Abstract
Leptin is an adipocyte-secreted hormone that has been proposed to regulate energy homeostasis as well as metabolic, reproductive, neuroendocrine, and immune functions. In the context of open-label uncontrolled studies, leptin administration has demonstrated insulin-sensitizing effects in patients with congenital lipodystrophy associated with relative leptin deficiency. Leptin administration has also been shown to decrease central fat mass and improve insulin sensitivity and fasting insulin and glucose levels in HIV-infected patients with highly active antiretroviral therapy (HAART)-induced lipodystrophy, insulin resistance, and leptin deficiency. On the contrary, the effects of leptin treatment in leptin-replete or hyperleptinemic obese individuals with glucose intolerance and diabetes mellitus have been minimal or null, presumably due to leptin tolerance or resistance that impairs leptin action. Similarly, experimental evidence suggests a null or a possibly adverse role of leptin treatment in nonlipodystrophic patients with nonalcoholic fatty liver disease. In this review, we present a description of leptin biology and signaling; we summarize leptin's contribution to glucose metabolism in animals and humans in vitro, ex vivo, and in vivo; and we provide insights into the emerging clinical applications and therapeutic uses of leptin in humans with lipodystrophy and/or diabetes.
Introduction
-
Leptin Biology
Leptin discovery
Leptin production and circulation
Leptin receptors
Leptin's antisteatotic and antilipotoxic role
Leptin's role in glucose homeostasis
Leptin's role in immune modulation
-
Leptin Signaling
Leptin and JAK2/STAT3/STAT5 signaling
Leptin and AMPK signaling
Leptin and PI3K/Akt/mTOR signaling
Leptin and FoxO1 signaling
Leptin and SHP2/MAPK signaling
Leptin and JAK2-independent signaling
Leptin and Insulin Signaling
-
Leptin Resistance or Tolerance
Impaired activation of leptin receptor signaling/inhibitors of leptin signaling
Impaired leptin transport across the BBB
Impaired ObRb trafficking
ER stress
Saturable nature of leptin signaling pathways
Impaired leptin neural circuitry
-
Effects of Leptin on Glucose Metabolism in Animals and Humans
States of leptin deficiency
States of leptin excess
Benefits, potential adverse effects, and challenges in relation to leptin administration
Future Directions
I. Introduction
Leptin has a crucial role in the regulation of energy homeostasis, insulin action and lipid metabolism (1). As a hormone secreted by adipocytes in quantities mainly correlated with fat cell mass, leptin serves as an important signal of body energy stores. Its importance is well illustrated by the physiological consequences of leptin deficiency in mice homozygous for a mutation in the obese (ob) gene, which prevents leptin production or leads to secretion of a truncated inactive leptin molecule (1–3). Ob/ob mice exhibit hyperphagia, early-onset obesity, insulin resistance, diabetes (1, 2), and several neuroendocrine abnormalities (3, 4). All these abnormalities can be corrected by exogenous leptin administration (1, 2, 5, 6), suggesting that leptin plays a role in glucose homeostasis and possibly in the pathogenesis of other obesity-related metabolic complications. Interestingly, leptin-induced normalization of hyperglycemia and hyperinsulinemia in ob/ob mice is observed even before body weight reduction, suggesting that leptin's effects on glucose homeostasis are, in part, independent of its weight-reducing effects (5, 6). Similar to ob/ob mice, other mouse models of obesity and leptin resistance or tolerance (7–9) present abnormalities of leptin deficiency due to subnormal leptin action (7, 10, 11).
The importance of leptin is also evident in human physiology (3, 4, 7–18). Leptin administration has been demonstrated to successfully treat obesity and its complications in individuals with congenital leptin deficiency, and thus, leptin is available on a compassionate basis for these patients (5–7). Moreover, leptin is effective in correcting neuroendocrine abnormalities and insulin resistance in patients with HIV-associated lipodystrophy (11–13) and congenital lipodystrophy (8–10). Therefore, an application has been submitted to the U.S. Food and Drug Administration (FDA) for approval of leptin in replacement doses for the treatment of congenital lipodystrophy. In contrast, in subjects with garden-variety obesity or diabetes (who exhibit hyperleptinemia presumably due to leptin tolerance), treatment with additional exogenous leptin has not been associated with significant weight loss or reduction in metabolic complications (19–21). This suggests that leptin tolerance or resistance exists in these subjects (22, 23). Hence, although great progress has been made in understanding the role of leptin in many physiological systems, much research is currently being directed toward elucidating the mechanisms and pathophysiology of leptin's effects on glucose metabolism. In this review, we describe the effects of leptin biology and signaling on glucose metabolism in animals and humans in vitro, ex vivo, and in vivo and provide novel insights into emerging clinical applications and therapeutic uses of recombinant leptin in the area of lipodystrophy, insulin resistance, and diabetes in humans.
