Abstract
Human bronchial epithelial cells exposed to synthetic double-stranded RNA (poly I:C) exhibited increased IL-6 and RANTES secretion and TLR2 expression that was inhibited following TLR3 silencing. Increased NF-κB and Stat3 phosphorylation were detected after poly I:C exposure and pretreatment with neutralizing antibody targeting IL-6 receptor α (IL-6Rα -nAb) or blocking Jak2 and Stat3 activity inhibited Stat3 phosphorylation. TLR2 up-regulation by poly I:C was also reduced by IL-6Rα-nAb and inhibitors of Jak2, Stat3 and NF-κB phosphorylation, whereas RANTES secretion was unaffected, but abolished following NF-κB inhibition. Treatment with exogenous IL-6 failed to increase TLR2. These findings demonstrate that TLR3 activation differentially regulates TLR expression through autocrine signaling involving IL-6 secretion, IL-6Rα activation and subsequent phosphorylation of Stat3. The results also indicate that NF-κB and Stat3 are required for TLR3-dependent up-regulation of TLR2 and that its delayed expression was due to a requirement for IL-6-dependent Stat3 activation.
Keywords: Inflammation, Viral infection, Cytokine
Introduction
Airway epithelial cells respond to viral infection through activation of multiple pathogen recognition receptors (PRRs) capable of detecting a variety of pathogen-associated molecular patterns (PAMPs). Double stranded RNA (dsRNA) generated during intracellular replication of viruses or the synthetic analog, polyinosinic polycytidylic acid (poly I:C) represents a viral molecular pattern that is recognized by Toll-like receptor 3 (TLR3). Additionally, airway epithelial cells express the cytoplasmic helicase proteins retinoic-acid-inducible gene I (RIG-I) and melanoma-differentiation-associated gene 5 (MDA5), jointly classified as RIG-like receptors (RLRs), which have also been implicated in the recognition of viral dsRNA and poly I:C (Yoneyama et al. 2004, 2005). RLRs exhibit RNA size-dependent activity, with RIG-I being activated by shorter, and MDA5 by longer dsRNA molecules. The longer synthetic dsRNA sequence of poly I:C appears to be preferentially recognized by MDA5 but not RIG-I (Yoneyama et al. 2004, 2005).
TLR3 interacts with the Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF) protein to mediate downstream signaling (Alexopoulou et al. 2001; Marshall-Clarke et al. 2007; Yoneyama and Fujita 2007). Conversely, both RIG-I and MDA5 possess caspase activation and recruitment domains (CARD) that signal through the IFN-β promoter stimulator-1 (IPS-1) pathway (Yoneyama et al. 2004, 2005; Yoneyama and Fujita 2007). Following ligation of viral dsRNA or poly I:C, both TLR3 and RLRs initiate signaling pathways that induce activation of multiple transcription factors including interferon (IFN)-regulatory factor 3 (IRF3), IRF7 and/or nuclear factor-κB (NF-κB). IRF and NF-κB pathways induce the transcription of type I interferons (IFNs) (IFNα and IFNβ) and pro-inflammatory cytokines/chemokines including the neutrophil chemoattractant, interleukin-8 (IL-8/CXCL8), Regulated on Activation, Normal T cells Expressed and Secreted (RANTES), pleiotropic antiviral cytokines IL-6 and IL-1β and pro-inflammatory cytokines such as TNF-α (Gern et al. 2003; Monick et al. 2003; Sasai et al. 2006; Matsukura et al. 2007; Bowie and Unterholzner 2008; Thompson and Iwasaki 2008; Melkamu et al. 2009). In vivo experiments with poly I:C revealed that MDA5 is essential for poly I:C-induced IFN-α production and that TLR3 signaling is critical for IL-12 production, whereas both MDA5 and TLR3 regulate IL-6 transcription and secretion (Kato et al. 2008) . Other signaling pathways including Janus kinase (JAK)/Signal Transducer and Activator of Transcription (Stat), mitogen-activated protein (MAP) kinases, and phosphoinositide-3-kinase (PI3K) have also been implicated in viral infection (Costa-Pereira et al. 2002; Bandyopadhyay et al. 2010).
In addition to eliciting the production of antiviral and proinflammatory cytokines, dsRNAs and poly I:C also induce the expression of TLRs (TLR2, TLR3) non-TLR (RIG-I, MDA5, PAR4, P2Y2) receptors, adaptor proteins and co-receptors including MyD88, TRIF, CD14 and Dectin-1 in various cell types including airway epithelial cells and smooth muscle cells (LeVine et al. 2001; Ritter et al. 2005; Sukkar et al. 2006; Lim et al. 2009; Melkamu et al. 2009). Most of these molecules serve as sensors of or signaling proteins for molecular patterns of viruses, bacteria, and fungal allergens, suggesting that dsRNAs can influence the immune response of the airway epithelium to bacterial and fungal infections. A recent study of esophageal epithelial cells demonstrated that poly I:C induced NF-ĸB-dependent TLR2 expression leading to enhanced epithelial responsiveness of cells to TLR2 ligands (Lim et al. 2009). Others have shown functional modulation of airway epithelial cells by dsRNA leading to an overall sensitization of the airway epithelium to TLR2 ligands, but the molecular mechanisms were not identified (LeVine et al. 2001; Melkamu et al. 2009).
In the present study, the mechanism by which dsRNAs activate the Stat3 pathway and induce TLR expression was investigated in human airway epithelial cells. The hypothesis was that dsRNAs stimulate TLR3 resulting in the release of specific cytokines that act as autocrine signaling molecules on receptors that signal through Stat3. This mechanism would serve to increase the diversity of signaling pathways activated during initial exposure to dsRNAs and increase the number and variety of target molecules regulated in response to viral infection. The results of the experiments identified IL-6 as a critical, rapidly released cytokine that functions as an autocrine signaling molecule to induce Stat3 activation required for increasing TLR2 expression.
Materials and methods
Materials
Poly I:C was obtained from InvivoGen (San Diego, CA), Stat3 blocker, 5,15-diphenylporphine (5, 15-DPP), NF-ĸB blocker, Bay11-7032, Jak2 blocker, Tyrphostin AG 490 (AG490) and DMSO were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Polyclonal anti-human IL-6Rα antibody (IL-6Rα-nAb, AB-227-NA) and polyclonal normal goat IgG as an isotype control for IL-6Rα-nAb (IL-6Rα-isotype cAb, AB-108-C) and all reagents for ELISA assays were purchased from R&D systems (Minneapolis, MN). All reagents were freshly prepared for each experiment. Pharmacological blockers or neutralizing antibody were pre-incubated with the cells for 2 h before activating with poly I:C or IL-6.
