Abstract
Advanced differentiated thyroid cancer (DTC), defined by clinical characteristics including gross extrathyroidal invasion, distant metastases, radioiodine (RAI) resistance, and avidity for 18-fluorodeoxyglucose (positron emission tomography-positive), is found in approximately 10–20% of patients with DTC. Standard therapy (surgery, RAI, TSH suppression with levothyroxine) is ineffective for many of these patients, as is standard chemotherapy. Our understanding of the molecular mechanisms leading to DTC and the transformation to advanced DTC has rapidly evolved over the past 15–20 years. Newer targeted therapy, specifically inhibitors of intracellular kinase signaling pathways, and cooperative multicenter clinical trials have dramatically changed the therapeutic landscape for patients with advanced DTC. In this review focusing on morbidities, molecules, and medicinals, we present a patient with advanced DTC, explore the genetics and molecular biology of advanced DTC, and review evolving therapies for these patients including multikinase inhibitors, selective kinase inhibitors, and combination therapies.
The Patient
Background on Advanced Thyroid Cancer
Genetics/Molecular Biology of Advanced DTC
-
Translation into Therapeutics
Multikinase inhibitors
Selective kinase inhibitors (Ras, BRAF, MEK, PI-3K)
Combination therapies
Summary of therapeutic recommendations
The Patient
I. The Patient
A 50-year-old woman underwent a right neck surgical exploration after presenting to an otolaryngologist with a palpable lower right neck mass. After identification of an enlarged right thyroid lobe, a right lobectomy was performed. Pathology demonstrated papillary thyroid carcinoma (PTC; classical type), with gross extrathyroidal extension into skeletal muscle, lymphovascular invasion, and multiple positive resection margins. After a completion thyroidectomy, she received radioiodine (RAI) therapy with 150 mCi of 131-I; diagnostic and posttreatment whole body scans both demonstrated only right thyroid bed uptake, without evidence of pathological uptake outside the neck. A computed tomography (CT) scan of the neck 1 week after RAI treatment revealed no gross evidence of disease, and further adjuvant therapy was not administered except for TSH-suppressive levothyroxine therapy. Subsequent stimulated serum thyroglobulin level was elevated, 15 ng/mL, with undetectable antithyroglobulin antibodies. A positron emission tomography (PET)-CT scan demonstrated multiple lesions with fluorodeoxyglucose (FDG)-avid uptake in the neck, mediastinum, and lungs, most measuring at least 1 cm in diameter. CT scanning confirmed significant disease in multiple cervical and mediastinal paratracheal locations, but palliative resection or external beam radiotherapy was deemed to be of minimal potential benefit, given the simultaneous presence of FDG-avid pulmonary metastases.
With bulky FDG-avid disease that radiographically progressed in less than 1 year after RAI treatment, in locations that had not demonstrated RAI uptake on her original posttreatment scan, and with a negative diagnostic RAI scan, the patient was assessed as having progressive, RAI-refractory PTC (1, 2). Because there was no approved effective systemic chemotherapy regimen available for this diagnosis, clinical trial options were discussed with the patient. She deferred consideration of investigational therapy, and treatment with the oral, multi-targeted kinase inhibitor (MKI) sorafenib was offered, based upon 3 recently published phase II studies reporting clinical benefit in similar patients (3–5). After informed consent for chemotherapy, treatment was initiated with sorafenib 400 mg twice daily. Serial CT imaging documented minimal decrease in the diameters of target lesions in the lungs and neck after 2 and 4 months of therapy, with no evidence of new or enlarging lesions. The patient tolerated therapy, only necessitating a 25% dose reduction due to severe diarrhea and palmar erythrodysesthesia on the full dose, and antihypertensive medication was required to maintain her blood pressure in the normal range.
II. Background on Advanced Thyroid Cancer
Differentiated thyroid cancer (DTC) accounts for more than 90% of all thyroid cancers and includes the papillary, follicular, and poorly differentiated histological types. The incidence of the disease continues to rise rapidly worldwide, especially in women (6), long-term survival is excellent, and most patients die of other causes. Consensus guidelines recommend that most patients with clinically significant cancer undergo primary surgical therapy with a total thyroidectomy, and adjuvant radioiodine treatment with 131I is often indicated for patients at higher risk for disease recurrence or mortality (7, 8). Levothyroxine therapy is administered to provide replacement therapy for postsurgical hypothyroidism, with higher doses that suppress serum thyrotropin to eliminate stimulation to any remaining microscopic tumor cells in those patients at risk for recurrence. Once initial treatment is completed, periodic follow-up is performed to detect residual or recurrent disease, based primarily upon measurement of serum thyroglobulin levels as a biomarker and neck ultrasonography. Locoregional recurrence is generally treated with further surgery, RAI, and in some cases external beam radiation therapy. Complete biochemical remission has been reported in 25–75% of patients with recurrent disease in lymph nodes, but recurrences in the thyroid bed are often associated with a poorer prognosis (9). Complete biochemical remission is variably defined by the primary papers cited in this review article.
Distant metastases are observed in about 15% of DTC patients, with half being detectable at initial disease presentation. They are located in the lungs (50%), bones (25%), lungs and bones (20%), or at other sites (5%). RAI uptake can be demonstrated in many of these patients with distant metastases, but complete remission of disease is observed in only about one-third despite multiple treatments (10). RAI activities to administer for metastatic disease can be determined 3 ways (empiric fixed-dose approach, quantitative tumor 131I dosimetry, and blood 131I dosimetry), but data to suggest that long-term clinical outcomes are improved by any particular approach are lacking.
Various terms have been used to characterize DTC patients with local and/or metastatic disease for whom further RAI therapy is inappropriate, describing the disease as “resistant,” “refractory,” “nonresponsive,” or “nonavid.” The first 3 terms all imply that RAI therapy has not or is unlikely to yield a clinically meaningful benefit. The last term is not necessarily equivalent, in that it describes tumors as not absorbing a significant amount of RAI typically defined on a diagnostic or posttherapy scintigram. However, for a variety of biological reasons, tumors may retain their “avidity” for RAI and yet do not receive a sufficient radiation dose from therapy to yield clinically meaningful benefit, eg, if tumor clearance of RAI is too rapid and retention is inadequate, or the tumor is resistant to the effects of the radiation. Nonetheless, tumors that are nonavid are highly unlikely to benefit from RAI treatment. Finally, there are patients whose tumors may in fact respond well to further RAI therapy, but for whom the risks of further RAI treatment may outweigh the benefits; examples could include patients with radiation-induced pulmonary fibrosis or marrow suppression from previous RAI exposure. In routine clinical care, patients can be defined as RAI resistant if they meet any of the following criteria: 1) RAI scans in the setting of gross or measurable disease reveal no significant RAI uptake in tumor; or 2) tumor that appears or progresses radiographically within a defined time frame after most recent RAI therapy, such as 1 year, regardless of the extent of RAI uptake in the tumor. Additional criteria that may be supportive or predictive of these definitions include previous cumulative administration of at least 600 mCi of 131I in treatment of disease (10), and the presence of high FDG uptake in tumors on PET imaging.
In the setting of metastatic disease, localized interventions, such as surgery, external beam radiotherapy, or laser photocoagulation can provide palliative relief. Bone metastases are often particularly difficult to treat, leading to significant morbidity and associated mortality (11). As with other malignancies, bisphosphonates and denosumab may reduce the frequency of skeletal-related events such as fracture and improve pain control from bone metastases through osteoclast inhibition (12, 13). There are, however, no large randomized trials showing clear evidence of reduced skeletal-related events with bisphosphonates or denosumab in patients with advanced thyroid cancer. Other palliative treatments that can benefit selected patients include percutaneous vertebroplasty, surgical excision (14, 15), transarterial embolization, and various catheter ablation techniques. As with other patients with advanced or metastatic malignancies, pain control, nutritional support, and end-of-life supportive measures can improve patient comfort even in the absence of specific anticancer intervention.
For those patients with metastatic DTC whose disease progresses despite these standard therapies, systemic chemotherapy has traditionally been an option of limited benefit and considerable morbidity (16). Initial reports described a possible role of monotherapy with doxorubicin, an inhibitor of DNA and RNA synthesis, for metastatic thyroid cancer (17). In 1 study, partial responses to doxorubicin were seen in 7 of 19 (37%) patients, and stable disease was reported in another 6. In a more recent report of patients with documented progressive disease prior to chemotherapy, partial response after 6 months of doxorubicin treatment was seen in only 5% of patients, and stable disease of between 1 and 22 months duration was described in another 42% (18). Overall, best responses occurred in patients with pulmonary metastases and high performance status, with less response noted in bone or nodal metastases. Common adverse events (AEs) included cardiomyopathy, granulocytopenia with accompanying infections, nausea, vomiting, infertility, and alopecia. Based on such limited studies demonstrating minimal benefit, doxorubicin has been the sole chemotherapy approved in the United States for treatment of metastatic thyroid carcinoma. Many other single cytotoxic chemotherapeutic agents have been described in case reports, small series, or occasional prospective trials, but without a suggestion that benefit outweighs toxicity. Combination regimens have failed similarly.
