Abstract
Human brain imaging has identified structural changes in gray and white matter that occur with learning. However, ascribing imaging measures to underlying cellular and molecular events is challenging. Here, we review human neuroimaging findings of structural plasticity and then discuss cellular and molecular level changes that could underlie observed imaging effects. We propose that greater dialogue between researchers in these different fields will help to facilitate cross talk between cellular and systems level explanations of how learning sculpts brain structure.
The brain is the source of behavior, but in turn it is modified by the behaviors it produces. This dynamic loop between brain structure and brain function is at the root of the neural basis of cognition, learning and plasticity. The concept that brain structure can be modified by experience is not new, but it has proven difficult to address experimentally. Recent developments in structural brain imaging techniques (Box 1), particularly magnetic resonance imaging (MRI), are now propelling such studies to the forefront of human cognitive neuroscience.
BOX 1. Structural brain imaging: available techniques.
Volumetry based on T1-weighted MRI was among the first techniques developed; it can work well with certain well-defined structures (e.g., Heschl’s gyrus, hippocampus), but delineating anatomical borders can often prove difficult, and analysis is limited to a pre-defined region. Voxel-based morphometry (VBM) proved to be a breakthrough88, as it allowed whole-brain, automatic, unbiased, semi-quantitative analysis of gray matter and white matter concentration. As an exploratory tool, VBM has become a standard technique. But VBM, and any other approaches which rely on inferring tissue boundaries from intensity gradients on T1-weighted images, are quite nonspecific with respect to the underlying tissue characteristics they measure. Although VBM-derived metrics are often described as ‘concentration’, ‘density’ or ‘volume’, they do not relate in a straightforward way to underlying neuronal densities, for example. Any tissue property that affects relaxation times (e.g., cell density, cell size, myelination), and hence affects voxel intensities on a T1-weighted image, will influence these measures.
VBM maps can also be ambiguous because they can reflect variations in some unknown combination of size, shape and or position of brain regions and their boundaries. Algorithms that model the cortical surface can also be applied to measure both surface area and thickness of gray matter. Surface models respect anatomical boundaries to a greater extent than voxelwise measures; for example cortex within the two banks of a sulcus will be differentiated to a greater extent.
Diffusion-weighted MRI has encouraged the analysis of specific white matter anatomical features89. By fitting a model, such as the diffusion tensor model, to diffusion measurements at each voxel, it is possible to estimate parameters that relate to features of the underlying tissue microstructure. For example, fractional anisotropy quantifies the directional dependence of water diffusion and depends on features such as axonal integrity, myelination, axon diameter and density18. Another useful parameter is the principal diffusion direction which, within a coherent fiber bundle, corresponds to the underlying fiber direction. By following these directional estimates it is possible to perform diffusion tractography and trace the pathways of underlying fiber bundles.
In addition to the approaches outlined above, techniques such as relaxometry82, magnetisation transfer90, deformation-based morphometry91 or analysis of sulcal morphology92, can provide complementary information on variation in brain structure.
Any technique that compares local imaging metrics over time across individuals or over time can be susceptible to error, bias, or variation, introduced by analysis steps such as region selection, spatial smoothing or image registration93 and so such steps must be carefully considered.
A connection between brain function and brain anatomy might be expected as neural information processing depends on the size, configuration, and arrangement of individual neurons; on the number and type of local synaptic connections they make; on the way that they are interconnected to distant neuronal populations; and on properties of non-neuronal cells, such as glia. Neuroimaging evidence, reviewed below, shows both differences in structural features among individuals and the relevant functions that these structures subserve, and changes in structural features when long-term neural activity patterns are changed by experience.
However, current neuroimaging techniques cannot directly inform us about the underlying cellular events mediating the observed effects. Moreover, phenomena visible via MRI are likely never the result of a single process happening independently, but probably involve multiple coordinated structural changes involving various cell types. Conversely, neuroimaging techniques offer certain advantages as they can be repeatedly performed in the same individual and provide whole-brain measures of brain structure and function. Contemporary neural models of cognition stress the idea of multiple interacting functional networks; it is therefore logical to search for network-level patterns in anatomical structures as well. Recent studies examining inter-regional correlations of cortical thickness reveal that gray matter anatomical networks parallel functional organizational patterns1, that they are modified during development2, and are sensitive to training3. The ability provided by macrostructural imaging methods to understand both function and anatomy in terms of regional interactions is likely to grow in importance, and can also help to create hypotheses where cellular and molecular probes can be applied.
Here we consider findings that have emerged from the human anatomical neuroimaging literature, discuss the questions raised by them, and propose some possible microstructural mechanisms that could underlie the observed macrostructural findings.
Brain anatomy and cognitive specialization
Many studies have exploited anatomical imaging to reveal group differences that reflect skill, knowledge or expertise. Among the first was the demonstration of larger posterior hippocampal volume in expert taxi drivers4. The obvious implication was that this finding represented experience-dependent plasticity of a structure involved in spatial navigation, a conclusion supported by a correlation between years of experience and hippocampal volume in this population.
Related findings have been reported in many other special populations. Musicians consistently show greater gray matter volume5 and cortical thickness3 in auditory cortices; they also show differences in motor regions, and in white matter organization of the spinothalamic tract6. The effects generally increase as a function of years of musical practice, again supporting an experience-dependent explanation. The cross-sectional design of such studies, however, cannot discern whether the anatomical effects are the cause or the consequence of the skill or knowledge that distinguishes the groups. Moreover, links to behavioral performance have not always been available, despite their importance to helping determine the relevance of structural effects to the presumed skill (Fig. 1, Box 2).
