Abstract
Thymic Foxp3-expressing regulatory T cells are activated by peripheral self antigen to increase their suppressive function, and a fraction of these cells survive as memory Tregs (mTregs). Memory Tregs persist in non-lymphoid tissue after cessation of antigen expression and have enhanced capacity to suppress tissue-specific autoimmunity. Here, we show that murine mTregs express specific effector memory T cell markers and localize preferentially to hair follicles in skin. Memory Tregs express high levels of both IL-2Rα and IL-7Rα. Using a genetic deletion approach, we show that IL-2 is required to generate mTregs from naive CD4+ T cell precursors in vivo. However, IL-2 is not required to maintain these cells in the skin and skin-draining lymph nodes. Conversely, IL-7 is essential for maintaining mTregs in skin in the steady state. These results elucidate the fundamental biology of mTregs and show that IL-7 plays an important role in their survival in skin.
INTRODUCTION
Regulatory T cells play an essential role in maintaining immune homeostasis in tissues. Both mice and humans harboring defects in these cells develop florid inflammation and autoimmunity, with a predilection for the gastrointestinal tract and skin (1, 2). Classically, two main subsets of Foxp3-expressing Tregs have been defined: those that develop in the thymus (thymus-derived Tregs; tTregs) and those that develop outside the thymus from naive CD4+ T cell precursors (peripherally-derived Tregs; pTregs). Recently, we have identified a population of Foxp3-expressing Tregs, termed memory Tregs (mTregs) (3). These cells are activated upon exposure to antigen in the periphery, persist in non-lymphoid tissues after antigen expression ceases, and have enhanced capacity to suppress autoimmune responses when tissue antigen is re-expressed. We have shown that this population plays a major role in attenuating autoimmune responses in skin upon repeated exposure to self antigen, and others have recently shown that mTregs are required to maintain regulatory memory upon successive alloantigen exposure expressed in the developing fetus (4). In addition, a similar population may exist in human blood (5). The fundamental biology of mTregs (i.e., how they are established and maintained in tissues) remains to be elucidated.
Interleukin-2 plays an essential role in the development of Tregs in the thymus and for the maintenance of both tTregs and pTregs in secondary lymphoid tissues. Mice deficient in IL-2 have significantly reduced numbers of Foxp3-expressing cells and develop autoimmunity (6). Mechanistically, IL-2 is known to provide survival signals to Tregs and promote their functional activity (7).
Interleukin-7 plays an essential role in the development and survival of both naive and memory T cells. Thymic T cell development is significantly impaired in IL-7-deficient mice and humans (8, 9). In addition, memory T cells fail to survive if they lack IL-7 receptor signaling or if they are adoptively transferred into IL-7-deficient hosts (10, 11).
Memory Tregs are a unique subset of regulatory cells that are maintained in non-lymphoid organs. Because they are both Foxp3-expressing cells and a subset of memory CD4+ T cells, we hypothesized that they would require IL-2, IL-7 or both for their maintenance in tissues. Here, we should that mTregs absolutely require IL-2 for their in vivo generation from peripheral naive CD4+ T cell precursors. However, IL-7 and not IL-2, is required to maintain this population in the skin. These results elucidate fundamental properties of mTregs in tissues and have potential therapeutic implications.
MATERIALS & METHODS
Mice
All animal studies were performed in compliance with institutional guidelines. K5/TGO and K5/TGO/DO11 mice were used as described (3). Hosts for adoptive transfer experiments were created by crossing K5/TGO mice with T cell receptor-alpha-deficient mice (TCRα-/-) on the Balb/c background. For adoptive transfer of DO11 T cells, DO11.10 TCR-transgenic mice were crossed onto Rag2-/-/CD90.1+ or Rag2-/-/CD90.2+/IL-2-/- backgrounds.
Adoptive transfer of T cells
Single cell suspensions from lymph nodes of DO11/Rag2-/-/CD90.1+ (i.e., DO11-WT) or DO11/Rag2-/-/CD90.2+/IL-2-/- (i.e., DO11-IL-2-/-) mice were prepared. Approximately 5×105 LN cells were adoptively transferred i.v. into gender-matched K5/TGO/TCRα-/- recipient mice, and mice were started on doxycycline chow the same day as adoptive transfer. For experiments involving co-transfer of cells from WT-DO11 and DO11-IL-2-/- mice, LN cells were pre-mixed in a ratio of 3:1 DO11-IL-2-/- to WT-DO11 cells. Sixty days later, mice were treated with a single injection of OX-7-SAP monoclonal antibody (5ug per mouse; Cat. # IT-2; Advanced Targeting Systems) to deplete CD90.1+ cells.