Cell culture
Immortalized human bronchial epithelial (HBE) cells were obtained from Dr. Fekadu Kassie (Masonic Cancer Center, University of Minnesota). HBE cells were originally immortalized by introduction of genes encoding cyclin-dependent kinase-4 and human telomerase reverse transcriptase (Ramirez et al. 2004). The cells were plated either into 6-well or 12-well plates containing bronchial epithelial growth medium (BEGM, Cambrex, Walkersville, MD) supplemented with defined growth factors and retinoic acid from a singleQuot kit (Cambrex, Walkersville, MD) and were incubated at 37 °C in a humidified 5 % CO2 atmosphere.
Silencing of TLR3 and Fluorescence-activated cell sorting (FACS)
Silencing TLR3 receptors in HBE cells was accomplished by lentiviral short hairpin (sh)RNA expression using shRNA constructs obtained from the RNA interference (RNAi) Consortium (TRC) library. Lentiviral constructs were prepared at the RNAi core facility maintained by the BioMedical Genomics Center at the University of Minnesota. The pGIPZ Lentiviral shRNAmir construct with Green Fluorescent Protein (GFP) reporter (clone V2LHS_171349, Accession # NM_003265) was used to silence TLR3 in HBE cells. HBE cells were also transfected with pGIPZ lentiviral vector without the shRNA insert (empty vector) for generating vector control cells. Incorporating GFP within the vector enabled flow cytometric sorting of cells expressing the TLR3-shRNA instead of relying on more time-consuming antibiotic selection methods. All flow cytometry sorting was performed in the Flow Cytometry Core Facility at the Stem Cell Institute, University of Minnesota. Cell sorting was performed using a FACS Vantage Sorter (Becton Dickinson) capable of three excitation lines: 488 nm, 340–360 nm, and 633 nm. HBE cells transfected with GFP-containing lentivirus were re-suspended at 3 × 106 cells/ml in HBSS in 1 % FBS. Samples were gated on live cells and collected in culture media appropriate to the designated experiment. Collected HBE cells expressing shRNA were further propagated and the level of TLR3 silencing determined by qRT-PCR.
Experimental conditions and collection of fluid samples and cell lysates
HBE cells were grown on 6-well or 12-well plates in medium containing BEGM until they were used in experiments. To determine the mechanism of poly I:C-induced TLR2 up-regulation, confluent HBE cells were pretreated with blockers for 2 h and then treated for a second time period with poly I:C and/or IL-6 in additive-free media. Conditioned media was then collected, snap frozen in liquid nitrogen, and stored at −80 °C until use. Monolayers were then washed with base medium and treated with Trizol or RLT-lysis buffer for RNA isolation and/or RIPA buffer to collect protein for Western blotting.
Quantitative RT-PCR (QRT-PCR)
Total RNA was extracted from the lysed cells using Trizol extraction (Invitrogen Inc., Carlsbad, CA) or RNeasy (Qiagen, Valencia, CA) and treated with DNase I (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Single stranded cDNA was prepared with 500 or 1,000 ng of RNA with SuperScript II reverse transcriptase (Invitrogen Inc., Carlsbad, CA) and random primers. Primer sets for the target genes were developed using Primer 3 software or taken from published work and all sequences developed for this study are reported in Table 1. Aliquots of cDNA equivalent to 25 ng of total RNA were used for qRT-PCR reactions, which were carried out using SYBER green detection from Strategene (La Jolla, CA). Real-time fluorescence was measured for 45 cycles and mRNA levels between treatment and controls were evaluated by comparing cycle thresholds (Ct) for target genes. Expression of mRNA was normalized to the median expression of GAPDH. Specificity and level of the different mRNA transcripts were assessed by analysis of melting point dissociation curves.
Table 1.
Primer sequences and PCR product characteristics
| Primer | Sequences (F and R 5′ to 3′) | Accession No. for the designed primer or Authors if taken from a published paper | mRNA Size (bp) | Position | Product Size (bp) | Tm (°c) |
|---|---|---|---|---|---|---|
| TLR2 | GGGTTGAAGCACTGGACAAT | NM_003264 | 3417 | 202–409 | 208 | 55.6 |
| TCCTGTTGTTGGACAGGTCA | 56.2 | |||||
| TLR3 | CCGTCTATTTGCCACACACTT | NM_003265 | 3057 | 15–244 | 230 | 55.5 |
| TCATCGGGTACCTGAGTCAAC | 56.2 | |||||
| RIG-I | AGGAAAACTGGCCCAAAACT | Source of the sequence (Le Goffic et al. 2007) | 55.0 | |||
| TTTCCCCTTTTGTCCTTGTG | 53.2 | |||||
| MDA5 | GTGCATGGAGGAGGAACTGT | Source of the sequence (Le Goffic et al. 2007) | 57.1 | |||
| GTTATTCTCCATGCCCCAGA | 54.4 | |||||
| GAPDH | ATGACATCAAGAAGGTGGTG | Source of the sequence (Lee et al. 2007) | 177 | 52.4 | ||
| CATACCAGGAAATGAGCTTG | 51.1 |
Western immunoblotting
Protein samples from monolayers of cells were prepared by homogenizing cell pellets in ice-cold RIPA lysis buffer (Santa Cruz biotechnologies Inc., Santa Cruz, CA), then centrifuged (14,000 × g for 20 min) and supernatants stored at −80 °C until use. The protein content of the samples was determined using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). For Western immunoblotting, 50 μg of protein was loaded onto 4–12 % Novex Trisglycine gels (Invitrogen Inc., Carlsbad, CA) and run for 60 min at 200 V. The proteins were then transferred onto a nitrocellulose membrane (Invitrogen Inc., Carlsbad, CA) for 1 h at 30 V. Subsequently, membranes were blocked in 5 % Blotto nonfat dry milk in Tris buffer containing 1 % Tween 20 for 1 h and probed overnight with the following primary antibodies: anti-phospho-Stat3, anti-phospho-NF-κB (obtained from Cell Signaling Technology, Beverly, MA and used at a dilution of 1:1,000.), anti-IL-6Rα (1 μg/mL of AB-227-NA from R&D systems, Minneapolis, MN) anti-TLR2 (C-19, sc-8690) and anti-beta-tubulin (purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA and used at a dilution of 1:200). After incubating the membranes with the respective secondary antibodies (goat anti-rabbit IgG; 1:5,000 for phospho-antibodies and donkey anti-goat IgG-HRP, sc-2020, 1:3000 for TLR2; and goat anti-mouse IgG-HRP, sc-2005, 1:3000 for ß-tubulin,) for 1 h, chemiluminescent immunodetection was used. Signals were visualized by exposing membranes to HyBolt CL autoradiography film (Metuchen, NJ). All membranes were stripped and reprobed with anti-β-tubulin to check for differences of protein loaded in each lane. For each protein, at least three Western blot assays were performed.
Measurement of IL-6 and RANTES secretion into the media and TLR2 protein in the lysate
Specific quantitation of selected cytokines (IL-6 and RANTES) present in the conditioned media was performed using appropriate ELISAs (Duoset ELISA kits for IL-6 and RANTES, R&D systems, Minneapolis, MN). We have previously shown that IL-6 is induced by poly I:C treatment of HBE cells (Melkamu et al. 2009) and RANTES is known to be a signature cytokine secreted in response to poly I:C. Moreover, the observation that no endogenous secretion of IL-6 or RANTES was detectable in HBE cells indicated that these cytokines would provide a sensitive measure of cell activation following poly I:C treatment. The Duoset ELISA assay was also employed to measure TLR2 levels in cell lysates.
