Table 3). Membranes were washed with 1% Tween-20 in PBS, incubated with species-appropriate, horseradish peroxidase-conjugated secondary antibodies (Table 3), and developed using enhanced chemiluminescence substrate (luminol/peroxide reagent, Pierce). Immunoreactive protein bands were visualized on film, digitized at 800 d.p.i. and quantitated using digital densitometry with rolling ball background subtraction (ImageQuant TL, GE Healthcare). Immunoblot data were normalized to whole-lane densitometric volumes determined from the total protein-stained standardization gel as described previously (VanGuilder et al., 2011a; VanGuilder et al., 2012), and the resultant data were evaluated by one-way ANOVA with Student Newman Keuls post hoc testing. Statistical relationships between protein expression and cognitive performance (mean proximity to platform) were assessed by Pearson product moment or Spearman rank order correlations depending on normality of datasets.
Table 3.
Antibody information
| Primary antibodies | Supplier | Catalog # |
Antibody host |
Immunogena |
Methodb and dilution |
| anti-Lingo-1 | Abcam | ab23631 | rabbit | synthetic peptide, aa600 to C-terminus | IB: 1/1000 IHC: 1/200 |
| anti-NFh | Sigma | n0142 | mouse | dephosphorylated carboxyterminal tail segment | IHC: 1/200 |
| anti-NgR1 | Santa Cruz Biotechnology | sc25659 | rabbit | aa31–150 | IHC: 1/200 |
| R&D Systems | af1208 | goat | recombinant peptide, aa27–447 | IHC: 1/100 | |
| anti-p75 | Abcam | ab38335 | rabbit | synthetic peptide, aa250–350 | IB: 1/2000 IHC: 1/500 |
| anti-RhoA | Abcam | ab54835 | mouse | recombinant full-length protein (aa1–194) | IB: 1/1000 |
| anti-TROY | Santa Cruz Biotechnology | sc13711 | goat | C-terminal peptide | IB: 1/250 IHC: 1/100 |
| Secondary antibodies | Supplier | Catalog # |
Antibody host |
Immunogena |
Methodb> and dilution |
| anti-goat (HRP-conjugated) | Santa Cruz Biotechnology | sc2768 | rabbit | goat IgG | IB: 1/2000 |
| anti-goat DyLight549-conjugated [F(ab’)2] | Jackson ImmunoResearch | 705506147 | donkey | goat IgG | IHC: 1/250 |
| anti-mouse (HRP-conjugated) | Thermo Scientific | 32430 | goat | mouse IgG | IB: 1/200 |
| anti-mouse DyLight488-conjugated [F(ab’)2] | Jackson ImmunoResearch | 715486150 | donkey | mouse IgG | IHC: 1/500 |
| anti-rabbit (HRP-conjugated) | GE Healthcare | na934V | donkey | rabbit IgG | IB: 1/2000 |
| anti-rabbit DyLight649-conjugated [F(ab’)2] | Jackson ImmunoResearch | 711496152 | donkey | rabbit IgG | IHC: 1/500 |
aa: amino acid
IB: immunoblotting; IHC: immunohistochemistry
Immunohistochemistry and microscopy
The distribution of NgR1 with LINGO-1, p75 and TROY was assessed to determine whether these proteins are co-expressed in the hippocampus of aged, cognitively impaired rats in a manner that would enable their interaction. While the extent of co-localization remains to be determined in adult and aged intact rats in future studies, the goal of this investigation was to establish the potential for functional consequences of NgR1 pathway overexpression in the hippocampus of aged cognitively impaired rats. After perfusion, rats used for immunohistochemical co-localization experiments (cohort 2, n=3, Table 1) were decapitated and their brains were extracted, sagittally hemisected, immersion-fixed in 4% paraformaldehyde, and cryoprotected with 30% sucrose in PBS. Hemispheres were embedded in Tissue-Tek OCT cryosectioning medium (Sakura Finetek, Torrance, CA) and frozen in isopentane on dry ice. 10µm sagittal cryosections were collected on charged glass slides (SuperFrost Plus, Fisher Scientific, Pittsburg, PA), post-fixed with 2% PBS-buffered paraformaldehyde, blocked with 10% donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.1% Triton X-100 in PBS, and incubated with primary antibodies overnight at 4°C (Table 3). After washing in 0.1% Triton X-100/PBS, slides were incubated fluorophore-conjugated secondary antibodies (Table 3) in blocking buffer. Non-specific secondary antibody signals and background fluorescence were controlled for by inclusion of negative control slides with primary antibodies omitted. To visualize reference structures, nuclei were stained with Hoechst 33258 (5µg/mL, Invitrogen) and axons were detected with an antibody to heavy neurofilament. Slides were washed, coverslipped with Aqua Poly/Mount (Polysciences, Warrington, PA), and dried overnight at room temperature for confocal imaging.
Images were acquired by a confocal laser scanning microscope (Leica TCS SP2 AOBS, Exton, PA) with ultraviolet-diode (Hoechst, 405nm), argon (488nm), and helium-neon (546nm and 649nm) lasers. Hippocampi were imaged as single optical sections at a resolution of 1024×1024 pixels using an HCX PL APO 63×, 1.4 numerical aperture oil-immersion objective (voxel width: 0.2325µm; voxel height: 0.2325µm; voxel depth: 0.1221µm; pinhole: 1 airy) with 2-fold line and frame averaging to reduce noise. Additional noise reduction and background subtraction were performed using Adobe Photoshop CS5 software (Adobe Systems, San Jose, CA). For each target, one representative image from our immunohistochemical assessment of three aged impaired rats is provided.
Principal component and support vector machine analyses
To visualize how expression of MAI/NgR1-associated proteins clusters individual rats and relates groups (CA1: adult n=7, aged intact n=7, aged impaired n=9; CA3: adult n=7, aged intact n=6, aged impaired n=10; DG: adult n=5, aged intact n=7, aged impaired n=10) to one another, a principal component analysis was performed using rats’ expression of MAG, Nogo-A, OMgp, NgR1 (previously described in (VanGuilder et al., 2012) in these same cohort 1 using these same hippocampal dissections, as well as LINGO-1, p75, TROY and RhoA. CA1, CA3 and DG subregions were processed separately, and datapoints representing individual rats were visualized as a two-dimensional function of protein expression using Genespring GX 12 software (Agilent Technologies), with components mean-centered and scaled as described previously (Freeman et al., 2009; VanGuilder et al., 2010). The ability of MAI/NgR1-associated protein expression profiles to correctly classify rats by cognitive status [Intact (adult and aged cognitively intact) or Impaired (aged cognitively impaired)] was tested using a support vector machine class-prediction modeling algorithm (Genespring GX 12) with a Gaussian kernel function, with sigma=1.5, as described previously (Freeman et al., 2010a; Freeman et al., 2010b).
Statistical analysis
Statistical analysis for significance was performed using SigmaStat 3.5 and SigmaPlot 11.0 for Windows (Systat Software, Inc., Chicago, IL). Morris water maze probe trial performance, protein expression, mRNA expression data were assessed for normality of distribution using the Shapiro-Wilk normality test. These data were assessed by one-way ANOVA with SNK pairwise multiple comparison post hoc testing to determine statistically significant group differences. Immunoblot bands with technical artifacts (e.g., air bubbles, ladder bleed-through) that confounded accurate quantitation, resulting in data points greater than two standard deviations from the group mean for a given protein target, were excluded from the study, as reflected in the degrees of freedom presented in the results. Morris water maze acquisition data for each group (adult, aged intact, aged impaired) was analyzed across testing blocks by one-way repeated-measures ANOVA with Holm Sidak post hoc testing to confirm that all groups acquired the maze task, and was analyzed between between groups for each block by one-way ANOVA with SNK post hoc testing to identify significant group differences in path length. To identify statistically significant relationships between protein expression and probe trial mean proximity values, Pearson product moment or Spearman rank order correlation analyses were performed for data with normal and non-normal distributions, respectively. Co-variance of NgR1 pathway component protein expression in hippocampal subregions was similarly assessed, using protein expression data from this work and our previous report (VanGuilder et al., 2012), including all age/cognitive groups.
Results
Spatial learning and memory
The cognitive assessment of these rats, and stratification of aged rats into cognitively intact (performing similarly to adults) and cognitively impaired (performing poorer than adults) groups based on water maze performance has been previously described (VanGuilder et al., 2011a; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). Behavioral data for cohort 1 (used for subregion dissections and quantitation of protein expression; Table 1) is summarized here to provide context for interpretation of protein expression data. Behavioral data associated with cohort 2 (used for protein localization studies in aged impaired rats) is available in Supplemental Table 1 and has been described elsewhere (VanGuilder et al., 2011a; VanGuilder et al., 2012). All rats were evaluated for hippocampal spatial learning and memory over four, two-day testing blocks, each consisting of five acquisition trials followed by one probe trial. Proximity to the escape platform location was averaged across all four probe trials, and this value was used to classify aged rats as cognitively intact or impaired. The data described here pertain to cohort 1. Adult mean probe trial performance ranged from 44.3cm to 52.5cm from the escape platform area, with a standard deviation of 2.9cm. Therefore, aged rats with mean proximity values between 41.4cm and 55.4cm were classified as cognitively intact, and those with mean proximity values greater than 55.4cm were classified as cognitively impaired. Probe trial performance of aged intact rats ranged from 43.5cm to 52.6cm, while probe trial performance of aged impaired rats ranged from 55.5cm to 65.0cm.