Given the generally poor outcomes associated with cytotoxic chemotherapy, considerable interest has evolved in applying discoveries about the pathophysiological basis of advanced thyroid cancer to development of antineoplastic therapies (19). Of prime importance has been the identification of specific oncogenic mutations that appear to be early genetic events in DTC and understanding the role of intercellular signaling between the tumor cell and the surrounding tumor microenvironment, especially the tumor vasculature. This review will focus on the underlying biological basis for today's novel therapies for advanced or metastatic DTC, the results of preclinical investigations and clinical trials, and potential future approaches to drug development.
III. Genetics/Molecular Biology of Advanced DTC
Our understanding of basic genetics and molecular biology of follicular cell-derived thyroid cancer (Figure 1) has changed dramatically over the past 20 years since a molecular model for thyroid cancer progression was first proposed (20). A major oncogenic driver of PTC is the MAPK pathway, with RET rearrangements accounting for 10–15%, Ras point mutations for 10–15%, and BRAF point mutations comprising the majority of 40–60% of all PTC > 1 cm and 60–75% of those with known mutations. Furthermore, BRAF mutations are associated with more aggressive disease and down-regulation of the sodium-iodide symporter, which may account for loss of RAI avidity in some tumors (21, 22). This mutation, however, cannot alone account for aggressive behavior in PTC because the proportion of PTC with BRAF mutations is much higher than those with high-risk features (gross invasion, significant structural recurrence, distant metastatic disease, disease-specific mortality). Follicular thyroid cancer has a somewhat different molecular etiology, with 10–15% harboring Ras point mutations and 30% being driven by the unique Pax8-peroxisome proliferator-activated receptor (PPAR)-γ genetic rearrangement. Finally, DTC can be associated with less common genetic syndromes by loss of tumor suppressor function including Cowden's syndrome (PTEN), Carney complex (PRKAR1A), and familial adenomatous polyposis (APC).
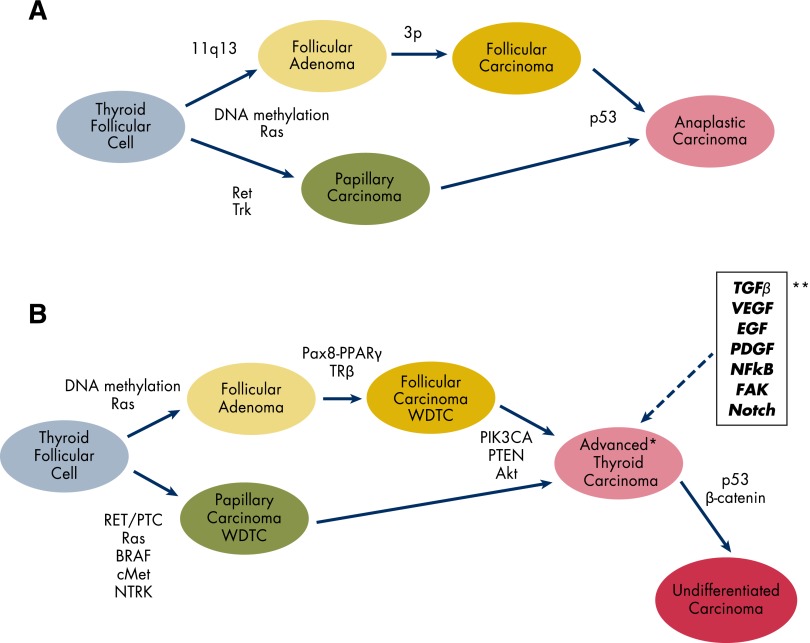
Figure 1.
Molecular biology of thyroid neoplasia development and progression. A, Adapted from J. A. Fagin: Genetic basis of endocrine disease 3: molecular defects in thyroid gland neoplasia. J Clin Endocrinol Metab. 1992;75:1398–1400 (20), with permission. © The Endocrine Society. B, *, Advanced thyroid carcinoma (poorly differentiated, grossly invasive, metastatic, RAI-resistant, PET-positive); **, activated signaling pathways that combine with genetic alterations to induce advanced thyroid carcinoma. 11q13, long arm of chromosome 11; 3p, short arm of chromosome 3; TRβ, thyroid hormone receptor β; cMET, proto-oncogene that encodes hepatocyte growth factor receptor; Trk, thropomysin receptor kinase; NTRK, Trk family genes.
Histopathologically, poorly differentiated thyroid cancer (PDTC) is characterized by an infiltrative pattern of growth, necrosis, a high mitotic index, and vascular invasion (23). Advanced DTC is defined more by behavior than specific histopathology. These tumors are generally RAI-resistant, FDG-PET- positive, locally invasive, and can form distant metastases. Although anaplastic thyroid cancer (ATC) and PDTC have higher mortality rates than DTC, most of the estimated 1780 deaths from thyroid cancer predicted for 2013 will be in patients with DTC (24). Rivera et al (25) examined metastases from patients with RAI resistant, PET-positive disease. They showed that 80% of these tumors had an aggressive histology (PDTC, tall cell variant, hepatocellular carcinoma, ATC), whereas only 20% were PTC. Interestingly, comparative analysis of primary tumors and distant metastases from 10 patients with primary well-differentiated thyroid cancer (WDTC) showed that the metastases had progressed to a more poorly differentiated histopathology in 70% of the patients. Activation of the MAPK pathway is commonly found in WDTC, PDTC, advanced, and ATC. Although this may be an attractive target for therapy, it does not appear to explain the differences between WDTC and advanced or PDTC.
One signaling pathway that appears to be genetically altered in advanced thyroid cancer is the phosphatidylinositol-3 kinase (PI-3K) pathway. PI-3K catalytic subunit (PIK3CA) mutations were found to be more common in ATC compared with WDTC, and this pathway was activated (pAkt) in the aggressive tumors (26). Furthermore, 35% of the tumors with PIK3CA mutations also had BRAF mutations, and 30% of these tumors had both PIK3CA and Ras mutations. Other groups have confirmed these observations and further shown that PIK3CA gene amplifications and inactivating mutations of the tumor suppressor PTEN were more common in the advanced cancers, suggesting that activation of the PI-3K pathway is a late event in thyroid cancer progression (27, 28). Ricarte-Filho et al (29) performed an extensive mutational analysis, using a mass spectrometry genotyping (Sequenom, San Diego, California) approach to characterize 111 mutations in patients with advanced primary thyroid cancer and RAI resistant distant metastases. They were the first group to identify Akt1 mutations in thyroid cancer and showed that PIK3CA and Akt1 mutations were discordant in different distant metastases from the same patient, suggesting that these mutations were a late event in the development of advanced thyroid cancer.
β-Catenin, a protein central to the Wnt signaling pathway in development, is also believed to be involved in aspects of Ras, MAPK, and PPAR signaling (30). Mutations in CTNNB1 (β-catenin gene) were identified in 19 of 31 (61%) ATC tumors (31). Many of the mutations involved phosphorylation sites required for protein degradation, and interestingly, multiple different mutations were found in some of the individual ATC tumors and mutations were not seen in matching DTC where examined. A subsequent study did not find β-catenin mutations in any of the 17 PDTC tumors examined (32). Kurihara et al (33) found abnormalities in the Wnt/β-catenin signaling pathway in a majority of 22 ATC tumor studied, with a mutation in the tumor suppressor protein, Axin 1, identified in 82% of tumors. Only one of the tumors had a CTNNB1 mutation. These data would indicate that the β-catenin pathway is activated in advanced thyroid cancer, and CTNNB1 mutations are late genetic events in the progression of thyroid cancer.
A unique feature of advanced thyroid cancer is inactivation of the p53 tumor suppressor through gene mutation. Although this mutation is rare in WDTC, it is more common in advanced and PDTC and is most commonly found in ATC (34, 35). Although β-catenin and p53 mutations are likely factors in the transition from differentiated to undifferentiated thyroid cancer, these advanced cancers likely contain multiple other yet unrecognized mutations.
The leading theory to explain differences between WDTC, advanced DTC, PDTC, and ATC is the progressive “multiple hit” theory (36). DTC is initiated by a mutation or rearrangement in the MAPK or Pax8-PPAR signaling pathways, and additional mutations (PI-3K/PTEN, Akt, p53), gene amplifications (EGFR, VEGFR), or epigenetic silencing leads to more aggressive disease. This theory is supported by comparative tissue studies (27–29, 37, 38) as well as transgenic animal models of thyroid cancer (39). A different theory, the fetal carcinogenesis model, has recently been proposed (40). In this model, WDTC arises from prothyrocytes and thyroblasts, whereas ATC forms in the earliest precursor thyroid stem cells. Persistence of these pools of tumor-initiating cells leads to cancers with higher recurrence rates and worse prognoses. Todaro et al (41) recently showed that a putative thyroid stem cell, expressing high levels of aldehyde dehydrogenase, had high levels of phosphorylated Akt and cMet, suggesting activation of these pathways, and was more commonly found in ATC than PTC or follicular thyroid cancer. Although this theory is less developed than the multiple hit theory, it may explain why some WDTC are more aggressive than others despite similar histopathological features. This stem cell hypothesis has been well-described for some germ cell tumors and leukemias, but controversies over validation of stem cell makers, the roles of quiescence and drug resistance, as well as tumor heterogeneity continue the debate over this hypothesis in solid tumors (42).