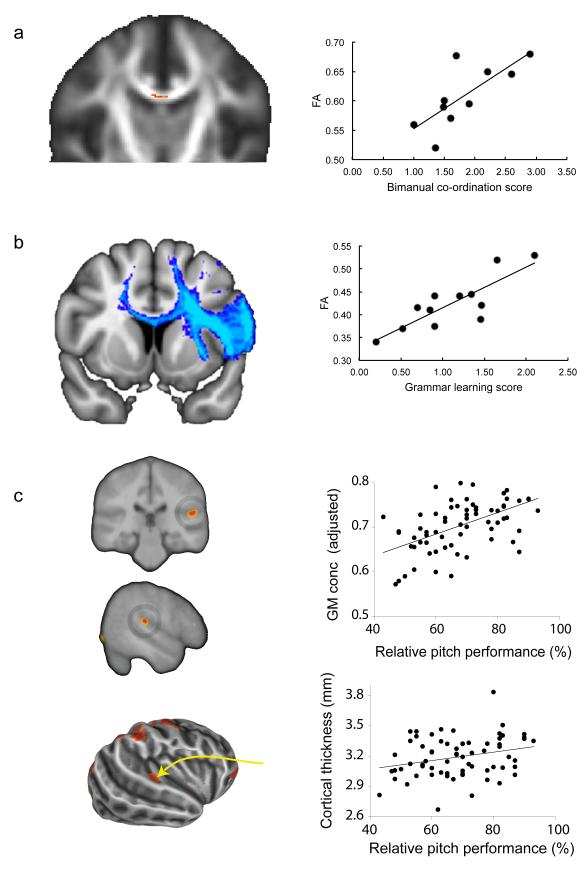
Figure 1. Behavioral relevance of brain structural variation.
Individual differences in performance of cognitive tasks correlates with variation in gray and white matter structure of task-relevant brain areas. Figures adapted from previous publications (with permission) as follows: (A) Performance on a bimanual co-ordination task correlates with fractional anisotropy in the body of the corpus callosum, a white matter region that contains transcallosal fibers liking supplementary and cingulate motor areas95. (B) Performance at acquiring the deep structure of an artificial grammar correlates with fractional anisotropy within pathways from Broca’s area, specifically in the left hemisphere96. (C) gray matter concentration (top) and cortical thickness (bottom) in areas of right auditory cortex covaries with behavioral ability specifically on pitch-based tests16.
BOX 2. Behavioral relevance of brain structural variation.
There are several reasons why it is important to establish relationships between MRI-based effects and behavior. First, in the context of learning, many different processes may be operating in parallel (for example learning words of a new language may entail auditory discrimination, motor articulatory skills, and semantic memory). If multiple brain regions show changes, interpretation is enhanced if they can be linked to separable behavioral effects. Second, individual differences in what is learned or how it’s learned could not be understood without measuring behavioral outcomes. Finally, in order for such research to have clinical relevance, it is essential to establish correlations between behavior and structure if one wants to understand disorders that are diagnosed on the basis of behavioral disturbances.
There is good evidence in the MRI literature that structural features can be directly linked to behavior94 (Fig. 1). For example, white matter microstructure correlates with behavior in task-relevant pathways for bimanual coordination95 or grammar learning96. In the auditory domain there is evidence that gray matter volume5, concentration, and cortical thickness16 in areas of right auditory cortex covary with behavioral ability specifically on pitch-based tests.
In the domain of memory we know that the volume of posterior hippocampus is enlarged in taxi drivers. Yet, performance in a navigation task amongst an unselected group of people was not predictable based on hippocampal volume97, indicating that the “taxi-driver effect” may be a specific consequence of extensive training, and not explainable as an aspect of population variance. Another possibility is that memory performance may be influenced by distinct navigation strategies, with different neural correlates98.
In longitudinal learning studies there has been mixed evidence for correlations between performance outcomes and brain changes. Whereas some report no such effects8,9,99, others have detected relationships; for example Hyde et al100 demonstrated morphometric changes in motor and auditory cortex following musical training which were predictive of final motor and auditory task performance, respectively. However, sometimes such relationships are found in a non-intuitive direction (e.g., greater performance improvements associated with smaller increases in gray matter in some cortical areas10). One white matter study, in which subjects varied in the amount of training completed, found that increases in fractional anisotropy were greatest in subjects who completed the most training11; whether this relationship is driven by variation in performance improvements (which also correlate with training time) or time spent training was not explicitly tested.
The relationship between macrostructural variation and behavior is presumably mediated by intermediate physiological properties that depend on structural substrates and influence behavioral responses50,94. To better understand these relationships it will be important in future to (1) develop better systems-level models of anatomical network properties that may be relevant to behavioral changes, (2) to develop better cognitive models of the behaviors of interest that take into account the relevant anatomical neural substrates, and (3) to ensure that sufficiently specific and sensitive behavioral probes are applied.
Finally, it is not always clear whether training or ability should be associated with increases or decreases in relevant brain regions because of the complex relationship between anatomical changes and underlying functionality. A solution to these problems comes from longitudinal studies (Fig. 2).