Group differences in probe trial performance were confirmed by one-way ANOVA with SNK post hoc testing (F2,21=33.96, P<0.001). Mean proximity values were as follows: adult group (48 ± 1.1 cm, n=7), aged intact group (48 ± 1.4 cm, n=7), aged impaired group (59 ± 0.9 cm, n=10). The probe trial performance of aged impaired rats was significantly poorer than both adult (SNK, p<0.001) and aged intact (SNK, p<0.001) groups. Retrospective analysis of acquisition trial data by repeated-measures ANOVA with Holm Sidak post hoc testing confirmed that all three groups learned the maze task, as demonstrated by significant reductions in path length over the four maze testing blocks (adult: F6,3=6.63, P=0.003; aged intact: F6,3=8.16, P=0.001; aged impaired: F9,3=10.75, P<0.001), as previously described (VanGuilder et al., 2011a; VanGuilder et al., 2012; VanGuilder Starkey et al., 2012). Nevertheless, a group difference was evident in path length to the escape platform in the fourth block of acquisition trials (F2,21=6.06, P=0.008). Aged impaired rats traveled significantly longer path lengths to the escape platform (701 ± 41.4 cm) than adult (424 ± 74.0 cm; SNK, p<0.05 vs. aged impaired) and aged intact rats (478 ± 79.1 cm; SNK, p<0.05 vs. aged impaired).
Accuracy of hippocampal CA1, CA3 and DG subregion dissections
To confirm the accuracy of subregion dissections, CA1, CA3 and DG samples derived from adult rats (n=7) were assessed for expression of transcripts characteristically enriched in specific subregions. In agreement with previous reports (Takeda et al., 2001; Fanselow & Dong, 2010), WFS1 mRNA was significantly enriched in CA1 (F2,18=115.37, P<0.001) compared to both CA3 (12-fold greater expression in CA1; SNK, p<0.001) and DG (9-fold greater expression in CA1; SNK p<0.001) (Figure 1A). Likewise, Nov mRNA (Zhao et al., 2001; Lein et al., 2004) was enriched CA1 (F2,18=101.53, P<0.001) by 6-fold compared to CA3 (SNK, p<0.001), and by 12-fold compared to DG (SNK, p<0.001). No differences in expression of WFS1 or Nov were evident between CA3 and DG. The CA3-associated transcripts NNAT (Greene et al., 2009; Oyang et al., 2011) and Spock1 (Bonnet et al., 1996; Lein et al., 2004) were also assessed for expression across subregions (Figure 1B). NNAT was significantly enriched in CA3 (F2,18=53.42, P<0.001) by 3.5- to 4-fold compared to CA1 (SNK, p<0.001) and DG (SNK, p<0.001). Spock1 was similarly enriched (F2,18=49.0, P<0.001), with expression levels in CA3 more than double those in CA1 (SNK, p<0.001) and DG (SNK, p<0.001). Neither NNAT nor Spock1 was differentially expressed between CA1 and DG. Both Tiam1 and DSP are reported to be highly enriched in DG compared to hippocampal subregions (Lein et al., 2004; Hagihara et al., 2009); (Greene et al., 2009). In our dissections, Tiam 1 was significantly enriched in DG (F2,18=95.41, P<0.001) compared to both CA1 (7-fold higher expression in DG; SNK, p<0.001) and CA3 (10-fold higher expression in DG; SNK, p<0.001) (Figure 1C). DSP was also highly enriched in DG (F2,18=33.46, P<0.001) by 14-fold compared to CA1 (SNK, p<0.001) and by nearly 17-fold compared to CA3 (SNK, p<0.001). There was no difference in expression of DSP or Tiam1 between CA1 and CA3. Together, these data validate the accuracy and reproducibility of our hippocampal subregion dissections.
Figure 1. Enrichment of hippocampal subregion-associated transcripts in CA1, CA3 and DG dissections.
To confirm the accuracy of our subregion dissections, transcripts previously characterized as enriched/restricted to either CA1, CA3 or DG were quantified in subregion samples dissected from adult rats. Aged intact and aged impaired rats were excluded from this quality control analysis to avoid the potential confound of age-related alterations in transcript expression or distribution. A) WFS1 and Nov were highly enriched in CA1 compared to both CA3 and DG, in agreement with the literature. B) NNAT1 and Spock1, which are reported to be overexpressed in CA3, were present in significantly greater amounts in CA3 compared to CA1 and DG. C) Tiam1 and DSP, both recognized markers of DG, were significantly enriched in DG compared to both CA1 and CA3. These data indicate the consistency and accuracy of our dissection of CA1, CA3, and DG. Data are presented as mean ± SEM; #p<0.001, one-way ANOVA with SNK post hoc testing (n=7 for each subregion)
Expression of NgR1 co-receptors is increased in aged rats with naturally-occurring cognitive impairment
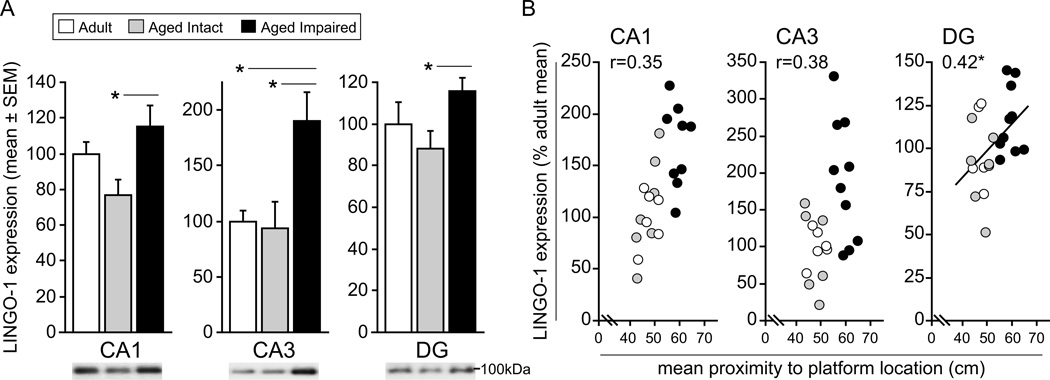
We have previously described hippocampal upregulation of the MAI ligands MAG, Nogo-A and OMgp, and their common receptor NgR1, in aged rats with deficits of spatial learning and memory and demonstrated that expression of these proteins correlates with water maze performance (VanGuilder et al., 2012). To determine whether NgR1 receptor complex components necessary for NgR1 signal transduction are concomitantly induced with cognitive decline, immunoblotting and densitometric quantitation of the NgR1 co-receptors LINGO-1, p75 and TROY was performed in hippocampal subregion dissections from adult (CA1: n=7; CA3: n=7; DG: n=5), aged intact (CA1: n=7; CA3: n=6; DG: n=7) and aged impaired (CA1: n=9; CA3: n=10; DG: n=10) rats. A significant effect of group on LINGO-1 protein expression was observed in multiple hippocampal subregions (CA1: F2,18=3.68, P=0.046; CA3: F2,19=5.73, P=0.011; DG: F2,19=3.54, P=0.049). Compared to cognitively intact aged rats, LINGO-1 protein content in age-matched, cognitively impaired rats was significantly elevated in CA1 (+50%; SNK, p<0.05), CA3 (+102%; SNK, p<0.05) and DG (+31%; SNK, p<0.05) (Figure 2A). An increase in LINGO-1 expression of nearly two-fold was observed in CA3 of aged impaired rats compared to mature adults (SNK, p<0.05). No differences in LINGO content were evident between aged impaired adult rats in CA1 or DG, or between aged intact and mature adults in any hippocampal subregion assessed. The potential relationship between increased hippocampal LINGO-1 expression and deficits of spatial learning and memory was assessed by statistical correlation analysis (Figure 2B). Pearson product moment correlation analysis demonstrated a significant association between LINGO-1 content and mean proximity-to-platform probe trial measures, with higher mean proximity values indicating poorer water maze performance, in DG (r=0.42, p<0.05). Correlations approached significance in CA1 and CA3.
Figure 2. Hippocampal LINGO-1 expression is upregulated with age-related cognitive decline.
A) LINGO-1 content is significantly increased in CA1, CA3 and DG of aged cognitively impaired rats compared to age-matched cognitively intact rats, and in CA3 compared to mature adults. No differences in LINGO-1 expression are evident between aged intact and adult rats. *p<0.05, one-way ANOVA, SNK post hoc testing. Representative blot images are shown. B) LINGO-1 content and water maze performance were significantly correlated in DG, and correlations approached statistical significance in CA1 and CA3, suggesting a relationship between increased LINGO-1 protein expression decreased hippocampus-dependent cognitive ability. *p<0.05, Pearson correlation.
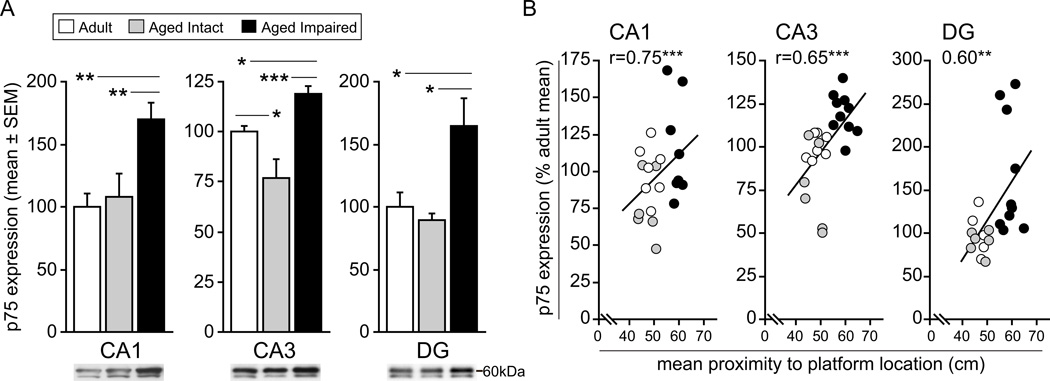
p75, the low-affinity neurotrophin receptor and TNF receptor superfamily member, which forms heterotrimeric co-receptor complexes with NgR1 and LINGO, was also quantified in hippocampal subregions and was observed to vary significantly in expression between groups (CA1: F2,19=7.26, P=0.005; CA3: F2,20=15.10, P<0.001; DG: F2,18=5.29, P=0.016). p75 was significantly elevated in aged impaired rats compared to aged intact rats, with increases ranging from nearly 60% in CA1 (SNK, p<0.01) and CA3 (SNK, p<0.001) to 85% in DG (SNK, p<0.05) (Figure 3A). Likewise, compared to mature adult rats, p75 content was significantly greater in CA1 (+70%; SNK, p<0.01), CA3 (+20%; SNK, p<0.05) and DG (+65%; SNK, p<0.05) of aged impaired rats. Expression of p75 was significantly decreased (−24%; SNK, p<0.05) CA3 of aged intact rats compared to adults, and expression was similar between aged intact and adult rats in CA1 and DG, suggesting that increased p75 expression occurs specifically with cognitive decline rather than as a general effect of aging. p75 content was significantly correlated with water maze performance, with greater p75 expression associated with greater mean proximity-to-platform measures (i.e., worse spatial learning and memory) (Figure 3B). This relationship was evident in CA1 (r=0.75, p<0.001) and CA3 (r=0.65, p<0.001) as well as in DG (r=0.60, p<0.01).
