Abstract
Objectives
Systemic candidal infections are a common problem in hospitalized patients due to central venous catheters fabricated using silicone biomaterial (SB). We therefore evaluated the effect of human serum on C. albicans biofilm morphology, growth, and the expression of virulence-related genes on SB in vitro.
Methods
We cultivated C. albicans SC5314 (wild-type strain, WT) and its derivative HLC54 (hyphal mutant, HM) for 48 h in various conditions, including the presence or absence of SB discs, and human serum. The growth of planktonic and biofilm cells of both strains was monitored at three time points by a tetrazolium salt reduction assay and by scanning electron microscopy. We also analyzed by RT-PCR its expression of the virulence-related genes ALS3, HWP1, EAP1, ECE1, SAP1 - SAP10, PLB1, PLB2, PLC and PLD.
Results
At each time point, planktonic cells of WT strain cultured in yeast nitrogen base displayed a much higher expression of EAP1 and HWP1, and a moderately higher ALS3 expression, than HM cells. In planktonic cells, expression of the ten SAP genes was higher in the WT strain initially, but were highly expressed in the HM strain by 48 h. Biofilm growth of both strains on SB was promoted in the presence of human serum than in its absence. Significant upregulation of ALS3, HWP1, EAP1, ECE1, SAP1, SAP4, SAP6 - SAP10, PLB1, PLB2 and PLC was observed for WT biofilms grown on serum-treated SB discs for at least one time point, compared with biofilms on serum-free SB discs.
Conclusions
Human serum stimulates C. albicans biofilm growth on SB discs and upregulates the expression of virulence genes, particularly adhesion genes ALS3 and HWP1, and hydrolase-encoding genes SAP, PLB1 and PLB2. This response is likely to promote the colonization of this versatile pathogen within the human host.
Introduction
Central venous catheters (CVCs) fabricated using silicone biomaterial (SB) are widely used for drawing fluids into or from hospitalized patients. These devices have emerged as the most common independent risk factor for implant-associated bloodstream candidal infections, due to their intraluminal or extraluminal colonization by this yeast [1], [2]. Candida species, and Candida albicans in particular, are the third-leading cause of such CVC-related fungemias [1], [3]. The increased drug resistance of biofilm yeast cells compared to planktonic yeast cells [4] and biofilm-associated transcriptional changes in virulence genes [5] are considered to be the major reasons for such recalcitrant yeast infections.
When a CVC is inserted into a blood vessel, its surfaces are constantly incubated in blood and serum components, including sugars, proteins, electrolytes, and other organic molecules [6], [7]. In this favorable environment, yeast attachment is followed by cell division, hyphal development, and extracellular matrix formation, which leads to development of a loosely packed three-dimensional biofilm structure with fluid channels that permit the exchange of nutrients and waste [8].
Microbial adherence to a substrate, whether biotic (e.g. endothelium) or abiotic (e.g. catheter material), is a prerequisite to biofilm formation [3]. C. albicans ability to adhere to substrates is important for virulence, and is mediated through large glycoproteins encoded by genes such as HWP1 and ALS (agglutinin-like sequence gene family) [9]–[12]. The interactions of C. albicans with biotic or abiotic surfaces and the subsequent alterations in gene expression have been well studied [13], [14]. Such interactions lead to changes in expression of genes encoding glycosylphosphatidylinositol-dependent cell wall proteins (GPI-CWPs), which mediate adhesion of C. albicans to human endothelial cells and epithelial cells; for this reason, GPI-CWPs are also known as adhesins. Furthermore, variations in expression levels of the following virulence-related genes have been described both in vivo and in vitro during biofilm development: adhesion genes ALS1, ALS2, ALS3, ALS4, ALS5, HWP1 and EAP1 [5, 11, 13, 15, and 16] and hydrolase-encoding genes SAPs (secreted aspartyl proteases), LIPs (lipases) and PLs (phospholipases) [17]–[20]. These studies showed that variations in biofilm model system, growth medium and/or other environmental conditions could have a considerable effect on the differential mRNA expression levels of surface-specific genes.
The morphologic transformation among the yeast, hyphal and pseudohyphal forms of C. albicans is often considered to be a factor that enhances its virulence. The hyphal phase is thought to promote tissue penetration and colonization of organs during early stages of infection, whereas the yeast form might be important for dissemination in the bloodstream [21]. Previous studies have also demonstrated that genes governing hyphal morphogenesis are co-regulated with those genes encoding virulence factors such as adhesins and hydrolytic enzymes [22]. In addition, yeast morphologic transformations can be induced by serum. However, there is scant information about the role played by human serum or its constituents in Candida colonization of CVCs and in their subsequent biofilm growth. Furthermore, any associated changes in expression of virulence-related genes have not yet been evaluated.
To determine the effect of human serum on the development of C. albicans biofilms on CVCs (essentially fabricated using silicone biomaterial), we characterized planktonic as well as biofilm growth on silicone biomaterial (SB) with and without human serum, of C. albicans SC5314 (a wild-type strain, WT) and its hyphal mutant (HM) (strain HLC54, efg1/efg1 cph1/cph1). This mutant strain lacks a functional EFG1 gene, which encodes a transcriptional regulator that mediates the expression of certain cell wall proteins, such as HWP1 or ALS3 [23], [24]. Both of these proteins are important in the yeast-to-hyphae transition and are, therefore, critical for virulence. EFG1-deleted strains are known to be growth defective, especially in the yeast phase [25], which may explain our observations above.
In addition, we monitored temporal changes in mRNA levels for genes encoding proteins related to adhesion (ALS3, HWP1, EAP1 and ECE1) and genes encoding hydrolytic enzymes (SAP1 - SAP10, PLB1, PLB2, PLC and PLD) in both strains during planktonic growth in vitro and in biofilms developed on SB discs in the presence and absence of human serum.
Materials and Methods
C. albicans Strains and Growth Conditions
C. albicans SC5314 (WT) and its hyphal mutant, (HM) HLC54 (efg1/efg1 cph1/cph1; kindly donated by Prof. NAR Gow, University of Aberdeen, UK) were used throughout the study. The strains were stored in vials with multiple glass beads (Microbank, Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada) at −70°C, subcultured monthly in Sabouraud’s dextrose agar (SDA, Oxoid Ltd., Hampshire, UK) and maintained at 4°C during the experimental period. Yeast nitrogen base (YNB, Difco) medium supplemented with 100 mM glucose was used for liquid cultures. The purity of the phenotypes was confirmed with commercially available API32C identification systems (Biomérieux, Mercy I’Etoile, France) and the germ tube test [26]. YNB medium was supplemented with or without 3% human serum (from male AB plasma, sterile-filtered, H4522, Sigma) throughout this study.
Preparation of the Standard Yeast Cell Suspension
Prior to each experiment, Candida strains were subcultured on SDA at 37°C for 18 h. To prepare the yeast inocula for biofilm growth, a loopful of the SDA culture was transferred into 100 ml of liquid YNB supplemented with 100 mM glucose (Sigma, St. Louis, MO, USA) and incubated at 37°C for 18 h in an orbital shaker (75 rpm). The resulting cells were harvested, washed twice in phosphate-buffered saline (PBS, pH 7.2), centrifuged (4000 × g; 5 min) and resuspended in YNB supplemented with 100 mM glucose to a concentration of 107 cells/ml, as assessed by spectrophotometry and confirmed by hemocytometric counting.
Preparation and Sterilization of SB Discs
Catheter discs of 0.5 cm diameter measured by a micrometer were cut off a catheter (Lily Medical Corporation, Chunan Town, Taiwan) using a lathe (The Colchester Lathe Company Ltd., Colchester, UK). The SB discs were sterilized by immersing in a 0.5% sodium hypochlorite solution for 3 minutes, and they were then washed four times in 100 ml of deionized sterile water for 10 min. The sterility of the discs was verified by rolling the discs on SDA plates, incubating the plates at 37°C for 24 h, and observing the plates for microbial growth.
Human Serum
Human serum (from male AB plasma, sterile-filtered, H4522) was obtained from Sigma, Aldrich, USA).
Planktonic Growth
Candida strains were grown in SDA medium for 18 h. The resulting yeast cells were suspended in 10 ml of PBS, washed twice by centrifugation at 4,000 × g for 5 min, and resuspended in 1 ml of PBS to obtain a dense suspension (equivalent to McFarland standard 4). This yeast suspension was then transferred to 20 ml of YNB supplemented with 100 mM glucose and incubated at 37°C in a water bath at 180 rpm. The cells were harvested following 90 min, 24 h and 48 h incubation by centrifugation at 4,000 × g for 5 min. The yeast pellets (approximately 1 ml) were directly used for RNA extraction.
Biofilm Development of C. albicans on Silicone Biomaterial
Biofilms of the two C. albicans strains were developed on SB discs as described by Thein et al. [27]. The catheter discs were first placed in individual wells of multiwell tissue culture plates (Nunclon Delta, Nunc, Kamstrum, Denmark), into which 1 ml of either 3% human serum solution or sterile distilled water was dispensed, and incubated at 37°C in an orbital shaker (80 rpm) for 1 h. After incubation, the serum solution and the water were aspirated and the serum-coated or control catheter discs were then ready to be immersed into microbial suspensions.