II. Leptin Biology
A. Leptin discovery
In 1994, the mouse ob gene was found to encode a 16-kDa 167–amino-acid secreted protein with a 4-helix bundle motif similar to that of a cytokine, which was named leptin (from the Greek word leptos, meaning thin) (10, 24). Leptin signals primarily the status of body energy reserves in fat to the brain and other tissues so that appropriate changes in food intake, energy expenditure, and nutrient partitioning can occur to maintain whole-body energy balance (24). This system is especially sensitive to energy deprivation. The discovery of leptin in 1995 also defined a novel endocrine role for adipose tissue and thus subsequently increased our understanding of how food intake and energy metabolism are regulated (10).
B. Leptin production and circulation
Leptin is primarily produced in adipose tissue but is also expressed in many other tissues, including the placenta, mammary gland, testes, ovary, endometrium, stomach, hypothalamus, pituitary, and others (25). Leptin concentrations in blood are pulsatile and follow a circadian rhythm, with lowest levels in the early to midafternoon and highest levels between midnight and early morning (26). The pulsatile characteristics of leptin secretion are similar in lean and obese individuals with the exception that the obese exhibit higher pulse amplitudes of leptin (26). Circulating leptin levels are directly proportional to the amount of body fat (23) and fluctuate with acute changes in caloric intake (27). Women tend to have higher leptin concentrations than men, although a significant reduction in the amount of circulating leptin occurs after menopause (28). This sexual dimorphism, which is independent of body mass index (BMI), is partly attributed to differences in fat mass, body fat distribution, and sex hormones (29). Subcutaneous fat expresses more leptin mRNA than omental (visceral) fat, and this may partially contribute to higher leptin levels in women compared with men (29). Thus, compared with visceral fat, accumulation of sc fat is associated with higher leptin levels (26) and steatotic and lipotoxic complications appear more frequently in visceral obesity (27). Table 1 depicts factors that affect circulating leptin levels in humans.
Table 1.
Factors That Regulate Serum Leptin Levels
| Factors Promoting Leptin Secretion | Factors Inhibiting Leptin Secretion |
|---|---|
| Excess energy stored as fat (obesity)a | Low energy states with decreased fat stored (leanness)a |
| Overfeedinga | Fastinga |
| Glucose | Thyroid hormones |
| Amino acids | Free fatty acids and other lipid metabolites |
| Insulin | Catecholamines and adrenergic agonists |
| Glucocorticoids | Androgens |
| Estrogens | PPARγ agonistsb |
| Inflammatory cytokines TNF-α and IL-6 (acute effect) | Inflammatory cytokine TNF-α (prolonged effect) |
Denotes major factor influencing leptin levels.
Unlike animals, PPARγ agonists in humans decrease leptin gene expression but increase sc fat mass, thus presenting a null effect.
C. Leptin receptors
Leptin exerts its effects via binding to specific leptin receptors (obese receptors [ObRs]) located throughout the central nervous system (CNS), including the hypothalamus, and several peripheral organs (4, 10, 28). The leptin receptor is a receptor in the class 1 cytokine receptor family, with a single membrane-spanning domain (10, 24, 28, 29). At present, 6 ObR isoforms, produced by alternative splicing of the leptin receptor gene, have been identified in rats (ObRa–ObRf) (30). In mice and humans, only 5 (ObRa–ObRe) and 4 (ObRa–ObRd) alternative spliced isoforms have been described, respectively (30, 31). These isoforms present homologous extracellular leptin-binding domains but distinct intracellular domains varying in length and sequence due to alternative mRNA splicing (32). The short isoforms ObRa and ObRc may play an important role in transporting leptin across the blood-brain barrier (BBB) (32).