Figure 3. p75 expression is induced in hippocampus of cognitively impaired rats.
A) p75 protein content is significantly elevated in CA1, CA3 and DG hippocampal subregions dissected from aged rats exhibiting cognitive decline compared to aged-matched, cognitively intact rats. Likewise, aged impaired rats demonstrate significantly greater p75 protein content compared to mature adults, although no differences in p75 expression were detected between aged intact rats and mature adults. *p<0.05, **p<0.01, ***p<.001, one-way ANOVA, SNK post hoc testing. Representative blots are depicted. B) In CA1, CA3, and DG, p75 protein expression correlated significantly with water maze performance, with higher protein levels associated with poorer probe trial performance. *p<0.01, **p<0.001, Pearson (normal distributions) or Spearman (non-normal distributions) correlation.
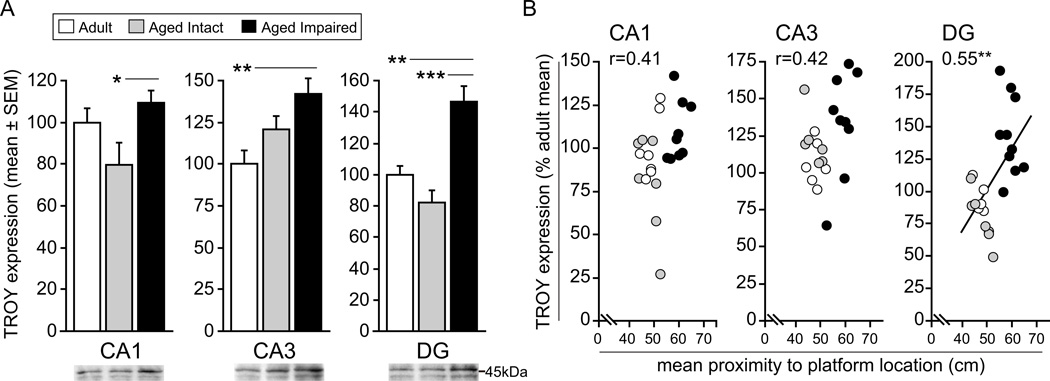
We also assessed hippocampal expression of TROY, another TNF receptor superfamily protein and NgR1/LINGO-1 heterotrimeric co-receptor complex component. Like LINGO-1 and p75, TROY expression was differentially expressed between groups in multiple hippocampal subregions (CA1: F2,20=3.82, P=0.039; CA3: F2,18=6.93, P=0.006; DG: F2,19=15.94, P<0.001). Significant increases in TROY were observed in aged impaired rats versus aged intact rats in CA1 (38%; SNK, p<0.05) and DG (83%; SNK, p<0.001) (Figure 4A). Additionally, significantly greater amounts of TROY protein were observed in CA3 (+42%; SNK, p<0.01) and DG (+42; SNK, p<0.001) of aged impaired rats compared to adults. No significant differences in TROY expression between aged intact and adult rats were observed. TROY expression in DG was significantly correlated with maze performance (r=0.55, p<0.01) (Figure 4B). A near-significant relationship was also evident in CA1 (r=0.41, p=0.052) and CA3 (r=0.42, p=0.057). Together, these data suggest that critical components of NgR1 co-receptor signaling complexes are induced with age-related cognitive decline.
Figure 4. Hippocampal TROY expression is increased with age-related cognitive decline.
A) TROY protein content is significantly upregulated in CA1 and DG of aged, cognitively impaired rats compared to aged, cognitively impaired rats. Increased protein expression is also evident in CA3 and DG of aged impaired rats compared to mature adults, while no significant differences exist between aged intact and adult rats. *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA, SNK post hoc testing. Representative blot images are shown. B) TROY expression was significantly correlated with spatial cognitive ability in CA3 and DG, and a relationship between protein content and maze performance approached significance in CA1. Increased TROY content was associated with cognitive impairment, indicated by larger mean proximity-to-platform values. **p<0.01, Pearson correlation.
NgR1 and NgR1 co-receptors are colocalized in hippocampus of aged cognitively impaired rats
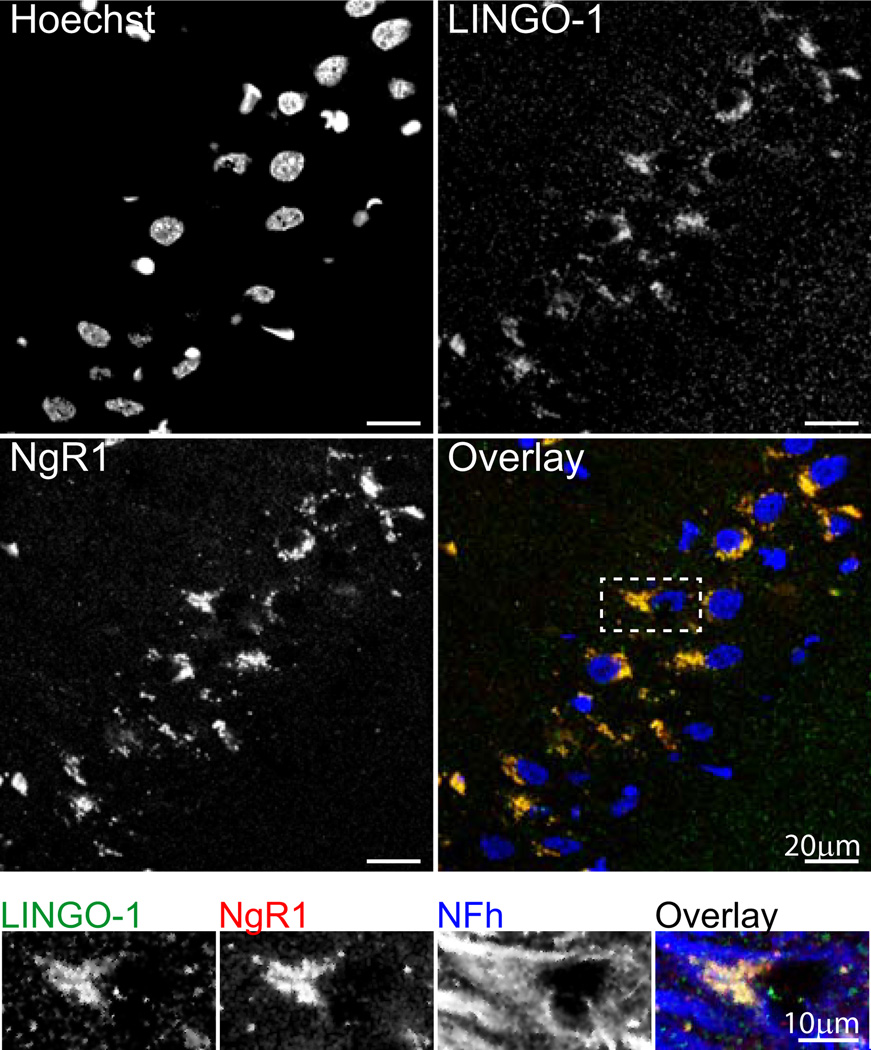
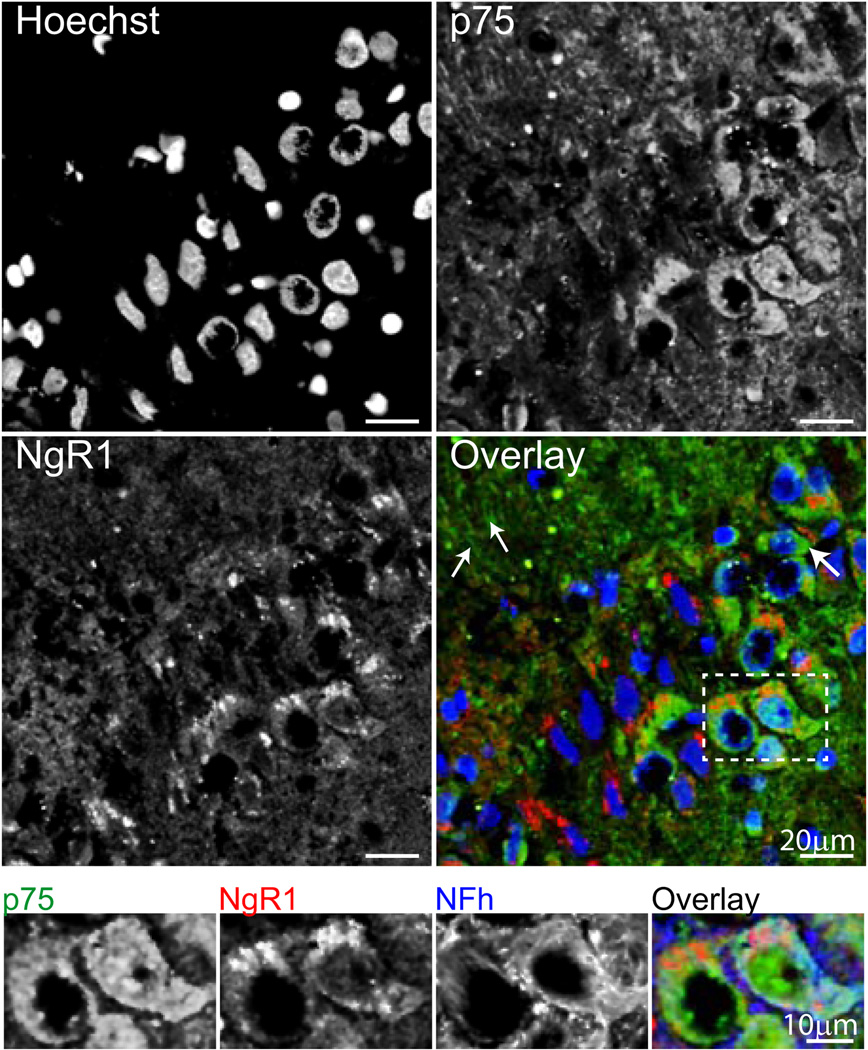
Because the neuronal NgR1 receptor lacks an intracellular domain, this protein requires interaction with co-receptors to transduce signals upon binding of its MAI ligands, Nogo-A, MAG and OMgp. Two heterotrimeric NgR1 receptor complexes, NgR1/LINGO-1/p75 and NgR1/LINGO-1/TROY, have been identified to date, but have been characterized largely in vitro. To determine whether the NgR1, LINGO-1, p75 and TROY are co-expressed in the hippocampus of aged, cognitively impaired rats, and therefore capable of transducing MAI-NgR1 pathway signals as a potential mechanism of cognitive decline, we visualized these proteins using multiplexed immunohistochemisty. This analysis demonstrated LINGO-1 expression as diffuse, punctate staining throughout the hippocampus (data not shown), with concentrated cytoplasmic staining evident in cell bodies, predominantly in the pyramidal cell layer, (CA3 shown, Figure 5, top panel). Cytoplasmic staining of LINGO-1 was demonstrated to colocalize with staining of NgR1 in the pyramidal cell layer, which we have previously described (VanGuilder et al., 2012). To determine whether cells co-expressing LINGO-1 and NgR1 were neurons, co-staining for the neuronal neurofilament protein, NFh, was performed. Axonal and perisomatic NFh staining typical of large neurons demonstrated neuronal co-expression of LINGO-1 and NgR1 (Figure 5, inset), suggesting the potential for receptor/co-receptor protein-protein interaction.