Statistical analysis
All data are reported as the mean ± SEM. Data were analyzed using either an unpaired, two tailed t test where appropriate or ANOVA with Tukey post-test for multiple comparisons. Differences between mean values were considered significant when p < 0.05.
Results
Silencing TLR3 reduced poly I:C-mediated induction of TLR2 expression
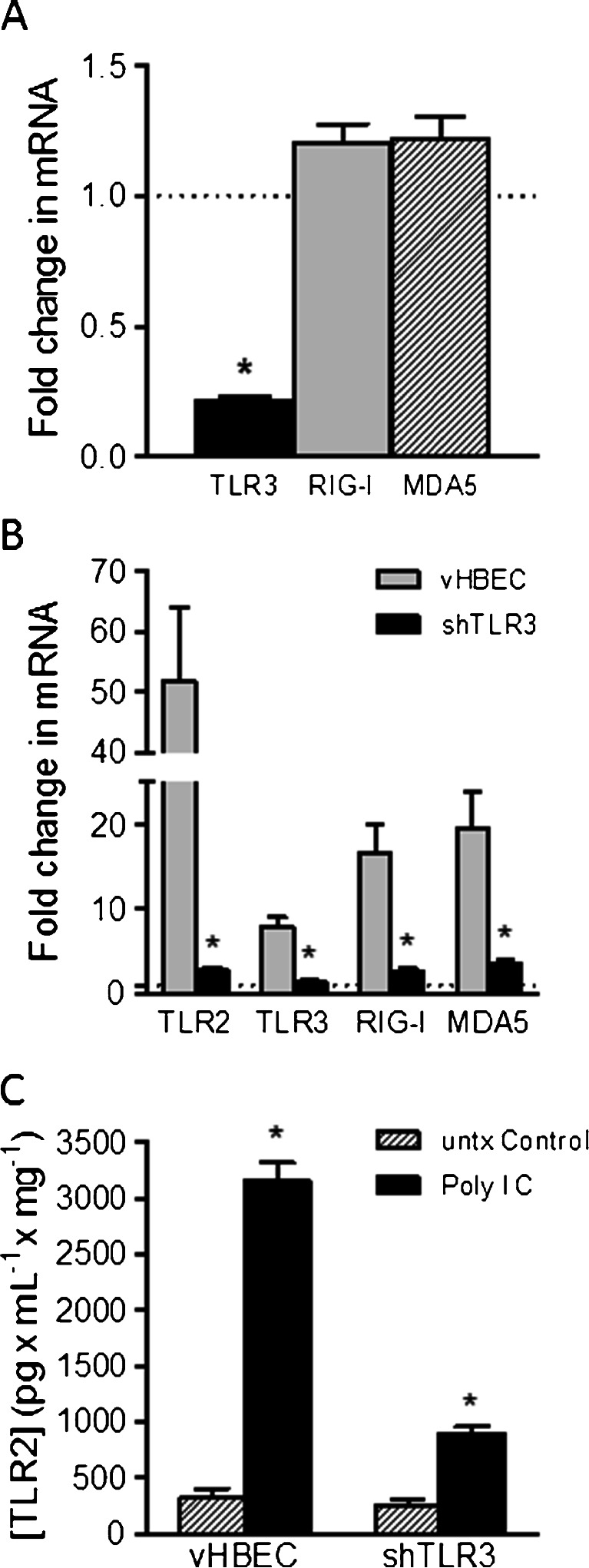
Short hairpin RNAs (shRNAs) targeting TLR3 (shTLR3) were introduced into immortalized HBE cells using a GFP-labeled lentiviral vector and selected by fluorescence-activated cell sorting to obtain an enriched population of shRNA-expressing cells. In addition, a cell line expressing the empty vector was also prepared to serve as a control. TLR3 mRNA expression, measured by qRT-PCR was reduced by 78 % compared to vector control cells (Fig. 1a). Expression of mRNA for non-TLR dsRNA-sensing cytoplasmic helicase proteins (RIG-I and MDA5) were also detected in HBE cells by qRT-PCR. However, TLR3 silencing was specific to the cognate receptor and did not significantly affect expression of RIG-I or MDA5 (Fig. 1a).
Fig. 1.
Effect of TLR3 Silencing on mRNA expression of RNA-helicases and TLR2 mRNA and protein expression: a; Levels of TLR3, RIG-I and MDA5 mRNA in HBE cells expressing shRNAs targeting TLR3 (n = 6; * significantly different from vHBEC controls) b; Comparison of TLR2, TLR3, RIG-I and MDA5 mRNA expression stimulated by Poly I:C (10 μg/ml for 24 h) in vector control cells (vHBE) and in TLR3 silenced (shTLR3) cells (n = 6–9; * significantly different from vHBEC controls). (c) TLR2 protein expression evoked by poly I:C (10 μg/mL 24 h) in vHBE and shTLR3 cells (n = 25; * significantly different from untreated (untx) control)
The effects of poly I:C and TLR3 silencing on TLR2 expression was evaluated by comparing shTLR3 expressing cells with vector control (vHBE) cells after exposure to 10 μg/mL poly I:C for 24 h. The results showed that poly I:C increased TLR2 mRNA expression in vHBE cells by 38.5 fold (Fig. 1b). In contrast, poly I:C induced TLR2 expression in shTLR3 cells was reduced by an order of magnitude to 3.2 fold (Fig. 1b) indicating that up-regulation of TLR2 mRNA following stimulation with poly I:C was primarily dependent on TLR3 activation. TLR2 protein expression, assessed by ELISA, also increased by 10 fold after poly I:C exposure in vHBE cells, however TLR3 silencing reduced this effect to 3.6 fold in shTLR3 cells (Fig 1c). Furthermore, mRNA expression of other receptors including TLR3, RIG-I, and MDA5 were also shown to be significantly increased by poly I:C and significantly reduced (by 2.6, 7.6, and 7.0 fold respectively) following TLR3 silencing (Fig. 1b).