In vivo neutralization experiments
For IL-2 neutralization studies, mice were then treated with intraperitoneal injections of a 1:1 mixture of JES6-1A12 (UCSF Monoclonal Antibody Core) and S4B6-1 (BioXCell, NH) IL-2 neutralizing monoclonal antibodies (50ug of each antibody per mouse, injected 3x/week for a total of 2-3 weeks). For IL-7Rα neutralization experiments, mice were treated with intraperitoneal injections of A7R34 (BioXCell, NH) monoclonal IL-7Rα antibody (500ug per mouse, injected 2x/week for a total of 3 weeks).
Statistics
Statistical analysis was done using GraphPad Prism (GraphPad Software). p values were calculated using a two-tailed unpaired t test.
RESULTS AND DISCUSSION
Characterization of mTregs in murine skin
We have recently established a mouse model of tetracycline-inducible self antigen expression in the skin (3). In K5/TGO/DO11 transgenic mice, Ovalbumin (Ova) is constitutively expressed in the thymus, leading to a modest deletion of Ova-specific CD4+ T cells (i.e., DO11 cells) and the generation of a significant number of Foxp3-expressing DO11 tTregs that populate secondary lymphoid tissues. Upon induction of cutaneous Ova expression with doxycycline, DO11 Tregs are activated, proliferate, acquire a more suppressive phenotype, and accumulate in the skin, where they play an obligatory role in resolving inflammation. When antigen expression is extinguished, a subset of Tregs stably resides in the skin and is capable of attenuating subsequent autoimmune responses when antigen is re-expressed. Comprehensive phenotypic analysis of DO11 mTregs that remain in the skin of K5/TGO/DO11 mice >40 days after cessation of Ova expression (mTregs) reveals that these cells express high levels of effector memory markers including IL-7R (CD127), CD44, CD27, CCR6, and low levels of CD62L (data not shown). When compared to Tregs in the skin-draining lymph nodes (SDLNs), mTregs in skin express significantly lower levels of the high affinity IL-2Rα (CD25) and higher levels of the IL-7Rα (CD127) (Fig. 1). These results suggest that IL-2, IL-7, or both play a role in maintaining mTregs in skin.
Figure 1. Memory Tregs have reduced IL-2Rα expression and increased IL-7Rα expression.
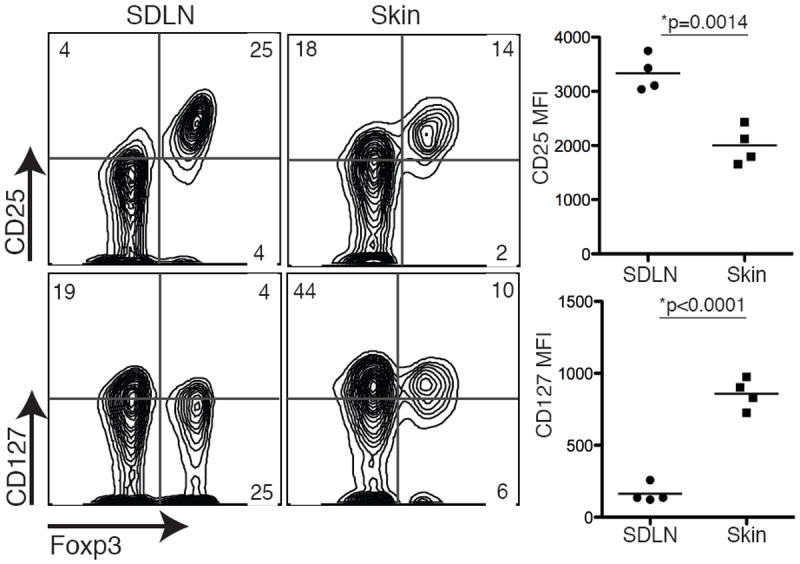
CD25 and CD127 expression was examined on mTregs isolated from the skin and SDLNs of K5/TGO/DO11 mice. Flow cytometry plots are gated on live CD4+DO11+ cells and column graphs are gated on live CD4+Foxp3+DO11+ cells. MFI, mean fluorescent intensity. Results are representative of 4 replicate experiments with > 3 mice per group.