Figure 5. LINGO-1 colocalizes with NgR1 in hippocampal neurons of aged impaired rats.
Top panel: Punctate LINGO-1 immunoreactivity is evident in the hippocampus, with areas of dense, putatively cytoplasmic staining observed to colocalize with NgR1 in the pyramidal cell layer (CA3 shown). Overlay: Blue=Hoechst, Green=LINGO-1, Red=NgR1. Inset: LINGO-1/NgR1 staining occurs in hippocampal neurons, as demonstrated by their co-expression in NFh-immunoreactive cells in the pyramidal cell layer. Immunoreactivity patterns are consistent with receptor/co-receptor colocalization in neuronal somata and proximal axon segments. Overlay: Green=LINGO-1, Red=NgR1, Blue=NFh.
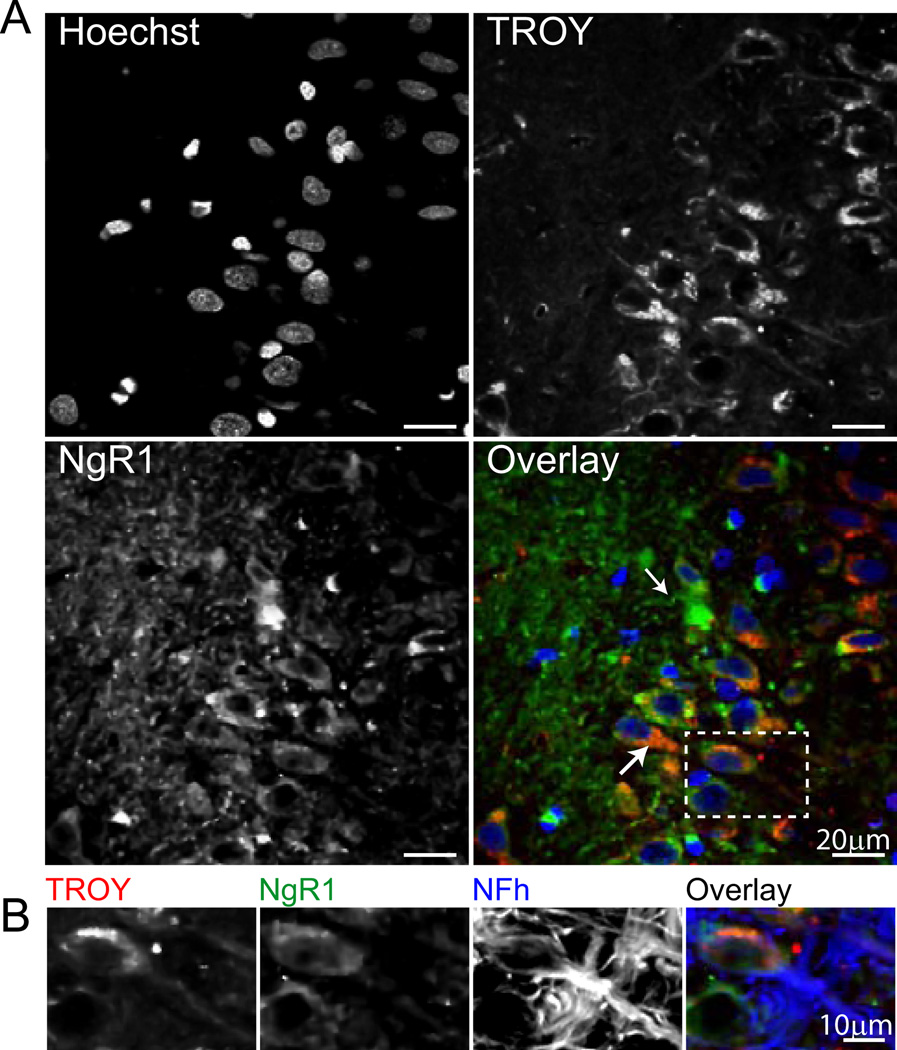
Similarly, p75 co-expression with NgR1 was evaluated by immunohistochemistry. Cytoplasmic p75 expression was evident throughout the hippocampus (data not shown), and was concentrated in the pyramidal cell layer as well as in processes projecting toward stratum radiatum (CA3 shown, Figure 6, top panel). Cytoplasmic p75 co-localized with NgR1, particularly in large cell bodies, although a subset of NgR1-expressing cells lacking p75 was also evident. p75/NgR1 colocalization was demonstrated in hippocampal neurons in the pyramidal cell layer by co-staining for NFh (Figure 6, inset). The hippocampal expression of TROY was also assessed, and was demonstrated in both NgR1-expressing and NgR1-lacking cell bodies (Figure 7, top panel). Likewise, a significant portion of NgR1 staining was not colocalized with TROY immunoreactivity. Both somatic and cellular projection-associated TROY/NgR1 co-expression was evident, particularly in large cell bodies in DG (shown). These cells were identified as neurons by co-staining for NFh, which demonstrated colocalization of TROY and NgR1 in both neuronal somata and axons (Figure 7, inset). These immunohistochemical demonstrations of NgR1/co-receptor co-localization in hippocampal neurons provide support for formation of NgR1 co-receptor complexes capable of effecting NgR1 pathway signaling in the hippocampus of aged, cognitively impaired rats.
Figure 6. p75 and NgR1 are co-expressed in hippocampal neurons of rats with cognitive decline.
Top panel: Diffuse hippocampal p75 expression is apparent in the hippocampus (CA3 shown), with concentrated areas of staining evident in cell bodies (large arrow) and processes (small arrows). Colocalization of p75 and NgR1 is evident in large cell bodies. p75 expression was also detected in a subset of nuclei. Overlay: Blue=Hoechst, Green=p75, Red=NgR1. Inset: Co-expression of p75 and NgR1 occurs in neurons in the pyramidal cell layer, where colocalization is evident in NFh-immunoreactive somata. Overlay: Green=p75, Red=NgR1, Blue=Nfh.
Figure 7. Hippocampal neurons of aged cognitively impaired rats co-express TROY and NgR1.
Top panel: Hippocampal TROY expression is evident in somatic cytoplasm (large arrow) and cellular processes (small arrow). Colocalization of TROY and NgR1 is evident both cell bodies and projections (see inset), although a significant proportion of NgR1-immunoreactive processes express little or no TROY. Overlay: Blue=Hoechst, Red=TROY, Green=NgR1. Inset: Neuronal co-expression of TROY and NgR1 is apparent in both somata and axons, as demonstrated by co-immunoreactivity with NFh. Overlay: Red=TROY, Green=NgR1, Blue=NFh.