To better understand the molecular biology leading to advanced thyroid cancer, investigators have performed direct comparative protein and gene expression studies between WDTC and advanced thyroid cancer arising in the same patient. A comparative tissue microarray from 12 patients with tumors that contained both DTC and ATC showed that the ATC component had significantly higher protein expression of VEGF and β-catenin, suggesting that these pathways may be important in the formation or maintenance of advanced thyroid cancer and they may be good therapeutic targets (38). This group also found p53 levels much higher in the ATC portion of the tumors, supporting previous studies showing that p53 mutations were more common in advanced thyroid cancer. Vasko et al (43) studied widely invasive PTC from 7 patients, comparing the invasive front with the center of the tumor using gene array analysis. They showed that the invasive front had undergone epithelial to mesenchymal transition (EMT), which is believed to be necessary for tumors to invade and metastasize. The gene expression analysis showed over-representation of TGFβ, nuclear factor κB (NFkB), integrin, notch, and PI-3K pathways on the invasive front, indicating that TGFβ inhibitors, NFkB inhibitors, focal adhesion kinase (FAK) and p21-activated kinase inhibitors (integrin signaling), γ-secretase inhibitors (Notch), and PI-3K inhibitors would be promising targets in advanced thyroid cancer therapy. Finally, Knauf et al (44) performed an elegant study in a mouse model of BRAF-induced thyroid cancer in which they used comparative microarray analysis on WDTC and PDTC arising in the same mice. Similar to the Vasko group, they showed that PDTC had changes consistent with EMT as well as changes in the NFkB-IL-8 immune response pathways. They further showed that tumor initiation by mutant BRAF rendered the cells susceptible to TGFβ-induced EMT, and both signaling pathways were required for this transition to a more advanced thyroid cancer phenotype, and that TGFβ was produced by the cancer cells in an autocrine fashion as well as tumor-associated macrophages through a paracrine pathway, highlighting the importance of the tumor microenvironment. These observations are supported by other studies comparing advanced thyroid cancer with WDTC from different patients that showed the TGFβ, MAPK, and FAK pathways were enriched in the advanced thyroid cancers (45). Riesco-Eizaguirre et al (21) have provided some mechanistic insights into the synergistic effects of BRAF and TGFβ signaling in thyroid cancer. They showed that expression of BRAFV600E in the nonmalignant PCCL3 rat thyroid cells induced TGFβ and decreased sodium iodide symporter expression and iodine uptake (21). Although others have shown that BRAFV600E inhibits iodine uptake, this group went on to show that this effect is dependent on TGFβ signaling because it could be blocked by a TGFβ antibody or a TGF receptor small molecule inhibitor. They further showed using human tissue immunohistochemistry that TGFβ levels were higher on the invasive front of WDTC and in tumor-involved lymph node with extranodal invasion.
Consistent with this observation of genetic and signaling pathway synergy in advanced cancer, Prahallad et al (46) used the observation that some cancers with the BRAFV600E mutation were sensitive to the selective inhibitor vemurafenib (melanoma), whereas other cancers (colon, thyroid) were resistant. They performed a synthetic lethal screen with a “kinome” library to look for synergistic pathways responsible for this resistance (46). They found that the epidermal growth factor receptor (EGFR) pathway was activated in the resistant cell lines and that this pathway was further activated by treatment with vemurafenib. A combination of vemurafenib and an EGFR inhibitor synergistically reduced tumor growth in vivo, suggesting that MAPK in combination with other specific pathway inhibitors would be rational directed treatment in some patients with advanced thyroid cancer. Using a completely different approach, Dar et al (47) used kinase-focused chemistry, kinome-wide profiling, and Drosophila genetics to show that targeting pathway synergy with rational polypharmacology will likely be important in advanced cancer therapy. Montero-Conde and colleagues recently showed that BRAFV600E mutant thyroid cancer cells were resistant to vemurafenib through upregulation of the HER3 signaling pathway. Treatment of these cells with the HER kinase inhibitor sensitized these cells to vemurafenib (111).
Based on these studies, a pattern is emerging that transition from WDTC to advanced thyroid cancer is likely a combination of genetic (mutations, gene amplification, gene rearrangements) and autocrine or paracrine (microenvironment—stromal cells, immune cells, vascular cells) signaling events. Although these advanced thyroid cancers clearly are a heterogeneous group of tumors, a fundamental understanding of genetic and signaling events can help guide tailored combination therapies to hopefully cure or control these aggressive tumors.
IV. Translation into Therapeutics
A. Multikinase inhibitors
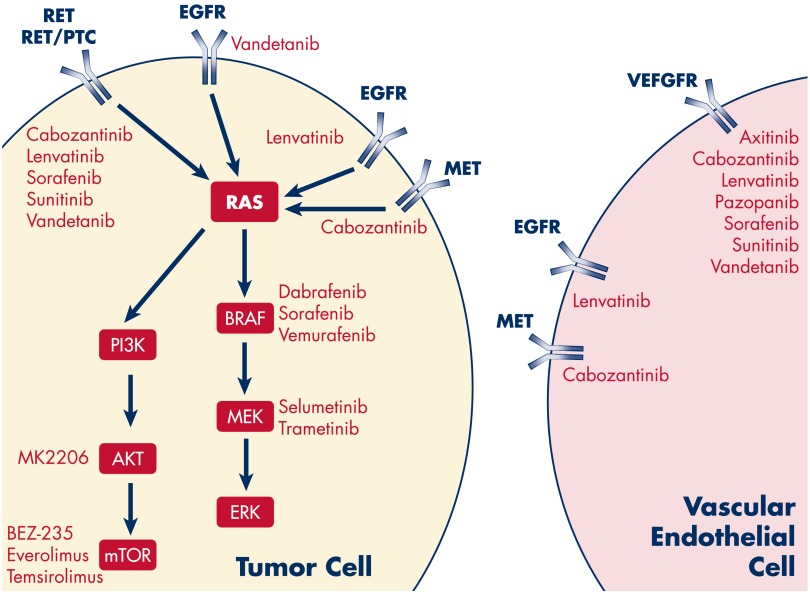
Two major advances over the past 5–10 years have provided new insights into systemic treatment of patients with advanced thyroid cancer: 1) an understanding of the genetics and molecular biology of thyroid cancer; and 2) cooperative, multicenter clinical trials. Aberrant kinase signaling through genetic alterations (mutations, rearrangements, gene amplification) and/or through increased paracrine or autocrine kinase signaling is a key driver of most advanced thyroid cancers (Figure 1B). Kinase inhibitors are therefore excellent therapeutic candidates for patients with advanced thyroid cancer. Figure 2 shows targets of many kinase inhibitors that are currently under clinical investigation or in clinical use. Current published clinical trials have focused on MKIs that target many of these signaling pathways (Table 1).
Figure 2.
Inhibitors of kinase signaling pathways in tumor cells and vascular endothelial cells.
Table 1.
Phase II Trials of Multikinase Inhibitors in Advanced DTC
| Drug | Targets | First Author, Year (Ref.) | n | RECIST Progressivea | PFS, mo | Discontinued drug (AE)b |
|---|---|---|---|---|---|---|
| Gefitinib | EGFR | Pennell, 2008 (112) | 17 | No | 3.9 | 7% |
| Axitinib | VEGFR, PDGFR, c-Kit | Cohen, 2008 (56) | 60 | No | 18.1 | 13% |
| Sorafenib | BRAF, VEGFR, PDGFR, RET | Gupta, 2008 (3) | 30 | Noc | 18.4 | 17% |
| Kloos, 2009 (4) | 41 | No | 15.0 | 26% | ||
| Hoftijzer, 2009 (5) | 31 | Yes | 13.4 | 19% | ||
| Ahmed, 2011 (49) | 34 | No | >19.0 | 6% | ||
| Motesanib | VEGFR, PDGFR, c-Kit | Sherman, 2008 (48) | 93 | Yes | 9.3 | 13% |
| Sunitinib | PDGFR, FLT3, c-Kit, VEGFR | Carr, 2010 (113) | 28 | No | 12.8 | 11% |
| Pazopanib | VEGFR, PDGFR, c-Kit | Bible, 2010 (52) | 37 | Yes | 11.4 | 5% |
Required RECIST progressive disease for enrollment.
Percentage of patients discontinuing drug due to AEs.
All had progressive disease.