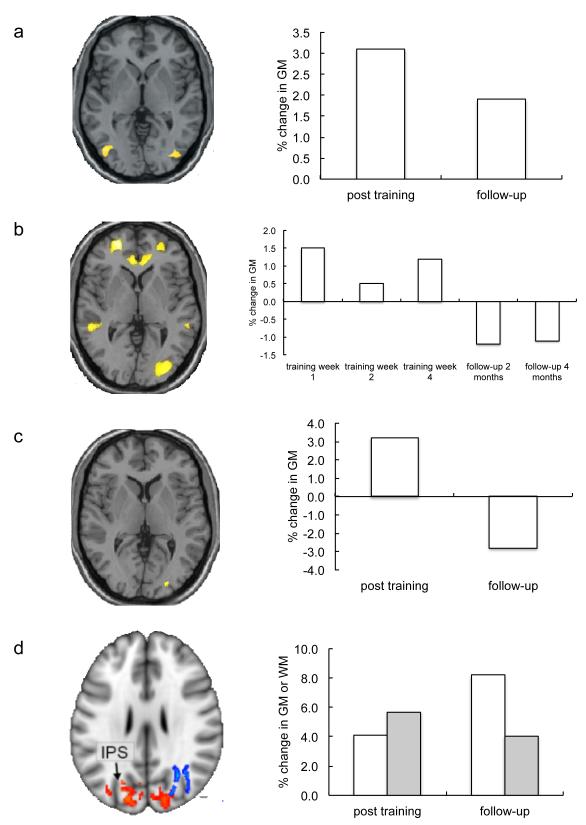
Figure 2. Longitudinal studies of structural changes in gray and white matter with learning.
A number of studies have used learning to juggle as a paradigm to test for brain structural plasticity in healthy adults. Figures adapted from previous publications (with permission) as follows: (A) 3 months of training results in increases in gray matter density bilaterally in the visual motion area, V57. (B) Serial scans throughout the training period show that such effects are apparent as early as one week after training begins8. (C) Given the same amount of training, older people learn less well on average than younger people, but those that are able to learn to juggle over the training period show similar brain structural changes99. (D) Not only gray matter (red clusters on brain, white bars), but also white matter (blue clusters on brain, grey bars), shows training-related changes. Both gray matter density and white matter fractional anisotropy increase around 5% over a 6 week training period9.
Longitudinal imaging studies
One of the first such longitudinal MRI studies used voxel-based morphometry (VBM, see Box 1) to demonstrate increased gray matter density in the visual motion area bilaterally when people learnt to juggle over a 3 month period7 and the same group later suggested that the changes were apparent after as little as 7 days of training8. Such experience-dependent macrostructural changes are not restricted to gray matter but can also be detected in white matter. Juggling training leads not only to increased gray matter concentration in occipito-parietal regions involved in visuo-motor co-ordination, reaching and grasping, but also to altered organization of underlying white matter pathways9 detected by fractional anisotropy (see Box 1). Similarly, practice of a complex whole-body balancing task resulted in increased gray matter in frontal and parietal cortex after just 2 days of training, and altered fractional anisotropy in corresponding white matter regions over the full 6 weeks of training10. However, in the latter study, fractional anisotropy changed in the opposite direction, showing reductions over time with training. While increases in fractional anisotropy are typically observed in association with maturation, development or learning, reduced fractional anisotropy might be observed if axon diameters increase or if a secondary fiber population matures in a region of fiber crossing (as was speculated to be the case here).
What aspect of learning experiences drives the observed brain changes? Both juggling and whole-body balancing are complex motor skills that involve procedural learning but other studies provide evidence that even purely cognitive tasks, such as working memory training11 result in measurable changes in brain structure.
Experience-dependent vs. pre-existing factors
Individual variation in anatomy affects perceptual and cognitive abilities (Box 2), but it is not known whether such correlations are related to differential environmental conditions, or if they reflect predispositions, that is, anatomical differences existing prior to the training or environmental event. The two options are not mutually exclusive, in that anatomical variation likely has many antecedents, including environmental, genetic and epigenetic ones.
Heritability studies in twin populations can quantify the degree to which environmental or genetic factors explain variation in gray or white matter measures. In gray matter, genetic influences are most notable in the frontal and temporal lobes, including areas related to language12. In the white matter, genetic factors explain about 75–90% of the variation in fractional anisotropy in large regions, particularly in parietal and frontal lobes; other white matter regions, such as the corpus callosum, showed much stronger evidence for environmental influence13.
Further evidence that not all of the relationship between brain anatomy and individual differences in behavioral performance can be accounted for by environmental experience comes from studies where there is little opportunity for experience to have an effect. For example, when volunteers were taught to discriminate unfamiliar foreign speech sounds14 pre-learning variability in left auditory cortex structure, or in related white matter pathways, predicted the rate and or outcome of learning. Similarly, while musical training probably influences auditory cortical anatomy, it does not entirely account for the relationship between auditory cortex volume and ability to learn pitch contours in a tone language15, or to discriminate melodies16. Together, these studies demonstrate that pre-existing anatomical features can affect learning rate and or attainment, but they leave open the question of how anatomical changes induced by training may be influenced by the initial anatomical state of the relevant structure.
Underlying cellular and molecular mechanisms
The preceding sections demonstrated the power of human neuroimaging studies for detecting effects of specific training regimes on brain structure and relating these to complex behavioral changes. However, neuroimaging measures are difficult to relate unambiguously to underlying biology. Studies at the cellular and molecular level can identify candidate mechanisms to help explain neuroimaging observations.