RhoA protein expression is increased with cognitive decline
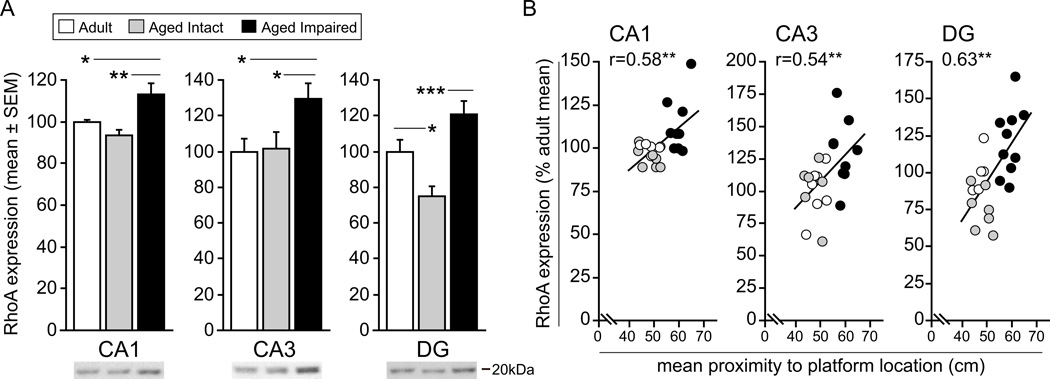
NgR1 pathway signaling converges on RhoA, a downstream effector that modulates structural plasticity among other processes. Given the coordinated induction of MAI ligands and NgR1, and our novel finding that the NgR1 co-receptors LINGO-1, p75 and TROY are upregulated in cognitively impaired aged rats, we sought to determine whether RhoA expression is likewise increased with cognitive decline. Immunoblotting for RhoA in the same hippocampal subregion dissections described above [adult (CA1: n=7; CA3: n=7; DG: n=5), aged intact (CA1: n=7; CA3: n=6; DG: n=7) and aged impaired (CA1: n=9; CA3: n=10; DG: n=10)] revealed small but significant increases in RhoA protein in aged impaired rats compared to cognitively intact adult and aged rats (CA1: F2,19=6.29, P=0.008; CA3: F2,19=4.32, P=0.028; DG: F2,19=11.87, P<0.001). Compared to age-matched cognitively intact rats, RhoA expression in aged impaired was increased by 21% in CA1 (SNK, p<0.01), 28% in CA3 (SNK, p<0.05), and by 60% in DG (SNK, p<0.001) (Figure 8A). Similarly, RhoA content was elevated in CA1 and CA3 of aged impaired rats compared to adults by 13% (SNK, p<0.05) and 30% (SNK, p<0.05), respectively. RhoA expression in aged intact rats differed from that in adults in DG only, in which RhoA protein was decreased by 25% (SNK, p<0.05). Like the MAI ligands, NgR1, and NgR1 co-receptors, RhoA content correlated significantly with cognitive performance among individual rats (Figure 8B). This relationship between protein expression and water maze deficits was evident in CA1 (r=0.58, p<0.01), CA3 (r=0.54, p<0.01) and DG (r=0.63, p<0.01). Future studies assessing RhoA activation status as well as expression and modification of downstream regulators of synaptic plasticity with aging and cognitive decline will provide added insight into the potential mechanistic role of MAI/NgR1 pathway induction with age-related cognitive impairment.
Figure 8. RhoA expression is significantly upregulated with in the hippocampus of aged, cognitively impaired rats.
A) RhoA protein content is significantly increased in CA1, CA3 and DG of aged impaired rats compared to aged intact rats, with similar increases evident between aged impaired and mature adult rats in CA1 and CA3. With the exception of DG, in which RhoA expression is decreased in aged impaired rats compared to adults, no differences between aged intact and adult rats were observed. *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA, SNK post hoc testing. Representative blots are shown. B) A statistically significant relationship between RhoA protein expression and cognitive performance was evident in CA1, CA3 and DG. Increased RhoA content was correlated with deceased spatial learning and memory ability. **p<0.01, Pearson (normal distributions) or Spearman (non-normal distributions) correlation.
Upregulation of NgR1 co-receptor and RhoA protein expression is not reflected at the transcript level
To determine whether NgR1 pathway induction with cognitive decline is reflected at the level of transcript expression, LINGO-1, p75, TROY and RhoA mRNA was quantified in hippocampal subregion dissections (adult n=7, aged intact n=7, aged impaired n=10; CA1, CA3 and DG subregions), which revealed a significant effect of age (both aged intact vs. adult and aged impaired vs. adult) on p75 and RhoA transcript content. This was evident in CA3 (p75: F2,20=12.59, P<0.001; RhoA: F2,20=14.52, P<0.001) and in DG (p75: F2,19=6.67, P=0.006; RhoA: F2,21=4.84, P=0.019). Specific pairwise comparisons are detailed in Table 3. Interestingly, no differences in transcript content for LINGO-1, p75, TROY or RhoA were observed between cognitively intact and cognitively impaired aged rats. This is in agreement with our previous findings that cognitive decline-associated increases in protein expression of MAI ligands and NgR1 occur in the absence of concomitant transcript induction (VanGuilder et al., 2012).
Protein-level induction of MAI/NgR1 pathway components is conserved across hippocampal subregions in aged cognitively impaired rats
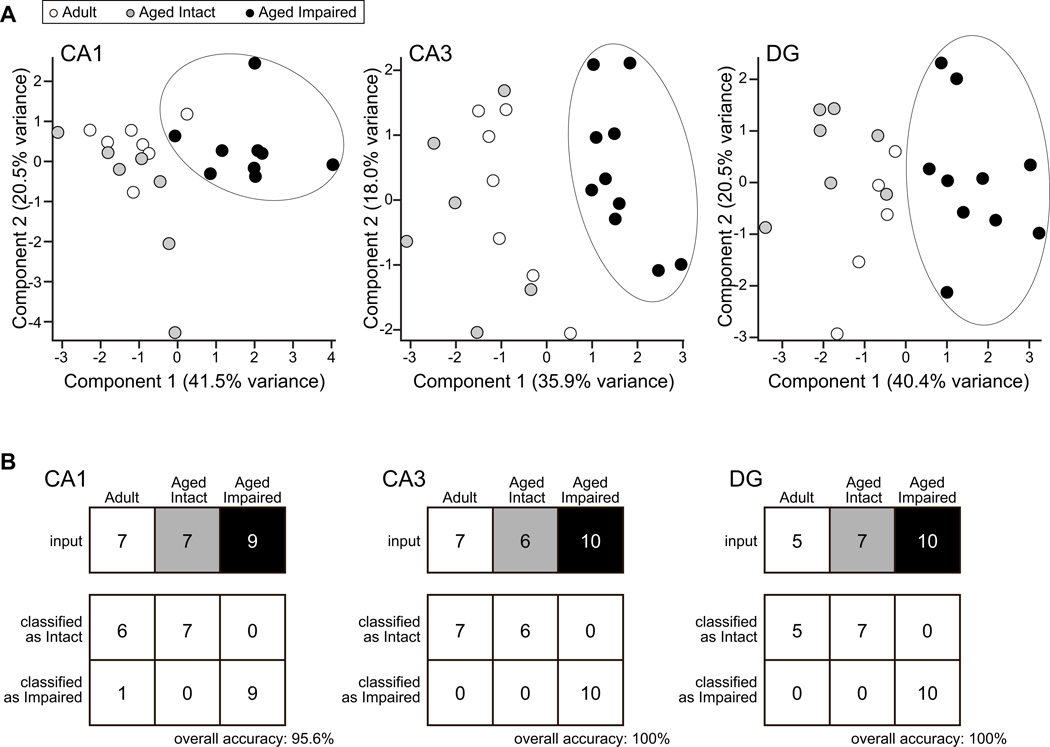
Given the consistent upregulation of NgR1 ligands (Nogo-A, MAG, OMgp), NgR1, NgR1 co-receptors (LINGO-1, p75, TROY), and RhoA that we have observed throughout the hippocampus of aged rats with impaired spatial learning and memory, both previously and in the present study, we assessed the covariance of MAI/NgR1 pathway components among adult, aged intact, and aged impaired rats. Protein expression data for these eight proteins in CA1 (adult: n=7; aged intact: n=7; aged impaired: n=9), CA3 (adult: n=7; aged intact: n=6; aged impaired: n=10), and DG (adult: n=5; aged intact: n=7; aged impaired: n=10) was assessed by statistical correlation analysis, which revealed strong covariance of pathway components (Table 5). Interestingly, p75 expression correlated more frequently than TROY with pathway components in CA1 and CA3, while TROY correlated more frequently than p75 in DG. We also visualized the relationship between cognitive status and MAI/NgR1 pathway component expression by principal component analysis. This demonstrated that MAI/NgR1 pathway protein expression segregates aged cognitively impaired rats from other groups when using either CA1, CA3 or DG expression profiles (Figure 9A), while aged cognitively intact and mature adult rats cluster together. To further examine the relationship of the MAI/NgR1 pathway to cognitive performance, the ability of pathway expression profiles, including protein expression of MAI ligands, NgR1, NgR1 co-receptors and RhoA, to correctly classify rats as cognitively intact or cognitively impaired was tested using a support vector machine class prediction algorithm. This machine learning approach classified individual adult (cognitively intact), aged intact and aged impaired rats with a high degree of accuracy (Figure 9B) based on the expression of MAI ligands, NgR1 and NgR1 co-receptors in individual hippocampal subregions. Overall classification accuracy, based on protein expression profiles, was 100% for CA3 and DG. Accuracy for CA1 was 95.6%, with one adult rat misclassified as cognitively impaired, and all aged intact rats correctly classified as cognitively intact.
Table 5.