Standard cell suspensions of C. albicans strains were prepared at a density of 1×107 cells/ml in YNB supplemented with or without 3% human serum. A 2-ml volume of these cell suspensions was then transferred into selected wells of a pre-sterilized, polystyrene, flat bottom 96-well microtiter plate (IWAKI, Tokyo, Japan), into which the serum-coated or control catheter discs (prepared as described above) were immersed. The plates were then incubated for 90 min at 37°C in an orbital shaker at 80 rpm to promote microbial adherence to the disc. In blank control wells, catheter discs were immersed into 2 ml of YNB.
Samples for biofilm growth analysis were taken at three time points (90 min, 24 h and 48 h) in the following manner. After 90 min (the adhesion phase), the cell suspensions were aspirated, and each disc was washed twice with PBS to remove loosely adherent cells. Then, we added 2 ml of either YNB or YNB supplemented with 3% human serum to each well, and re-incubated the discs at 37°C for 24 h and for 48 h. The plates were then removed from the incubator, and the wells were washed twice with PBS at the respective time intervals to eliminate traces of the growth medium. Finally, we quantified biofilm growth using a tetrazolium salt reduction assay (see below) and investigated biofilm ultrastructure with SEM.
Quantification of Cell Growth
Biofilm growth was monitored by a metabolic assay based on the reduction of 2, 3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), a tetrazolium salt [28]. Briefly, XTT (Sigma, MO, USA) was dissolved in PBS to a final concentration of 1 mg ml−1. The solution was filter-sterilized using a 0.22-µm pore-size filter and then stored at -70°C until required. Prior to each assay, the XTT solution was thawed and mixed with a freshly prepared 0.4 mM menadione solution (an electron-coupling agent that accelerates the reaction; Sigma, MO, USA) at a volume ratio of 20∶1.
Prior to the XTT reduction assay SB discs with biofilms were washed twice with 2 ml of PBS placed inside the sterile wells of microtitre plates, to remove loosely adherent cells. Afterwards, the biofilm-containing SB discs were transferred into 2-ml plastic vials containing 316 µl of PBS, 80 µl of XTT solution, and 4 µl of menadione solution. After incubation in the dark for 3 h at 37°C, the vials were centrifuged at 13,200 rpm for 10 min; 100 µl of the solution was transferred to a well in a microtiter plate, and the color change in the solution (indicating XTT reduction) was measured at 492 nm using a microtiter plate reader (Spectra-Max 340 Tunable Microplate Reader, Molecular Devices Ltd., Sunnyvale, CA, US). The mean and standard deviation (SD) of the results of three independent experiments were analyzed using a Student’s t-test to identify significant differences in biofilm growth at each time point (90 min, 24 h and 48 h).
Scanning Electron Microscopy
Morphology of planktonic and biofilm cells, as well as the ultrastructure of biofilms, was examined at various stages and under varying conditions of growth by scanning electron microscopy (SEM) as follows. At 90 min, 24 h and 48 h, 100 µls of the planktonic cultures were placed on glass slides and air dried, whereas selected biofilm-containing SB discs were removed from the microtiter plates, fixed in 2% glutaraldehyde for 2 hrs at room temperature. The glass slides and discs were subsequently washed in 70% ethanol, dehydrated in increasing concentrations of ethanol (70% for 30 min, 80% for 30 min, and 95% for 30 min), and stored in 1∶1 hexamethyldisilazane and absolute ethanol in a desiccator. Then, the specimens were air dried and mounted on aluminum stubs with copper tape and coated with gold under low pressure with an ion sputter coater (JEOL JFC1 100: JEOL, Tokyo, Japan). The surface topography of the biofilm was visualized with a scanning electron microscope (Philips XL30CP) in high-vacuum mode at 10 Kv.
Gene Expression Analysis
Extraction and Quantification of Total RNA
Planktonic-phase cultures of the C. albicans strains were harvested and then washed twice in PBS by centrifugation at 3500 rpm. The yeast pellet was collected for RNA extraction.
Biofilm-containing SB discs were washed three times in PBS to remove loosely adherent cells, and then biofilm cells were recovered from the SB discs by transferring the disc into 1 ml of PBS in eppendorf tubes and vortexing at 180 rpm for 2 minutes to disperse the biofilm. These biofilm cells were collected by centrifugation at 13,200 rpm for 5 minutes.
Total RNA of planktonic and biofilm cells was extracted using an SV Total RNA Isolation system (Promega, Madison, WI, USA) according to the manufacturer’s manual. RNA concentrations were quantified using a Beckman spectrophotometer, and an A260/A280 ratio between 1.8 and 2.0 ensured RNA purity. Additionally, gel electrophoresis was performed to ensure intact RNA.
cDNA Preparation and Quantification
Reverse transcription (RT) was performed on 5 µg of RNA in 11 µls of Diethylpryocarbonate water (Sigma, UK). To this mixture, 1 µl of oligo (dT) primer (a concentration of 0.5 µg/µl; Gibco BRL; Life Technologies, Gaithersburg, MD, USA) was added at 70°C for 10 min as previously described by Samaranayake et al. [29]. The annealed product was chilled on ice and mixed with 4 µl of ‘first-strand buffer’ (250 mM Tris-HCl, pH 8.3), 2 µl of 0.1 M DTT and 1 µl of 10 mM dNTPs. After incubation at 43°C for 2 min, 1 µl of 200 U µL−1 of SuperScript II reverse transcriptase (Gibco BRL; Life Technologies, Gaithersburg, MD, USA) was added to make up a final volume of 20 µl, which was then incubated at 43°C for 90 min to carry out cDNA synthesis [29].
Quantitative Real-time PCR
Real-time was performed as described in an earlier study [30] to quantify cDNAs (which had been synthesized as described above) corresponding to the following C. albicans genes: EFB1, ALS3, EAP1, HWP1, ECE1, SAP1 - SAP10, PLB1, PLB2, PLC and PLD. Forward and reverse primers (listed in Table 1) were designed with Primer Express (Applied Biosystems, Foster City, USA). Real time PCR was carried out with an ABI Step One Real Time PCR System using 2 × SYBR Green Master Mix (Applied Biosystems). The conditions were optimized for 40 cycles of amplification, each cycle consisting of denaturation at 95°C for 15 s followed by annealing at 60°C for 1 min. Quantitation standards, composed of 2-fold serial dilutions of PCR products, were run in conjunction with each set of samples, as well as a master-mix negative control (water). Each experiment was carried out with EFB1 as the house keeping gene. The tests were performed in duplicate and repeated at least once on different days for reproducibility. A melt curve assay was carried out for each experiment to confirm the specificity of the primers [31]. The relative fold change of each of the virulence genes was ascertained by comparing the gene expression levels of the test and control (with and without human serum) samples.
Table 1. Forward and reverse primers used in real-time PCR for the quantification of the expression of C. albicans virulence-related genes.
| ALS3-RT-F | CTG GAC CAC CAG GAA ACA CT |
| ALS3-RT-R | ACC TGG AGG AGC AGT GAA AG |
| ECE1-RT-F | GTC GTC AGA TTG CCA GAA ATT G |
| ECE1-RT-R | CTT GGC ATT TTC GAT GGA TTG T |
| EAP1-RT-F | TGT GAT GGC GGT TCT TGT TC |
| EAP1-RT-R | GGT AGT GAC GGT GAT GAT AGT GAC A |
| HWP1-RT-F | CGGAATCTAGTGCTGTCGTCTCT |
| HWP1-RT-R | CGACACTTGAGTAATTGGCAGATG |
| SAPRT1-F | GAA CCA AGG AGT TAT TGC CAA GA |
| SAPRT1-R | TTT GTC CAG TGG CAG CAT TG |
| SAPRT2-F | GTC ACT TTA AAA AAA CAA GGA GTC ATT G |
| SAPRT2-R | TAT TTG TCC CGT GGC AGC AT |
| SAP3RT-F | CAG CTT CTG AAT TTA CTG CTC CAT T |
| SAP3RT-R | TCC AAA AAG AAG TTG ACA TTG ATC A |
| SAPRT4-F | CGC TGG TGT CCT CTT AGA TTC TG |
| SAPRT4-R | AGG CAT AGA TAA TGC TAC GAG CAA |
| SAPRT5-F | CCA GCA TCT TCC CGC ACT T |
| SAPRT5-R | TTT AGC GTA AGA ACC GTC ACC AT |
| SAPRT6-F | GAT TGT AAA ACT TCA GGT ACC GTT GA |
| SAPRT6-R | CGA AGC AGG AAC GGA GAT CT |
| SAP7RT-F | TTC TCG TGA TGC TGT CCA AG |
| SAP7RT-R | AAA GCC TTC AAA TCC CCA GT |
| SAPRT8-F | GGT GTT AGT AGA GAT CTG GCC ACT ATT |
| SAPRT8-R | GGT GTT CCC ATC AAG ATC ATA AAC T |
| SAPRT9-F | TTC GGG TTC AGG AAC AAC ATC T |
| SAPRT9-R | GCT GAA TGA CGT GTG CTG GTA |
| SAPRT10-F | GGT TTT CGA TAG GCG ATT GAG A |
| SAPRT10-R | CCG TCC TTT TCA GTC TTG AGA TC |
| PLB1RT-F | GGT GGA GAA GAT GGC CAA AA |
| PLB1RT-R | AGC ACT TAC GTT ACG ATG CAA CA |
| PLB2RT-F | TGA ACC TTT GGG CGA CAA CT |
| PLB2RT-R | GCC GCG CTC GTT GTT AA |
| PLCRT-F | AGC CAC CAA TTG GCA AAC TTA |
| PLCRT-R | ACT GCT TGA TTT TAA AGT TGG TTT CC |
| PLDRT-F | TGT TTA CGG TGA AGG GTT GGA |
| PLDRT-R | CAC TGC TAA CCC TTG CTC TCT TG |
| EFB1RT-F | AAG AAG GCT GCT AAA GGT CCA A |
| EFB1RT-R | ATC CCA TGG TTT GAC ATC CAA |
Statistical Analysis
Statistical analysis was conducted by a statistical analysis computer software package (SPSS 20.0 for Windows©, SPSS Inc., Chicago, IL, USA) using the normality test (checked by using the Kolmogorow–Smirnov test) and the equal variance assumptions test (checked by the modified Levene test). T-tests were used to compare a) the biofilm growth of C. albicans strains SC5314 (WT) and HLC54 (HM) on silicone biomaterial and, b) expression levels of virulence-related genes in the C. albicans strains SC5314 (WT) and HLC54 (HM) on silicone biomaterial in the presence or absence of human serum at three time points (90 min, 24 h and 48 h). A p value less than 0.05 was considered statistically significant.
