Abstract
The Nlrp3 inflammasome has recently been implicated in the development of the metabolic syndrome through the impairment of adipose tissue insulin sensitivity. Recent literature associates Nlrp3 activation with much pathology caused by prolonged insulin resistance state, therefore establishing Nlrp3 as a promising therapeutic target for type-2-diabetes treatment.
More than 27% of the U.S. population suffers from the metabolic syndrome,1 which includes several of our most common health problems, such as central obesity, glucose intolerance/type 2 diabetes, dyslipidemia with accelerated atherosclerosis, hypertension, nonalcoholic hepatosteatosis, and elevated uric acid with increased risk of gout.2 Despite the varied clinical presentation, many of the components of the metabolic syndrome are driven by one single etiologic factor---insulin resistance.3,4 Indeed, mouse models with insulin resistance in various tissues have been shown to mimic many aspects of the metabolic syndrome.5,6
Despite the growing body of evidence that demonstrates the importance of diet and obesity to the onset of insulin resistance and the metabolic syndrome, the molecular mechanisms underlying this phenomenon are not completely understood. Inflammation at the level of adipose tissue and liver,7--9 oxidative stress,10 and mitochondrial dysfunction11 have all been shown to promote insulin resistance. However, how these act to give rise to the multifactorial pathophysiology of insulin resistance and how alterations in the energy balance can be sensed by cells of the organism and translated into signals that could affect insulin sensitivity are not known.
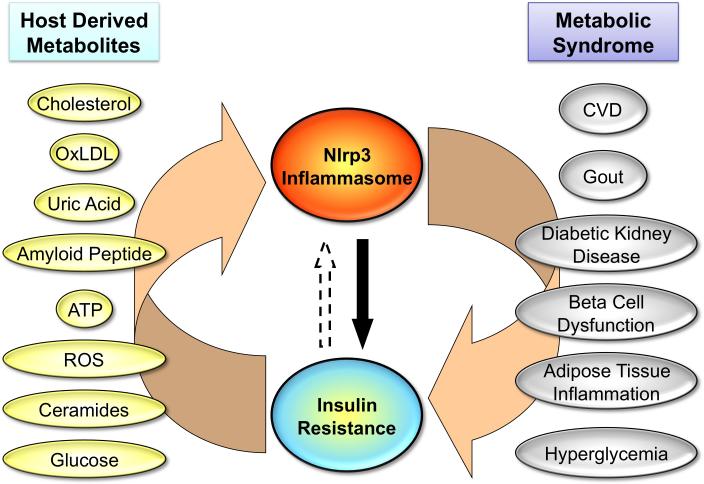
In a recent report, Vandanmagsar et al12 demonstrate that danger signals originating with obesity are sensed by the Nlrp3 class of cytosolic pattern recognition receptors, which are expressed in cells involved in the innate immune response, such as macrophages, and can activate a proinflammatory response mediated by interleukin (IL)-1β that results in insulin resistance at the level of fat, liver, and muscle of obese animals. Different from other pattern recognition receptors in the immune system, which are responsive to pathogen-derived antigens, the Nlrp3 inflammasome can be activated by host-derived molecules that are abundant in obese individuals, including excess ATP, glucose, ceramides, reactive oxygen species, oxidized LDL, uric acid, as well as crystals of cholesterol and monosodium urate.13 Activation of the Nlrp3 inflammasome by these endogenous signals results in caspase-1--mediated processing and activation of IL-1β in macrophages13. This promotes classical M1 activation of macrophages that has been linked to the development of inflammation in adipose tissue and metabolic diseases.14,15
These investigators further demonstrated that mice with ablation of the Nlrp3 receptor are resistant to diet-induced insulin resistance and hepatosteatosis and that this correlates with reduced activation of T cells in adipose tissue. They also showed that macrophages from the adipose tissue of Nlrp3 knockout mice have a blunted response to ceramide, which may explain the reduced M1 polarization of macrophages in the fat tissue in response to obesity. These findings suggest that the Nlrp3 inflammasome is a missing piece in the puzzle of the complex mechanisms involved in the onset of the metabolic syndrome and could be a potential target for treatment of insulin resistance.
