Abstract
The specificity of the Pseudomonas phaseolicola toxin for enzyme inhibition and its relationship to toxin-induced chlorosis in bean leaves (Phaseolus vulgaris L.) was examined. The toxin showed no significant inhibitory activity against glutamine synthetase, glutamine transferase, carbamyl phosphate synthetase, aspartate carbamoyltransferase, or arginase at concentrations 100-fold higher than that needed to inhibit ornithine carbamoyltransferase by 50%.
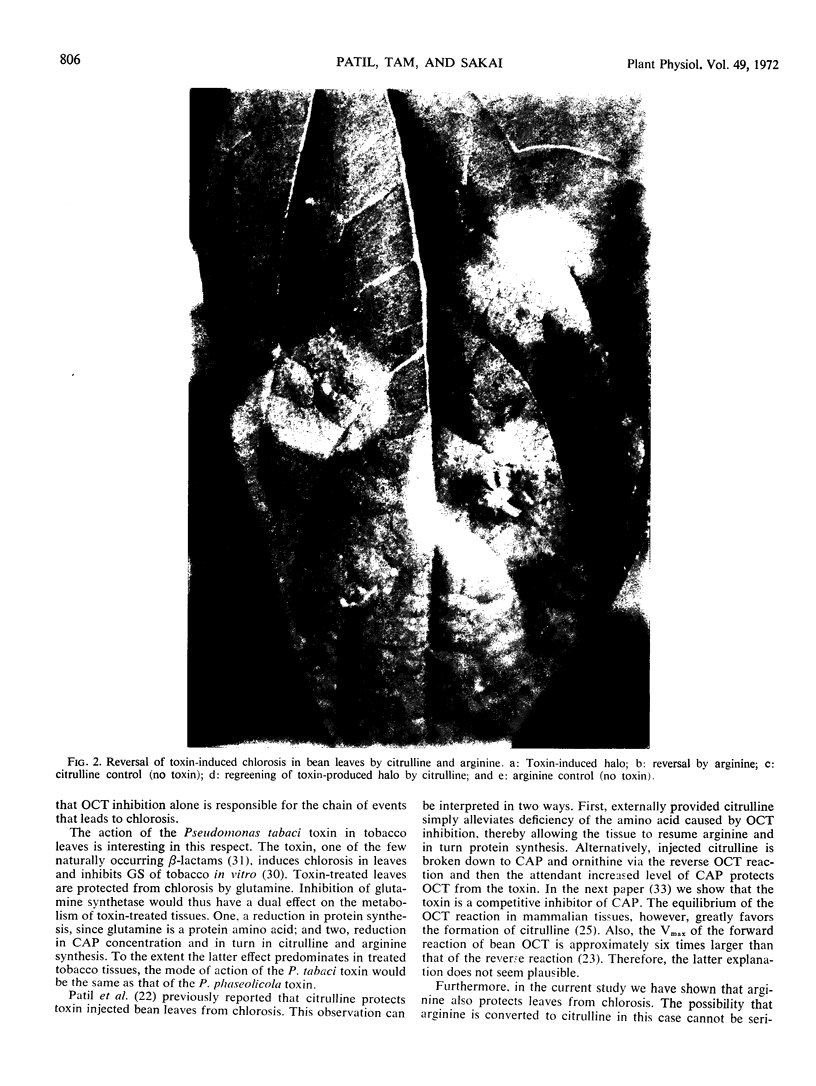
Protection from and reversal of toxin-induced chlorosis in bean leaves was attempted with several amino acids. Aside from protection with l-citrulline which was previously reported, only l-arginine-HCl and to a minor extent l-leucine and l-glutamine showed protection from chlorosis. l-Citrulline and l-arginine-HCl (but not l-glutamine and l-leucine) also reversed toxin-induced chlorosis.
Ultrastructurally, cells from toxin-treated chlorotic tissues showed no observable changes as compared to nontreated tissues. This, together with the ability of the two amino acids to reverse chlorosis, indicated that the toxin causes a reversible biochemical lesion in treated tissue.