Results
Biofilm Growth of C. albicans on SB Discs
Data comparing biofilm growth of C. albicans strains SC5314 (WT) and HLC54 (HM) on SB discs in the absence (control) or presence of 3% human serum were obtained by using a tetrazolium salt reduction assay (Table 2). In the WT strain, significant differences in biofilm growth were observed between serum-coated and control SB discs at both 24 h (p<0.001) and 48 h (p<0.01). In terms of biofilm growth significant inter-strain variations between the WT and HM strains were observed in the presence of human serum, with the WT strain demonstrating higher metabolic activity at 24 h when compared to the HM strain at 48 h (p = 0.001) (Table 2).
Table 2. Growth of C. albicans biofilms on SB discs measured by the XTT reduction assay.
| 90 min | 24 h | 48 h | |||||||||||||||||||
| Control1 | +3% human serum | Control | +3% human serum | Control | +3% human serum | ||||||||||||||||
| Mean2 | ± SD | Mean | ± SD | p-value | % | Mean | ± SD | Mean | ± SD | p-value | % | Mean | ± SD | Mean | ± SD | p-value | % | ||||
| SC5314(WT) | 0.08 | (0.011) | 0.26 | (0.051) | <0.001 | ↑ | 236.17 | 0.50 | (0.032) | 0.63 | (0.063) | 0.001 | ↑ | 26.17 | 0.68 | (0.064) | 0.79 | (0.056) | 0.011 | ↑ | 15.89 |
| HLC54(HM) | 0.07 | (0.010) | 0.21 | (0.050) | <0.001 | ↑ | 204.33 | 0.71 | (0.106) | 0.86 | (0.081) | 0.024 | ↑ | 20.26 | 0.71 | (0.069) | 0.92 | (0.076) | <0.001 | ↑ | 30.05 |
| p-value | 0.175 | 0.309 | 0.001 | 0.001 | 0.366 | <0.001 | |||||||||||||||
Strains SC5314 (WT) and HLC54 (HM) were grown in YNB medium with SB discs in the presence or absence of 3% human serum, and growth evaluated for the adhesion phase (90 min) and the biofilm phase (24 h and 48 h). Results are the mean ± SD of three independent experiments.
Control : C. albicans biofilm developed on silicone biomaterial discs in YNB (without human serum).
OD values depicting metabolic activity of C. albicans biofilm.
SEM Studies on Candida Planktonic Growth
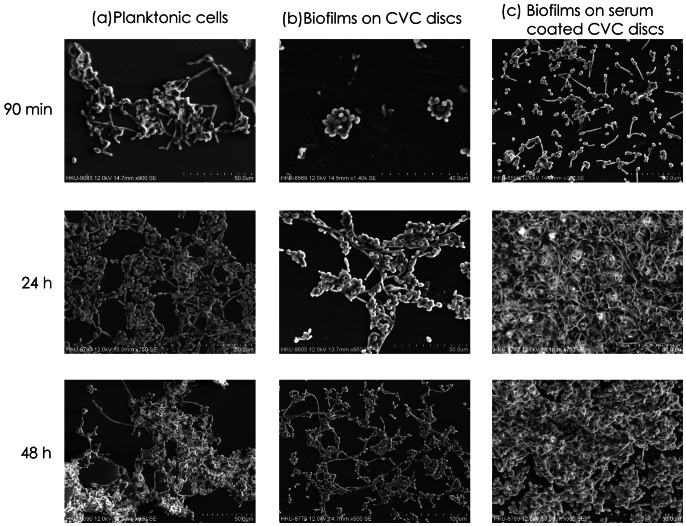
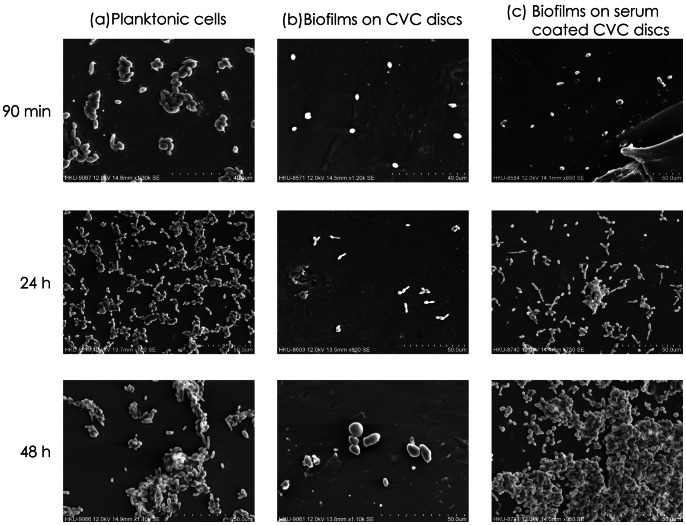
After 90 min of incubation, C. albicans WT planktonic cells consisted of yeast and germ tubes (Figure 1a), whereas HM planktonic cells were predominantly in the yeast phase (Figure 2a). Following 48 h of incubation, WT cells consisted of co-aggregated yeast cells and a few long strands of hyphae; however, the HM samples at 24 h and at 48 h contained only co-aggregates of yeast cells and they lacked filamentous cells (Figures 1a and 2a).
Figure 1. Cell morphology and biofilm ultrastructure of C. albicans SC5314 (WT).
(a) Planktonic cells incubated in YNB. (b) Biofilms on CVC discs incubated in YNB. (c) Biofilms on serum-coated CVC discs incubated in YNB supplemented with 3% human serum.
Figure 2. Cell morphology and biofilm ultrastructure of C. albicans HLC54 (HM).
(a) Planktonic cells incubated in YNB. (b) Biofilms on CVC discs incubated in YNB. (c) Biofilms on serum-coated CVC discs incubated in YNB supplemented with 3% human serum.
SEM Studies on Candida Biofilm Development on SB Discs
During the adhesion phase (90 min), WT C. albicans in YNB adhered to the catheter surface and divided to form microcolonies with germ tubes (Figure 1b). At 24 h, the biofilm was characterized by loosely packed yeast and hyphal cells with a thin, heterogeneous architecture. At 48 h, a similar thin biofilm was noted to be devoid of extracellular material (Figure 1b).
WT biofilms on human serum-coated SB discs exhibited numerous germ tubes both at 90 min and at 24 h (Figure 1c). The presence of pseudohyphae and hyphae, covered with a thick extracellular matrix, was evident. A similar biofilm architecture was observed at 48 h, with increased yeast growth (in addition to filamentous forms) (Figure 1c).
By contrast, the HM strain did not seem to form obvious biofilms on SB discs in the absence of human serum (Figure 2b), but it showed sparse biofilm growth in serum-containing YNB at all three time points (Figure 2c). At 24 h, the HM samples exhibited elongated yeast cells and scant growth, whereas large aggregates of cells were observed at 48 h. Furthermore, the biofilm growth of HM at 48 h in human serum, as observed by SEM (Figure 2c), was much lower than that of the WT strain (Figure 1c), thereby confirming the data derived from the tetrazolium salt reduction assay (Table 2).
Expression of Virulence-related Genes in C. albicans Planktonic Cells
The expression levels of various adhesion genes (ALS3, HWP1, EAP1 and ECE1) and hydrolase genes (SAP1 - SAP10, PLB1, PLB2, PLC and PLD) for planktonic WT and HM cells was measured at 90 min, 24 h and 48 h using RT-PCR (Table 3).