NgR1 signaling pathway component co-variance
| CA1 | NgR1 | MAG | Nogo-A | OMgp | RhoA | TROY | p75 | LINGO-1 |
|---|---|---|---|---|---|---|---|---|
| LINGO-1 | - | 0.58** | 0.50* | 0.54* | - | - | 0.46* | |
| p75 | 0.47* | 0.67** | 0.61** | 0.60** | 0.44* | - | ||
| TROY | - | - | - | - | - | |||
| RhoA | 0.68** | - | - | 0.61** | ||||
| OMgp | 0.50* | 0.73*** | 0.52* | |||||
| Nogo-A | - | 0.67** | ||||||
| MAG | 0.49* | |||||||
| NgR1 | ||||||||
| CA3 | NgR1 | MAG | Nogo-A | OMgp | RhoA | TROY | p75 | LINGO-1 |
|---|---|---|---|---|---|---|---|---|
| LINGO-1 | - | 0.53* | 0.50* | - | 0.43* | - | 0.42* | |
| p75 | 0.57** | - | - | 0.53** | 0.53* | - | ||
| TROY | - | - | - | - | 0.58* | |||
| RhoA | - | - | 0.53* | - | ||||
| OMgp | 0.43* | - | - | |||||
| Nogo-A | 0.54* | - | ||||||
| MAG | - | |||||||
| NgR1 | ||||||||
| DG | NgR1 | MAG | Nogo-A | OMgp | RhoA | TROY | p75 | LINGO-1 |
|---|---|---|---|---|---|---|---|---|
| LINGO-1 | - | - | - | - | - | 0.50* | 0.60** | |
| p75 | - | - | - | - | 0.65** | 0.74*** | ||
| TROY | - | - | 0.60** | - | 0.69*** | |||
| RhoA | 0.52* | - | - | - | ||||
| OMgp | - | - | - | |||||
| Nogo-A | - | 0.55* | ||||||
| MAG | - | |||||||
| NgR1 | ||||||||
Using NgR1 pathway component protein expression levels determined in the current study (LINGO-1, p75, TROY, RhoA) and previously assessed and reported using the same dissected CA1, CA3 and DG hippocampal subregion samples from these animals (NgR1, MAG, Nogo-A, OMgp; VanGuilder et al., 2012), we sought to determine the degree of pathway component covariance across adult, aged intact, and aged impaired rats. Co-variance of protein expression of the NgR1 receptor, co-receptors (LINGO-1, p75 TROY), primary ligands (MAG, Nogo-A, OMgp), and RhoA was determined by Pearson product moment analysis.
Correlation coefficients are presented; *p<0.05, **p<0.01, ***p<0.001.
CA1: adult n=7, aged intact n=7, aged impaired n=9; CA3: adult n=7, aged intact n=6, aged impaired n=10; DG: adult n=5, aged intact n=7, aged impaired n=10.
Figure 9. MAI/NgR1 pathway components co-vary in expression and correctly classify rats by cognitive status.
A) Principal component analysis using adult mean-centered expression values of MAG, Nogo-A, OMgp, NgR1, LINGO-1, p75, TROY and RhoA, obtained through immunoblot analyses of CA1, CA3 and DG hippocampal subregion dissections, segregates aged impaired rats from aged intact and mature adult rats. Aged intact and adult rats cluster more closely together, providing visualization of the cognitive decline-specific nature of MAI/NgR1 pathway induction. B) Informatic class-prediction analysis demonstrates that the expression patterns of the eight MAI/NgR1 pathway proteins assessed to date classify adult, aged intact and aged impaired rats as cognitively intact or cognitively impaired with high accuracy.
Discussion
In this work, we demonstrate significant protein-level induction of the signal-transducing NgR1 co-receptors, LINGO-1, p75, and TROY, and a downstream effector GTPase, RhoA, in aged cognitively impaired rats compared to both age-matched cognitively intact and adult rats. Interestingly, increased protein expression with cognitive decline is not accompanied by a concomitant upregulation of NgR1 pathway component transcripts. These data build on our previous finding that NgR1 and its ligands are upregulated in the hippocampus with age-related cognitive decline. Further, we demonstrate that NgR1 and its co-receptors are co-localized in hippocampal neurons of aged impaired rats, indicating that this pathway is not only excessively expressed with cognitive decline, but also possesses the capacity for NgR1 signal transduction through interaction of NgR1 with LINGO-1, p75 and TROY. Bioinformatic analysis of hippocampal protein expression data generated in this study and our previous examinations of these same animals, revealed that NgR1 pathway component expression is highly covariant across adult, aged intact, and aged impaired rats. Lastly, we demonstrate that pathway expression data accurately segregates aged impaired rats from aged intact and adult rats. Together, these data indicate a coordinated induction of the NgR1 signaling pathway with cognitive decline, and suggest that this pathway may be capable of transducing plasticity-suppressing signals in hippocampal neurons.
Age-related cognitive decline is a prevalent health concern for the aging population, and can diminish independence and health-span to a disabling degree (Hedden & Gabrieli, 2004). Dysregulation of multiple processes, including increased neuroinflammation, impaired metabolic and trophic support, and impaired neurotransmission have been described in the aging hippocampus of rodent and primate model organisms, as well as in humans (VanGuilder & Freeman, 2011). Rather than being specific to cognitive decline, which impacts an estimated 40% of the aging human population (Koivisto et al., 1995; Schaie, 1996; Small, 2002), alteration of these processes is often evident among aged subjects regardless of cognitive status. The MAI/NgR1 pathway appears to be one of relatively few signaling systems dysregulated preferentially with cognitive decline and is therefore promising as a novel mechanism of age-related spatial learning and memory deficits and as a potential target for therapeutic intervention. We have previously demonstrated hippocampal upregulation of neuronal NgR1 and its primary plasticity-suppressing ligands in cognitively impaired aged rats, and the correlation of these proteins to severity of cognitive deficits (VanGuilder et al., 2011b; VanGuilder et al., 2012). Whether this induction represents an increase in hippocampal NgR1 signaling capacity remains to be established. NgR1, a glypiated surface protein lacking a membrane-spanning domain (Fournier et al., 2001), requires multiple co-receptors for signal transduction and expression of these co-receptors have not been assessed in aged, behaviorally phenotyped subjects.
NgR1 was initially identified as a high-affinity receptor for the Nogo-66 domain of Nogo-A (Fournier et al., 2001) and later determined to interact with the additional ligands MAG (Domeniconi et al., 2002; Liu et al., 2002) and OMgp (Wang et al., 2002b). In addition to the canonical function of the MAI/NgR1 signaling pathway in neurite outgrowth inhibition and growth cone collapse (Prinjha et al., 2000; Giger et al., 2008; Schnell et al., 2011), this pathway also functions to modulate ultrastructural and functional aspects of synaptic plasticity (Zagrebelsky et al., 2005; Aloy et al., 2006; Lee et al., 2008; Michaelsen et al., 2010; Raiker et al., 2010; Zagrebelsky et al., 2010; Delekate et al., 2011) with demonstrated effects on learning and memory (Karlen et al., 2009). It is our hypothesis that overexpression of MAI/NgR1 pathway components, including the NgR1 co-receptors and RhoA described here, confers abnormal rigidity to hippocampal microcircuitry in aged cognitively impaired rats and therefore prevents acquisition and consolidation of new spatial memories. This is likely achieved by preventing the enhancement of synaptic connectivity associated with learning (Caroni et al., 2012) through suppression of structural rearrangement of dendritic spines (Lee et al., 2008; Raiker et al., 2010; Zagrebelsky et al., 2010) and decreased recruitment of signaling molecules such as glutamate receptors (Raiker et al., 2010; Peng et al., 2011).
NgR1 heterotrimeric receptor/co-receptor complexes mediate MAI/NgR1 signaling by activating RhoA (Niederost et al., 2002), which cannot be achieved by NgR1 alone. Upon MAI ligand binding to NgR1, p75 transduces the pathway signal using the Rho GDP/GTP exchange factor kalirin9 as an intermediary (Harrington et al., 2008), which displaces RhoA from chaperone Rho-GDP dissociation inhibitors (Rho-GDI) (Yamashita & Tohyama, 2003). TROY-mediated activation of RhoA has also been demonstrated (Shao et al., 2005; Wills et al., 2012), but the specific intermediary through which this occurs remains to be determined. Because p75 and TROY are interchangeable in these tripartite receptor complexes, LINGO-1 has been postulated to serve merely as an adaptor protein that modulates the interaction of NgR1 with p75 and TROY (Bandtlow & Dechant, 2004). It has recently been demonstrated however that LINGO-1 itself, when interacting with MAI ligand-bound NgR1, activates RhoA through the serine-threonine kinase WNK1 (with no lysine [K] 1), which displaces RhoA from Rho-GDI (Zhang et al., 2009). This signaling redundancy, combined with the functional redundancy of the three MAI ligands known to activate the NgR1 signaling pathway with similar effects on neuronal morphology and plasticity, may serve as a safeguard in the mature brain that prevents aberrant outgrowth and spontaneous fluctuation of synaptic structure to promote healthy cognition. As a potential mechanism of age-related deficits of learning and memory, however, the multiple stages of functional redundancy in the MAI/NgR1 pathway poses an obstacle for development of treatments targeting pathway elements upstream of RhoA, which itself may not be an ideal target due to its numerous additional functions in inflammation, cancer, and cardiovascular function (Beckers et al., 2010; Rathinam et al., 2011; Rougerie & Delon, 2012).
Our results demonstrate that p75, TROY and LINGO-1 co-localize with NgR1 in hippocampal neurons of aged cognitively impaired rats, indicating the capacity for MAI/NgR1 signaling in these cells. The redundant signaling functions of p75 and TROY may be necessary because these co-receptors are suggested to be expressed by distinct neuron populations. This is based on empirical demonstrations that p75 is co-expressed with NgR1 in most, but not all CNS neurons, and that while loss of p75 renders most neurons unresponsive to MAG, Nogo-A and OMgp, it does not eradicate all neuronal MAI/NgR1 action (Wang et al., 2002a; Yamashita et al., 2002; Lauren et al., 2003; Barrette et al., 2007). TROY expression in CNS neurons is likewise selective, and accounts for MAI/NgR1 signaling in neurons lacking p75, in which blockade of TROY abolishes NgR1 pathway action (Park et al., 2005). Interestingly, we observed a higher degree of p75 covariance with other MAI/NgR1 pathway components than of TROY in CA1 and CA3, suggesting that NgR1 signaling of neurons in these subregions may rely predominantly on p75. In this study, we observed coordinated upregulation of LINGO-1, p75 and TROY in CA1, CA3 and DG hippocampal subregions in aged, cognitively impaired rats, but no broad age-related changes in expression (i.e., in aged cognitively intact rats compared to middle-aged adults). This suggests that the induction of these, and other MAI/NgR1 pathway components (VanGuilder et al., 2012), with age-related cognitive decline represents a phenomenon distinct from typical developmental regulation (Llorens et al., 2011) of the pathway, although a comprehensive timecourse analysis of this pathway from maturity to advanced aging remains to be completed.