The first MKI agent to be systematically evaluated in DTC was motesanib, which was observed to induce tumor shrinkage in several DTC patients treated in a phase I trial. A multicenter, open-label phase II trial was subsequently performed, testing the efficacy of motesanib in 93 patients with radiographically progressive DTC (48). Partial response (determined by the standard Response Evaluation Criteria in Solid Tumors [RECIST]) was confirmed by subsequent imaging and independent radiological review in 14% of the DTC patients, and another 35% of these previously progressive disease patients maintained stable disease for at least 24 weeks on therapy. Given the historical lack of sufficient patient recruitment to multicenter clinical trials to permit adequate assessment of chemotherapies, this trial represented a “proof of concept,” demonstrating the feasibility of successful pursuit of chemotherapy clinical trials for DTC as well as the potential for disease control with antiangiogenic drugs.
Another of these MKIs, sorafenib, has activity against RET and BRAF, both key molecules in the MAPK pathway that is commonly activated in PTC (3–5, 49). A total of 136 patients with advanced DTC were enrolled in these 4 studies with a starting dose of 400 mg oral sorafenib twice daily. Progression-free survival (PFS) ranged from 15 to more than 19 months, compared with historical PFS of 6–9 months (50). There were no complete responses, and 40% of patients had a partial response to sorafenib therapy. Two major limitations of these studies were lack of a direct comparator control group and inclusion of patients with stable disease. Furthermore, many patients experienced AEs from the drug (diarrhea, skin rash, hypertension, fatigue, plantar-palmar erythrodysesthesia) requiring dose reduction in >50% of the patients, and >10% of patients discontinued the drug due to AEs. A phase III, randomized, placebo-controlled, multicenter trial has completed enrollment of 380 patients with advanced DTC and will likely overcome the limitations of the phase II trials to assess the ability of sorafenib to significantly increase PFS (51). Results of this trial should be available within the next 6 months. Most of the MKIs studied in advanced thyroid cancer do not specifically target the MAPK pathway (Table 1). In fact, all except for gefitinib inhibit the VEGFR and platelet-derived growth factor receptor (PDGFR) pathways, which are thought to be important autocrine and paracrine pathways leading to advanced thyroid cancer (Figure 1B). Three of the studies evaluating the efficacy of sorafenib, motesanib, and pazopanib included only patients with RECIST progressive disease (5, 48, 52). PFS ranged from 9.3 to 13.4 months, and no complete responses were observed. Partial responses to therapy with sorafenib, motesanib, and pazopanib were 25, 14, and 49%, respectively. A clinically meaningful response (partial response and stable disease) was observed in 59, 81, and 96% of these patients. Many patients ultimately had progressive disease, indicating that these targeted agents can stabilize, but not eradicate disease in patients with advanced thyroid cancer. It is still unclear whether the primary target of these MKIs is the epithelial cells of the cancer or the supporting microenvironment (stromal cells, vascular endothelium, etc.). VEGFR and PDGFR, among other important receptors, are expressed on many of these cell types. The effects are likely at many cell types in these complex tumors, and it will be important to better understand this complex microenvironment if we hope to eradicate or chronically control these advanced cancers. Vandetanib, which is approved for treatment of progressive medullary thyroid carcinoma, is an example of a kinase inhibitor with multiple targets, each of which may contribute to its efficacy. Targeting VEGFR, Ret, and EGFR, vandetanib was evaluated in a randomized, placebo-controlled phase II trial in patients with locally advanced or metastatic DTC (53). In the 72 vandetanib-treated patients, PFS was 11.1 months, compared with only 5.9 months in the 73 placebo-treated patients (hazard ratio, 0.63), although objective responses were rarely seen.
A few novel MKIs have completed phase II clinical trials and have been presented in abstract form but are not yet published in peer-reviewed format. Lenvatinib (E7080), an oral kinase inhibitor targeting VEGFR, PDGFR, fibroblast growth factor receptor, Ret, and Kit, was given once daily (24 mg starting dose, given to 58 patients with advanced, RECIST-progressive DTC) (54). Fifty percent of patients had confirmed partial response, whereas 35% of patients required dose reduction due to toxicity and 23% of patients were withdrawn from therapy due to toxicity (proteinuria was a common AE not seen as frequently with other MKI). Cabozantinib (XL184), an oral MKI that targets c-MET (the receptor for hepatocyte growth factor, an important mediator of both tumor proliferation and angiogenesis), VEGFR, and Ret, was given once daily (starting dose, 140 mg daily) to 14 patients with advanced, progressive DTC (55). Confirmed partial response was observed in 8 (53%), including 5 of 8 patients previously treated with 1 other MKI.
To develop a more personalized approach to targeted therapy in patients with advanced thyroid cancer, some of these studies have assessed genetic alterations in a subset of the tumors. Axitinib significantly altered serum levels of VEGF and soluble VEGFR, while having little effect on c-Kit levels (56). There were not, however, enough events of progressive disease to determine whether VEGF and soluble VEGFR are good peripheral biomarkers of axitinib efficacy. A genotype analysis of tumors in the motesanib study showed that 30% of tumors had a BRAFV600E mutation, 18% had one of the Ras mutations, and 52% of tumors had no identified mutation of gene rearrangement (48). The investigators went on to show that 60% of patients with a BRAFV600E tumor had a clinically meaningful (partial response or stable disease) response to motesanib treatment, whereas 33% of patients with tumors that were BRAF wild-type responded to this therapy. The number of patients in each group was small, and the observed differences in response were not significant. In the phase II lenvatinib trial, partial responses were more common, and prolonged PFS was observed in patients whose tumors contained RAS mutations (54). Combining RAS and BRAF mutation status with baseline VEGF and ANG-2 or treatment-associated changes in FGF-2 and IL-10 levels provided superior prediction of lenvatinib treatment response (57).
Kloos et al (4) examined pharmacodynamic responses (activated MAPK, VEGF, and Akt pathways) as well as imaging responses (tumor perfusion by dynamic contrasted-enhanced magnetic resonance imaging and FDG-PET imaging) in 10–14 patients receiving sorafenib therapy. They observed variable responses of decreased pathway signaling, tumor perfusion, and glucose uptake to sorafenib treatment, but no correlation with subsequent RECIST response to this therapy or BRAF status. Significant effects may have again been masked by small sample size. Bible et al (52) performed an exploratory analysis of a series of plasma biomarkers (VEGF, platelet-derived growth factor, hepatocyte growth factor, E-selectin, IL-6, and IL-8) as response to 1 cycle of pazopanib therapy in 24 patients. Baseline or change in plasma levels of these biomarkers did not correlate with tumor response or need for dose reduction in these patients.
Although it is disappointing that no specific biomarkers have yet been identified to predict which patients with advanced thyroid cancer will respond to a specific targeted therapy, this personalized approach will likely yield fruitful biomarkers in larger future studies.
B. Selective kinase inhibitors (Ras, BRAF, MEK, PI-3K)
In addition to inhibition of receptor tyrosine kinases, selective inhibition of the dysregulated kinases of the MAPK signaling pathway is a particularly attractive therapeutic strategy for differentiated thyroid carcinomas. In addition to the upstream activation of signaling by mutant RET/PTC seen in some PTC, mutations of K-RAS, N-RAS, or H-RAS genes are seen in 2, 7, and 4% of thyroid cancers, respectively, and activating mutations in BRAF are identified in up to 40% of cases (58). Downstream activation of MAPK kinase (MEK) is a common mediator of mutant RET/PTC, RAS, or BRAF kinases, leading to the numerous stimulated effects of MAPK signaling, including cell proliferation, survival, differentiation, motility, and angiogenesis, and thus selective therapeutic targeting of either the specific mutated upstream oncogenic kinase or MEK itself is being extensively explored.
1. RAS inhibitors
Given the central role of activated RAS superfamily proteins in many cancers, targeted inhibition has been a goal of many drug development programs. Efforts to identify selective inhibitors of RAS kinases, however, have been unsuccessful, in large part due to the difficulty in overcoming the very high affinity for binding of GTP (59). A more indirect approach, targeting the posttranslational modifications that are required for successful RAS function, yielded agents that were tested in clinical trials, but results remained disappointing. The covalent attachment of the farnesyl isoprenoid group to RAS proteins by farnesyltransferase (FT), which results in the stable localization of the protein at the inner surface of the cell membrane, was an obvious and early target for the design of rational therapies against the RAS pathway. Despite preclinical studies indicating that FT inhibitors can lead to reductions in tumor cell proliferation, blockade of cell cycle in G1 arrest, induction of apoptosis, and inhibition of hypoxia-induced VEGFA production, monotherapy with FT inhibitors in more than 60 clinical trials produced few objective responses (60). One major criticism of these studies was the relatively high frequency of patients whose tumors had K-RAS mutations, given that blockade of FT leads to alternative modification of K-RAS protein by geranylgeranyl transferase 1 (61). This outcome of the “law of unintended consequences,” in which inhibition of a rational target leads to unexpected up-regulation of pathways that effectively undo the initial inhibition, has been recreated multiple times during the subsequent development of molecularly targeted therapies, and highlights the need for a comprehensive strategy to combinatorial therapeutics.