Table 3. Expression levels of virulence-related genes in planktonic cells of C. albicans SC5314 (WT) and C. albicans HLC54 (HM).
| (A) | (B) | (C) | ||||||||||||||||
| 90 min | 24 h | 48 h | ||||||||||||||||
| HLC54 (HM) | SC5314 (WT) | HLC54 (HM) | SC5314 (WT) | HLC54 (HM) | SC5314 (WT) | |||||||||||||
| Mean | Mean | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | ||||
| ALS3 | 1.08 | (0.009) | 14.07 | (0.852) | *** | ↑ | 3.04 | (0.163) | 3.30 | (0.175) | ns | ↑ | 3.91 | (0.322) | 2.67 | (0.144) | ** | ↓ |
| ECE1 | 0.48 | (0.053) | 0.64 | (0.038) | * | ↑ | 1.69 | (0.138) | 1.22 | (0.052) | ** | ↓ | 1.50 | (0.068) | 0.88 | (0.042) | *** | ↓ |
| EAP1 | 0.60 | (0.134) | 24.96 | (6.355) | ** | ↑ | 0.82 | (0.072) | 39.50 | (1.075) | *** | ↑ | 2.56 | (0.184) | 27.99 | (1.126) | *** | ↑ |
| HWP1 | 0.62 | (0.108) | 421.11 | (36.091) | *** | ↑ | 0.85 | (0.183) | 45.80 | (2.635) | *** | ↑ | 3.00 | (0.068) | 14.53 | (1.048) | *** | ↑ |
| SAP1 | 0.37 | (0.039) | 0.70 | (0.101) | * | ↑ | 11.63 | (1.125) | 0.42 | (0.021) | *** | ↓ | 3.24 | (0.274) | 2.51 | (0.083) | * | ↓ |
| SAP2 | 0.37 | (0.023) | 0.80 | (0.021) | *** | ↑ | 15.27 | (0.846) | 0.78 | (0.093) | *** | ↓ | 3.72 | (0.118) | 5.95 | (0.599) | ** | ↑ |
| SAP3 | 0.41 | (0.094) | 0.83 | (0.065) | ** | ↑ | 19.49 | (3.742) | 0.37 | (0.082) | ** | ↓ | 10.46 | (2.768) | 3.09 | (0.220) | * | ↓ |
| SAP4 | 0.28 | (0.016) | 0.69 | (0.045) | *** | ↑ | 15.66 | (1.247) | 0.50 | (0.013) | *** | ↓ | 9.87 | (0.694) | 5.09 | (0.716) | ** | ↓ |
| SAP5 | 0.47 | (0.001) | 1.65 | (0.048) | *** | ↑ | 8.20 | (1.749) | 1.16 | (1.422) | ** | ↓ | 3.30 | (0.052) | 1.48 | (0.080) | *** | ↓ |
| SAP6 | 0.44 | (0.020) | 1.44 | (0.084) | *** | ↑ | 11.03 | (0.224) | 0.45 | (0.034) | *** | ↓ | 7.54 | (0.259) | 3.80 | (0.250) | *** | ↓ |
| SAP7 | 0.72 | (0.041) | 1.84 | (0.317) | ** | ↑ | 28.01 | (8.081) | 0.37 | (0.114) | ** | ↓ | 13.10 | (0.113) | 4.81 | (0.568) | *** | ↓ |
| SAP8 | 1.40 | (0.098) | 2.09 | (0.052) | *** | ↑ | 46.07 | (2.286) | 0.70 | (0.068) | *** | ↓ | 11.75 | (0.922) | 4.77 | (0.540) | *** | ↓ |
| SAP9 | 0.11 | (0.027) | 0.54 | (0.042) | *** | ↑ | 2.20 | (0.305) | 0.23 | (0.026) | *** | ↓ | 0.97 | (0.052) | 1.69 | (0.061) | *** | ↑ |
| SAP10 | 0.88 | (0.048) | 1.76 | (0.195) | ** | ↑ | 6.32 | (0.846) | 2.58 | (0.248) | ** | ↓ | 3.28 | (0.137) | 4.45 | (1.063) | ns | ↑ |
| PLB1 | 0.85 | (0.161) | 11.59 | (0.477) | *** | ↑ | 57.14 | (9.819) | 1.63 | (0.514) | ** | ↓ | 27.60 | (6.148) | 8.79 | (0.640) | ** | ↓ |
| PLB2 | 0.25 | (0.031) | 0.61 | (0.050) | *** | ↑ | 9.69 | (2.626) | 0.23 | (0.034) | ** | ↓ | 4.48 | (1.009) | 2.04 | (0.106) | * | ↓ |
| PLC | 0.85 | (0.065) | 1.41 | (0.129) | ** | ↑ | 22.17 | (0.601) | 0.62 | (0.083) | *** | ↓ | 4.43 | (0.118) | 2.25 | (0.061) | *** | ↓ |
| PLD | 0.60 | (0.088) | 3.02 | (0.230) | *** | ↑ | 7.52 | (1.903) | 0.35 | (0.018) | ** | ↓ | 0.91 | (0.068) | 0.83 | (0.046) | ns | ↓ |
The data were obtained by quantitative real time RT-PCR at three time points (90 min, 24 h and 48 h). Results are the mean ± SD values of three independent experiments conducted on different days.
ns : p>0.05;
p<0.05;
p<0.01;
p<0.001.
The arrows refer to up/down regulation of mRNA expression levels of virulence-related genes between the wild type C. albicans SC5314 (WT) and its corresponding hyphal mutant HLC54 (HM).
a) Adhesion genes
The mRNA levels of the adhesion genes EAP1 and HWP1 were significantly higher in WT planktonic cells than in HM planktonic cells at all three time points (p<0.01) (Table 3). Transcript levels for ALS3 and ECE1, however, were in all cases low and did not show significant differences between the two strains (Table 3).
b) SAP genes
After 90 min of incubation, the expression of all ten SAP genes studied was significantly higher in WT planktonic cells than in HM planktonic cells (Tabel 3). Interestingly, however, at 24 h, the expression levels of all ten SAP genes became significantly lower in the WT strain than in the HM strain (p<0.001). After 48 h of incubation, most of the SAP genes (SAP1 and SAP3 - SAP8) exhibited lower transcript levels in WT cells than in HM cells, whereas the opposite was true for SAP2, SAP9 and SAP10 (Table 3).
c) PL genes
Significantly higher mRNA levels for all four phospholipase genes (PLB1, PLB2, PLC and PLD) were detected at the 90-min time point in WT cells in comparison to HM cells (p<0.001) (Table 3). However, all these genes were significantly downregulated in the WT strain at 24 h and 48 h of incubation (p<0.05), whereas they were upregulated (especially PLB1) at the same time points in the HM strain (Table 3).
Expression of Virulence-related Genes in C. albicans Biofilms on SB
a) Adhesion genes
For biofilms of the WT strain, levels of the ALS3 mRNA were moderately higher at 24 h, but was significantly higher at 48 h (p<0.001), in human serum-coated SB discs than in the serum-free controls (Table 4). A stimulatory effect of human serum on ALS3 expression was also observed for the HM strain at 90 min and at 48 h (Table 4). ECE1 mRNA levels of the WT biofilms were significantly higher at all three time points (p<0.05) in human serum-incubated SB discs than in the serum-free controls. A significant increment in ECE1 mRNA levels was also observed, but only at 24 h, for HM biofilms on human serum-incubated SB discs compared with the control (p<0.05) (Table 4).
Table 4. Expression levels of virulence-related genes in biofilms of C. albicans SC5314 (WT) grown on SB discs in the presence or absence of human serum.