Immunofluorescent confocal microscopy revealed that mTregs occupy a unique spatial niche in skin. Prior to antigen induction in K5/TGO/DO11 mice, there are very few DO11 cells in the skin (3). Approximately 10-14 days after induction of cutaneous Ova expression, K5/TGO/DO11 mice develop a florid inflammatory dermatitis. At the height of the autoimmune response, both effector DO11 cells and DO11 Tregs localize with CD11c+ cells diffusely throughout the papillary dermis and at the junction between the dermis and epidermis, with some cells infiltrating the epidermis (Supplemental Figure S1). However, >40 days after cessation of Ova expression in the skin, mTregs localize preferentially to hair follicles (Supplemental Figure S1). Notably, few mTregs were observed in the interfollicular epidermis or interfolliclular dermis, and were found preferentially in the dermis surrounding the lower segments of hair follicles. Some mTregs were observed in close proximity to hair follicle-associated CD11c+ cells. Interestingly, hair follicles have been called the “gatekeepers of the epidermis”, as keratinocytes that comprise distinct regions of the follicular epidermis secrete specific chemokines that play a critical role in the migration of Langerhans cell precursors to hair follicles and subsequent entry into the epidermis (12, 13). It is possible that hair follicle keratinocytes and/or follicle-associated dendritic cells secrete chemokines that recruit mTregs to this anatomic niche.
IL-2 is required to generate pTregs in vivo
Given the well-defined role of IL-2 in Treg biology and the fact that mTregs express high levels of CD25, we sought to determine whether IL-2 plays a role in establishing mTregs. To do so, we utilized an adoptive transfer approach. In this model, adoptive transfer of DO11 cells into K5/TGO mice crossed onto a TCRα-deficient background (K5/TGO/TCRα-/-) with subsequent induction of cutaneous Ova expression, results in a self-resolving acute inflammatory disease that recapitulates the inflammation in K5/TGO/DO11 mice (Fig. 2A). In addition, as in K5/TGO/DO11 mice, a stable mTreg population is generated that persists in the skin for >80 days after cessation of Ova expression. When T cells from DO11/Rag-/- mice are utilized for adoptive transfer, every DO11 Treg that is generated upon Ova induction in the skin is a pTreg (i.e., derived from a naive CD4+ T cell precursor) as naive DO11 cells from DO11/Rag-/- mice are devoid of thymus-derived Foxp3-expressing Tregs (data not shown). Consequently, this model represents the most robust system available for pTreg generation in vivo. To elucidate the role of IL-2 in the generation of pTregs (and the subsequent formation of mTregs), lymph node cells from DO11/Rag-/- or DO11/Rag-/-/IL-2-/- mice were adoptively transferred into K5/TGO/TCRα-/- hosts and Ova expression induced in the skin. When compared to LN cells from wildtype DO11/Rag-/- mice, transfer of DO11/Rag-/-/IL-2-/- LN cells resulted in a significant delay in the onset of cutaneous inflammation that failed to resolve (Fig. 2A). WT DO11 Tregs were observed in both the SDLNs and skin between 10 and 15 days after Ova induction and plateaued at >40 days, where Foxp3-expressing cells comprised 40% and 80% of DO11 cells in the SDLNs and skin, respectively (Fig. 2B). In contrast, Foxp3-expressing DO11 cells were never observed to comprise > 0.15% in either the SDLNs or skin at all time points examined when DO11/Rag-/-/IL-2-/- LN cells were used for adoptive transfer (Fig. 2B). Despite the fact that pTregs essentially failed to develop, DO11 cells lacking IL-2 were able to expand and differentiate into effector cells capable of inducing cutaneous inflammation, albeit with delayed kinetics when compared to WT DO11 cells (Fig. 2A). These results suggest that although cytokine(s) other than IL-2 can compensate for the expansion and differentiation of effector T cells in response to peripheral antigen (presumably IL-15 and/or IL-7), IL-2 is absolutely required for differentiation and expansion of pTregs in vivo.
Figure 2. IL-2 is required to generate pTregs in vivo.