The long leptin receptor isoform, ObRb, which contains a full-length intracellular domain required for cell signal transduction, is primarily responsible for leptin signaling (30, 31, 33) (Figure 1). ObRb is strongly expressed throughout the CNS and particularly in the hypothalamus, where it regulates energy homeostasis (food intake and energy expenditure) and neuroendocrine function (30, 32–35). In the db/db mouse model, the ObRb is dysfunctional, resulting in obesity and the metabolic syndrome (36). ObRb mRNA is highly expressed in the hypothalamus, particularly in the arcuate nucleus (ARC), the ventromedial hypothalamus (VMH), the lateral hypothalamic area, the dorsomedial hypothalamus (DMH), and the ventral premammillary nucleus (32). Via its receptor, leptin directly targets hypothalamic neurons, particularly ARC neurons (proopiomelanocortin [POMC] and agouti-related protein [AgRP] neurons), which are critical mediators of leptin action (37). POMC neurons are anorexigenic (appetite suppressor) neurons coexpressing POMC and cocaine- and amphetamine-regulated transcript. After proteolytic cleavage, POMC generates α-MSH, which stimulates melanocortin-3 and -4 receptors (MC3R and MC4R), which are important in energy homeostasis (37). Genetic defects in the melanocortin system (POMC or MCR genes) are related to obesity in humans (38). Conversely, AgRP neurons are orexigenic (appetite stimulator) neurons that inhibit POMC activity by releasing inhibitory γ-aminobutyric acid and coexpress the orexigenic AgRP and neuropeptide Y (NPY), antagonists of both MC3R and MC4R (39). Leptin inhibits AgRP/NPY expression and AgRP neuronal excitability as well as γ-aminobutyric acid release from AgRP neurons (39). Moreover, in the VMH, leptin stimulates the expression of anorexigenic brain-derived neurotrophic factor (BDNF) through MC4R signaling (40). Finally, in the hypothalamus, leptin acts on neurons that directly or indirectly regulate levels of other circulating hormones (eg, thyroid hormone, sex steroids, and GH) (30, 32, 33, 41). Leptin action on these hypothalamic neurons also controls the activity of the autonomic nervous system; direct effects of leptin on ObRb-containing neurons in the brainstem and elsewhere may also play an important role (31, 33).
Figure 1.
Intracellular signaling pathways recruited by leptin. The full-length isoform, ObRb, contains intracellular motifs required for activation of the JAK2/STAT3 signal transduction pathway. ObRb contains 4 important tyrosine residues that provide docking sites for signaling proteins with SHP2 domains. Activation of JAK2 occurs by transphosphorylation and subsequent phosphorylation of tyrosine residues in the cytoplasmic region of the receptor. Phosphorylation of STAT3 leads to their dissociation from the receptor and the formation of active dimers, which translocate to the nucleus to regulate gene expression, binding to the promoter regions of target genes. The leptin-regulated STAT3 signaling may interact with STAT5, which may regulate STAT3-dependent gene expression. The ability of SOCS3 to inhibit leptin-stimulated phosphorylation of JAK2 provides a negative-feedback mechanism on the leptin signaling system. The ERK members of the MAPK family are components of the well-defined AMPK-Ras/Raf/MAPK signaling cascade and have become activated by leptin. Leptin activates PI3K signaling via IRS-1 and/or IRS-2 phosphorylation. The PTP1B inhibits leptin-stimulated phosphorylation of IRS-1/2 and/or JAK2. Leptin administration results further in Akt/mTOR phosphorylation followed by regulation of FOXO and S6 expression.
ObRb is also expressed in multiple peripheral tissues, including pancreatic islets, adipose tissue, skeletal muscle, liver, and immune cells (36, 37, 42). In the pancreatic islets, leptin directly inhibits insulin expression and secretion (42). In liver and white adipose tissue, leptin inhibits lipogenesis and stimulates lipolysis (36, 37) and adipocyte-specific overexpression of ObRb prevents diet-induced obesity (DIO) in mice (38). Leptin directly promotes fatty acid oxidation in isolated adipocytes and skeletal muscle (39, 40) and decreases lipid levels in isolated livers (43). Liver-specific overexpression of ObRb prevents hepatic steatosis in ObR-deficient fa/fa rats (44, 45). However, deletion of the ObRb in these peripheral tissues has no effect on energy balance, body weight, or glucose homeostasis in mice, indicating that these processes are mainly regulated by the central effects of leptin in the brain (46).