| (A) | (B) | (C) | ||||||||||||||||
| 90 min | 24 h | 48 h | ||||||||||||||||
| YNB | YNB+3% human serum | YNB | YNB+3% human serum | YNB | YNB+3% human serum | |||||||||||||
| Mean | ± SD | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | ||||
| ALS3 | 2.45 | (0.190) | 1.92 | (0.297) | ns | ↓ | 1.63 | (0.119) | 2.01 | (0.282) | ns | ↑ | 0.70 | (0.035) | 0.90 | (0.012) | ** | ↑ |
| ECE1 | 11.84 | (1.025) | 20.08 | (2.551) | ** | ↑ | 2.23 | (0.483) | 5.26 | (0.327) | *** | ↑ | 2.56 | (0.312) | 3.18 | (0.160) | * | ↑ |
| EAP1 | 0.36 | (0.017) | 0.31 | (0.015) | * | ↓ | 0.72 | (0.088) | 0.90 | (0.034) | * | ↑ | 0.73 | (0.051) | 0.95 | (0.168) | ns | ↑ |
| HWP1 | 16.83 | (1.151) | 13.11 | (0.031) | ** | ↓ | 1.10 | (0.233) | 1.04 | (0.130) | ns | ↓ | 0.52 | (0.042) | 0.92 | (0.178) | * | ↑ |
| SAP1 | 0.10 | (0.006) | 0.15 | (0.005) | *** | ↑ | 0.49 | (0.012) | 0.76 | (0.003) | *** | ↑ | 0.86 | (0.053) | 1.62 | (0.433) | * | ↑ |
| SAP2 | 0.31 | (0.004) | 0.23 | (0.021) | ** | ↓ | 1.01 | (0.028) | 1.59 | (0.129) | ** | ↑ | 1.43 | (0.165) | 1.82 | (0.175) | * | ↑ |
| SAP3 | 0.17 | (0.037) | 0.22 | (0.023) | ns | ↑ | 0.79 | (0.185) | 1.00 | (0.103) | ns | ↑ | 0.76 | (0.073) | 1.02 | (0.184) | ns | ↑ |
| SAP4 | 0.09 | (0.012) | 0.21 | (0.039) | ** | ↑ | 0.54 | (0.023) | 1.03 | (0.033) | *** | ↑ | 0.97 | (0.124) | 1.56 | (0.019) | ** | ↑ |
| SAP5 | 0.11 | (0.021) | 0.14 | (0.007) | ns | ↑ | 0.59 | (0.047) | 0.72 | (0.035) | * | ↑ | 0.75 | (0.087) | 0.88 | (0.085) | ns | ↑ |
| SAP6 | 0.12 | (0.004) | 0.18 | (0.018) | ** | ↑ | 0.58 | (0.081) | 0.81 | (0.082) | * | ↑ | 0.80 | (0.061) | 1.21 | (0.021) | *** | ↑ |
| SAP7 | 0.56 | (0.039) | 0.40 | (0.064) | * | ↓ | 0.52 | (0.182) | 0.61 | (0.041) | ns | ↑ | 0.54 | (0.123) | 0.81 | (0.133) | ns | ↑ |
| SAP8 | 0.21 | (0.016) | 0.39 | (0.064) | * | ↑ | 0.74 | (0.035) | 1.14 | (0.071) | ** | ↑ | 1.01 | (0.199) | 1.78 | (0.230) | * | ↑ |
| SAP9 | 0.20 | (0.019) | 0.25 | (0.018) | * | ↑ | 0.62 | (0.013) | 0.67 | (0.017) | * | ↑ | 1.31 | (0.088) | 1.62 | (0.134) | * | ↑ |
| SAP10 | 0.45 | (0.016) | 0.56 | (0.069) | * | ↑ | 0.09 | (0.011) | 0.71 | (0.091) | *** | ↑ | 1.39 | (0.125) | 2.01 | (0.159) | ** | ↑ |
| PLB1 | 0.18 | (0.061) | 0.17 | (0.012) | ns | ↓ | 0.69 | (0.064) | 0.90 | (0.077) | * | ↑ | 0.68 | (0.090) | 0.77 | (0.148) | ns | ↑ |
| PLB2 | 0.06 | (0.008) | 0.08 | (0.002) | * | ↑ | 0.27 | (0.051) | 0.35 | (0.035) | ns | ↑ | 0.40 | (0.035) | 0.47 | (0.081) | ns | ↑ |
| PLC | 0.19 | (0.006) | 0.25 | (0.010) | ** | ↑ | 0.59 | (0.044) | 0.54 | (0.032) | ns | ↓ | 0.72 | (0.020) | 0.62 | (0.098) | ns | ↓ |
| PLD | 0.38 | (0.036) | 0.44 | (0.055) | ns | ↑ | 0.82 | (0.237) | 1.18 | (0.223) | ns | ↑ | 0.81 | (0.043) | 1.39 | (0.178) | ** | ↑ |
Samples were taken during the adhesion phase (90 min) and biofilm growth (24 h and 48 h). Results are the mean ± SD values of three independent experiments.
ns : p>0.05;
p<0.05;
p<0.01;
p<0.001.
The arrows refer to up/down regulation of mRNA expression levels of virulence genes of C. albicans SC5314 (WT) in the presence of human serum relative to the mRNA expression levels devoid of serum.
In contrast to ALS3 and ECE1 transcript levels, human serum did not seem to have a significant effect on mRNA levels of EAP1 and HWP1 in SB biofilms of either strain (Table 4). In WT biofilms, the HWP1 mRNA levels were very high at 90 min and were drastically downregulated at 24 h and 48 h on SB discs with or without serum, while in HM biofilms HWP1 mRNA levels were negligible under all test conditions (Table 4).
In summary, transcript levels of the adhesion genes in the HM biofilms were different from those in the WT biofilms. In the HM strain, there was a significant upregulation of ALS3, ECE1 and EAP1 transcripts in serum-coated SB discs at 90 min (p<0.01). However, at 24 h, only ECE1 expression was upregulated, and the other transcripts were downregulated (p<0.001). To conclude, the mRNA expression levels of the four adhesion genes in WT strain biofilms in serum coated discs were comparatively higher than the HM strain biofilms (Table 4).
b) SAP genes
Transcript levels for six of 10 SAP genes (SAP1, SAP4, SAP6, SAP8–SAP10) were significantly higher at all three time points in the WT biofilms from human serum-treated SB discs than in the serum-less WT controls (p<0.05) (Table 4). However, WT biofilms on control discs demonstrated significantly higher levels of transcripts for SAP2 and SAP7 at 90 min. (p<0.05), lower levels of SAP3 and SAP7 transcripts at 24 h, and lower levels of SAP3, SAP5 and SAP7 transcripts at 48 h, compared with biofilms on human serum coated discs (Table 4).
The levels of six SAP genes (SAP1, SAP2, SAP4, SAP5, SAP6 and SAP8) were higher in HM biofilms on human serum-coated SB discs than in HM controls at 90 min. The expression of seven SAP genes (SAP2–SAP6, SAP8 and SAP10) was also significantly higher in HM serum-coated biofilms than in the HM controls both at 24 h and at 48 h (p<0.005) (Table 5).
Table 5. Expression levels of virulence-related genes in biofilms of C. albicans HLC54 (HM) grown on SB discs in the presence or absence of human serum.
| (A) | (B) | (C) | ||||||||||||||||
| 90 min | 24 h | 48 h | ||||||||||||||||
| YNB | YNB+3% human serum | YNB | YNB+3% human serum | YNB | YNB+3% human serum | |||||||||||||
| Mean | ± SD | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | Mean | ± SD | Mean | ± SD | p-value | ||||
| ALS3 | 0.29 | (0.026) | 0.38 | (0.026) | * | ↑ | 1.17 | (0.152) | 1.15 | (0.056) | ns | ↓ | 0.62 | (0.021) | 0.80 | (0.019) | *** | ↑ |
| ECE1 | 0.60 | (0.134) | 0.84 | (0.009) | * | ↑ | 0.82 | (0.072) | 2.09 | (0.216) | ** | ↑ | 2.56 | (0.184) | 1.59 | (0.184) | ** | ↓ |
| EAP1 | 0.17 | (0.005) | 0.21 | (0.013) | ** | ↑ | 0.66 | (0.062) | 0.75 | (0.025) | ns | ↑ | 0.99 | (0.054) | 0.98 | (0.047) | ns | ↓ |
| HWP1 | 0.28 | (0.053) | 0.37 | (0.082) | ns | ↑ | 0.14 | (0.016) | 0.40 | (0.047) | ** | ↑ | 0.23 | (0.007) | 0.15 | (0.062) | ns | ↓ |
| SAP1 | 0.26 | (0.008) | 0.42 | (0.038) | ** | ↑ | 1.02 | (0.078) | 1.29 | (0.227) | ns | ↑ | 1.40 | (0.145) | 1.42 | (0.042) | ns | ↑ |
| SAP2 | 0.51 | (0.018) | 0.61 | (0.052) | * | ↑ | 0.72 | (0.046) | 0.91 | (0.018) | ** | ↑ | 1.00 | (0.079) | 1.32 | (0.144) | * | ↑ |
| SAP3 | 0.71 | (0.073) | 0.78 | (0.054) | ns | ↑ | 0.84 | (0.260) | 1.29 | (0.033) | * | ↑ | 1.56 | (0.129) | 1.67 | (0.233) | ns | ↑ |
| SAP4 | 0.33 | (0.013) | 0.55 | (0.023) | *** | ↑ | 0.86 | (0.019) | 1.67 | (0.099) | *** | ↑ | 1.11 | (0.006) | 1.44 | (0.029) | *** | ↑ |
| SAP5 | 0.43 | (0.008) | 0.71 | (0.032) | *** | ↑ | 0.37 | (0.034) | 0.72 | (0.017) | *** | ↑ | 0.46 | (0.058) | 0.65 | (0.045) | * | ↑ |
| SAP6 | 0.42 | (0.023) | 0.73 | (0.013) | *** | ↑ | 0.63 | (0.056) | 1.12 | (0.070) | ** | ↑ | 0.79 | (0.022) | 0.98 | (0.081) | * | ↑ |
| SAP7 | 1.86 | (0.051) | 1.75 | (0.258) | ns | ↓ | 0.82 | (0.052) | 2.49 | (0.335) | ** | ↑ | 0.85 | (0.133) | 0.91 | (0.031) | ns | ↑ |
| SAP8 | 0.79 | (0.051) | 1.21 | (0.063) | ** | ↑ | 2.41 | (0.037) | 2.91 | (0.279) | * | ↑ | 1.60 | (0.242) | 2.33 | (0.259) | * | ↑ |
| SAP9 | 0.50 | (0.025) | 0.82 | (0.296) | ns | ↑ | 0.96 | (0.072) | 0.90 | (0.044) | ns | ↓ | 1.54 | (0.063) | 2.33 | (0.383) | * | ↑ |
| SAP10 | 2.10 | (0.376) | 2.30 | (0.222) | ns | ↑ | 0.87 | (0.029) | 1.14 | (0.104) | * | ↑ | 1.38 | (0.104) | 2.66 | (0.218) | ** | ↑ |
| PLB1 | 0.24 | (0.015) | 0.46 | (0.121) | * | ↑ | 1.54 | (0.182) | 3.43 | (0.137) | *** | ↑ | 1.93 | (0.147) | 1.49 | (0.079) | * | ↓ |
| PLB2 | 0.31 | (0.054) | 0.38 | (0.093) | ns | ↑ | 0.56 | (0.116) | 0.74 | (0.053) | ns | ↑ | 0.61 | (0.121) | 0.64 | (0.017) | ns | ↑ |
| PLC | 1.00 | (0.074) | 1.05 | (0.088) | ns | ↑ | 2.28 | (0.529) | 1.79 | (0.216) | ns | ↓ | 0.71 | (0.054) | 1.04 | (0.079) | ** | ↑ |
| PLD | 1.12 | (0.082) | 1.35 | (0.107) | * | ↑ | 1.39 | (0.331) | 1.31 | (0.123) | ns | ↓ | 0.93 | (0.133) | 2.44 | (0.476) | ** | ↑ |
Samples were taken during the adhesion phase (90 min) and biofilm growth (24 h and 48 h). Results are the mean ± SD values of three independent experiments.
ns : p>0.05;
p<0.05;
p<0.01;
p<0.001.