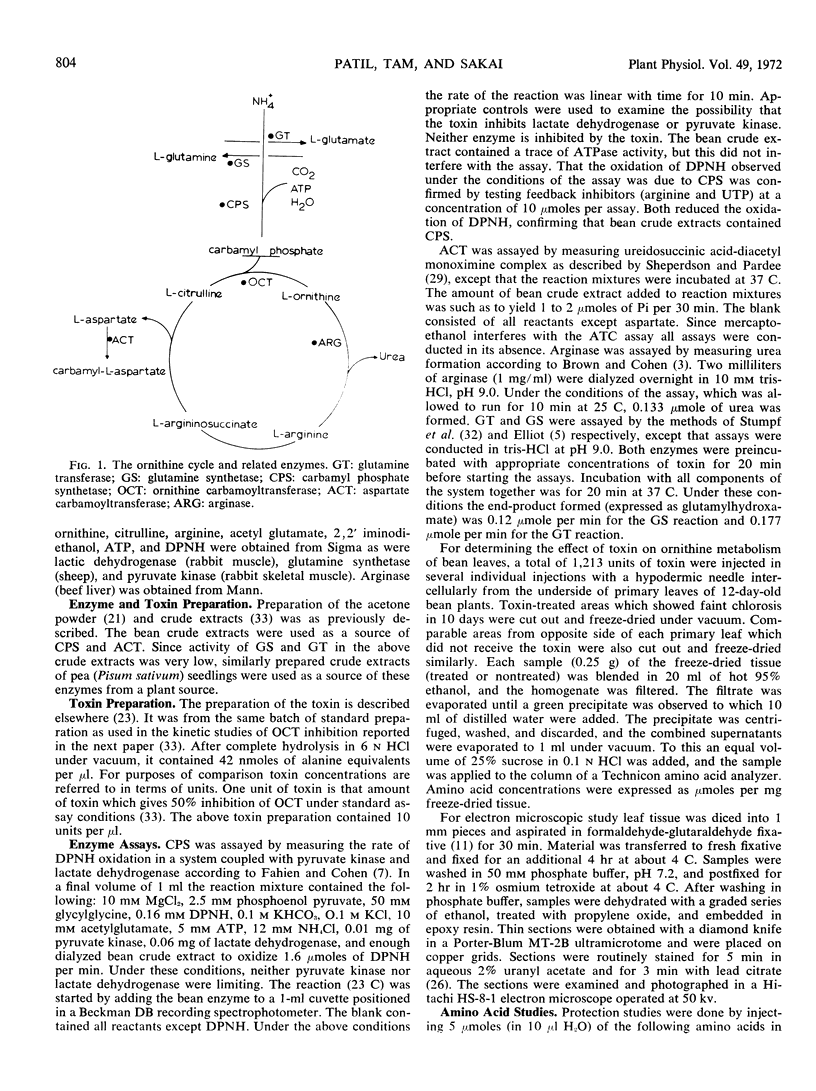
While tissues from bean plants inoculated with P. phaseolicola showed a large accumulation of ornithine, toxin-treated tissues showed no accumulation of ornithine. The latter finding indicated that in addition to the ornithine carbamoyltransferase inhibitor, the pathogen may produce inhibitors of other ornithine metabolizing enzymes in inoculated tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN G. W., Jr, COHEN P. P. Comparative biochemistry of urea synthesis. I. Methods for the quantitative assay of urea cycle enzymes in liver. J Biol Chem. 1959 Jul;234(7):1769–1774. [PubMed] [Google Scholar]
- Bone D. H. Metabolism of Citrulline and Ornithine in Mung Bean Mitochondria. Plant Physiol. 1959 Mar;34(2):171–175. doi: 10.1104/pp.34.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. Isolation of glutamine synthetase and glutamotransferase from green peas. J Biol Chem. 1953 Apr;201(2):661–672. [PubMed] [Google Scholar]
- Ellis R. J., Hartley M. R. Sites of synthesis of chloroplast proteins. Nature. 1971 Oct 13;233(5320):193–196. [PubMed] [Google Scholar]
- FAHIEN L. A., COHEN P. P. A KINETIC STUDY OF CARBAMYL PHOSPHATE SYNTHETASE. J Biol Chem. 1964 Jun;239:1925–1934. [PubMed] [Google Scholar]
- JOSEPH R. L., BALDWIN E., WATTS D. C. Studies on carbamoyl phosphate-L-ornithine carbamoyltransferease from ox liver. Biochem J. 1963 May;87:409–416. doi: 10.1042/bj0870409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski K. L-Glutamine as donor of carbamoyl group nitrogen for the enzymic synthesis of citrulline in green pea seedlings. Acta Biochim Pol. 1965;12(3):243–249. [PubMed] [Google Scholar]
- LEVENBERG B. Role of L-glutamine as donor of carbamyl nitrogen for the enzymatic synthesis of citruline in Agaricus bisporus. J Biol Chem. 1962 Aug;237:2590–2598. [PubMed] [Google Scholar]
- Margulies M. M., Parenti F. In vitro Protein Synthesis by Plastids of Phaseolus vulgaris. III. Formation of Lamellar and Soluble Chloroplast Protein. Plant Physiol. 1968 Apr;43(4):504–514. doi: 10.1104/pp.43.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Naylor A. W. Purine and pyrimidine nucleotide inhibition of carbamyl phosphate synthetase from pea seedlings. Biochem Biophys Res Commun. 1968 May 10;31(3):322–327. doi: 10.1016/0006-291x(68)90478-6. [DOI] [PubMed] [Google Scholar]
- Patil S. S. Inhibition of Ornithin Carbamyl Transferase from Bean Plants by the Toxin of Pseudomonas phaseolicola. Plant Physiol. 1970 Nov;46(5):752–753. doi: 10.1104/pp.46.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. S., Zucker M. Potato phenolases. Purification and properties. J Biol Chem. 1965 Oct;240(10):3938–3943. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K. Ein phytotoxisches Polysaccharid von Pseudomonas phaseolicola. Naturwissenschaften. 1969 Nov;56(11):569–570. doi: 10.1007/BF00597283. [DOI] [PubMed] [Google Scholar]
- STUMPF P. K., LOOMIS W. D., MICHELSON C. Amide metabolism in higher plants. I. Preparation and properties of a glutamyl transphorase from pumpkin seedling. Arch Biochem. 1951 Jan;30(1):126–137. [PubMed] [Google Scholar]
- Sinden S. L., Durbin R. D. Glutamine synthetase inhibition: possible mode of action of wildfire toxin from Pseudomonas tabaci. Nature. 1968 Jul 27;219(5152):379–380. doi: 10.1038/219379a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Isolation and proof of structure of wildfire toxin. Nature. 1971 Jan 15;229(5281):174–178. doi: 10.1038/229174a0. [DOI] [PubMed] [Google Scholar]
- Tam L. Q., Patil S. S. Mode of Action of the Toxin from Pseudomonas phaseolicola: II. Mechanism of Inhibition of Bean Ornithine Carbamoyltransferase. Plant Physiol. 1972 May;49(5):808–812. doi: 10.1104/pp.49.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]