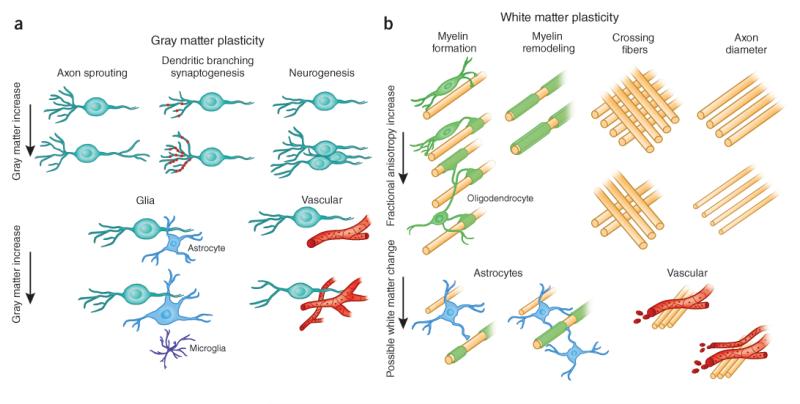
Numerous approaches can be used to gain molecular and cellular evidence on experience-dependent microstructural changes, ranging from cell cultures to studies in behaving animals. Each experiment is typically only able to test for a limited set of structural changes so building up a clear picture of how to understand systems level effects requires integration across a wide literature. Observed changes can be broadly categorized into neuronal changes in gray matter and in white matter, and extra-neuronal change (Fig. 3). Neuronal changes in gray matter may include neurogenesis, synaptogenesis and changes in neuronal morphology. In white matter, changes in the number of axons, axon diameter, the packing density of fibers, axon branching, axon trajectories and myelination can be found. Extra-neuronal changes include increases in glial cell size and number and angiogenesis.
Figure 3. Candidate cellular and molecular mechanisms.
(A) Cellular events underlying changes detected by MRI during learning include axon sprouting (a), dendritic branching and synaptogenesis (b), neurogenesis (c), changes in glial number and morphology (d), and angiogenesis (e) in gray matter regions. (B) Changes in white matter include axon branching, packing density, axon diameter, fiber crossing, and the number of axons (a), myelination of unmyelinated axons (b), myelin thickness and morphology (c), changes in astrocyte morphology or number (d), and angiogenesis (e).
Any of these cellular changes may influence MRI signals (see Box 1). For example, variations in neuronal, glial and synaptic density may impact on modalities sensitive to the proportion of cellular material versus extracellular space within a voxel, such as proton density imaging or relaxometry. Such features would therefore influence commonly-used methods to assess gray matter change (voxel-based or tensor-based morphometry, cortical thickness) that rely on image intensity boundaries in T1-weighted images. Myelin will modulate measures sensitive to lipid content, such as relaxation times17 (and hence any method based on T1-weighted images), and measures that reflect the presence of barriers to water diffusion, such as fractional anisotropy18. Changes in the trajectory of white matter pathways could impact on fractional anisotropy values in white matter, and on quantitative measures from modelling of complex diffusion profiles19. Angiogenesis could be detected by techniques such as contrast-enhanced imaging of blood volume or perfusion imaging of cerebral blood flow.
Ultimately, histological studies are required to make direct links between imaging measures and underlying mechanisms. For example, in one elegant study of gray matter plasticity, groups of mice were trained on different versions of a water maze, designed to depend on distinct brain systems, and volume measures were used to assess structural differences between groups20. As predicted, animals trained on a spatial version had growth in the hippocampus whereas those trained on a cued version had growth in the striatum. The MRI-derived measures of growth correlated with GAP-43 (growth-associated protein-43) staining, a marker for axonal growth cones, and not with measures of neuronal size or number, suggesting that the MRI volume change reflected remodeling of neuronal processes, rather than neurogenesis.
Candidate mechanisms for gray matter changes
The vast majority of neuroimaging studies are motivated by hypotheses concerning neuronal structure or function. Yet non-neuronal components, such as vasculature and glial cells, will also impact on MRI signals. Vasculature accounts for about 5% of gray matter21. In human gray matter, glia are believed to outnumber neurons by approximately 6:1, with varying ratios in different brain regions. In this section we will discuss evidence for both neuronal and non-neuronal activity-dependent changes in gray matter and will speculate on whether such changes may contribute to observed neuroimaging effects.
Neurogenesis
If a neuroimaging study detects increases in volume of a particular structure then an attractive explanation is that there has been growth of new neurons. There is good evidence for adult neurogenesis occurring with learning in the hippocampus. Learning accelerates the maturation of the dendritic trees of new-born neurons and promotes their integration into functional hippocampal neural networks22. Transiently reducing the number of adult-born hippocampal neurons in mice impairs performance in memory tasks23, and conversely, increasing adult hippocampal neurogenesis, by genetic manipulation, improves pattern separation learning24.
What is the likelihood that neurogenesis underlies some of the observed neuroimaging changes with experience? Although adult neurogenesis produces thousands of new granule cells in the dentate gyrus every month25, this is a relatively small increase in total number of hippocampal neurons. Furthermore, although there have been reports of neurogenesis in the adult neocortex26, this is controversial. Thus, neurogenesis is likely a minor factor in MRI changes, particularly those found outside the hippocampus in association with learning. Animal studies using ferritin-based reporters27 and labeling precursor cells with iron-oxide nanoparticles28 to visualize neuroblast migration with MRI may be helpful in answering this question.
Gliogenesis
Another explanation for MRI volume increases is increase in the number of non-neuronal cells. Unlike mature neurons, which cannot divide, astrocytes and oligodendrocyte progenitor cells (OPCs) retain the ability to divide in the adult brain. Indeed, it has been argued that all new cells in adult neocortex are non-neuronal; including glial cells and endothelial cells29. Gliogenesis, and structural plasticity of non-neuronal cells, occurs in response to learning and experience30 and might therefore be an important candidate mechanism for some of the MRI findings discussed above. The role of astrocytes in synaptic function, ion homeostasis, neuroenergetics, and regulating blood flow in response to neuronal activity implicates these cells in changes detected by functional and structural MRI31.
In addition, microglia, the resident immune cells of the brain, have traditionally been considered only in the context of pathology, but new research is pointing to microglial involvement in structural and functional plasticity of synapses and dendrites during development and learning, and hence could have direct relevance for MRI-based measures. For example, in-vivo microscopy shows that microglia have highly motile cell processes that continually survey the brain parenchyma and form transient contacts with synapses32. This process is experience-sensitive, because light deprivation reduces the motility of microglial processes, whereas re-exposure to light reverses this response32 and is regulated by glutamate and adenosine triphosphate (ATP) in an activity-dependent manner33.