The arrows refer to up/down regulation of mRNA expression levels of virulence genes of C. albicans HLC54 (HM) in the presence of human serum relative to the mRNA expression levels devoid of serum.
c) PL genes
PLB1 transcript levels in serum-treated WT biofilms were higher than the levels in serum-free controls both at 24 h and 48 h time points. By contrast, PLB1 expression in serum-treated HM biofilms was significantly much higher than in the controls both at 90 min and 48 h (p<0.001) (Table 5).
Both up- and downregulation of PLB2, PLC and PLD mRNA transcripts during biofilm growth for both the WT and HM strains in human serum-coated/uncoated SB discs during the total incubation period were evident (Table 5). These results demonstrate that the effect of serum on gene expression was variable, depending on time point and strain.
Discussion
Candida albicans Biofilms on Central Venous Catheters
Following insertion into blood vessels, CVC surfaces become covered with a film of proteins, sugars, electrolytes and other blood components that promote the development of a biofilm; a complex microbial community of bacteria and yeasts enveloped in an extracellular matrix of proteins and polymeric material [32]. Indeed, electron microscopy studies have demonstrated the abundance of biofilm-associated microorganisms on most, if not all, CVCs [33].
Biofilm features can be reproduced to some extent in in vitro experimental models pre-conditioned with host proteins [34]. Previous reports have demonstrated that in vitro growth of Candida biofilms can be affected by serum components [35], [36], and that human serum promotes and modifies biofilm growth of C. albicans in particular by initiating germ-tube production [37]. Increased biofilm growth of C. albicans has also been reported on catheter surfaces pre-conditioned with body fluids, such as serum and blood [1], [36]. Many have reported that such biofilm activity could be reliably quantified using the XTT assay provided appropriate controls are used [28]. In the current study, we set up to assess the effect of human serum on the formation of C. albicans biofilms on SB discs in vitro. We also aimed to determine how human serum affects the differential expression of C. albicans virulence-related genes (encoding adhesins, hyphae and extracellular hydrolases) in such biofilms. For this purpose, we used a C. albicans WT strain and a derived HM strain. In contrast to the wild type C. albicans SC5314, the double mutant efg1/efg cph1/cph neither produces hyphae nor invades a reconstituted human oral epithelium model supporting the theory that the hyphal phase in an important virulence attribute of C. albicans isolates [38]. Hence, we examined the HM strain in addition to the WT strain to investigate if human serum modifies the virulence of Candida by virtue of its hyphal appendages.
C. albicans Biofilm Growth on Silicone Biomaterial
In our in vitro study, we noted a thick biofilm on human serum-coated SB discs only in the WT and not the HM strain during a 48 h incubation period. These biofilms consisting of a dense network of filamentous cells embedded in an extracellular matrix have been previously documented on surfaces such as denture acrylic or polystyrene [39], [40]. Furthermore, we found that human serum stimulated cell proliferation and filamentation in WT biofilms grown on SB discs. Indeed, the serum-free SB discs showed scant growth of WT cells during the initial 90 min, although, filamentation was also observed after 24 h. Our findings are consistent with those of an earlier study that showed a role for serum in the increased formation of hyphae in C. albicans biofilms developed on microtiter plates and catheters [13].
We observed minimal adhesion of the HM strain to SB discs and it was completely defective in filamentous growth in the absence of human serum throughout the 48 h incubation period. The HM strain displayed pseudo-hyphal growth on serum-coated SB discs, both at 24 h and 48 h, thus indicating the powerful effect that human serum exerts on yeast filamentation.
C. albicans biofilms, when grown on static denture acrylic surfaces, have a thickness of 25 µm [39], whereas those grown using an in vitro model with a flowing growth medium can have a thickness of up to 70 µm [41]. Interestingly, the thickness of biofilms on a central venous catheter has been shown to exceed 100 µm in vivo in a rat model [42], the authors attributed this increased thickness to the flow characteristics of the model and to the host-derived conditioning film covering the device. Fully mature biofilms developed within 24 h in this rat model and were composed of a dense multi-layered network of yeast cells and hyphae [42].
C. albicans Virulence Gene Expression on Silicone Biomaterial
Virulence-related gene expression in biofilms has predominantly been investigated in bacterial infections [43], and little is known about virulence gene expression in fungal biofilms. However, there are several studies available on the differential expression of various mRNAs and some studies on the transcriptomic analyses during Candida adherence and hyphal formation in vitro and in vivo [7], [13], [42], [44]–[46].
Adhesion Genes
In our experiments, the expression of ALS3, a member of the ALS gene family, was low during planktonic growth in the WT strain. Nevertheless, the ALS3 gene was highly expressed in the WT strain at all three time points in the SB discs incubated in human serum. It appears, therefore, that ALS3 is important both for adhesion and for biofilm growth of C. albicans on SB discs and that human serum enhances the upregulation of ALS3 expression. Furthermore, a significant increase in ALS3 expression in WT biofilms at 48-h, when compared with the HM strain, suggests that ALS3 is important for biofilm maturation. Previous studies have demonstrated the importance of ALS3 expression in biofilm formation on silicone substrates [45, 47 and 48]. Similar expression patterns of ALS3 have been revealed by RT-PCR of RNA samples from C. albicans biofilms developed on reconstituted human buccal epithelium, denture acrylic, silicone-elastomer catheter material and other abiotic surfaces [49], thus suggesting that this gene has a role in the formation of biofilms on diverse surface types. Furthermore, expression of ALS3 (and ALS2) and HWP1 has been observed in biofilms associated with abiotic surfaces [5, 13, 45 and 50]. In general, our findings are consistent with the previous studies on ALS3 expression related to abiotic surfaces.
It is noteworthy that Nobile et al. [45] and Zhao et al. [47] have shown that ALS3-defective hyphal mutants of C. albicans are known to form disorganized, thin biofilms on catheter material. This was also the case for the biofilms formed by the HM strain in our study, as observed by SEM. Interestingly, however, we noted that the HM strain produced increased levels of ALS3 transcripts in biofilms on human serum-treated SB discs than in serum-free controls at 90 min and 48 h.
Previous investigations have not revealed expression of ALS3 transcripts in C. albicans biofilms “in vivo”, (in biofilms developed on catheters in live rats) although high expression of ALS1 and ALS2 has been observed [7]. It has been reported that certain ALS mutants of C. albicans cannot produce fully developed biofilms on silicone-elastomer catheter disks [11], the authors hypothesized that the different ALS genes may be active at different times and that they may complement each others’ functions.
HWP1
The HWP1 protein is expressed on the surface of germ tubes and hyphae but not during the yeast phase [51], and is required for filamentation, normal biofilm formation and virulence [45]. Furthermore, HWP1 is a substrate for mammalian transglutaminase, which seems to form cross-links between HWP1 and host-cell surface proteins in vitro, thus mediating stable attachment of hyphae to host epithelial cells [15].
We noted that HWP1 transcript levels were much higher in WT planktonic cells than in HM planktonic cells. This result agrees with those of Sindl & Sundstrom, [52], who noted that HWP1 mRNA levels might increase in the yeast form. Indeed, in the SEM analysis, C. albicans, which displays morphologically heterogenic forms (yeast, hyphae and pseudohyphae) [53], was abundant in planktonic phase cultures. This result corroborates the increased expression of the filament-inducing HWP1 in these heterogeneic WT planktonic Candida cultures. HWP1, together with the hyphal proteins ALS3 and ALS1, is an adhesin that might promote cell-cell or cell-substrate binding [54].
We also noted a dramatic decrease in HWP1 transcript levels in both serum treated and untreated WT biofilms by 24 h. In the biofilm phase of the WT strain, hyphal-producing genes HWP1 and ECE1 were significantly downregulated during the experimental period, whereas these genes were not highly expressed in the HM strain.
EAP1
In contrast to ALS3 and HWP1, which are specifically expressed during hyphal development [54], EAP1 is expressed in both yeast and hyphal cells [3], [16]. EAP1 encodes a glycosyl-phosphatidylinositol-anchored, glucan-cross-linked cell wall protein that has a role in cell adhesion to surfaces. We observed much lower levels of EAP1 expression at all three time points in HM planktonic cells than in WT planktonic cells. The decreased EAP1 expression in HM planktonic cells might contribute to their apparently diminished cell-cell adhesion properties suggested by our SEM analysis. Nevertheless, EAP1 expression in the biofilms of both strains was lower than in the corresponding planktonic cells in all the conditions tested, implying that EAP1 may not be important for biofilm development, at least in this system.