The earliest suggestion that FT inhibition might have a role in the treatment of thyroid cancer came from studies of cell lines and tumor xenografts in which treatment with the prototypic FT inhibitor manumycin combined with paclitaxel enhanced apoptosis (62). Further studies suggested that this effect might be mediated by multiple FT substrates, not just RAS specifically (63). Other studies that also showed synergism between FT inhibitors and taxanes found evidence of enhanced accumulation of microtubule-associated taxane, mediated through RAS-independent FT inhibition of microtubule-associated histone deacetylase 6 (64). Nonetheless, the theoretical possibility that an FT inhibitor that might affect RAS signaling could be synergistic with a MKI that affects BRAF led to a phase I trial of tipifarnib combined with sorafenib (65). Twenty-two patients with progressive metastatic DTC (pretherapy median time to progression, 2.6 months) were treated as part of a larger phase I study, using a standard 3+3 drug dose escalation schema. Only 1 DTC patient experienced a partial response (partial response rate, 4.5%), and another 8 (36%) had stable disease lasting 6 months or longer. Median time to failure was 9 months, and median PFS was 20 months. Although two-thirds of tested tumors carried BRAFV600E mutations, there was no obvious relationship between mutation status and tumor response; RAS mutations were not found in 2 tumors from patients with follicular carcinoma who had samples available. The most common toxicities included rash, fatigue, diarrhea, and elevations in serum amylase and lipase. These results suggest that FT inhibition may yet have a clinical role in treating thyroid cancer, but more extensive studies of the effect of these drugs (and geranylgeranyl transferase inhibitors) on the entire prenylated proteome must be explored to understand the underlying mechanism of action far beyond RAS signaling.
2. BRAF inhibitors
With recognition of the marked frequency of BRAF mutations in malignancies such as PTC and melanoma, evidence that targeting these mutant BRAF kinases can effectively treat these cancers has mounted. Using 2 selective inhibitors of BRAF, it was shown that both drugs blocked RAS- and RAF-dependent MEK phosphorylation in cell lines bearing mutations in either BRAF or RET/PTC (66). Similarly, treatment of BRAF-mutant PTC cells with anti-RAF small interfering RNA suppressed tumor cell growth by depletion of BRAF protein (67).
The various small molecule inhibitors of activated BRAF serine/threonine kinase that have been developed are categorized on the basis of their mechanism of action (68). Type I inhibitors target a highly conserved motif containing a catalytic aspartate residue in the so-called “DFG-in” conformation, leading to interactions with the kinase hinge region and ATP binding site of the activated form of the kinase. Type II inhibitors bind to the ATP binding site itself and an adjacent hydrophobic pocket created by the activation loop. Type II inhibitors were the first compounds introduced into the clinic for cancer therapy, but type I inhibitors may provide the necessary specificity to target successfully mutant BRAF kinases.
3. Type II inhibitors: sorafenib, regorafenib, XL281
The initial drug proposed as a type II RAF inhibitor was sorafenib, previously discussed as a MKI, which had activity against both BRAF and V-raf-1 murine leukemia viral oncogene homolog 1 (C-RAF). However, further studies suggested that its effects might not be mediated through BRAF inhibition, given that the potency of sorafenib to block signaling through mutant BRAF was abrogated by gatekeeper mutations but without affecting ability to inhibit tumor growth (69). Clinically, the failure of sorafenib to result in significant objective responses in BRAF-mutant melanoma has been interpreted as consistent with the non-BRAF mediated mechanism of action of the drug (70). Whether the drug's activity in DTC is mediated primarily through its antiangiogenic targets or through BRAF remains to be determined, but by analogy to other tumors, there has been little to suspect that it works as an effective BRAF inhibitor in thyroid cancer. The related drug regorafenib, a fluorinated analog of sorafenib that can inhibit both wild-type and mutant BRAFV600E with similar potency (IC50, 20–30 nm), was studied in a phase I trial of continuous daily dosing that has been preliminarily reported (71). Of 38 patients with progressive solid malignancies, 5 had thyroid cancer, but none of them demonstrated objective response. Further trials in other tumor types have not shown enhanced benefit in BRAF mutant tumors compared with wild-type (72).
XL281 (BMS-908662) is an oral small molecule that inhibits both wild-type and mutant BRAF kinases (IC50, 5 and 6 nm, respectively) as well as C-RAF (IC50, 3 nm). The drug has been evaluated in a phase I trial that recruited 30 patients with progressive solid tumors during the dose escalation portion of the study (73). Preliminary data from that phase I study described stable disease in 5 PTC patients; of the 2 patients whose tumors were documented to contain BRAF mutations, both remained stable after more than 1 year of therapy, as did a third PTC patient whose mutation status was unknown. An additional 2 patients with Hurthle cell carcinomas also were treated with prolonged stable disease, but 1 patient with anaplastic carcinoma progressed despite treatment. No partial response was reported in any of the thyroid cancer patients. An expansion cohort in PTC was initiated, but results have not yet been reported. The most common side effect reported among all 48 solid tumor patients in the trial was fatigue in nearly half of patients, and other common toxicities included nausea, diarrhea, and vomiting, all of which were occasionally severe. Four patients were also described as having developed either cutaneous squamous cell carcinomas or premalignant keratoacanthomas.
4. Type I inhibitors: vemurafenib, dabrafenib
Type I inhibitors with preferential binding to the kinase domain of BRAF in the active conformation demonstrate greater inhibitory potency against the BRAFV600E mutant kinase than the wild-type (68). In thyroid cancer cells, where C-RAF and RAF-1 expression is thought to be minimal, selective targeting of the mutant BRAF most associated with aggressive disease is a highly attractive therapeutic option.
a. Vemurafenib (PLX4032; RG7204).
Vemurafenib is a potent kinase inhibitor of BRAFV600E with IC50 of 31 nm and C-RAF with IC50 of 48 nm; potency against wild-type BRAF kinase is 3-fold less (74). Along with its sister compound PLX4720, vemurafenib was identified through a crystallography-guided scaffold-based drug design approach optimized for binding to the mutant kinase (75). In preclinical models of melanoma, vemurafenib inhibited proliferation and ERK phosphorylation in cell lines bearing activating mutations in codon 600 in a dose-dependent manner, but no inhibition was noted in wild-type cell lines (76). Similarly, vemurafenib treatment produced partial and complete remissions of BRAFV600E mutated tumor xenografts but not of wild-type tumors.
In a first-in-human phase I study of vemurafenib, 81% of patients had a significant tumor reduction, with a confirmed response rate of 56% among patients with metastatic melanoma harboring BRAF mutation who received treatment in a dose-extension cohort at a dose of 960 mg twice daily (77). The median PFS duration was at least 7 months. This clinical efficacy among melanoma patients with the relevant mutated BRAF kinase drastically contrasts with a complete absence of clinical response among those lacking the BRAFV600E mutation (77). These results underscore the importance of selecting patients whose tumors bear the appropriate molecular target for the success of molecularly targeted drugs. A recently reported randomized phase III trial demonstrated improved overall survival, PFS, and response rate after treatment with vemurafenib for patients with previously untreated BRAFV600E-mutant melanoma, compared with dacarbazine, and has led to the drug's recent approval (78). Common toxicities of vemurafenib therapy include noninflammatory arthralgias, skin rash, fatigue, alopecia, photosensitivity, nausea, and diarrhea. In addition, keratoacanthomas and squamous cell carcinomas are seen frequently, and acquired RAS mutations in these non-BRAF mutated keratinocytes are being recognized as a contributing mechanism (79, 80).
Preclinical studies in PTC have mimicked the experience with melanoma. Both vemurafenib and PLX4720 were shown to block cellular proliferation of multiple cell lines demonstrated to contain the BRAFV600E mutation, but an EC50 was not reached for cell lines containing mutations in RET/PTC or RAS (74, 81). MEK and ERK phosphorylation was also inhibited in a dose-dependent fashion in BRAF mutant cells. Notably, however, neither proliferation nor downstream kinase phosphorylation could be completely inhibited despite maximum drug concentrations, and feedback down-regulation of ERK phosphatases was demonstrated as a potential mechanism (81). In addition, MEK and ERK phosphorylation was unexpectedly increased in cell lines containing upstream mutations in RET/PTC or RAS, likely due to paradoxical transactivation of dimerized RAF kinases (82). Cell cycle arrest at the G1-S transition was noted in 1 BRAF mutant cell line, without evidence of cytotoxicity of treatment. In a xenograft model, BRAF mutant tumor growth was slowed but not completely blocked by vemurafenib, and MEK and ERK phosphorylation was reduced 2- to 4-fold (81). A subsequent study of BRAF mutant orthotopic xenografts treated for 4 weeks with PLX4720 demonstrated 97% less tumor volume compared with controls (83). Furthermore, the tumors treated with PLX4720 were markedly less invasive, produced 99% fewer microscopic lung metastases, and contained increased nuclear localization of thyroid-specific transcription factors, but no differences were seen in differentiation markers such as the sodium-iodine symporter, thyroglobulin, or the TSH receptor (83).