Synaptogenesis and changes in neuronal morphology
Although we would argue that neurogenesis is unlikely to play a large role in MRI-detected experience dependent change outside the hippocampus, other changes in neuronal morphology may nevertheless contribute. For example, motor skill learning is associated with synaptogenesis34 and changes in dendritic spine morphology35. One study of cerebellar changes in rats showed that while an increase in synapse number persisted for 4 weeks, initial astrocytic growth (hypertrophy) declined in the absence of continued training, indicating differences in glial vs. neuronal responses to experience36. Changes in dendritic spine structure can also persist after learning. For example, monitoring spine formation and elimination over time in the mouse cerebral cortex by in-vivo microscopy shows that the extent of spine remodeling correlates with behavioral improvement after learning37. A small fraction of new spines are preserved after learning and these appear to provide a structural basis for long-term memory retention.
These distinctions in persistence of different types of structural change suggest that observing the time course of training-evoked change in neuroimaging studies may help to narrow down candidate mechanisms, but results thus far are mixed. Some studies on juggling, for example, found that gray matter changes reverted to baseline levels7, consistent with the time course of glial change observed in animal studies, but others have observed a persistence or even continued increases in these changes after the end of training9,38, more consistent with synaptogenesis and spine formation.
Vascular changes
Training studies suggest that experience can alter the vasculature, particularly with regimes that increase physical activity. For example, experiments on middle-aged monkeys show that physical exercise increases histologically-quantified vascular volume in the cerebral cortex in parallel with improved performance on cognitive tests; both effects were lost after a 3-month sedentary period39. Such vascular changes likely contribute to activity-dependent differences observed by structural MRI after training. One compelling study performed in both mice and humans showed that imaging measures of increased blood volume in the dentate gyrus of the hippocampus of exercising mice correlated with postmortem measures of neurogenesis within this structure40. The authors argue that similar increases in blood volume observed using imaging in the hippocampus of exercising humans therefore likely also reflect neurogenesis, but this remains to be directly tested and it is plausible that vascular changes could occur in some contexts even in the absence of neurogenesis.
Signalling pathways for gray matter changes
A broad range of activity-dependent signaling molecules and transcription factors are involved in regulating dendritic morphology and development of neurons and glia, most notably neurotransmitters, cytokines, and growth factors. Summarizing the voluminous literature on signaling in neuronal plasticity is beyond the scope of this review. One example, with supporting evidence from cellular to human imaging studies, is brain-derived neurotrophic factor (BDNF) and its high-affinity receptor TrkB (tropomyosin-receptor-kinase B), which have been widely implicated in neurogenesis, and morphological changes in dendrites during environmental experience and learning41. In human studies, variation in the polymorphism for the BDNF gene are associated with variations in hippocampal volume42, memory performance43 and susceptibility to plasticity-inducing brain stimulation protocols44. BDNF can regulate development of oligodendrocyte progenitor cells and affect myelination45, however, a possible role in activity-dependent regulation of myelination has not been shown.
Far less attention has been given to activity-dependent regulation of glial development. Blocking neural impulse activity with tetrodotoxin (TTX) reduces the number of astrocytes that develop in hippocampal cell cultures, in part through release of the neurotransmitter ATP from neurons, which in turn stimulates release of the cytokine leukemia-inhibitory factor (LIF) from astrocytes46. Immune system signaling molecules affecting microglia have been implicated in activity-dependent structural plasticity and remodeling of brain circuits, including the major histocompatibility complex (MHC)47 and C1q48.
Functional activity in neurons, astrocytes and blood vessels is tightly coupled and regulated by several signaling molecules. Among these, Vascular endothelial growth factor (VEGF), has multiple activities on blood vessels, neurons, astrocytes, neurogenesis, and cognition. Overexpressing VEGF or blocking endogenous VEGF in the hippocampus of adult mice affects neurogenesis, angiogenesis, long-term potentiation, and memory49. However, this study found that the effects of VEGF manipulation on memory were evident before newly added neurons could have become functional, thus implicating effects of VEGF on mature neurons in the formation of memory.
Candidate mechanisms for white matter changes
Although it is clear that cellular changes in gray matter participate in learning, it is less obvious how structural changes in white matter might do so. However, any complex task requires transmission of information through a series of distant cortical regions with distinct task-relevant functions. Optimizing the speed or synchrony of impulse transmission could therefore be an important aspect of learning50. Changes in white matter, including axon diameter, the number of myelinated axons in a tract, the thickness of myelin, or other morphological features such as internodal distance, determine the speed of impulse propagation and thus could contribute to increased functional performance with learning.
These structural properties of white matter influence neuroimaging measures. For example, diffusion imaging measures are sensitive to multiple tissue properties18 including variation in myelin51, axon diameter and packing density52, axon permeability18 and fiber geometry19.
Myelin
Many diffusion imaging studies of experience-dependent white matter plasticity propose change in myelin as a potential mechanism. This is a departure from the traditional view of myelin as passive electrical insulation, static and irrelevant to nervous system plasticity outside the context of injury or disease53. However, myelination is dynamic through development and into early adulthood; notably in the cerebral cortex where the frontal lobes are the last regions to myelinate. Could activity-dependent modulation of myelin persist throughout adulthood?