TLR3 silencing abrogated poly I:C-stimulated secretion of selected cytokines
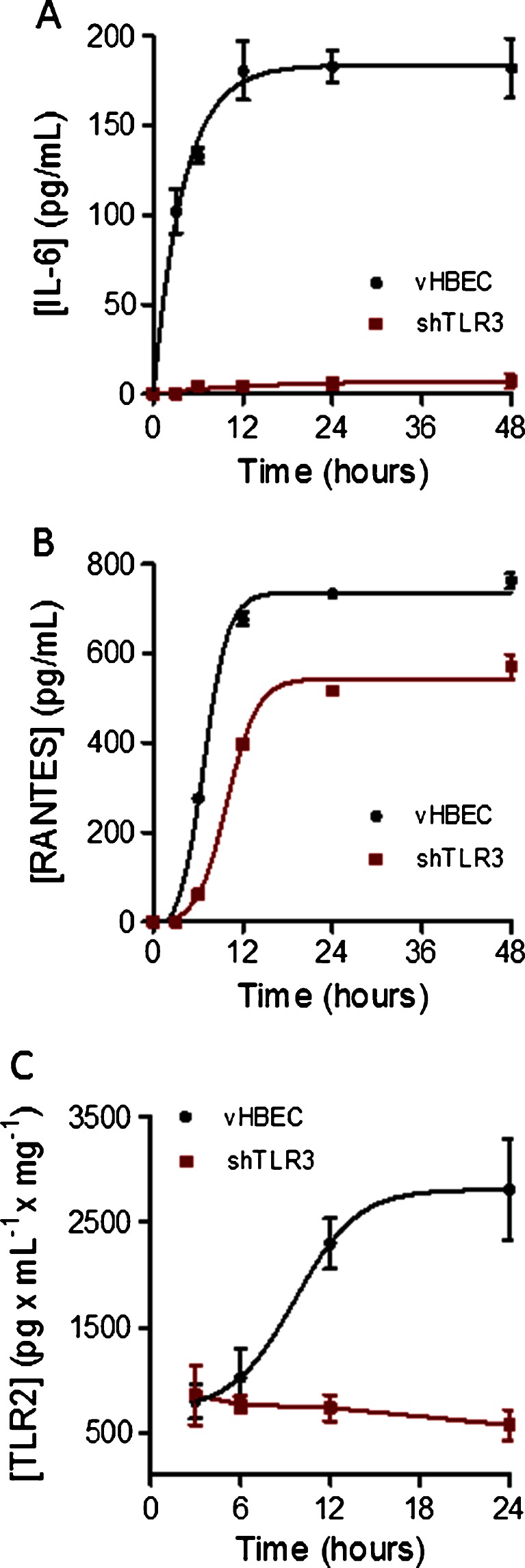
To assess the time course of IL-6, RANTES and TLR2 protein expression in response to poly I:C exposure, vHBE and shTLR3 cells were incubated in additive-free tissue culture media with or without poly I:C (10 μg/mL). Conditioned media and cell-lysates were collected from monolayers after 0, 3, 6, 12, 24, and 48 h of incubation. Concentrations of IL-6 (Fig. 2a) and RANTES (Fig 2b) in the media and TLR2-protein levels in cell lysates (Fig. 2c) were assayed by ELISA using commercially available kits. In vHBE cells, detectable levels of IL-6 secretion were observed within 3 h after addition of poly I:C and reached a maximum and sustained level of secretion at 12 h. The effect of poly I:C on IL-6 secretion was essentially abolished after TLR3 silencing. RANTES secretion was detectable 6 h after poly I:C stimulation and remained maximally elevated from 12 to 48 h. However, maximal secretion was reduced by 30 % in shTLR3 cells as compared to vHBE cells (Fig. 2b). Unlike IL-6 or RANTES where no basal secretion was detected in the media, basal TLR2 protein expression was measurable in the cell lysates. Induction of TLR2 expression following poly I:C treatment was evident after 12 h, reaching its maximum at 24 h (Fig. 2c). Poly I:C induction of TLR2 expression was significantly blocked in shTLR3 cells (Fig. 2c).
Fig. 2.
Effect of TLR3 silencing on the kinetics of selected cytokine and TLR2 secretion stimulated by Poly I:C (10 μg/ml): a; IL-6 and b; RANTES released into the media over 48 h were measured by ELISA. TLR2-protein expression (c) was measured by ELISA in cell lysates. Each data point represents as the mean ± SE (n = 6) for vHBE and shTLR3 cells
Poly I:C elicited Stat3 and NF-ĸB activation critical for up-regulation of TLR2 expression
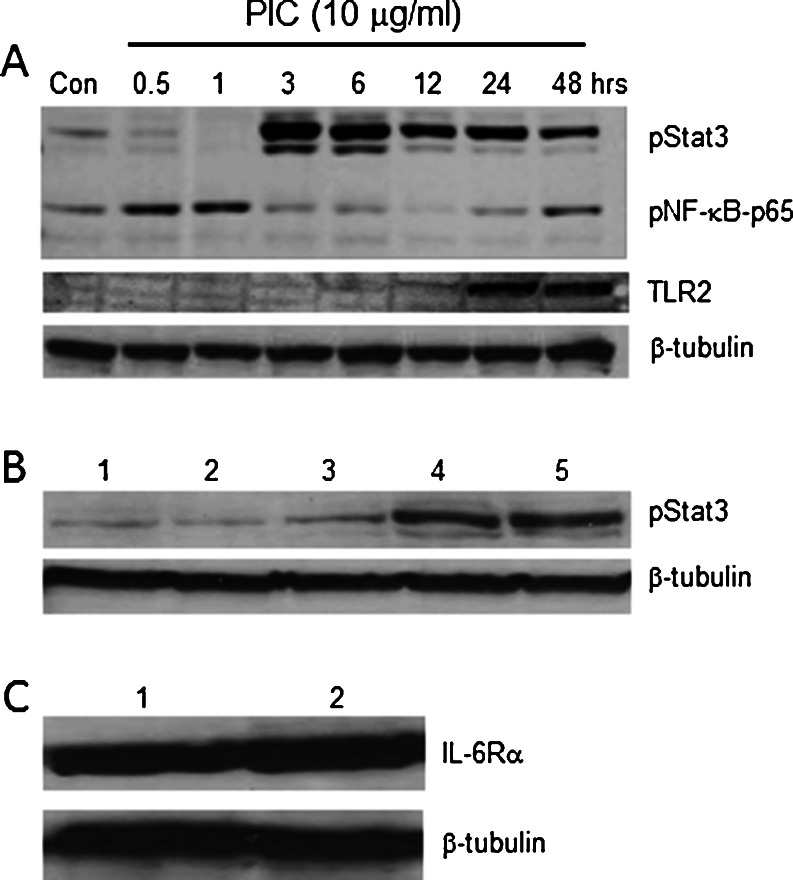
The kinetics of NF-ĸB and Stat3 activation in relation to TLR2 protein expression was analyzed by immunoblotting cell-lysates from HBE cells without and with poly I:C (10 μg/ml) stimulation at various time points (0.5, 1, 3, 6, 12, 24, 48 h) using anti-phospho-Stat3 (Tyr705) and anti-phospho-NF-ĸB-p65 antibodies, followed by stripping and re-probing with anti-TLR2 and anti-β tubulin antibodies (Fig. 3a). Phosphorylation of NF-ĸB-p65 was detected at early time points and maximum activation was attained within 1 h, then steadily declining until recovering activity again at 48 h. Similarly, poly I:C stimulation strongly induced Stat3 phosphorylation, but not until 3 h after stimulation and was sustained for the entire duration of poly I:C exposure (48 h). The effect of varying concentrations of IL-6 on Stat3 phosphorylation is shown in Fig. 3b. The threshold concentration for Stat3 activation was estimated at 1 ng/ml, approximately 5 fold higher than the maximum concentration of IL-6 detected in the extracellular media 12 h following Poly I:C stimulation (Fig 2a). A western blot showing IL-6Rα protein expression before and after Poly I:C stimulation is presented in Fig. 3c. The results indicate that Poly I:C does not alter the expression of the IL-6 receptor.