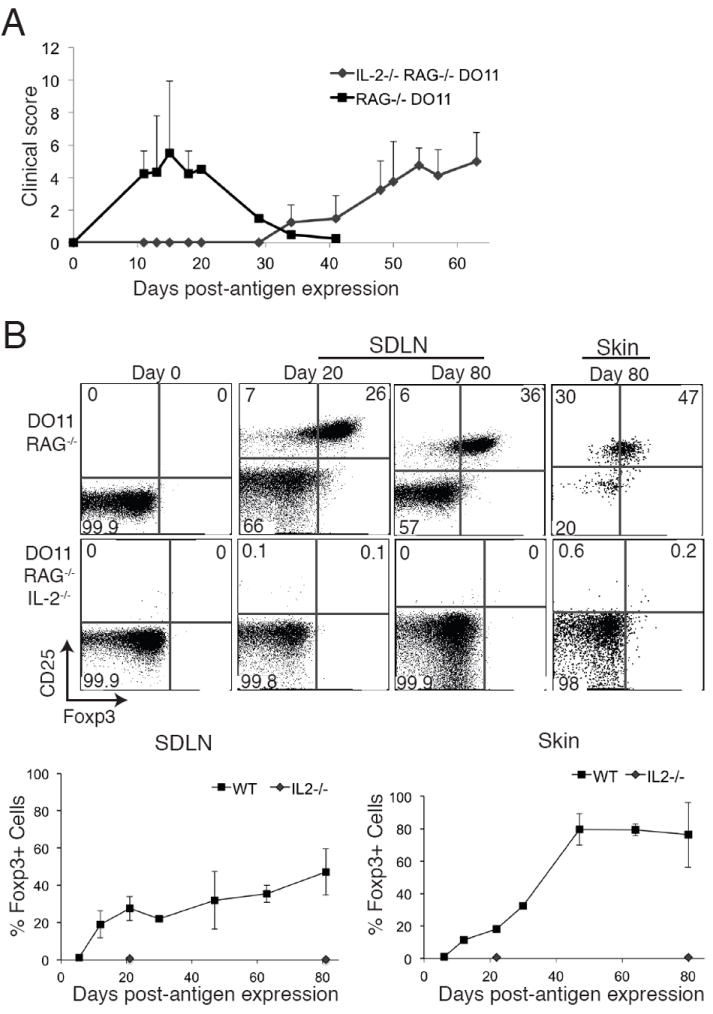
(A) Lymph node cells from DO11/Rag-/- or DO11/Rag-/-/IL-2-/- mice were adoptively transferred into K5/TGO/TCRα-/- hosts, Ova expression was induced in the skin, and severity of clinical skin disease measured. (B) Skin and SDLNs were harvested at specific times after antigen induction and DO11 cells analyzed by flow cytometry. Live CD4+DO11+ cells are shown. Error bars in 2A represent range. Results are representative of 2 replicate experiments with > 2 mice per group.
IL-2 is not required to maintain mTregs in the SDLNs and skin
To determine whether IL-2 is required to maintain mTregs in peripheral tissues, we employed both neutralizing antibody and genetic deletion approaches. To generate a stable mTreg population in the skin, cutaneous Ova expression was induced in K5/TGO/DO11 mice and approximately 30 days later, antigen expression was extinguished. Mice were then maintained for >40 days in the absence of Ova. We have previously shown that Ova expression is not detectable 20-30 days after removal of doxycycline (3). To determine if IL-2 is required to maintain mTregs in the skin and SDLNs, mice were treated with a well-characterized cocktail of IL-2 neutralizing antibodies (14, 15). When compared to mice treated with control antibody, there was no difference in the number of Foxp3-expressing DO11 cells in the skin and SDLNs in mice treated with IL-2 neutralizing antibodies (Supplemental Figure S2a). In these experiments, IL-2 neutralization was confirmed by down-regulation of the IL-2R on Tregs in SDLNs (Supplemental Figure S2b).