Myelination of unmyelinated axons, or modification of the myelin sheath on myelinated axons, could participate together with synaptic remodeling in altering brain circuitry according to experience. Oligodendrocyte progenitor cells (OPC) remain resident in substantial numbers in the adult brain; indeed 1/3 of OPCs in the adult mouse brain originate after adolescence54. These cells participate in repair after myelin damage, but they could in theory participate in learning if myelination of unmyelinated axons is stimulated by functional activity. Internodal lengths decrease in visual cortex of rhesus monkeys55 during normal aging, suggesting active remyelination throughout life.
Activity-dependent changes in myelin would provide a mechanism for experience-dependent regulation of impulse conduction velocities. Physical activity is known to affect conduction velocity, as conditions of inactivity, such as during bed rest or outer space missions, temporarily reduce conduction velocities56. Increasing motor activity in rats is associated with altered myelin thickness and axon diameter in peripheral nerves57. The results suggest that activity not only influences the formation of myelin, but also influences the maintenance and morphology of the sheath after myelination is complete.
A number of neuroimaging studies have reported changes in white matter structure with learning in adults9-11 yet sensitivity of myelination to environmental experience appears to be reduced in adulthood. Although the volume of the splenium of the corpus callosum increases by 10 % in adult rats exposed to an enriched environment, histological analysis shows that this was caused by an increase in number of astrocyte cell processes and branching of unmyelinated axons, rather than an increase in myelin58.
Combined histological and MRI studies on animals are required to answer the question of whether myelin changes underlie white mater plasticity observed with imaging. A recent study of rats trained in the Morris water maze showed changes in diffusivity or anisotropy in several brain regions, including cingulate, piriform, and somatosensory cortex, dentate gyrus, and corpus callosum59. Similar effects were detected, albeit at lower magnitude, in older rats. Histological analysis confirmed that grey matter regions with decreased diffusivity also showed an increase in astrocyte cell volume while the increased fractional anisotropy observed in corpus callosum was associated with increased staining for myelin basic protein.
Activity-dependent axonal sprouting, pruning or re-routing
In hippocampus, sprouting of mossy fiber axons has been observed after induction of long-term potentiation60, and spatial learning61, but similar changes are induced by forced and voluntary physical exercise in the absence of learning62. Pruning of axons is guided by activity-dependent competition to refine functional circuits. Using a mouse genetic system in which restricted populations of neurons in the hippocampus can be inactivated, Yasuda et al, (2011) show a similar activity-dependent competition participates in establishment of functional memory circuits63. This study reports that inactive axons in the hippocampus are eliminated by activity-dependent competition with active axons, and in the dentate gyrus, which undergoes neurogenesis throughout life, axon refinement is achieved by competition between mature and young neurons.
There is some evidence for changes in long-range cortico-cortical connectivity occurring with learning and with recovery from damage64. For example, when macaque monkeys learn to use a rake to retrieve food pellets, cells in the parietal cortex, where new bimodal responses are found, also show a novel pattern of anatomical connectivity: inputs from certain visual areas were detected in trained animals but not in untrained animals, suggesting the possibility of a re-branching of fibers in response to training, to allow particular types of visual information to reach parietal regions. Similar rewiring has been observed in response to damage in a squirrel monkey model65. Such changes in the route of fiber bundles should impact on imaging measures reflecting the directional preferences of water diffusion. For example, diffusion MRI models of complex fiber structure19 could be used to detect subtle changes in tract geometry.
Signalling pathways for white matter changes
Recent in vitro studies are beginning to elucidate the molecular signals and neurotransmitter release mechanisms that could allow for activity in an axon to influence myelinating glia and white matter microstructure. Synapses do form transiently on some OPCs in white matter66,67, but their function is unknown. Recently a nonsynaptic mechanism of neurotransmitter (ATP) release from axons has been described taking place through volume-regulated anion channels in axons that become activated by trains of action potentials68. Activity-dependent release of ATP from axons has been shown to regulate myelination in the peripheral69 and central nervous systems70,71. The diverse range of membrane receptors expressed in oligodendrocytes suggest that other types of cell-cell communication molecules72-74, including diffusible and cell surface molecules, could influence OPC proliferation, migration, differentiation, survival and myelin formation, in an activity-dependent manner.
In addition to effects on OPC development, new evidence shows that electrical activity in axons can control the complex sequence of cellular events necessary for myelination. Immature oligodendrocytes populate the human cerebral white matter throughout the later half of gestation, yet the majority do not commit to myelinogenesis until three months later75, demonstrating a dissociation between events that regulate maturation of oligodendrocytes and their commitment to myelinogenesis. Myelin formation requires cell recognition to myelinate the appropriate axon, the formation of adhesive contacts, elaboration of vast areas of cell membrane to form myelin sheets, wrapping multiple layers of membrane around axons, and the removal of cytoplasm from between the wraps of myelin to form compact stacks of lipid membrane, all of which might be influenced by signaling from electrical activity in axons. Impulse activity regulates expression of a cell adhesion molecule on neurons, L1-CAM (L1 cell adhesion molecule), that is essential for myelination76, and recently vesicular release of the neurotransmitter glutamate along axons has been shown to stimulate the initial events in myelination. Both the cholesterol-rich signaling domains between axons and oligodendrocytes and the local synthesis of myelin basic protein from mRNA in the oligodendrocyte process are stimulated by the activity-dependent release of glutamate from axons77. This would preferentially myelinate axons that are electrically active and increase the speed of conduction through these functionally active circuits. This process could therefore underlie some of the changes in white matter seen in MRI studies.
Interrelations between neuron and glial changes
Considering activity-dependent changes in neurons and glia independently is highly artificial as the two cell types are tightly coupled in both gray and white matter tissue through multiple interactions and pathways of communication. Myelination is regulated by axon diameter, for example. Thus, changes in axon diameter during learning could in turn cause oligodendrocytes to alter the thickness of the myelin sheath. Conversely, myelinating glia can regulate axon diameter and even the survival of axons73. Axons that become demyelinated can die and this leads to the death of neurons78. Regardless of which cell initiates the response, both axons and glia may be affected by impulse activity (directly or indirectly) through their close association.