In the dose-escalation phase of the vemurafenib phase I trial, 3 patients with metastatic PTC harboring BRAFV600E mutation were included (K. B. Kim, submitted for publication). Among the 3 patients, 1 had a confirmed partial response with reduction of pulmonary target lesions by 31%, and the duration of response was 7.6 months before the disease progressed in the lungs and the bones. The time to progression was 11.7 months. The other 2 patients had stable disease, and the time to progression was 13.2 and 11.4 months, respectively. Two of the patients eventually died of their disease, 1 of whom had developed anaplastic transformation about 1 year after discontinuing vemurafenib. On the basis of these results, a phase II trial of vemurafenib has recently been initiated in patients with progressive metastases from BRAFV600E mutant PTC.
Among melanoma patients, acquired resistance to vemurafenib therapy has been observed, associated with a variety of proposed mechanisms other than secondary BRAF mutations. In cell lines, activation of RAS is often seen, due either to RAS mutations themselves or increased signaling from cell surface receptors such as c-MET or SRC (84, 85). Enhanced signaling through C-RAF or RAF-1 has also been reported (86). Failure of patients with BRAFV600E mutant colon carcinoma to respond to therapy with vemurafenib has also been reported, with frequent upstream activation of RAS signaling hypothesized as the mechanism (46). As BRAF inhibitor therapy evolves for DTC, it is likely that similar mechanisms of resistance will emerge, suggesting that rational combination regimens will probably be necessary (87). Vemurafenib (Zelboraf; Genentech, South San Francisco, California) has recently been approved by the US Food and Drug Administration for treatment of melanoma and is now available for clinical use. Patients treated with vemurafenib in a phase III trial had a 24% incidence of cutaneous squamous cell carcinoma, likely through a Ras-mediated mechanism, so careful dermatological monitoring is suggested for patients receiving this drug.
b. Dabrafenib (GSK2118436).
One of a series of drugs developed to bind to the kinase hinge region and ATP binding site, dabrafenib inhibits several of the codon 600 variants of BRAF, including V600E (IC50, 0.5 nm), V600K (0.6 nm), and V600D (1.9 nm) (88). In a first-in-human dose escalation phase I trial, 36 patients with BRAF-mutant melanoma were treated with dabrafenib at the recommended phase 2 dose of 150 mg twice daily. Partial response was recorded in 25 patients (69%), including those with V600K and V600G mutations, and significant tumor reductions were seen in 90% of patients with intracerebral metastases. Of the 9 patients with BRAF-mutant PTC included in the trial, 3 (33%) achieved partial response (89).
5. MEK inhibitors
Because MEK is the common downstream effector of activated signaling from either RAS or RAF, it has been an attractive potential therapeutic target for many solid tumors dependent upon the MAPK pathway, particularly those with BRAF mutations. A series of drugs emerged with potential clinical application that noncompetitively inhibit ATP binding by both MEK isoforms, with increasing potency and selectivity for MEK kinases. Clinical trials of these compounds have moved forward rapidly in BRAF mutant melanoma, but PTC may represent novel therapeutic applications of these drugs (90).
Among the first MEK inhibitors to enter clinical trials, CI-1040 selectively inhibits purified MEK (IC50, 17 nm) (91). Clinical trials yielded disappointing results in various solid tumors, likely due to a lack of documenting activated MAPK signaling in the tumors. However, in thyroid cancer cell lines and tumor xenografts, CI-1040 selectively inhibited proliferation in the presence of BRAF and RAS mutations, but wild-type and RET/PTC mutant cells did not respond (92). Similar results were seen with cell lines treated with a CI-1040 derivative, PD0325901, but some sensitivity to the drug was identified in orthotopic tumor models bearing the RET/PTC mutation (93, 94). However, this drug also did not progress beyond phase I due to unacceptable toxicity.
a. Selumetinib (AZD6244, ARRY-142886).
Selumetinib inhibits MEK at concentrations several orders of magnitude lower than other kinases (IC50, 12 nm) (95). Despite preclinical demonstration of efficacy in multiple tumor models, clinical responses have been few in phase I and II trials.
In multiple PTC cell lines, growth inhibition was demonstrated at clinically achievable concentrations in the presence of BRAF mutation, but concentrations severalfold higher were required in the setting of wild-type BRAF (96). Cytostatic inhibition of tumor growth was observed in a xenograft model, despite marked inhibition of ERK phosphorylation. Using the phase I recommended dose of 100 mg twice daily, a phase II study was initiated in patients with RAI refractory PTC that had progressed within 12 months (97). Of 39 patients enrolled, partial response was observed in only 1 (3%), and stable disease for at least 24 weeks in another 36%. The median PFS was 32 weeks. Although the analysis was limited by small numbers, the PFS was 33 weeks in patients with tumors bearing BRAF mutation, compared with only 11 weeks in BRAF wild-type (P = .3). Significant AEs included rash, fatigue, diarrhea, and peripheral edema.
To explore further the role of BRAF mutations in thyroid oncogenesis, a mouse model of doxycycline-inducible BRAFV600E expression in thyroid follicular cells was developed (98). PTC developed quickly after mutant BRAF induction. Of particular note, treatment of these mice with either the BRAF inhibitor PLX4720 or the MEK inhibitor PD0325901 triggered expression of thyroid-specific genes, including the sodium iodide symporter (which localized appropriately at the cell membrane). RAI uptake and retention were similarly enhanced by treatment. Based on these results, a pilot study was launched to explore the hypothesis that therapy with selumetinib could reinduce RAI uptake and facilitate RAI treatment of metastatic tumors that were FDG-avid but RAI refractory (99). In a recent study, 20 evaluable patients had completed the 4-week treatment with selumetinib, 75 mg twice daily, and underwent 124I-PET scans to assess RAI uptake. Twelve patients (60%) experienced increased uptake, of whom 8 subsequently received therapeutic 131I treatment, 5 had either confirmed or unconfirmed partial responses, and 2 had stable disease on subsequent tomographic imaging. Serum thyroglobulin levels declined by >90% in the 131I-treated patients. Somewhat surprisingly, no difference was suggested in response between BRAF mutant and wild-type tumors, but all of the patients with NRAS mutations reacquired uptake.
6. PI-3K pathway inhibitors
Targeted inhibition of the PI-3K pathway has been an attractive therapeutic concept, given that many advanced DTC tumors demonstrate activation of signaling due to diminished expression of PTEN or mutation, amplification, or overexpression of either PI-3K or AKT, resulting in enhanced tumor proliferation, migration, and survival (100). In a broad array of thyroid cell lines bearing typical MAPK pathway oncogenic mutations and activation of AKT, treatment with rapamycin rapidly blocks mammalian target of rapamycin (mTOR) complex 1 phosphorylation (101). Growth inhibition and cell cycle arrest were also seen, except in cell lines bearing BRAF mutations. However, feedback activation of both ERK and AKT was observed, suggesting that compensatory mechanisms might rapidly bypass the drug's effect. Xenograft models demonstrated prolongation of time to tumor progression, but tumor growth quickly resumed after therapy discontinuation, consistent with a cytostatic rather than cytotoxic effect of mTOR inhibition. Interestingly, treatment in similar models with the dual PI-3K/mTOR inhibitor resulted in marked growth inhibition in cell culture, but minimal effect on tumor progression in xenografts, suggesting that alternative mechanisms to promote tumor growth must be up-regulated in the presence of PI-3K pathway blockade (102). In other studies, targeted inhibition of PI-3K or AKT was more effective at growth inhibition in cell lines bearing PTEN deletion or activating PI-3K mutations, although these are uncommonly encountered in DTC (103).
Given this preclinical evidence of potential benefit, 3 phase II clinical trials are now exploring monotherapy with the mTOR inhibitor everolimus in advanced thyroid cancer, including patients who have failed prior MKI treatment. Additional trials are evaluating the value of combining PI-3K pathway inhibition with targeted treatment against other signaling pathways (see Section IV.C).
C. Combination therapies
Numerous factors have led to the recognition that existing novel monotherapies described above represent a first step in improving patient outcomes but were insufficient to eradicate advanced and metastatic disease. The absence of complete responses, the unacceptable rate of failure to respond at all, and the emerging evidence of resistance mechanisms to these treatments are driving research to identify rational ways to combine individual therapies for more effective outcomes. In this respect, thyroid cancer research is mirroring ongoing work in multiple other malignancies, where individual MKIs or other targeted therapies are being merged together, or with selected traditional cytotoxic agents, to attempt to improve patient survival.
Early preclinical studies focused on combining inhibition of the MAPK and PI-3K pathways, both to synergize blockade of proliferation pathways as well as to bypass mechanisms of resistance. Examples include the demonstration of synergistic effects of: 1) the MEK inhibitor RDEA119 and the mTOR inhibitor temsirolimus in cell lines carrying either BRAF or PTEN mutations; 2) the pan-RAF inhibitor RAF265 and the mTOR complex 1/mTOR complex 2 inhibitor BEZ-235 (102); and 3) the Akt inhibitor MK2206 combined with either the V600EBRAF inhibitor vemurafenib or the MEK inhibitor selumetinib (103). Preliminary data from a phase II study of temsirolimus (25 mg iv weekly) and sorafenib (200 mg orally twice daily) were recently presented (104). Of 37 eligible patients, 8 (22%) experienced partial responses, and 21 (57%) remained stable; partial responses were seen in 38% of the patients who had never received prior systemic chemotherapy.