IL-2 neutralization with antibodies most likely results in partial inhibition of all bioavailable IL-2. Thus, it is plausible that in these studies some IL-2 was available to maintain mTregs in the skin and SDLNs. To circumvent this caveat, we utilized an adoptive transfer approach with IL-2-deficient DO11 cells into T cell-deficient hosts. Because adoptive transfer of DO11/Rag-/-/IL-2-/- cells into K5/TGO/TCRα-/- hosts fails to generate Tregs upon induction of Ova expression (Fig. 2B), we provided IL-2 in trans to enable Treg generation and subsequent development of mTregs. In these experiments, CD90.2+DO11/Rag-/-/IL-2-/- cells were co-transferred with WT CD90.1+DO11/Rag-/- cells into K5/TGO/TCRα-/- mice and Ova expression was induced in the skin. Approximately 30 days later, Ova expression was extinguished and mice were maintained for >30 days in the absence of antigen to generate mTregs. WT CD90.1+DO11/Rag-/- cells were then depleted using a toxin-conjugated anti-CD90.1 depleting antibody. After deletion of IL-2 producing cells, mice were maintained for approximately 40 more days, after which, skin and SDLNs were harvested and mTregs quantified (Fig. 3A). In contrast to transfer of IL-2-deficient DO11 cells alone, co-transfer with WT DO11 cells resulted in Treg generation, as Tregs derived from IL-2-deficient DO11 cells were readily detected in both the SDLNs and skin after induction of Ova expression (Fig. 3B). Depletion of CD90.1+ cells was robust and specific, as these cells were not detected in either the SDLNs or skin in all mice treated with the toxin-conjugated anti-CD90.1 antibody; however, CD90.2+ cells were readily detected (Fig. 3B). Consistent with anti-IL-2 neutralization studies, depletion of IL-2 producing T cells in this adoptive transfer model resulted in no change in the percentage or absolute number of mTregs in the skin or SDLNs (Fig. 3C). Interestingly, although WT DO11 cells were readily detected in SDLNs prior to depletion, these cells were never observed in the skin, before or after deletion (Fig. 3B). We speculate that this is secondary to a competitive disadvantage, as WT DO11 cells were co-transferred with IL-2-deficient DO11 cells at a ratio of 1:3 (i.e., WT DO11: IL-2-deficient DO11 cells). Thus, IL-2 producing T cells were most likely never present in the skin in this adoptive transfer model. Taken together, these results indicate that IL-2 is not required to maintain mTregs in skin.
Figure 3. IL-2 is not required to maintain mTregs in the periphery.
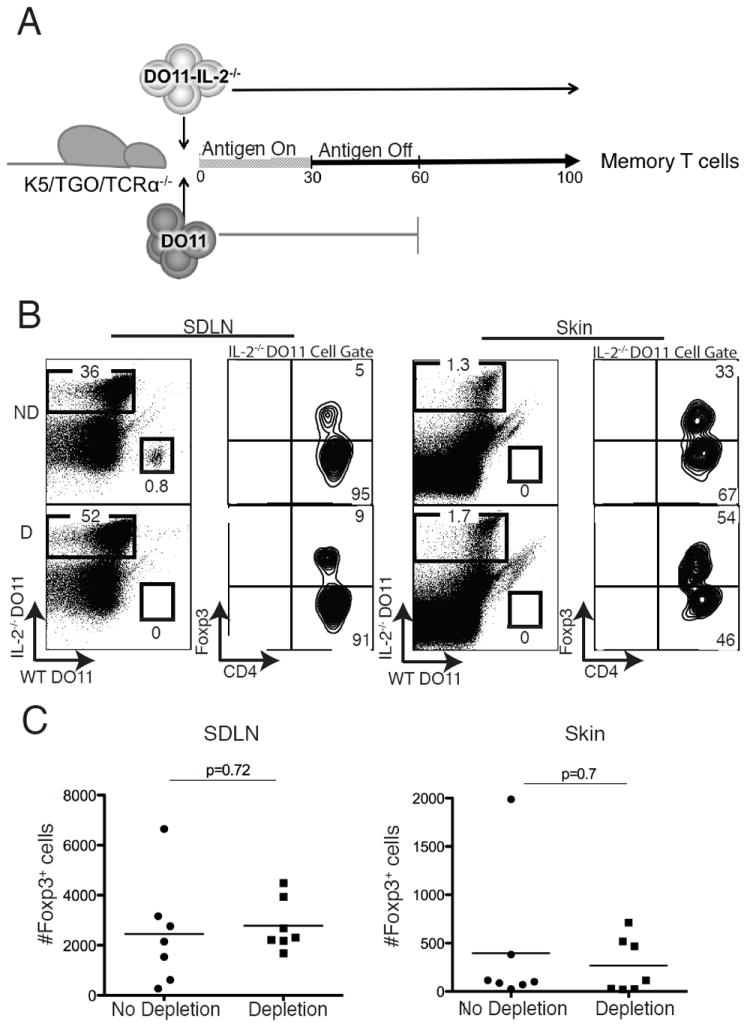
(A) To provide IL-2 in trans, WT CD90.1+DO11/Rag-/- cells (“DO11”) were co-transferred with CD90.2+DO11/Rag-/-/IL-2-/- cells (“DO11-IL-2-/-”) into K5/TGO/TCRα-/- hosts and Ova expression was induced in the skin for 30 days. Antigen was subsequently extinguished and 30 days later (i.e., day 60), WT DO11 cells were depleted or not depleted in control animals. DO11 memory cells were quantified 40 days after depletion (i.e., day 100). (B) Flow cytometry of DO11 cells harvested from skin and SDLNs at day 100 in mice where WT DO11 cells had been depleted (D) or not depleted (ND). (C) Absolute numbers of DO11-IL-2-/- cells expressing Foxp3 in the SDLN and skin in depleted and non-depleted mice on day 100. p = p value from 2-tailed unpaired t test. Results are pooled data from 3 replicate experiments with > 2 mice per group.