An example of this intimate relationship is provided by the protein Nogo-A. Nogo-A is a myelin protein that interacts with the Nogo-66 receptor 1 (NgR1) in axons to inhibit growth cone motility and axon sprouting. Several other myelin proteins, including MAG (myelin-associated glycoprotein), and OMgp (Oligodendrocyte myelin glycoprotein), interact with the Nogo receptor, making myelin a potent inhibitor of axon sprouting, fasciculation, branching, axon extension79, as well as affecting synapse formation, morphology, and activity-dependent synaptic strength80. The function of myelin proteins in suppressing axon sprouting is thought to limit structural plasticity of neural circuits after refinement through environmental experience, and thus preserve the refinements. Myelin is therefore important in determining the critical period for learning and it is central to activity-dependent development of neural circuits.
More recently, it has been determined that Nogo-A is also expressed in some neurons. Ablation of this gene in neurons leads to longer neurites, increased fasciculation and decreased branching of cultured dorsal root ganglion neurons, and anti-Nogo-A antibodies lead to aberrant innervation of the hind limb of chick embryos79. In Nogo-A we see a molecule coupling neurons and glia, white matter and gray matter to activity-dependent structural modifications of brain circuits during learning of the type that likely underlie the changes seen with MRI during learning. Nogo-A may be exceptional in this respect, or simply the first of many molecules yet to be recognized controlling activity-dependent interactions between neurons and glia in gray and white matter.
Concluding remarks
Human imaging studies identifying experience-dependent structural changes in brain gray and white matter have rightly generated much excitement in recent years. A future challenge is to determine the cellular changes that underlie these macrostructural observations. Meeting this challenge requires greater cross-talk between those studying human populations and those working with animal models, and greater integration of techniques. Animal studies in which both imaging and histological measures can be taken in parallel, in particular, will help to establish the relative contribution of different cellular processes to the MRI effects, keeping in mind that multiple, coordinate cellular responses may be associated with a single MRI-based variable.
In future, greater use of multi-modal imaging approaches in humans should provide increased specificity to better discriminate specific types of cellular changes during learning and in relation to behavior. The MRI technique of magnetization transfer provides a good example of the potential for complementarity across modalities, because it is thought to be differentially sensitive to myelination. In magnetization transfer, the magnetization of macromolecules, such as those contained in myelin, is selectively altered (saturated), so that its effect can be detected via exchange with observable liquid spins. Magnetization transfer has already been used to examine natural variation of white matter composition in healthy populations81 and therefore has distinct potential to be used as a more specific probe of neural plasticity associated with learning. Similarly, myelin measures can be derived from maps of multi-exponential T2 relaxation times82 and vital stains for myelin sheaths can be imaged with positron emission tomography83. These myelin-specific measures could complement measures derived from MRI techniques such as DTI or VBM that are sensitive to multiple features of tissue organization and microstructure.
Pushing the boundaries of image acquisition with sophisticated hardware can provide a new window on tissue microstructure at a level not previously achievable in human studies. Within gray matter, for example, imaging at ultra-high resolution, and with multiple signal modalities, allows for measures to be taken in specific cortical layers84 or hippocampal subfields85. New developments in modeling of complex tissue architecture can provide increased sensitivity to specific cellular features. In white matter, for example, diffusion imaging can be adapted to generate axon diameter distributions86, or estimates of myelin microstructure87. Such advances offer great potential for furthering our understanding of brain structural variation with learning and behaviour. Despite the many obstacles that will have to be overcome, human neuroimaging and cellular and molecular neuroscience have much to gain from further interactions in both directions.
Acknowledgements
RJZ is supported by the Canadian Institutes of Health Research, and the Natural Sciences and Engineering Research Council of Canada; RDF is supported by funds for intramural research at the US National Institutes of Health; HJB is supported by the Wellcome Trust.