A second strategy involves sequential inhibition along the MAPK pathway, blocking both BRAF and MEK simultaneously. Recent studies with BRAF-mutant melanoma have contributed to this approach, driven by the need to overcome acquired resistance to monotherapy with BRAF inhibitors mediated by reactivation of MAPK signaling (105). Addition of either selumetinib or the MEK inhibitor trametinib (GSK1120212) blocked ERK phosphorylation and G0/G1 cell cycle progression in melanoma cell lines that developed resistance to PLX4720 (106). In a phase I trial of the combination regimen of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib, an overall objective response rate of 81% was observed in BRAF mutant melanoma, which increased to 90% in patients treated at the recommended phase II dose (107). A randomized trial of dabrafenib vs dabrafenib plus trametinib is under way in BRAF mutant PTC. Other possible approaches that have been advanced include combining BRAF and EGFR inhibitors, BRAF with IGF-I receptor inhibitors, and BRAF with HER kinase inhibitors based on observations in BRAF mutant colon carcinoma, melanoma, and thyroid, respectively (46, 106, 111).
D. Summary of therapeutic recommendations
Despite a rapidly expanding armamentarium of chemotherapeutic options described here, use of these agents should clearly be limited to a highly selected group of appropriate patients. Toxicities of therapy are numerous, frequently serious, and occasionally fatal. Common side effects can include skin rash, diarrhea, nausea, fatigue, anorexia, hypertension, mucositis, and cytopenias (108). More significant but less common problems can arise from heart failure, cardiac arrhythmias, enteric fistulas and perforations, abnormal hepatic function, bleeding, proteinuria, neurological dysfunction, and squamous malignancies. On the other hand, efficacy, as defined by PFS and “disease control rate” (sum of frequency of complete responses, partial responses, and stable disease as best outcomes), is observed in most patients who are treated. However, complete responses are rare, and no therapy has yet been demonstrated to improve overall survival in any cohort or subset of treated patients in any trial. Thus, use of these chemotherapies should be limited to patients with significant burden of RAI refractory, metastatic, or unresectable locoregional disease that is progressing despite adequate TSH suppressive thyroid hormone therapy (1, 2). Once the decision to treat has been made, participation in a therapeutic clinical trial should be the initial consideration for those patients who are eligible for such research studies. For those patients to be treated with noninvestigational therapies, the absence of direct comparison between any 2 agents or regimens prevents formal recommendation of an “optimal” drug to select as first-line therapy. Informed consent should be obtained before initiation of treatment, and a careful plan of safety monitoring and disease reassessment should be established at the outset of therapy, consistent with standard recommendations for the safe use of oral chemotherapy (109, 110).
V. The Patient
After 6 months of therapy with sorafenib, the patient's restaging exams demonstrated significant progression of disease. Many of her cervical and pulmonary metastases enlarged by 50–100% compared with baseline measurements, with diameters up to 2.5 cm. New metastases were also identified in the lungs bilaterally, but no abdominal, pelvic, skeletal, or intracerebral disease was found. Sorafenib therapy was discontinued, and the patient was now willing to consider investigational treatment options. To determine eligibility for a phase I clinical trial, her primary tumor from her previous right lobectomy was analyzed by PCR-based DNA sequencing. A point mutation in the BRAF gene was identified, changing the DNA sequence of codon 600 in exon 15 from GTG to GAG, and thus predicting the oncogenic BRAF V600E amino acid substitution from valine to glutamic acid. After providing informed consent, the patient was treated on a phase I trial with a small molecule type II inhibitor of BRAF. A radiographic partial response was recorded after 6 months of therapy, with >30% reduction of the sum of the longest diameters of her target metastatic lesions, and her serum thyroglobulin had declined by >50% from baseline. At her most recent follow-up, she continued to maintain a partial response after 18 months of therapy.
Acknowledgments
Funding was provided by the National Institutes of Health/National Cancer Institute (CA 155512) to B.R.H.
Disclosure Summary: B.R.H. has received research support from Veracyte, Inc. S.I.S. has consulted for Exelixis, Eisai, AstraZeneca, Roche, and Bayer, and has received Pfizer Research support from Pfizer.
Footnotes
- AE
- adverse event
- ATC
- anaplastic thyroid cancer
- CT
- computed tomography
- DTC
- differentiated thyroid cancer
- EGFR
- epidermal growth factor receptor
- EMT
- epithelial to mesenchymal transition
- FAK
- focal adhesion kinase
- FDG
- fluorodeoxyglucose
- FT
- farnesyltransferase
- MEK
- MAPK kinase
- MKI
- multi-targeted kinase inhibitor
- NFkB
- nuclear factor κB
- PDGF
- platelet-derived growth factor
- PDGFR
- PDGF receptor
- PDTC
- poorly differentiated thyroid cancer
- PET
- positron emission tomography
- PFS
- progression-free survival
- PI-3K
- phosphatidylinositol-3 kinase
- PIK3CA
- PI-3K catalytic subunit
- PPAR
- peroxisome proliferator-activated receptor
- PTC
- papillary thyroid cancer
- RAI
- radioiodine
- WDTC
- well-differentiated thyroid cancer.
References
- 1. Schlumberger M, Sherman SI. Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol. 2012;166:5–11. [DOI] [PubMed] [Google Scholar]
- 2. Haugen BR, Kane MA. Approach to the thyroid cancer patient with extracervical metastases. J Clin Endocrinol Metab. 2010;95:987–993. [DOI] [PubMed] [Google Scholar]
- 3. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. [DOI] [PubMed] [Google Scholar]
- 6. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 7. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 8. Pacini F, Castagna MG, Brilli L, Pentheroudakis G. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v214–v219. [DOI] [PubMed] [Google Scholar]
- 9. Steward DL. Update in utility of secondary node dissection for papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:3393–3398. [DOI] [PubMed] [Google Scholar]
- 10. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. [DOI] [PubMed] [Google Scholar]
- 11. Muresan MM, Olivier P, Leclere J, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15:37–49. [DOI] [PubMed] [Google Scholar]
- 12. Vitale G, Fonderico F, Martignetti A, et al. Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br J Cancer. 2001;84:1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21:31–35. [DOI] [PubMed] [Google Scholar]
- 14. Bernier MO, Leenhardt L, Hoang C, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568–1573. [DOI] [PubMed] [Google Scholar]
- 15. Zettinig G, Fueger BJ, Passler C, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma—surgery or conventional therapy? Clin Endocrinol (Oxf). 2002;56:377–382. [DOI] [PubMed] [Google Scholar]
- 16. Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol. 2010;22:464–468. [DOI] [PubMed] [Google Scholar]
- 17. Gottlieb JA, Hill CS., Jr Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med. 1974;290:193–197. [DOI] [PubMed] [Google Scholar]
- 18. Matuszczyk A, Petersenn S, Bockisch A, et al. Chemotherapy with doxorubicin in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res. 2008;40:210–213. [DOI] [PubMed] [Google Scholar]
- 19. Sherman SI. Targeted therapies for thyroid tumors. Mod Pathol. 2011;24:S44–S52. [DOI] [PubMed] [Google Scholar]
- 20. Fagin JA. Genetic basis of endocrine disease 3: molecular defects in thyroid gland neoplasia. J Clin Endocrinol Metab. 1992;75:1398–1400. [DOI] [PubMed] [Google Scholar]
- 21. Riesco-Eizaguirre G, Rodriquiz I, De la Vieja A, et al. The BRAFV600E oncogene induces transforming growth factor β secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009;69:8317–8325. [DOI] [PubMed] [Google Scholar]
- 22. Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiltzik D, Carlson DL, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286. [DOI] [PubMed] [Google Scholar]
- 24. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 25. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008;113:48–56. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Rostan G. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2012;65:10199–10207. [DOI] [PubMed] [Google Scholar]
- 27. Hou P, Liu D, Sha Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. [DOI] [PubMed] [Google Scholar]
- 28. Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. [DOI] [PubMed] [Google Scholar]
- 29. Ricarte-Filho JC, Ryder M, Chitale Da, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbosh PH, Nephew KP. Multiple signaling pathways converge on β-catenin in thyroid cancer. Thyroid. 2005;15:551–561. [DOI] [PubMed] [Google Scholar]
- 31. Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcanqiu ML, Rimm DL. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–1815. [PubMed] [Google Scholar]
- 32. Rocha AS, Soares P, Fonseca E, Cameselle-Teijeiro J, Oliveira MC, Sobrinho-Simoes M. E-Cadherin loss rather than β-catenin alterations is a common feature of poorly differentiated thyroid carcinomas. Histopathology. 2003;42:580–587. [DOI] [PubMed] [Google Scholar]
- 33. Kurihara T, Ikeda S, Ishizaki Y, et al. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid. 2004;14:1020–1029. [DOI] [PubMed] [Google Scholar]
- 34. Ito T, Seyama T, Mizuno T, et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992;52:1369–1371. [PubMed] [Google Scholar]
- 35. Fagin JA, Matsuo K, Karmarkar A, Chen DL, Tang SH, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:955–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. [DOI] [PubMed] [Google Scholar]