Interestingly, upregulation of NgR1 signaling components with cognitive decline does not appear to be regulated at the level of transcript expression in these animals. Through this work and our previous study (VanGuilder et al., 2012), we have demonstrated that hippocampal transcript content for many pathway components (NgR1, MAG, OMgp, Nogo-A, LINGO-1, TROY) is largely unchanged with both aging and cognitive decline. RhoA and p75 transcripts increase significantly with aging, but we observed no difference in their mRNA expression between cognitively intact and cognitively impaired aged rats. This suggests a post-transcriptional level of NgR1 pathway induction with age-related cognitive decline. The dissociation of protein and mRNA levels that we have observed is particularly intriguing given that previous studies of rodents and humans have observed a marked regulation of NgR1 pathway component transcript expression associated with high levels of neuronal activity (Josephson et al., 2003; Bandtlow et al., 2004; Trifunovski et al., 2004). It remains to be determined whether decreased neuronal activity occuring with aging or cognitive decline is a causative mechanism of NgR1 pathway upregulation. Additional studies are needed to determine whether translation rates of these proteins are increased in cognitively impaired animals, potentially through increased association of transcripts with polysomes or miRNA regulation, and whether environmental or pharmacological manipulations are sufficient to normalize their expression.
Age-related cognitive decline likely reflects a reduced ability of the aged brain to modify synaptic connectivity in response to changing task demands. Behaviorally-induced synaptogenesis and synapse rearrangement, such as destabilization and subsequent long-term re-stabilization of existing synapses, is closely related to functional plasticity, (De Roo et al., 2008a; De Roo et al., 2008b; Wilbrecht et al., 2010). The signaling effects of NgR1 co-receptors in the healthy CNS are beginning to be characterized, and include the regulation of neuronal structure and function previously attributed to MAIs and NgR1 (Hannila & Kawaja, 1999; Kohn et al., 1999; Walsh et al., 1999; Mi et al., 2004; Park et al., 2005; Shao et al., 2005; Ji et al., 2006; Zhang et al., 2009). For example, treatment with soluble TROY and TROY knockout block the inhibitory effects of myelin and promote neurite outgrowth in vitro (Park et al., 2005), and similar effects are observed following genetic p75 deletion (Wang et al., 2002a). Likewise, antibody-mediated LINGO-1 inactivation increases neurite length and decreases branching in dorsal root ganglion cultures (Petrinovic et al., 2010). Zagrebelsky et al (2005) have demonstrated an inverse relationship between p75 expression and neuronal spine density in the hippocampus in vivo. In organotypic hippocampal slice preparations, prolonged antibody-mediated antagonism of p75 increases spine density (Egashira et al., 2010), while knockdown of TROY increases synapse density in cultured hippocampal neurons (Wills et al., 2012). The actions of p75 and TROY are similar to the effects associated with NgR1 in the developing hippocampus, including restriction of synapse number and inhibition of excitatory synapse formation (Wills et al., 2012), and occur via RhoA activation. Diminished hippocampal synaptogenesis and suppression of synaptic strengthening by MAI/NgR1 signaling, mediated by NgR1 co-receptor signal transduction through RhoA, are likely contributing factors in the deficits of spatial learning and memory characteristic of cognitive decline.
In addition to promoting structural rigidity of synapses, NgR1 co-receptors also modulate synaptic signaling dynamics and cognitive function. p75 facilitates the synaptic suppression induced by repeated initiation of long-term depression (Egashira et al., 2010), which can be prevented by anti-p75 antibody treatment. Consistent with our correlation analysis demonstrating a significant relationship between increased hippocampal p75 expression and impaired spatial cognition, the inhibitory effects of hippocampal p75 on LTP and spatial learning and memory are dose dependent (Barrett et al., 2010). Homozygous p75-null mice exhibit both enhanced spatial learning and memory and increased LTP at Schaffer collateral synapses compared to wild-type controls (Barrett et al., 2010). Similarly, LINGO-1 inactivation promotes dopaminergic neuron function and improves behavioral function in parkinsonian rats (Inoue et al., 2007). Whether these effects are specific to signal transduction of the MAI/NgR1 pathway or reflect additional functions of NgR1 co-receptors, remains to be determined.
Given the effects of MAI/NgR1 signaling on both electrophysiological and anatomical correlates of synaptic plasticity in the undamaged CNS and demonstrations that suppressing various stages of MAI/NgR1 pathway signaling improves cognitive function, this pathway is attractive as a potential causative mechanism of non-neurodegenerative age-related deficits of learning and memory. To date, we have demonstrated the protein-level induction of NgR1 signaling machinery including the co-receptors LINGO-1, p75 and TROY, and the downstream effector upon which NgR1 signaling converges, RhoA, in a rat model of naturally-occurring cognitive decline. We also demonstrate that expression of these pathway components inversely correlates with spatial learning and memory performance, and that NgR1 is co-localized with its necessary co-receptors in the hippocampus of aged, cognitively impaired rats. Additionally, expression of the MAI/NgR1 pathway components investigated to date in this model, including ligands (MAG, Nogo-A, OMgp), the NgR1 receptor, NgR1 co-receptors (LINGO-1, p75, TROY), and RhoA, are highly co-variant in individual animals, suggesting that this pathway may be intrinsically involved in cognitive decline.
In conclusion, our findings indicate a consistent induction of NgR1 signaling machinery, including ligands, co-receptors and downstream effectors, throughout the hippocampus with cognitive decline. Combined with our previous demonstration that NgR1 and its MAI ligands are induced in the hippocampus with cognitive decline, the increased expression of LINGO-1, p75, TROY, and RhoA and co-localization of NgR1 with its critical co-receptors in hippocampal neurons that we describe here indicates hyperactivation and functional signaling capacity of this pathway. The specificity of pathway upregulation to aged rats with impaired spatial learning and memory, and its absence of regulation as a general effect of aging, provide the rationale for targeting this pathway for development of therapeutics that preserve or restore cognitive function in the aging population. Together, the abnormally-elevated expression of MAI/NgR1 signaling machinery, the functional redundancy of MAI ligands and NgR1 co-receptors, and the convergence of MAI/NgR1 signaling on RhoA suggests that antagonizing NgR1 to prevent downstream activation of RhoA may serve as the most promising targets to increase structural and functional plasticity in the aged brain and improve learning and memory deficits associated with cognitive decline. Future work will focus on determining potential mechanisms of NgR1 pathway expression regulation, and will assess the relationship between reported effects of MAI/NgR1 signaling on electrophysiological correlates of learning and memory (i.e., LTP, long-term depression, paired-pulse facilitation), anatomical and biochemical correlates of synaptic plasticity, and animal behavior.
Supplementary Material
Table 4.
Hippocampal expression of NgR1 signaling pathway transcripts
| Transcript | CA1 | CA3 | DG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adult | Aged Intact |
Aged Impaired |
Adult | Aged Intact |
Aged Impaired |
Adult | Aged Intact |
Aged Impaired |
|
| LINGO-1 | 100 ± 3.5 | 99 ± 3.1 | 92 ± 3.0 | 100 ± 2.8 | 121 ± 8.5 | 113 ± 5.7 | 100 ± 6.0 | 124 ± 14.8 | 131 ± 15.4 |
| p75 | 100 ± 5.7 | 105 ± 5.9 | 110 ± 9.5 | 100 ± 6.9 | 252 ± 36.1*** | 196 ± 11.2** | 100 ± 8.7 | 134 ± 9.5* | 154 ± 12.5** |
| TROY | 100 ± 5.3 | 110 ± 6.3 | 107 ± 4.7 | 100 ± 5.2 | 152 ± 17.2 | 133 ± 15.6 | 100 ± 11.9 | 181 ± 32.8 | 155 ± 24.4 |
| RhoA | 100 ± 3.8 | 101 ± 4.2 | 108 ± 5.0 | 100 ± 15.7 | 274 ± 26.3*** | 269 ± 28.5*** | 100 ± 11.8 | 196 ± 22.2* | 190 ± 26.9* |
Data are presented as % adult mean ± SEM.
p<0.05 vs. adult group;
p<0.01 vs. adult group;
p<0.001 vs. adult group.
No significant differences were observed between aged intact and aged impaired groups. One-way ANOVA with SNK post hoc testing; adult: n=7, aged intact: n=7, aged impaired: n=10 (for each subregion).
Acknowledgements
This work was supported by NIA grants R01AG026607 and P01AG11370 to WES, and generous support from the Donald W. Reynolds Foundation. We thank Julie Farley for assistance with behavioral phenotyping, Han Yan and Junie Warrington for performing hippocampal subregion dissections, Georgina Bixler for performing qPCR studies, and Brian Davidson and Isaac Washington for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CA1
Cornu Ammonis area 1
- CA3
Cornu Ammonis area 3
- DG
dentate gyrus
- Dsp
desmoplakin
- LTP
long-term potentiation
- MAG
myelin-associated glycoprotein
- MAI
myelin-associated inhibitor
- NFh
heavy neurofilament
- Nnat
neuronatin
- NgR1
Nogo-66 receptor 1
- Nogo-A
Neurite outgrowth inhibitor A
- Nov
nephroblastoma overexpressed
- OMgp
Oligodendrocyte Myelin glycoprotein
- OUHSC
University of Oklahoma Health Sciences Center
- PBS
phosphate-buffered saline
- SNK
Student Newman Keuls post hoc testing
- Spock1
sparc-osteonectin, cwcv and kazal like domains proteoglycan
- Tiam1
T-cell lymphoma invasion and metastasis 1
- Wfs1
Wolfram syndrome 1
Footnotes
The authors have no conflicts of interest to declare.