IL-7 is required to maintain mTregs in skin
IL-7 has long been recognized as a survival factor for memory T cells, and mTregs in the skin express high levels of IL-7Rα (Fig. 1). We tested whether IL-7R-mediated signaling plays a role in maintaining these cells in the skin and SDLNs. To do so, we utilized the A7R34 anti-IL-7Rα antibody clone, which does not cause antibody-dependent cell-mediated cytotoxicity and has been shown to mediate its effects specifically by blocking IL-7 signaling (16, 17). To generate a stable mTreg population in the skin, Ova expression was induced in K5/TGO/DO11 mice for 30 days, after which doxycycline was removed for >30 days to extinguish antigen expression. Mice were then treated for 3-4 weeks with anti-IL-7Rα or control antibody, and both skin and SDLNs were harvested for assessment of DO11 T cell numbers. Consistent with previous reports, the A7R34 antibody clone stably binds to cells expressing IL-7Rα, thereby inhibiting staining of this receptor by flow cytometry (Supplementary Figure S2c) (16). Mice treated with anti-IL-7Rα had a significant reduction in the number of DO11 mTregs and non-Treg memory T cells in the skin, but not in the SDLNs (Fig. 4). There was no change in the ratio of Tregs to non-Tregs in the skin or SDLNs after anti-IL-7Rα treatment (data not shown). Similar results were observed when IL-7R signaling was blocked in our adoptive transfer model (i.e., when mTregs are generated by antigen recognition by naive CD4 T cells in the periphery), suggesting that IL-7 is required to maintain mTregs in the skin regardless of whether they are derived from tTregs or pTregs (data not shown). Collectively, these results suggest that IL-7 plays an essential role in maintaining effector memory Tregs and non-Treg effector memory cells in the skin.
Figure 4. IL-7 is required to maintain mTregs in skin.
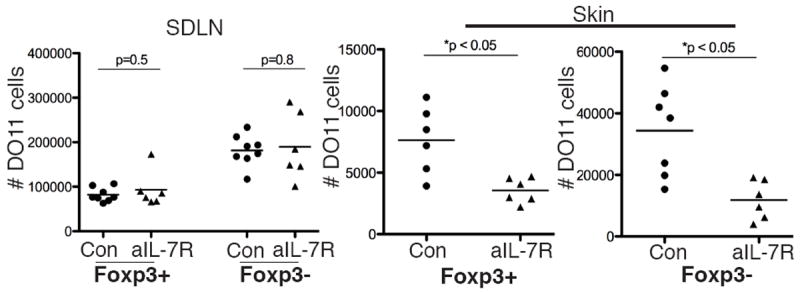
Memory Tregs were generated by inducing Ova expression in the skin of K5/TGO/DO11 mice for 30 days and subsequently turning off antigen for > 30 days. Mice were subsequently treated with anti-IL-7Rα blocking antibody or control antibody for 21-28 days. Absolute number of Foxp3+ and Foxp3- DO11 cells in the skin and SDLNs after treatment with anti-IL-7Rα blocking antibody or control antibody are shown. p value from 2-tailed unpaired t test. Results are pooled data from 2 of 3 replicate experiments with > 3 mice per group.