- 38. Wiseman SM, Griffith OL, Deen S, et al. Identification of molecular markers altered during transformation of differentiated into anaplastic thyroid carcinoma. Arch Surg. 2007;142:717–729. [DOI] [PubMed] [Google Scholar]
- 39. Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takano T. Fetal cell carcinogenesis of the thyroid: theory and practice. Semin Cancer Biol. 2007;17:233–240. [DOI] [PubMed] [Google Scholar]
- 41. Todaro M, Iovino F, Eterno V, et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. [DOI] [PubMed] [Google Scholar]
- 42. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vasko V, Espinosa AV, Scouten W, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA. 2007;104:2803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knauf JA, Sartor MA, Medvedovic M, et al. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFβ signaling. Oncogene. 2011;30:3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montero-Conde C, Martin-Campos JM, Lerma E, et al. Molecular profiling related to poor prognosis in thyroid carcinoma. Combining gene expression data and biological information. Oncogene. 2008;27:1554–1561. [DOI] [PubMed] [Google Scholar]
- 46. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. [DOI] [PubMed] [Google Scholar]
- 47. Dar AC, Das TK, Shokat KM, Cagan RL. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature. 2012;486:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sherman SI, Wirth LJ, Droz JP, et al. ; Motesanib Thyroid Cancer Study Group. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed M, Barbachano Y, Riddel A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–322. [DOI] [PubMed] [Google Scholar]
- 50. Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–2160. [DOI] [PubMed] [Google Scholar]
- 51. Brose MS, Nutting CM, Sherman SI, et al. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bible KC, Suman VJ, Molina JR, et al. ; Endocrine Malignancies Disease Oriented Group, Mayo Clinic Cancer Center, Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. [DOI] [PubMed] [Google Scholar]
- 54. Sherman SI, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC). J Clin Oncol. 2011;29:5503. [Google Scholar]
- 55. Cabanillas ME, Brose MS, Ramies DA, Lee Y, Miles D, Sherman SI. Antitumor activity of cabozantinib (XL184) in a cohort of patients with differentiated thyroid cancer (DTC). J Clin Oncol. 2012;30(suppl):5547 (Meeting Abstract). [Google Scholar]
- 56. Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ball DW, Sherman SI, Jarzab B, et al. Lenvatinib treatment of advanced RAI-refractory differentiated thyroid cancer (DTC): cytokine and angiogenic factor (CAF) profiling in combination with tumor genetic analysis to identify markers associated with response. J Clin Oncol. 2012;30(suppl):5518 (Meeting Abstract). [Google Scholar]
- 58. Santarpia L, Lippman SM, El Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young A, Lyons J, Miller AL, Phan VT, Alarcon IR, McCormick F. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. [DOI] [PubMed] [Google Scholar]
- 60. Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11:775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Whyte DB, Kirschmeier P, Hockenberry TN, et al. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272:14459–14464. [DOI] [PubMed] [Google Scholar]
- 62. Yeung SC, Xu G, Pan J, Christgen M, Bamiagis A. Manumycin enhances the cytotoxic effect of paclitaxel on anaplastic thyroid carcinoma cells. Cancer Res. 2000;60:650–656. [PubMed] [Google Scholar]
- 63. Pan J, She M, Xu ZX, Sun L, Yeung SC. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005;65:3671–3681. [DOI] [PubMed] [Google Scholar]
- 64. Zhou J, Vos CC, Gjyrezi A, et al. The protein farnesyltransferase regulates HDAC6 activity in a microtubule-dependent manner. J Biol Chem. 2009;284:9648–9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hong DS, Cabanillas ME, Wheler J, et al. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab. 2011;96:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ouyang B, Knauf JA, Smith EP, et al. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006;12:1785–1793. [DOI] [PubMed] [Google Scholar]
- 67. Mitsiades CS, Negri J, McMullan C, et al. Targeting BRAFV600E in thyroid carcinoma: therapeutic implications. Mol Cancer Ther. 2007;6:1070–1078. [DOI] [PubMed] [Google Scholar]
- 68. Zambon A, Niculescu-Duvaz I, Niculescu-Duvaz D, Marais R, Springer CJ. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789–792. [DOI] [PubMed] [Google Scholar]
- 69. Whittaker S, Kirk R, Hayward R, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci Transl Med. 2010;2:35–41. [DOI] [PubMed] [Google Scholar]
- 70. Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shimizu T, Tolcher AW, Patnaik A, et al. Phase I dose-escalation study of continuously administered regorafenib (BAY 73–4506), an inhibitor of oncogenic and angiogenic kinases, in patients with advanced solid tumors. J Clin Oncol. 2010;28:3035.20458031 [Google Scholar]
- 72. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 73. Schwartz GK, Robertson S, Shen A, et al. A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients (Pts) with advanced solid tumors. J Clin Oncol. 2009;27:3513. [Google Scholar]
- 74. Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res. 2008;6:751–759. [DOI] [PubMed] [Google Scholar]
- 75. Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. [DOI] [PubMed] [Google Scholar]
- 77. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oberholzer PA, Kee D, Dziunycz P, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salerno P, De Falco V, Tamburrino A, et al. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab. 2010;95:450–455. [DOI] [PubMed] [Google Scholar]
- 82. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nucera C, Nehs MA, Nagarkatti SS, et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist. 2011;16:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vergani E, Vallacchi V, Frigerio S, et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia. 2012;13:1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Su F, Bradley WD, Wang Q, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969–978. [DOI] [PubMed] [Google Scholar]
- 86. Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res. 2012;72:779–789. [DOI] [PubMed] [Google Scholar]
- 88. Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:8503. [Google Scholar]
- 89. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leboeuf R, Baumgartner JE, Benezra M, et al. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab. 2008;93:2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–5293. [DOI] [PubMed] [Google Scholar]
- 92. Liu D, Liu Z, Jiang D, Dackiw AP, Xing M. Inhibitory effects of the mitogen-activated protein kinase kinase inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab. 2007;92:4686–4695. [DOI] [PubMed] [Google Scholar]
- 93. Liu D, Xing M. Potent inhibition of thyroid cancer cells by the MEK inhibitor PD0325901 and its potentiation by suppression of the PI3K and NF-κB pathways. Thyroid. 2008;18:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Henderson YC, Chen Y, Frederick MJ, Lai SY, Clayman GL. MEK inhibitor PD0325901 significantly reduces the growth of papillary thyroid carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2010;9:1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. [DOI] [PubMed] [Google Scholar]
- 96. Ball DW, Jin N, Rosen DM, et al. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab. 2007;92:4712–4718. [DOI] [PubMed] [Google Scholar]
- 97. Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma (IRPTC) with or without follicular elements. Clin Cancer Res. 2012;18:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Saji M, Ringel MD. The PI3K-Akt-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW. Dual inhibition of mitogen-activated protein kinase kinase and mammalian target of rapamycin in differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:4107–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jin N, Jiang T, Rosen DM, Nelkin BD, Ball DW. Synergistic action of a RAF inhibitor and a dual PI3K/mTOR inhibitor in thyroid cancer. Clin Cancer Res. 2011;17:6482–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu R, Liu D, Xing M. The Akt inhibitor MK2206 synergizes, but perifosine antagonizes, the BRAFV600E inhibitor PLX4032 and the MEK1/2 inhibitor AZD6244 in the inhibition of thyroid cancer cells. J Clin Endocrinol Metab. 2012;97:E173–E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sherman EJ, Ho AL, Fury MG, et al. A phase II study of temsirolimus/sorafenib in patients with radioactive iodine (RAI)-refractory thyroid carcinoma. J Clin Oncol. 2012;30(suppl):5514 (Meeting Abstract). [Google Scholar]
- 105. Nissan MH, Solit DB. The “SWOT” of BRAF inhibition in melanoma: RAF inhibitors, MEK inhibitors or both? Curr Oncol Rep. 2011;13:479–487. [DOI] [PubMed] [Google Scholar]
- 106. Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Infante JR, Falchook GS, Lawrence DP, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol. 2011;29(suppl):8503 (Meeting Abstract). [Google Scholar]
- 108. Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. [published online October 4, 2011]. J Thyroid Res. doi:10.4061/2011/985780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Weingart SN, Brown E, Bach PB, et al. NCCN task force report: oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S1–S14. [PubMed] [Google Scholar]
- 110. Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013;98:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF mutant thyroid carcinomas. [published online January 29, 2013]. Cancer Discov. doi:10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18:317–323. [DOI] [PubMed] [Google Scholar]
- 113. Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]