References
- Aloy EM, Weinmann O, Pot C, Kasper H, Dodd DA, Rulicke T, Rossi F, Schwab ME. Synaptic destabilization by neuronal Nogo-A. Brain Cell Biol. 2006;35:137–156. doi: 10.1007/s11068-007-9014-3. [DOI] [PubMed] [Google Scholar]
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE. 2004:pe24. doi: 10.1126/stke.2352004pe24. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Dlaska M, Pirker S, Czech T, Baumgartner C, Sperk G. Increased expression of Nogo-A in hippocampal neurons of patients with temporal lobe epilepsy. Eur J Neurosci. 2004;20:195–206. doi: 10.1111/j.1460-9568.2004.03470.x. [DOI] [PubMed] [Google Scholar]
- Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, Trieu J, Murphy M. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20:145–152. doi: 10.1002/hipo.20598. [DOI] [PubMed] [Google Scholar]
- Barrette B, Vallieres N, Dube M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007;34:519–538. doi: 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- Bixler GV, VanGuilder HD, Brucklacher RM, Kimball SR, Bronson SK, Freeman WM. Chronic insulin treatment of diabetes does not fully normalize alterations in the retinal transcriptome. BMC Med Genomics. 2011;4:40. doi: 10.1186/1755-8794-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Perin JP, Charbonnier F, Camuzat A, Roussel G, Nussbaum JL, Alliel PM. Structure and cellular distribution of mouse brain testican. Association with the postsynaptic area of hippocampus pyramidal cells. J Biol Chem. 1996;271:4373–4380. doi: 10.1074/jbc.271.8.4373. [DOI] [PubMed] [Google Scholar]
- Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008a;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008b;6:e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci USA. 2011;108:2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Egashira Y, Tanaka T, Soni P, Sakuragi S, Tominaga-Yoshino K, Ogura A. Involvement of the p75(NTR) signaling pathway in persistent synaptic suppression coupled with synapse elimination following repeated long-term depression induction. J Neurosci Res. 2010;88:3433–3446. doi: 10.1002/jnr.22505. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Bixler GV, Brucklacher RM, Lin CM, Patel KM, VanGuilder HD, LaNoue KF, Kimball SR, Barber AJ, Antonetti DA, Gardner TW, Bronson SK. A multistep validation process of biomarkers for preclinical drug development. Pharmacogenomics J. 2010a;10:385–395. doi: 10.1038/tpj.2009.60. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Salzberg AC, Gonzales SW, Grant KA, Vrana KE. Classification of alcohol abuse by plasma protein biomarkers. Biol Psychiatry. 2010b;68:219–222. doi: 10.1016/j.biopsych.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, VanGuilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci USA. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Venkatesh K, Chivatakarn O, Raiker SJ, Robak L, Hofer T, Lee H, Rader C. Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems. Restor Neurol Neurosci. 2008;26:97–115. [PMC free article] [PubMed] [Google Scholar]
- Gillani RL, Tsai SY, Wallace DG, O'Brien TE, Arhebamen E, Tole M, Schwab ME, Kartje GL. Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behav Brain Res. 2010;208:415–424. doi: 10.1016/j.bbr.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, Borges K, Dingledine R. Quantitative transcriptional neuroanatomy of the rat hippocampus: evidence for wide-ranging, pathway-specific heterogeneity among three principal cell layers. Hippocampus. 2009;19:253–264. doi: 10.1002/hipo.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp. 2009;33:1543. doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannila SS, Kawaja MD. Nerve growth factor-induced growth of sympathetic axons into the optic tract of mature mice is enhanced by an absence of p75NTR expression. J Neurobiol. 1999;39:51–66. [PubMed] [Google Scholar]
- Harrington AW, Li QM, Tep C, Park JB, He Z, Yoon SO. The role of Kalirin9 in p75/nogo receptor-mediated RhoA activation in cerebellar granule neurons. J Biol Chem. 2008;283:24690–24697. doi: 10.1074/jbc.M802188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Inoue H, Lin L, Lee X, Shao Z, Mendes S, Snodgrass-Belt P, Sweigard H, Engber T, Pepinsky B, Yang L, Beal MF, Mi S, Isacson O. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson's disease models. Proc Natl Acad Sci USA. 2007;104:14430–14435. doi: 10.1073/pnas.0700901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF, McCoy JM, Pepinsky RB, Mi S, Relton JK. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Josephson A, Trifunovski A, Scheele C, Widenfalk J, Wahlestedt C, Brene S, Olson L, Spenger C. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 2003;311:333–342. doi: 10.1007/s00441-002-0695-8. [DOI] [PubMed] [Google Scholar]
- Karlen A, Karlsson TE, Mattsson A, Lundstromer K, Codeluppi S, Pham TM, Backman CM, Ogren SO, Aberg E, Hoffman AF, Sherling MA, Lupica CR, Hoffer BJ, Spenger C, Josephson A, Brene S, Olson L. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci USA. 2009;106:20476–20481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto K, Reinikainen KJ, Hanninen T, Vanhanen M, Helkala EL, Mykkanen L, Laakso M, Pyorala K, Riekkinen PJ., Sr Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology. 1995;45:741–747. doi: 10.1212/wnl.45.4.741. [DOI] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 2003;24:581–594. doi: 10.1016/s1044-7431(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzlinger PM, Shimizu S, Marklund N, Thompson HJ, Schwab ME, Saatman KE, Hoover RC, Bareyre FM, Motta M, Luginbuhl A, Pape R, Clouse AK, Morganti-Kossmann C, McIntosh TK. Delayed inhibition of Nogo-A does not alter injury-induced axonal sprouting but enhances recovery of cognitive function following experimental traumatic brain injury in rats. Neuroscience. 2005;134:1047–1056. doi: 10.1016/j.neuroscience.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Llorens F, Gil V, del Rio JA. Emerging functions of myelin-associated proteins during development, neuronal plasticity, and neurodegeneration. FASEB J. 2011;25:463–475. doi: 10.1096/fj.10-162792. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Michaelsen K, Zagrebelsky M, Berndt-Huch J, Polack M, Buschler A, Sendtner M, Korte M. Neurotrophin receptors TrkB.T1 and p75NTR cooperate in modulating both functional and structural plasticity in mature hippocampal neurons. Eur J Neurosci. 2010;32:1854–1865. doi: 10.1111/j.1460-9568.2010.07460.x. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR. Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging. 2005;26:683–688. doi: 10.1016/j.neurobiolaging.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyang EL, Davidson BC, Lee W, Poon MM. Functional characterization of the dendritically localized mRNA neuronatin in hippocampal neurons. PLoS One. 2011;6:e24879. doi: 10.1371/journal.pone.0024879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Peng X, Kim J, Zhou Z, Fink DJ, Mata M. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J Neurochem. 2011;119:1183–1193. doi: 10.1111/j.1471-4159.2011.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinovic MM, Duncan CS, Bourikas D, Weinman O, Montani L, Schroeter A, Maerki D, Sommer L, Stoeckli ET, Schwab ME. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci. 2011;16:2561–2571. doi: 10.2741/3872. [DOI] [PubMed] [Google Scholar]
- Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett. 2012;142:1–13. doi: 10.1016/j.imlet.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Intellectual Development in Adulthood: The Seattle Longitudinal Study. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Schnell L, Hunanyan AS, Bowers WJ, Horner PJ, Federoff HJ, Gullo M, Schwab ME, Mendell LM, Arvanian VL. Combined delivery of Nogo-A antibody, neurotrophin-3 and the NMDA-NR2d subunit establishes a functional 'detour' in the hemisected spinal cord. Eur J Neurosci. 2011;34:1256–1267. doi: 10.1111/j.1460-9568.2011.07862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW. What we need to know about age related memory loss. BMJ. 2002;324:1502–1505. doi: 10.1136/bmj.324.7352.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Inoue H, Tanizawa Y, Matsuzaki Y, Oba J, Watanabe Y, Shinoda K, Oka Y. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10:477–484. doi: 10.1093/hmg/10.5.477. [DOI] [PubMed] [Google Scholar]
- Trifunovski A, Josephson A, Ringman A, Brene S, Spenger C, Olson L. Neuronal activity-induced regulation of Lingo-1. Neuroreport. 2004;15:2397–2400. doi: 10.1097/00001756-200410250-00019. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation. 2011a;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Sonntag WE, Freeman WM. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J Neurochem. 2012;121:77–98. doi: 10.1111/j.1471-4159.2012.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011b;43:201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder Starkey HD, Van Kirk CA, Bixler GV, Imperio CG, Kale VP, Serfass JM, Farley JA, Yan H, Warrington JP, Han S, Mitschelen M, Sonntag WE, Freeman WM. Neuroglial Expression of the MHCI Pathway and PirB Receptor Is Upregulated in the Hippocampus with Advanced Aging. J Mol Neurosci. 2012;48:111–126. doi: 10.1007/s12031-012-9783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Krol KM, Crutcher KA, Kawaja MD. Enhanced neurotrophin-induced axon growth in myelinated portions of the CNS in mice lacking the p75 neurotrophin receptor. J Neurosci. 1999;19:4155–4168. doi: 10.1523/JNEUROSCI.19-10-04155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Holtmaat A, Wright N, Fox K, Svoboda K. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J Neurosci. 2010;30:4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ZP, Mandel-Brehm C, Mardinly AR, McCord AE, Giger RJ, Greenberg ME. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 2012;73:466–481. doi: 10.1016/j.neuron.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nature Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nature Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Schweigreiter R, Bandtlow CE, Schwab ME, Korte M. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 2010;30:13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu X, Zhang Y, Zhou J, Yu Z, He C. LINGO-1 interacts with WNK1 to regulate nogo-induced inhibition of neurite extension. J Biol Chem. 2009;284:15717–15728. doi: 10.1074/jbc.M808751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.