ECE1
ECE1 expression has been shown to correlate with the extent of cell elongation [55]. In our study, ECE1 mRNA levels were low during planktonic growth, although with moderate increases at 24 h and 48 h. WT strain on SB discs in the presence of human serum demonstrated an upregulation of ECE1 during the adhesion phase and throughout the biofilm growth for 48 h, whereas the HM strain showed much lower expression levels of ECE1 during the first 24 h to 48 h. Interestingly, the intensity of ECE1 expression was observed to coincide with cell elongation morphologies seen on scanning electron micrographs of the WT and HM biofilms on serum coated SB discs.
Overall, expression of the adhesion genes in HM planktonic cells was generally lower than in WT planktonic cells. The reason for this low expression might be the fact that the HM strain lacks EFG1, a key transcriptional factor of the cAMP pathway that is essential for formation of hyphae [56]. EFG1 is involved in the expression of HWP1 and SAP4 - SAP6 [22], [50], [57].
SAP Gene Expression
In CVC-associated infection, site-specific co-regulated expression of enzymes, such as secreted aspartyl proteases (SAPs) and phospholipases (PLs), has a key role in yeast colonization [58], [59]. The various SAP genes (SAP1–SAP10) are differentially and selectively regulated in yeast and filamentous forms at different stages of infection, contributing to the virulence of the organism [60]. For example, SAP1–SAP3 are predominantly expressed in yeast cells, whereas SAP4–SAP6 are expressed in hyphae, the predominant cell type in Candida biofilms [61]. SAP1 expression is also thought to contribute to the adherence of C. albicans [62], [63].
In the early planktonic phase (90 min), the WT strain exhibited significantly higher levels of all 10 SAP genes compared with the HM strain. However, this trend was reversed at 24 h, with HM showing higher expression levels for all 10 SAP genes compared with the WT strain. One possible explanation is that the upregulation of SAP genes in the HM strain could be partially compensating for the lack of the EFG1 and CPH1 genes, which encode two transcription factors required for hyphae formation. In particular, the upregulation of SAP genes could be contributing to adherence of the HM mutant, because it is known that the expression of SAP1–SAP3 may contribute to C. albicans adherence [62], [63], SAP4–SAP6 are expressed in the hyphal form [64], [65], which is known to be more adherent than the yeast form [21], [22]. It is noteworthy that C. albicans planktonic cultures consisted mostly of blastospores and slightly elongated yeast cells throughout the experimental period. Although most studies support the notion that the hyphal form contributes to virulence per se, several authors have noted that co-regulation of genes controlling hyphal morphogenesis with genes encoding other virulence factors confounds analysis [21], [66]. For instance, SAP5 and SAP6 have been described as being associated with the yeast-to-hypha morphological conversion, which is responsible for invasive infections [57]. Therefore, the formation of hyphae together with SAP expression may be a component of the overall virulence strategy of C. albicans.
Interestingly, we found that WT biofilms on serum-treated SB discs displayed an increased biofilm growth and showed higher levels of SAP1–SAP3 and SAP7–SAP10 transcripts than biofilms grown on untreated SB discs. Previous in vitro studies have shown that SAP1–SAP3 contribute to the damage of host cells and tissues [67]–[69]. Although the roles of SAP7–SAP10 in C. albicans infections are not fully understood [18], [60], both SAP9 and SAP10 expression are associated with fungal adherence, cell wall integrity and cell separation during budding [70]. In addition, it has been reported that SAP7 is expressed in an intravenous infection model of candidiasis, thereby indicating that SAP7 expression may be important in catheter infections [71]. Therefore, our findings, together with previous research, support the hypothesis that SAP1–SAP3 and SAP7–SAP10 may contribute to biofilm formation on silicone biomaterial.
PL Gene Expression
We also investigated the expression of mRNA transcripts of PL genes in planktonic and biofilm cells. In a recent study, the expression levels of PLB1 and PLB2 were observed to be model-dependent [5]. The authors found low levels of PLB1 and PLB2 expression in biofilms grown on silicone in microtiter plates and on RHE (reconstituted human epithelium) up to 12 h, whereas PLB2 was highly expressed in biofilms grown on RHE on silicone disks in a continuous flow system (the CDC reactor) [5]. In our study, PLB1 and PLB2 were upregulated in the WT planktonic cells; significant upregulation of both genes was also observed at 24 h in WT biofilms formed on human serum-coated SB discs (only PLB1 was considerably upregulated at 48 h). The HM strain, however, demonstrated significant upregulation of PLB1 in planktonic cultures and in biofilms incubated in human serum at all three time points. These results are in contrast to a previous study that reported that planktonic yeast cells produced more phospholipases than biofilm cells [72]. In summary, previous research have produced variable results regarding PL gene expression and our investigations also demonstrated that the different members of the PL gene family were expressed at different times in biofilms.
Conclusions
Taken together, our results indicate that in vitro biofilm formation in C. albicans, in the presence of human serum, is accompanied by variable and progressive changes in the expression of several virulence-related genes relative to incubation without human serum. Significant upregulation of virulence-related genes included those associated with adherence, hyphal growth and secretory aspartyl proteinases and phospholipases; ALS3, HWP1, EAP1, ECE1, SAP1, SAP4, SAP6–SAP10, PLB1, PLB2 and PLC. However, the expression patterns of these virulence gene families under laboratory growth conditions do not seem to be representative of in vivo gene expression, which is probably attributable to the complex and variable environmental conditions in the host. Therefore, the roles of individual virulence genes during catheter infection need to be established in animal models using the respective gene-deficient candidal strains. The observations of this study, however, will be useful as fundamental data for such future studies.
Acknowledgments
The authors thank Professor N. A. R. Gow (The University of Aberdeen, UK) for providing C. albicans SC5314 strain and its hyphal mutant C. albicans HLC54.
Funding Statement
This study was funded by the Research Grants Council of the University of Hong Kong (HKU, Project code: 201109176041). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kojic EM, Darouiche RO (2004) Candida infections of medical devices. Clin Microbiol Rev 17: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cauda R (2009) Candidaemia in patients with an inserted medical device. Drugs 69: 33–38. [DOI] [PubMed] [Google Scholar]
- 3. Li F, Svarovsky MJ, Karlsson AJ, Wagner JP, Marchillo K, et al. (2007) Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo . Eukaryot Cell 6: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55: 999–1008. [DOI] [PubMed] [Google Scholar]
- 5. Nailis H, Kucharikova S, Ricicova M, Van Dijck P, Deforce D, et al. (2010) Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and –independent gene expression. BMC Microbiol 10: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denstedt JD, Wollin TA, Rede G (1998) Biomaterials used in urology: current issues of biocompatibility, infection and encrustation. J Endourol 12: 493–500. [DOI] [PubMed] [Google Scholar]
- 7. Nett JE, Lepak AJ, Marchillo K, Andes DR (2009) Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis 200: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramage G, Martinez JP, Lopez-Ribot JL (2006) Minireview: Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6: 979–986. [DOI] [PubMed] [Google Scholar]
- 9. Hoyer LL (2001) The ALS gene family of Candida albicans . Trends Microbiol 9: 176–180. [DOI] [PubMed] [Google Scholar]
- 10. Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, et al. (2002) Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol 44: 61–72. [DOI] [PubMed] [Google Scholar]
- 11. Zhao X, Oh SH, Yeater KM, Hoyer LL (2005) Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function with the Als family. Microbiol 151: 1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tronchin G, Pihet M, Lopes-Bezerra LM, Bouchara JP (2008) Adherence mechanisms in human pathogenic fungi. Med Mycol 46: 749–772. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Sanchez S, Aubert S, Iraqui I, Janbon G, Ghigo J-M, et al. (2004) Candida albicans Biofilms: A developmental state associated with specific and stable gene expression patterns. Eukaryot Cell 3: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchais V, Kempf M, Licznar P, Lefrancois C, Bouchara JP, et al. (2005) DNA array analysis of Candida albicans gene expression in response to adherence to polystyrene. FEMS Microbiol Lett 245: 25–32. [DOI] [PubMed] [Google Scholar]
- 15. Staab JF, Bradway SD, Fidel PL, Sundstrom P (1999) Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283: 1535–1538. [DOI] [PubMed] [Google Scholar]
- 16. Li F, Palecek SP (2003) EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell 2: 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kunze D, Melzer I, Bennett D, Sanglard D, MacCallum D, et al. (2005) Functional analysis of the phospholipase C gene CaPLC1 and two unusual phospholipase C genes, CaPLC2 and CaPLC3, of Candida albicans . Microbiol 151: 3381–3394. [DOI] [PubMed] [Google Scholar]
- 18. Aoki W, Kitahara N, Miura N, Morisaka H, Yamamoto Y, et al. (2011) Comprehensive characterization of secreted aspartic proteases encoded by a virulence gene famiy in Candida albicans . J Biochem 150: 431–438. [DOI] [PubMed] [Google Scholar]
- 19. Mendes A, Mores AU, Carvalho AP, Rosa RT, Samaranayake LP, et al. (2007) Candida albicans biofilms produce more secreted aspartyl protease than the planktonic cells. Biol Pharm Bull 30: 1813–1815. [DOI] [PubMed] [Google Scholar]