Fig. 3.
Time course of Poly I:C-phosphorylation of Stat3, NF-ĸB and up-regulation of TLR2 and IL-6Rα protein expression: a; Western blot showing the kinetics of Stat3 (pStat3-Tyr705) and NF-ĸB (p-NF-ĸB-p65) phosphorylation and TLR2 protein expression in cell-lysates from monolayers of HBE cells without (control) and with poly I:C (10 μg/ml) stimulation at different time points 0.5, 1, 3, 6, 12, 24, 48 h). ß-tubulin was used as the loading control. b Effects of exogenously added IL-6 (lane 1: untreated control; lane 2: 0.5 ng/ml; lane 3: 1 ng/ml; lane 4: 5 ng/ml; lane 5: 10 ng/ml) on Stat3 phosphorylation, with β-tubulin used as a loading control. c; Western blot comparing the expression of IL-6Rα before (lane 1) and after (lane 2) treatment with 10 μg/ml poly I:C. β-tubulin was used as the loading control
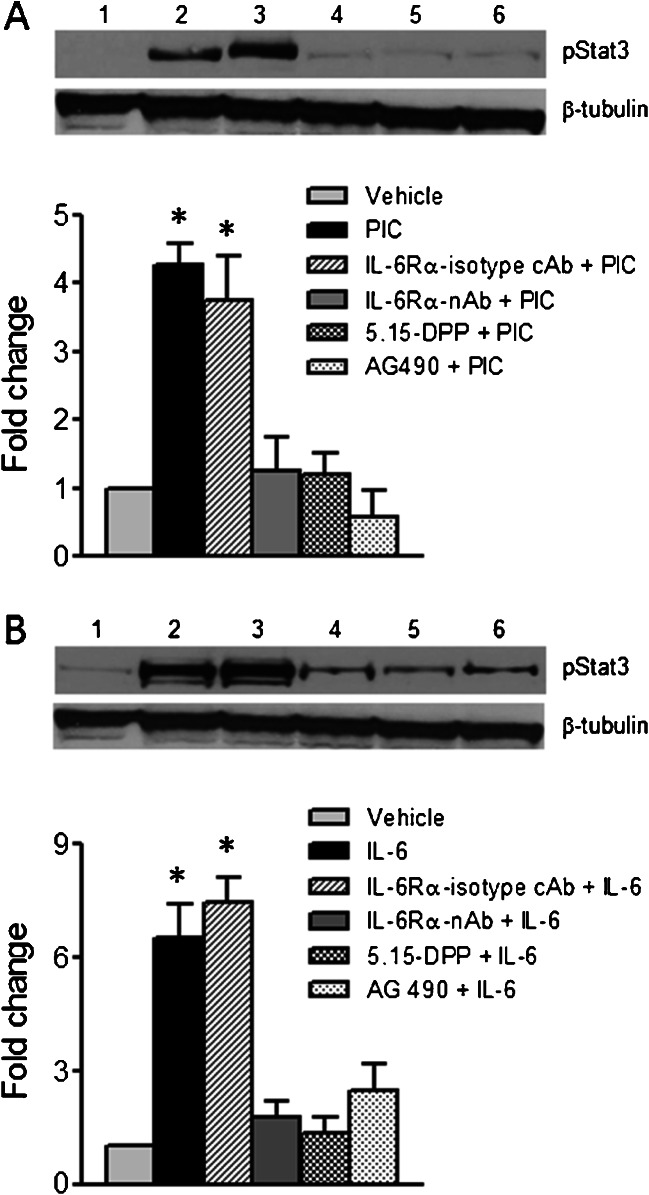
The effect of blocking the IL-6-Jak2-Stat3 signaling pathway on Stat3 phosphorylation is shown in Fig. 4a. HBE cells were pretreated with either IL-6Rα neutralizing antibody (IL-6Rα-nAb, 10 μg/ml), IL-6Rα isotype control antibody (IL-6Rα-isotype cAb, AB-108-C, 10 μg/ml) the Stat3 blocker, 5,15-diphenylporphine (5,15-DPP-50 μM) or the Jak2 blocker, AG490 (50 μM) and the NK-kB inhibitor (Bay11-7032, 1 μM) for 2 h, followed by stimulation with IL-6 (20 ng/ml) or poly I:C (10 μg/ml) for 3 h when cell lysates were collected and compared to untreated control cells and cells treated with IL-6 (20 ng/ml) and poly I:C (10 μg/ml) for 3 h without IL-6Rα-nAb, 5,15-DPP or AG490. Stat3 phosphorylation was then determined by western blotting. Densitometry analysis revealed that treatment with poly I:C augmented Stat3-phosphorylation by 4.27 fold, compared to untreated controls. However, when cells were incubated with IL-6Rα-nAb, but not IL-6Rα-isotype cAb prior to poly I:C stimulation, Stat3 phosphorylation was significantly blocked (Fig. 4a). Pharmacological inhibitors of Stat3 phosphorylation (5,15-DPP) and Jak2 (AG490) essentially abolished poly I:C-induced Stat3 activation. Similarly, exogenously added IL-6 also increased Stat3 phosphorylation by 6.5 fold compared to untreated controls and pretreatment with IL-6Rα-nAbn but not IL-6Rα-isotype cAb, significantly inhibited the increase in Stat3 phosphorylation evoked by IL-6. Treatment with 5,15-DPP or AG490 also blocked the effects of IL-6 on Stat3 phosphorylation (Fig 4b).
Fig. 4.