Foxp3-expressing Tregs are a dynamic and heterogeneous population. Different subsets of these cells have different requirements for their establishment and maintenance, which most likely depends on both their stage of differentiation and their local environment. We speculate that at any one time the secondary lymphoid organs of mice and humans contain a mixture of tTregs, pTregs and mTregs in an as yet undefined ratio.
In the skin, effector mTregs do not require IL-2 for their maintenance (Fig. 3). This may be because of an inherent difference in signaling pathways required to maintain memory vs. naive T cells or may be specific to the tissue in which they reside. Given the abundance of effector T cells in secondary lymphoid tissues compared to the skin, it is likely that there is relatively more constitutive IL-2 production in lymph nodes when compared to the skin in the steady state. In addition, it is well known that keratinocytes are a rich source of constitutive IL-7 production (18). It is interesting to speculate that effector mTregs (and non-Treg effector memory cells) have adapted to be dependent on IL-7 in tissues where IL-2 is less abundant. This is consistent with previous studies showing that Tregs increase expression of IL-7Rα in the absence of IL-2 (19). An alternative explanation is that mTregs in the skin are dependent on relatively low levels of IL-2 that come from a source other than T cells, such as cutaneous dendritic cells. Although possible, we feel this explanation is less likely given that dendritic cells need to be activated to make IL-2, as they have not been shown to constitutively secrete this cytokine in the steady state (20, 21). However, even if low levels of non-T cell derived IL-2 are capable of mediating signals to mTregs in the skin, our data suggest that this is not the dominant pathway for maintenance of these cells, as specifically blocking IL-7 signaling significantly reduces the number of cutaneous mTregs in IL-2 competent hosts (Fig. 4).
Collectively, our results provide direct evidence that signaling through the IL-7R is a major pathway required to maintain effector mTregs in the skin. We speculate that factors (other than IL-2) also play a role in maintaining these cells. Further elucidating the fundamental biology of this unique population will undoubtedly provide insight on how Tregs maintain immune homeostasis in peripheral tissues.
Supplementary Material
Acknowledgments
We thank Carlos Benetiz for assistance with animal husbandry, Johnthan Paw and Mike Lee for cell sorting, and Dr. Ann Rothstein, Dr. Katya Ravid and Greg Martin for derivation of TRE-TGO transgenic mice.
Footnotes
M.D.R. is supported by an NIH K08 grant (1K08AR062064-01), a Burroughs Wellcome Career Award for Medical Scientists (CAMS), the Scleroderma Research Foundation, and the UCSF Department of Dermatology. This work was partially funded through NIH grants P01 AI35297 (A.K.A), R01 AI73656 (A.K.A), and U19 AI56388 (A.K.A.). I.K.G is supported by an Erwin Schroedinger Fellowship from the Austrian Science Fund (FWF).
References
- 1.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 7.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 10.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 11.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, Kubo A, Cho Y, Clausen BE, Matsushima K, Suematsu M, Furtado GC, Lira SA, Farber JM, Udey MC, Amagai M. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath WR, Mueller SN. Hair follicles: gatekeepers to the epidermis. Nat Immunol. 2012;13:715–717. doi: 10.1038/ni.2374. [DOI] [PubMed] [Google Scholar]
- 14.Le Saout C, Villard M, Cabasse C, Jacquet C, Taylor N, Hernandez J. IL-2 mediates CD4+ T cell help in the breakdown of memory-like CD8+ T cell tolerance under lymphopenic conditions. PLoS ONE. 2010;5:e12659. doi: 10.1371/journal.pone.0012659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penaranda C, Kuswanto W, Hofmann J, Kenefeck R, Narendran P, Walker LSK, Bluestone JA, Abbas AK, Dooms H. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc Natl Acad Sci U S A. 2012;109:12668–12673. doi: 10.1073/pnas.1203692109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 18.Matsue H, Bergstresser PR, Takashima A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J Immunol. 1993;151:6012–6019. [PubMed] [Google Scholar]
- 19.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feau S, Facchinetti V, Granucci F, Citterio S, Jarrossay D, Seresini S, Protti MP, Lanzavecchia A, Ricciardi-Castagnoli P. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood. 2005;105:697–702. doi: 10.1182/blood-2004-03-1059. [DOI] [PubMed] [Google Scholar]
- 21.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol. 2003;170:5075–5081. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