- 20. Dolan JW, Bell AC, Hube B, Schaller M, Warner TF, et al. (2004) Candida albicans PLD1 activity is required for full virulence. Med Mycol 42: 439–447. [DOI] [PubMed] [Google Scholar]
- 21. Gow NA, Brown AJ, Odds FC (2002) Fungal morphogenesis and host invasion. Curr Opin Microbiol 5: 366–371. [DOI] [PubMed] [Google Scholar]
- 22. Kumamoto CA, Vinces MD (2005) Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol 7: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 23. Sharkey LL, McNemar MD, Saporito-Irwin SM, Sypherd PS, Fonzi WA (1999) HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1 and RBF1 . J Bacteriol 181: 5273–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S (1998) Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet 33: 451–459. [DOI] [PubMed] [Google Scholar]
- 25. Sohn K, Urban C, Brunner H, Rupp S (2003) EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol Microbiol 47: 89–102. [DOI] [PubMed] [Google Scholar]
- 26. Hilmioglu S, Ilkit M, Badak Z (2007) Comparison of 12 liquid media for germ tube production of Candida albicans and C. tropicalis . Mycoses 50: 282–285. [DOI] [PubMed] [Google Scholar]
- 27. Thein ZM, Samaranayake YH, Samaranayake LP (2007) Characteristics of dual species Candida biofilms on denture acrylic surfaces. Arch Oral Biol 52: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 28. Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP (2003) Biofilm forming ability of Candida albicans is unlikely to contribute to high oral yeast carriage in human immunodeficiency virus-infection. J Clin Microbiol 41: 2961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samaranayake YH, Dassanayake RS, Jayatilake JAMS, Cheung BPK, Yau JYY, et al. (2005) Phospholipase B enzyme expression is not associated with other virulence attributes in Candida albicans isolates from patients with human immunodeficiency virus infection. J Med Microbiol 54: 583–593. [DOI] [PubMed] [Google Scholar]
- 30. Samaranayake YH, Dassanayake RS, Cheung BPK, Jayatilake JAMS, Yeung KWS, et al. (2006) Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS 2006 114: 857–866. [DOI] [PubMed] [Google Scholar]
- 31. Lachke SA, Srikantha T, Tsai LK, Daniels K, Soll DR (2000) Phenotypic switching in Candida glabrata involves phase-specific regulation of the metallothionein gene MT-II and the newly discovered haemolysin gene HLP. . Infect Immun 68: 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finkel JS, Mitchell AP (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, et al. (1993) Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 168: 400–407. [DOI] [PubMed] [Google Scholar]
- 34. Francois P, Vaudaux P, Lew PD (1998) Role of plasma and extracellular matrix proteins in the physiopathology of foreign body infections. Ann Vasc Surg 12: 34–40. [DOI] [PubMed] [Google Scholar]
- 35. Samaranayake LP, McCourtie J, MacFarlane TW (1980) Factors affecting the in vitro adherence to Candida albicans to acrylic surfaces. Arch Oral Biol 25: 611–615. [DOI] [PubMed] [Google Scholar]
- 36. Nikawa H, Nishimura H, Makihira S, Hamada T, Sadamori S, et al. (2000) Effect of serum concentration on Candida biofilm formation on acrylic surfaces. Mycoses 43: 139–143. [DOI] [PubMed] [Google Scholar]
- 37. Shepherd MG, Yin CY, Ram SP, Sullivan PA (1980) Germ tube induction in Candida albicans . Can J Microbiol 26: 21–26. [DOI] [PubMed] [Google Scholar]
- 38. Jayatilake JAMS, Samaranayake YH, Cheung LK, Samaranayake LP (2006) Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J Oral Pathol Med 35: 484–491. [DOI] [PubMed] [Google Scholar]
- 39. Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, et al. (2001) Antifungal resistance of Candida biofilms formed on denture acrylic in vitro . J Dent Res 80: 903–908. [DOI] [PubMed] [Google Scholar]
- 40. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA (2002) Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramage G, Wickes BL, Lopez-Ribot JL (2001) Biofilms of Candida albicans and their associated resistance to antifungal agents. Am Clin Lab 20: 42–44. [PubMed] [Google Scholar]
- 42. Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, et al. (2004) Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun 72: 6023–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang L, Mah TF (2008) Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol 190: 4447–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cao YY, Cao YB, Xu Z, Ying K, Li Y, et al. (2005) cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother 49: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nobile CJ, Nett JE, Andes DR, Mitchell AP (2006) Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukarot Cell 5: 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeater KM, Chandra J, Cheung G, Mukherjee PK, Zhao X, et al. (2007) Temporal analysis of Candida albicans gene expression during biofilm development. Microbiol 153: 2373–2385. [DOI] [PubMed] [Google Scholar]
- 47. Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, et al. (2006) Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiol 152: 2287–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coleman DA, Oh SH, Zhao X, Zhao H, Hutchins JT, et al. (2009) Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J Microbiol Methods 78: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Green CB, Zhao X, Yeater KM, Hoyer LL (2005) Construction and real-time RT-PCR validation of Candida albicans PALS-GFP reporter strains and their use in flow cytometry analysis of ALS gene expression in budding and filamenting cells. Microbiol 151: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 50. O’Connor L, Lahiff S, Casey F, Glennon M, Cormican M, et al. (2005) Quantification of ALS1 gene expression in Candida albicans biofilms by RT-PCR using hybridization probes on the Light Cycler. Mol Cell Probes 19: 153–162. [DOI] [PubMed] [Google Scholar]
- 51. Sundstrom P (2002) Adhesion in Candida spp. Cell Microbiol 4: 461–469. [DOI] [PubMed] [Google Scholar]
- 52.Snide JL, Sundstrom P (2006) Characterisation of HWP1 promoter activation in pseudohyhal cells in Candida albicans Presentation at the Eighth ASM Conference on Candida and Candidiasis, Denver, CO. Washington, DC: American Society for Microbiology.
- 53. Odds FC (1994) Candida species and virulence. ASM News 60: 313–318. [Google Scholar]
- 54. Liu Y, Filler SG (2011) Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 10: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Birse CE, Irwin MY, Fonzi WA, Sypherd PS (1993) Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans . Infect Immun 61: 3648–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stoldt VR, Sonneborn A, Leuker CE, Ernst JF (1997) Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J 16: 1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Felk A, Kretschmar M, Albrecht A, Schaller M, Beinhauer S, et al. (2002) Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun 70: 3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schaller M, Borelli C, Korting HC, Hube B (2005) Hydrolytic enzymes as virulence factors of Candida albicans. . Mycoses 48: 365–377. [DOI] [PubMed] [Google Scholar]
- 59. Hube B, Naglik J (2001) Candida albicans proteinases: resolving the mystery of a gene family. Microbiol 147: 1997–2005. [DOI] [PubMed] [Google Scholar]
- 60. Naglik JR, Challacombe SJ, Hube B (2003) Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 67: 400–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen YC, Wu CC, Chung WL, Lee FJ (2002) Differential secretion of Sap4–6 proteins in Candida albicans during hyphae formation. Microbiol 148: 3743–3754. [DOI] [PubMed] [Google Scholar]
- 62. Borg-von Zepelin M, Meyer I, Thomssen R, Wurzner R, Sanglard D, et al. (1999) HIV-Protease inhibitors reduce cell adherence of Candida albicans strains by inhibition of yeast secreted aspartic proteases. J Invest Dermatol 113: 747–751. [DOI] [PubMed] [Google Scholar]
- 63. Kvaal CS, Lachke SA, Srikantha T, Daniels K, McCoy J, et al. (1999) Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicnas confers increased virulence in a mouse model of cutaneous infection. Infect Immun 67: 6652–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hube B, Monod M, Schofield DA, Brown AJ, Gow NA (1994) Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans . Mol Microbiol 14: 87–99. [DOI] [PubMed] [Google Scholar]
- 65. White TC, Agabian N (1995) Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J Bacteriol 177: 5214–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu H (2002) Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int J Med Microbiol 292: 299–311. [DOI] [PubMed] [Google Scholar]
- 67. Ibrahim AS, Filler SG, Sanglard D, Edwards JE Jr, Hube B (1998) Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun 66: 3003–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schaller M, Korting HC, Schafer W, Bastert J, Chen W, et al. (1999) Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol 34: 169–180. [DOI] [PubMed] [Google Scholar]
- 69. Schaller M, Bein M, Korting HC, Baur S, Hamm G, et al. (2003) The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect Immun 71: 3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Albrecht A, Felk A, Pichova I, Naglik JR, Schaller M, et al. (2006) glycosylphosphatidyl -inositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host pathogen interactions. J Biol Chem 281: 688–694. [DOI] [PubMed] [Google Scholar]
- 71. Taylor BN, Hannemann H, Sehnal M, Biesemeier A, Schweizer A, et al. (2005) Induction of SAP 7 correlates with virulence in an intravenous infection model of candidiasis but not in a vaginal infection model in mice. Infect Immun 73: 7061–7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taniguchi L, de Fatima Faria B, Rosa RT, de Paula E, Carvalho A, et al. (2009) Proposal of a low cost protocol for colorimetric semi-quantification of secretory phospholipase by Candida albicans grown in planktonic and biofilm phases. J Microbiol Methods 78: 171–174. [DOI] [PubMed] [Google Scholar]