Effects of IL-6Rα neutralizing antibody, isotype control antibody, Jak2 and Stat3 inhibitors on Poly I:C/IL-6-induced Stat3 phosphorylation: a; Western blot showing Stat3 activation (Tyr705) in lane 1) vehicle treated HBE cells; lane 2) poly I:C (10 μg/ml) treated cells for 3 h; lane 3) IL-6Rα isotype control antibody (IL-6Rα isotype cAb) + poly I:C (10 μg/ml); lane 4) IL-6Rα neutralizing antibody (IL-6Rα-nAb-10 μg/ml) + poly I:C (10 μg/ml);, lane 5) Stat3 blocker-5,15-diphenylporphine (5,15-DPP-50 μM) + poly I:C (10 μg/ml) and lane 6) Jak2 blocker-(AG490, 50 μM) + poly I:C (10 μg/ml). The relative signal intensity was quantified using image J 1.42 software (NIH) and the mean ± SE of three independent blots were plotted in the bar graph (* significantly different from vehicle control, IL-6Rα-nAb, 5,15-DPP and AG490 treated conditions). b; Western blot showing Stat3 (Tyr705) phosphorylation in response to exogenously added IL-6; lane 1) vehicle treated HBE cells; lane 2) IL-6 (20 ng/ml) treated for 3 h; lane 3) IL-6Rα isotype cAb (10 μg/ml) + IL-6 (20 ng/ml); lane 4) IL-6Rα neutralizing antibody (IL-6Rα-nAb-10 μg/ml) + IL-6 (20 ng/ml); lane 5) Stat3 blocker-5,15-diphenylporphine (5,15-DPP-50 μM) + IL-6 (20 ng/ml) and lane 6) Jak2 blocker-(AG490, 50 μM) + IL-6 (20 ng/ml). Relative signal intensity was determined using image J 1.42 software and the mean ± SE of three independent blots are shown in the bar graph (* significantly different from vehicle control, IL-6Rα-nAb, 5,15-DPP and AG490 treated conditions)
TLR2 up-regulation by poly I:C was dependent on IL-6-Jak2-Stat3 and NF-kB pathways
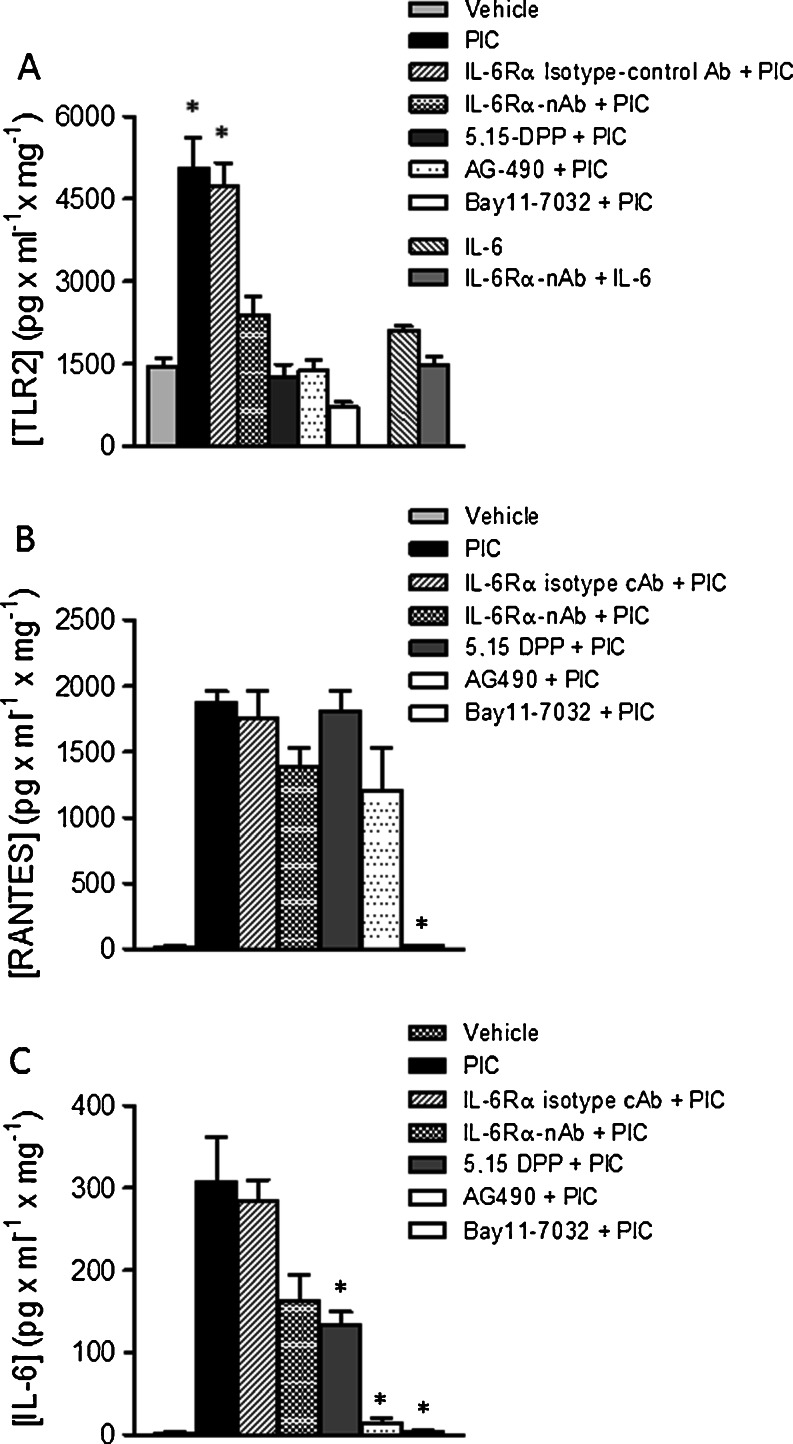
Initially, HBE cells were pretreated with IL-6Rα-nAb (10 μg/ml), IL-6Rα-isotype cAb (10 μg/ml) Stat3 blocker (5,15-DPP; 50 μM) or Jak2 blocker (AG490; 50 μM) for 2 h, followed by poly I:C (10 μg/ml) treatment for 24 h before whole cell lysates were collected. Cell lysates were also obtained from vehicle-treated control cells and poly I:C (10 μg/ml) stimulated cells for 24 h without pretreatment. TLR2-protein expression was then measured by ELISA (Fig. 5a). Poly I:C-induced TLR2 expression was significantly reduced after pharmacological inhibition of NF-ĸB and Jak2-Stat3 pathways. In addition, treatment with IL-6Rα-nAb also blocked the effect of poly I:C on TLR2 up-regulation (Fig. 5a) as did 5,15-DPP AG490 and Bay11-7032, indicating that increased expression of TLR2 evoked by poly I:C is dependent on the activation of the IL-6-Jak2-Stat3 signaling pathway. In contrast, stimulation with exogenous IL-6 failed to significantly increase TLR2 expression, indicating that activation of the Stat3 pathway without stimulating NF-κB phosphorylation is not sufficient to regulate TLR2 expression.
Fig. 5.