Interestingly, the sulfonylurea glyburide---an anti-diabetic drug that stimulates insulin secretion---has recently been shown to act as an inhibitor of Nlrp3.16 More importantly, therapeutic agents that block IL-1β signaling improve glycemic control of diabetic patients, demonstrating that targeting the Nlrp3 inflammasome is a promising therapeutic strategy in type 2 diabetes clinical trials.17
With this in mind, the next obvious question is whether manipulating the Nlrp3 inflammasome may also affect other components of the metabolic syndrome? Even though the answer to this question is not clear, there is a growing body of evidence that supports a potential role of the Nlrp3 inflammasome in the development of multiple aspects of the metabolic syndrome. As noted by Vandanmagsar et al, the Nlrp3 inflammasome is activated in adipose tissue in mouse models of obesity and attenuated by calorie restriction. It also correlates with glycemia in type 2 diabetes patients after weight loss interventions.12 Reactive oxygen species and mitochondria dysfunction in adipose tissue, which have been implicated in the cellular stress cascades leading to insulin resistance, are activating signals of the Nlrp3 inflammasome.18 Others have shown that islet amyloid polypeptide in diabetic pancreas can activate Nlrp3 inflammasome and induce IL-1β secretion and inflammation of the islets, leading to the development of pancreatitis.19 Crystalline cholesterol present in atherosclerotic lesions has also been shown to be a potent activator of Nlrp3, thereby participating in the inflammatory process present in atherosclerotic plaques.20 Two recent genome-wide association studies have identified associations between SNPs in the Nlrp3 gene with high circulating levels of C-reactive protein and fibrinogen, two predictors of coronary heart disease.21,22 Uric acid crystals involved in gout are also capable of activating Nlrp3.23 Moreover, uric acid and IL-1β levels are both correlated with osteoarthritis progression and pathology,24 suggesting a role of the Nlrp3 inflammasome. Finally, in diabetic nephropathy, local IL-1β secretion has been shown to participate in the onset of kidney inflammation, and Nlrp3 inflammasome activation is observed in chronic kidney disease.25,26
Despite the potential role of the Nlrp3 inflammasome in metabolic syndrome, activation of this pathway in fat occurs late in obesity (7 months from the beginning of the high-fat diet in the mouse), suggesting that the Nlrp3 inflammasome is not a primary etiologic factor in the disease.12 In fact, many other pathways have been shown to promote insulin resistance and the metabolic syndrome before activation of caspase-1 and IL-1β during obesity. These include the numerous pathways known to induce recruitment of T cells and activation of macrophages in adipose tissue and liver7--9 and alterations in insulin receptor levels and insulin signaling through its receptor and substrate proteins.27 It is also not clear if the most common danger signals that are elevated with obesity, such as changes in nutrient metabolism,28,29 mitochondria dysfunction,11 and endoplasmic reticulum stress10,30 require the Nlrp3 inflammasome to promote insulin resistance. It also remains to be determined if insulin resistance itself can activate the Nlrp3 inflammasome, therefore creating one of many vicious cycles involved in the insulin resistance/inflammation axis.
Figure.
Cross-talk between insulin resistance and Nrlp3 inflammasome. OxLDL indicates oxidized low density lipoprotein; ATP, adenosine triphosphate; ROS, reactive oxygen species; CVD, cardiovascular disease.
References
- 1.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–-2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 2.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–-822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–-158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 4.Haas JT, Biddinger SB. Dissecting the role of insulin resistance in the metabolic syndrome. Curr Opin Lipidol. 2009;20:206–-210. doi: 10.1097/MOL.0b013e32832b2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–-134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14:778–-782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–-107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 8.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2010 doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 9.Mori MA, Liu M, Bezy O, Almind K, Shapiro H, Kasif S, Kahn CR. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes. 2010 doi: 10.2337/db10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–-948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 11.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–-387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 12.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–-188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–-832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–-309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–-184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–-70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–-321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–-225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 19.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–-904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–-1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassel CL, Lange LA, Keating BJ, Taylor KC, Johnson AD, Palmer C, Ho LA, Smith NL, Lange EM, Li Y, Yang Q, Delaney JA, Tang W, Tofler G, Redline S, Taylor HA, Jr., Wilson JG, Tracy RP, Jacobs DR, Jr., Folsom AR, Green D, O'Donnell CJ, Reiner AP. Association of genomic loci from a cardiovascular gene SNP array with fibrinogen levels in European Americans and African-Americans from six cohort studies: the Candidate Gene Association Resource (CARe). Blood. 2011;117:268–-275. doi: 10.1182/blood-2010-06-289546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, Pellikka N, Wallaschofski H, Kettunen J, Henneman P, Baumert J, Strachan DP, Fuchsberger C, Vitart V, Wilson JF, Pare G, Naitza S, Rudock ME, Surakka I, de Geus EJ, Alizadeh BZ, Guralnik J, Shuldiner A, Tanaka T, Zee RY, Schnabel RB, Nambi V, Kavousi M, Ripatti S, Nauck M, Smith NL, Smith AV, Sundvall J, Scheet P, Liu Y, Ruokonen A, Rose LM, Larson MG, Hoogeveen RC, Freimer NB, Teumer A, Tracy RP, Launer LJ, Buring JE, Yamamoto JF, Folsom AR, Sijbrands EJ, Pankow J, Elliott P, Keaney JF, Sun W, Sarin AP, Fontes JD, Badola S, Astor BC, Hofman A, Pouta A, Werdan K, Greiser KH, Kuss O, Meyer Zu Schwabedissen HE, Thiery J, Jamshidi Y, Nolte IM, Soranzo N, Spector TD, Volzke H, Parker AN, Aspelund T, Bates D, Young L, Tsui K, Siscovick DS, Guo X, Rotter JI, Uda M, Schlessinger D, Rudan I, Hicks AA, Penninx BW, Thorand B, Gieger C, Coresh J, Willemsen G, Harris TB, Uitterlinden AG, Jarvelin MR, Rice K, Radke D, Salomaa V, Willems vD, Boerwinkle E, Vasan RS, Ferrucci L, Gibson QD, Bandinelli S, Snieder H, Boomsma DI, Xiao X, Campbell H, Hayward C, Pramstaller PP, van Duijn CM, Peltonen L, Psaty BM, Gudnason V, Ridker PM, Homuth G, Koenig W, Ballantyne CM, Witteman JC, Benjamin EJ, Perola M, Chasman DI. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple Loci for C-reactive protein levels. Cirulation. 2011;123:731–-738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–-241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 24.Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, Coleman RE, Kraus VB. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011;108:2088–-2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–-1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satirapoj B. Review on pathophysiology and treatment of diabetic kidney disease. J Med Assoc Thai. 2010;93(suppl 6):S228–-S241. [PubMed] [Google Scholar]
- 27.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–-569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 28.Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Phys. 1999;111:241–-248. doi: 10.1046/j.1525-1381.1999.99220.x. [DOI] [PubMed] [Google Scholar]
- 29.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr., Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–-326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–-461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]