References
- 1.He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- 2.Raznahan A, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez P, Evans AC, Lerch JP, Zatorre RJ. Neuro-anatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cerebral Cortex. 2009;19:1583–1596. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- 4.Maguire EA, et al. Navigation-related structural change in the hippocampi of taxi drivers. PNAS. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider P, et al. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nature Neuroscience. 2002;5:688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 7.Draganski B, et al. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 8.Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning--revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taubert M, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi H, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson PM, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 13.Chiang MC, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain Structure Predicts the Learning of Foreign Speech Sounds. Cereb. Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- 15.Wong PC, et al. Volume of left Heschl’s Gyrus and linguistic pitch learning. Cereb Cortex. 2008;18:828–836. doi: 10.1093/cercor/bhm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster NE, Zatorre RJ. Cortical structure predicts success in performing musical transformation judgments. NeuroImage. 2010;53:26–36. doi: 10.1016/j.neuroimage.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Laule C, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI: From quantitative measurement to in-vivo neuroanatomy. Elsevier; London: 2009. [Google Scholar]
- 19.Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 20.Lerch JP, et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. NeuroImage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 21.Barbier EL, Lamalle L, Decorps M. Methodology of brain perfusion imaging. J Magn Reson Imaging. 2001;13:496–520. doi: 10.1002/jmri.1073. [DOI] [PubMed] [Google Scholar]
- 22.Tronel S, et al. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci U S A. 2010;107:7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 27.Iordanova B, Ahrens ET. In vivo magnetic resonance imaging of ferritin-based reporter visualizes native neuroblast migration. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieman BJ, et al. In vivo MRI of neural cell migration dynamics in the mouse brain. NeuroImage. 2010;50:456–464. doi: 10.1016/j.neuroimage.2009.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 30.Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Ment Retard Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Takano T, Nedergaard M. Astrocytic calcium signaling: mechanism and implications for functional brain imaging. Methods Mol Biol. 2009;489:93–109. doi: 10.1007/978-1-59745-543-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleim JA, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 35.Kolb B, Cioe J, Comeau W. Contrasting effects of motor and visual spatial learning tasks on dendritic arborization and spine density in rats. Neurobiol Learn Mem. 2008;90:295–300. doi: 10.1016/j.nlm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Kleim JA, et al. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res. 2007;178:244–249. doi: 10.1016/j.bbr.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draganski B, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhyu IJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Pezawas L, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 44.Cheeran B, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao J, et al. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- 46.Cohen JE, Fields RD. Activity-dependent neuron-glial signaling by ATP and leukemia-inhibitory factor promotes hippocampal glial cell development. Neuron Glia Biol. 2008;4:43–55. doi: 10.1017/S1740925X09000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datwani A, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi M, et al. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A. 2002;99:16192–16196. doi: 10.1073/pnas.252249999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fields RD. Neuroscience. Change in the brain’s white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters A, Verderosa A, Sethares C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia. 2008;56:1151–1161. doi: 10.1002/glia.20686. [DOI] [PubMed] [Google Scholar]
- 56.Ruegg DG, Kakebeeke TH, Gabriel JP, Bennefeld M. Conduction velocity of nerve and muscle fiber action potentials after a space mission or a bed rest. Clin Neurophysiol. 2003;114:86–93. doi: 10.1016/s1388-2457(02)00329-2. [DOI] [PubMed] [Google Scholar]
- 57.Canu MH, Carnaud M, Picquet F, Goutebroze L. Activity-dependent regulation of myelin maintenance in the adult rat. Brain Res. 2009;1252:45–51. doi: 10.1016/j.brainres.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 58.Markham JA, Herting MM, Luszpak AE, Juraska JM, Greenough WT. Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain Res. 2009 doi: 10.1016/j.brainres.2009.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. 2011;6:e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams B, Lee M, Fahnestock M, Racine RJ. Long-term potentiation trains induce mossy fiber sprouting. Brain Res. 1997;775:193–197. doi: 10.1016/s0006-8993(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez-Amaya V, Escobar ML, Chao V, Bermudez-Rattoni F. Synaptogenesis of mossy fibers induced by spatial water maze overtraining. Hippocampus. 1999;9:631–636. doi: 10.1002/(SICI)1098-1063(1999)9:6<631::AID-HIPO3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 62.Toscano-Silva M, et al. Hippocampal mossy fiber sprouting induced by forced and voluntary physical exercise. Physiol Behav. 2010;101:302–308. doi: 10.1016/j.physbeh.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Yasuda M, et al. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 2011;70:1128–1142. doi: 10.1016/j.neuron.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansen-Berg H. Structural plasticity: rewiring the brain. Curr Biol. 2007;17:R141–144. doi: 10.1016/j.cub.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 65.Dancause N, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 67.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fields RD, Ni Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci Signal. 2010;3:ra73. doi: 10.1126/scisignal.2001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 70.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 74.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 75.Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol. 2002;61:197–211. doi: 10.1093/jnen/61.2.197. [DOI] [PubMed] [Google Scholar]
- 76.Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 77.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–31. doi: 10.1212/01.wnl.0000275229.13012.32. discussion S43-54. [DOI] [PubMed] [Google Scholar]
- 79.Petrinovic MM, et al. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- 80.Lee H, et al. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang X, Herron TJ, Woods DL. Regional variation, hemispheric asymmetries and gender differences in pericortical white matter. NeuroImage. 2011;56:2011–2023. doi: 10.1016/j.neuroimage.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Laule C, et al. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4:460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stankoff B, et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-(1)(1)C]-2-(4′-methylaminophenyl)- 6-hydroxybenzothiazole. Ann Neurol. 2011;69:673–680. doi: 10.1002/ana.22320. [DOI] [PubMed] [Google Scholar]
- 84.Duyn JH, et al. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104:11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Leemput K, et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barazany D, Basser PJ, Assaf Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain. 2009;132:1210–1220. doi: 10.1093/brain/awp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avram AV, Guidon A, Song AW. Myelin water weighted diffusion tensor imaging. NeuroImage. 2010;53:132–138. doi: 10.1016/j.neuroimage.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 88.Wright IC, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. NeuroImage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 89.Johansen-Berg H, Behrens TEJ. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. Elsevier; London: 2009. [Google Scholar]
- 90.Horsfield MA. Magnetization transfer imaging in multiple sclerosis. J Neuroimaging. 2005;15:58S–67S. doi: 10.1177/1051228405282242. [DOI] [PubMed] [Google Scholar]
- 91.Ashburner J, et al. Identifying global anatomical differences: deformation-based morphometry. Hum Brain Mapp. 1998;6:348–357. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<348::AID-HBM4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- 93.Thomas AG, et al. Functional but not structural changes associated with learning: An exploration of longitudinal Voxel-Based Morphometry (VBM) NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johansen-Berg H. Behavioural relevance of variation in white matter microstructure. Curr Opin Neurol. 2010;23:351–358. doi: 10.1097/WCO.0b013e32833b7631. [DOI] [PubMed] [Google Scholar]
- 95.Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Floel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca’s area predicts grammar learning success. NeuroImage. 2009;47:1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 97.Maguire EA, et al. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13:250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- 98.Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci. 2007;27:10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hyde KL, et al. Musical training shapes structural brain development. J Neurosci. 2009;29:3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]