Effects of IL-6Rα neutralizing antibody, IL-6Rα isotype cAb, Jak2 and transcription factor inhibitors on poly I:C-induced TLR2, RANTES and IL-6 protein expression: a; TLR2 expression in cell lysates: Stimulation of HBE cells with vehicle, poly I:C(10 μg/ml); IL-6Rα-nAb (10 μg/ml) + poly I:C (10 μg/ml); IL-6Rα isotype cAb (10 μg/ml) + poly I:C (10 μg/ml); Stat3 blocker (5,15-DPP-50 μM + poly I:C (10 μg/ml), Jak2 blocker (AG490, 50 μM + poly I:C (10 μg/ml) or NF-kB blocker (Bay11-7032, 1 μM + poly I:C (10 μg/ml) for 24 h inhibited TLR2 protein expression. Stimulation with IL-6 (20 ng/ml) or pretreatment with IL-6Rα-nAb + IL-6 (20 ng/ml) did not significantly affect TLR2 expression (n ≥ 6; (* significantly different from vehicle control, IL-6Rα-nAb, 5,15-DPP, AG490, Bay11-7032 and IL-6 treated conditions). b; Effects of pretreatment with IL-6Rα-nAb (10 μg/ml) + poly I:C (10 μg/ml), Stat3 blocker (5,15-DPP-50 μM + poly I:C (10 μg/ml), Jak2 blocker (AG490, 50 μM + poly I:C (10 μg/ml)) and NF-κB blocker (Bay11-7032, 1 μM + poly I:C (10 μg/ml) for 24 h on RANTES protein secretion (n ≥ 6 for each condition; (* significantly different from poly I:C, IL-6Rα-nAb + poly I:C, IL-6Rα isotype cAb + poly I:C, 5,15-DPP + poly I:C conditions). c; Effects of pretreatment conditions described in part B for 24 h on IL-6 protein secretion (n ≥ 6 for each condition; * significantly different from poly I:C and IL-6Rα isotype cAb + poly I:C, conditions)
Poly I:C induced RANTES and IL-6 secretion is differentially regulated by Stat3 and NF-κB
HBE cells pretreated with IL-6Rα -nAb (10 μg/ml), IL-6Rα isotype cAb (10 μg/ml), 5,15-DPP (50 μM), AG490 (50 μM) or Bay11-7032 (1 μM) for 2 h were stimulated with poly I:C (10 μg/ml) for 24 h before collecting the culture media. Media was also collected from vehicle-treated control cells and cells treated with poly I:C (10 μg/ml) for 24 h without pretreating them with IL-6Rα -nAb, IL-6Rα isotype cAb, Stat3, Jak2 and NF-κB blockers. The level of RANTES (Fig. 5b) and IL-6 (Fig. 5c) protein secretion into the media was determined by ELISA using commercially available kits. Treatment of the cells with the NF-κB blocker significantly reduced IL-6 and RANTES secretion in HBE cells exposed to poly I:C. Unlike IL-6, RANTES induction by poly I:C was not affected by Stat3 or Jak2 inhibition, or by the neutralizing antibody directed against the IL-6 receptor.
Discussion
In a previous investigation involving the effects of hog confinement dust on human airway epithelial cells, TLR2 mRNA and protein expression were shown to increase by 3–5 fold. Inhibition of IL-6 production significantly reduced TLR2 mRNA expression evoked by hog dust extract, suggesting a role for IL-6 in the regulation of TLR2 expression (Bailey et al. 2008). In addition, TNFα and INF-γ were shown to act synergistically to increase TLR2 expression, a response that was markedly enhanced following treatment with dexamethasone (Homma et al. 2004). Although the precise mechanism responsible for potentiation by dexamethasone remains to be elucidated, the data from this study and others (Matsuguchi et al. 2000; Matsumura et al. 2003; Kumar et al. 2006; Matsukura et al. 2006; Satta et al. 2008; Lim et al. 2009) have suggested the involvement of the NF-κB signaling pathway in TLR2 up-regulation. In the present study, inhibition of NF-κB activation with Bay11-7032 abolished the poly I:C-dependent increase in TLR2 protein expression as well as IL-6 and RANTES, but did not affect basal levels of TLR2 protein within the cell lysate. Moreover, inhibition of Stat3 phosphorylation also abrogated poly I:C evoked up-regulation of TLR2, suggesting an important role for this transcription factor in the control of TLR2 expression. At the present time, a direct signaling pathway linking TLR3 activation to Stat3 phosphorylation has not been defined in airway epithelial cells. In contrast to what was observed for TLR2 expression, Stat3 phosphorylation was not required to stimulate RANTES. Previous studies in BEAS-2B cells showed that double stranded RNA activated both NF-κB and IRF3, which function cooperatively to regulate RANTES mRNA transcription (Ieki et al. 2004). Although the results of the present study show that inhibition of NF-κB phosphorylation in HBE cells is sufficient to abolish RANTES expression, they do not exclude the possibility that IRF3 is also required for stimulation of RANTES following treatment with poly I:C.
Earlier experiments using human squamous cell carcinoma cell lines (HN6, HN12, HN13, and HN30) and immortalized human keratinocytes, showed that NF-κB blockade inhibited Stat3 activation, suggesting the existence of cross-talk between NF-κB and Stat3 signaling pathways (Squarize et al. 2006). In the present study, we tested the hypothesis that the dependence of TLR2 expression on Stat3 activation involved the release of IL-6 and subsequent autocrine stimulation of endogenous IL-6 receptors that directly control the phosphorylation state of Stat3. Analysis of the time course results following poly I:C stimulation reveals rapid IL-6 mRNA transcription and protein synthesis leading to significant levels of secretion after a period of 3 h and maximal, sustained secretion after 12 h. Associated with elevated IL-6 secretion was an increase in Stat3 phosphorylation at 3 h followed by a time-dependent decrease in phosphorylation over the next 48 h of poly I:C exposure. We speculate that this time-dependent decrease in Stat3 phosphorylation could be due to receptor down-regulation, or perhaps uncoupling of the receptor from downstream activation of Jak2, or an increase in phosphatase activity. In spite of this effect, it is worth noting that Stat3 phosphorylation was enhanced throughout the following 48 h time period when increased TLR2 expression was detected. Moreover, we observed that stimulation of Stat3 phosphorylation by exogenously added IL-6 required a minimum concentration of 1 ng/ml while the maximum concentration of IL-6 in the bulk extracellular media measured after poly I:C stimulation was less than 0.2 ng/ml. This level of IL-6 receptor sensitivity would indicate that autocrine release of IL-6 by airway epithelial cells results in a much higher ligand concentration within the unstirred fluid layer adjacent to the plasma membrane which appears to be necessary for receptor activation.
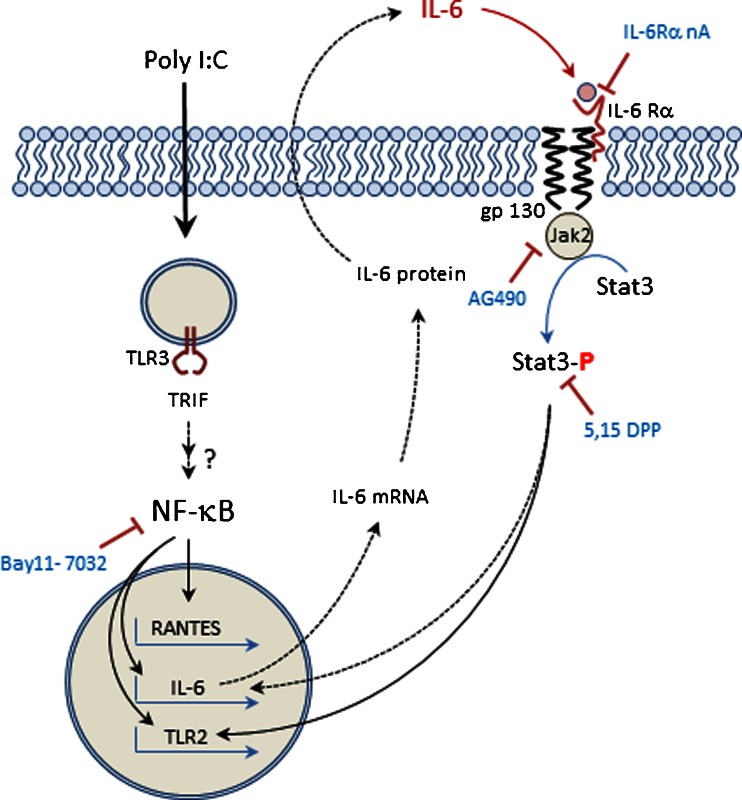
A model summarizing a possible mechanism accounting for the effects of poly I:C on TLR2 expression and cytokine secretion is shown in Fig. 6. The data indicate that poly I:C binds to TLR3, stimulating a signaling cascade that results in activation of NF-κB which in turn stimulates the transcription, translation and subsequent secretion of IL-6. Once released into the extracellular media, IL-6 binds to its receptor on the plasma membrane, stimulating Jak-dependent phosphorylation of Stat3 and subsequent transcription of TLR2. Addition of exogenous IL-6 alone without TLR3 activation, produced phosphorylation of Stat3, but did not significantly increase TLR2 protein expression, indicating that Stat3 activation alone could not mimic the effects of poly I:C on TLR2 up-regulation. In contrast, inhibition of Stat3 phosphorylation did not affect RANTES secretion, demonstrating that autocrine feedback mediated by IL-6/IL-6R produced differential regulation of cytokine and TLR expression. These findings support the conclusion that IL-6 is an essential, rapidly released cytokine that functions as an autocrine signaling molecule to induce Stat3 activation required for up-regulation of TLR2 expression in bronchial epithelial cells.
Fig. 6.
Model describing the role of autocrine signaling mediated by IL-6 in the regulation of TLR2 expression in HBE cells
Acknowledgments
This work was supported by grants [8K01OD010970 (to TM) and HL110539 (to HK and SMO)] from the NIH.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-ĸB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-like receptor 2 is up-regulated by hog confinement dust in an IL-6 dependent manner in the airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1049–L1054. doi: 10.1152/ajplung.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay SK, de la Motte CA, Majors AK, Strong SA. Inhibition of the phosphatidylinositol-3-kinase pathway abrogates polyinosinic:polycytidylic acid–stimulated hyaluronan-mediated human mucosal smooth muscle cell binding of U937 monocytic cells. J Interferon Cytokine Res. 2010;30(11):809–816. doi: 10.1089/jir.2009.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signaling. Nat Rev Immunol. 2008;8(12):911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pereira AP, Williams TM, Strobl B, Watling D, Briscoe J, Kerr IM. The antiviral response to gamma interferon. J Virol. 2002;76(18):9060–9068. doi: 10.1128/JVI.76.18.9060-9068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S, Busse WW. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2003;28:731–737. doi: 10.1165/rcmb.2002-0055OC. [DOI] [PubMed] [Google Scholar]
- Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- Ieki K, Matsukura S, Kokubu F, Kimura T, Kuga H, Kawaguchi M, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Schleimer RP, Adachi M. Double-stranded RNA activates RANTES gene transcription through co-operation of nuclear factor-jB and interferon regulatory factors in human airway epithelial cells. Clin Exp Allergy. 2004;34:745–752. doi: 10.1111/j.1365-2222.2004.1941.x. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting edge: influenza a virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- Lee SY, Palmer ML, Maniak PJ, Jang SH, Ryu PD, O’Grady SM. P2Y receptor regulation of sodium transport in human mammary epithelial cells. Am J Physiol [Cell Physiol] 2007;293:C1472–C1480. doi: 10.1152/ajpcell.00068.2007. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of streptococcus pneumoniae following influenza A infection in mice. J Virol Methods. 2001;94:173–186. doi: 10.1016/S0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- Lim DM, Narasimhan S, Michaylira CZ, Wang ML. TLR3-mediated NF-ĸB signaling in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1172–G1180. doi: 10.1152/ajpgi.00065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Clarke S, Downes JE, Haga IR, Bowie AG, Borrow P, Pennock JL, Grenics RK, Rothwell P. Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem. 2007;282:24759–24766. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y. Gene expressions of toll-like receptor 2, but not toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol. 2000;165:5767–5772. doi: 10.4049/jimmunol.165.10.5767. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Jeki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Kasama T, Adachi M. Synthetic double-stranded RNA induces multiple genes related to inflammation through toll-like receptor 3 depending on NF-κB and/or IRF-3 in airway epithelial cells. Clin Exper Allergy. 2006;36(8):1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Homma T, Takeuchi H, Nohtomi K, Adachi M. Role of RIG-I, MDA-5, and PKR on the expression of inflammatory Chemokines induced by synthetic dsRNA in airway epithelial cells. Int Arch Allergy Immunol. 2007;143(1):80–83. doi: 10.1159/000101411. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Degawa T, Takii T, Hayashi H, Okamoto T, Inoue J, Onozaki K. TRAF6-NF-kappaB pathway is essential for interleukin-1- induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology. 2003;109:127–136. doi: 10.1046/j.1365-2567.2003.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkamu T, Squillace D, Kita H, O’Grady SM. Regulation of TLR2 expression and function in human airway epithelial cells. J Membr Biol. 2009;229(2):101–113. doi: 10.1007/s00232-009-9175-3. [DOI] [PubMed] [Google Scholar]
- Monick MM, Yarovinsky TO, Powers LS, Butler NS, Carter AB, Gudmundsson G, Hunninghake GW. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278:53035–53044. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64(24):9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- Ritter M, Mennerich D, Weith A, Seither P. Characterization of toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of toll-like receptors, adaptor proteins and inflammatory response. J Inflamm. 2005;29:12–16. doi: 10.1186/1476-9255-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M, Shingai M, Funami K, Yoneyama M, Fujita T, Matsumoto M, Seya T. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177:8676–8683. doi: 10.4049/jimmunol.177.12.8676. [DOI] [PubMed] [Google Scholar]
- Satta N, Kruithof EKO, Reber G, de Moerloose P. Induction of TLR2 expression by inflammatory stimuli is required for endothelial cell responses to lipopeptides. Mol Immunol. 2008;46:145–157. doi: 10.1016/j.molimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Jr, Gutkind JS. Molecular cross-talk between the NFκB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8(9):733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118(3):641–648. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Iwasaki A. Toll-like receptors regulation of viral infection and disease. Adv Drug Deliv Rev. 2008;60(7):786–794. doi: 10.1016/j.addr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18(5–6):545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]