D. Leptin's antisteatotic and antilipotoxicity role
Whenever leptin action is not present either due to hypoleptinemia or leptin resistance, overfeeding could lead to fat deposition to nonadipose tissues characterized by generalized steatosis, lipotoxicity, and lipoapoptosis. Generalized steatosis leading to a dysfunction of nonadipose tissues, ie, lipotoxicity, may affect pancreatic β-cells (47, 48), myocardium (49), liver, kidneys, skeletal muscle (50), and other nonadipose tissues (27). When there is ectopic lipid accumulation during overfeeding, the surplus of fatty acids follows the nonoxidative metabolic pathway resulting in metabolic trauma characterized by lipotoxicity and lipoapoptosis (51). Also, apoptosis results from excessive ceramide synthesis coupled with underexpression of the antiapoptotic factor bcl-2 (52). Ceramidotoxicity, which is not clearly identified in metabolic syndrome, has been shown to occur spontaneously in the pancreatic islets of Zucker diabetic fatty (ZDF) rodents with type 2 diabetes and metabolic syndrome (53). In humans, the constellation of lipotoxic abnorrmalities that leads to insulin resistance, type 2 diabetes mellitus, and fatty liver is referred to as the metabolic syndrome, suggesting that this syndrome includes lipotoxicity of pancreatic β-cells, liver, myocardium, and skeletal muscle in a manner similar to lipotoxicity observed in rodents (27, 54, 55).
Leptin constitutes a liporegulatory hormone that controls lipid homeostasis in nonadipose tissues particularly during periods of overfeeding (27). It has been suggested that leptin regulates intracellular fatty acid homeostasis and prevents tissues from lipotoxicity, similar to insulin, which regulates intracellular glucose homeostasis and prevents glucotoxicity (56). Leptin activates AMP-activated protein kinase (AMPK), arresting lipogenesis and promoting fatty acid oxidation by inactivating acetyl coenzyme A (CoA) carboxylase (ACC) (99). In nonobese diabetic mice with uncontrolled diabetes type 1, leptin therapy normalizes the levels of a wide array of hepatic intermediary metabolites comprising acylcarnitines, tricarboxyl acid cycle intermediates, amino acids, and acyl CoAs (58). Leptin reduces both lipogenic and cholesterologenic transcription factors and enzymes and lowers plasma and tissues lipids (58).
E. Leptin's role in glucose homeostasis
Leptin may also directly regulate glucose homeostasis independently of its effects on adiposity; leptin regulates glycemia at least in part via the CNS, but it may also directly regulate the physiology of pancreatic β-cells (59, 60) and peripheral insulin-sensitive tissues (61, 62). Low doses of leptin that do not reduce food intake and body weight almost normalize the severe hyperglycemia observed in ob/ob mice, demonstrating that leptin actions in glucose homeostasis are independent from its actions in food intake and body weight (2). In the context of severe obesity and insulin resistance, the potent leptin actions in glucose homeostasis are predominantly mediated by POMC-expressing neurons within the arcuate nucleus of the hypothalamus (64), although the AgRP-expressing neurons and other hypothalamic and extrahypothalamic neurons may also have important roles (65).
Recent studies have shown remarkable antidiabetic properties of leptin as a powerful suppressor of glucagon (58, 66, 67). Leptin suppresses glucagon hypersecretion in rodents with diabetes type 1 at least as effectively as somatostatin and insulin, without producing undesirable adverse effects (58, 67, 68). Administration of recombinant leptin to mice with uncontrolled diabetes type 1 reversed the catabolic syndrome in a similar manner as insulin did. Moreover, when leptin was added to low-dose insulin therapy at 10% of the optimal dose, the volatility of insulin monotherapy disappeared (58). This leptin action was ascribed to the tonic suppression of hyperglucagonemia, affecting hyperglycemia (66). The antihyperglycemic, glucagon-suppressing action of peripherally administered leptin has been duplicated via leptin infusion into the intracerebral ventricle (68). Therefore, leptin suppresses α-cells directly or via a leptin-responsive hypothalamic pathway for glucagon production or both (66, 69).
Diabetes type 1 is characterized by loss of the endocrine and paracrine functions of insulin. α-Cells lack constant regulation of high insulin levels from juxtaposed β-cells and hypersecrete glucagon, which causes directly diabetic symptomatology (70). It has been proposed that insulin monotherapy corrects the endocrine insulin deficiency but may not fully correct the paracrine insulin deficiency that is responsible for the glycemic volatility and the catabolic syndrome of diabetes type 1 (66). If glucagon hypersecretion is the major cause of metabolic aberrations in human diabetes, glucagon inactivation or suppression could provide an attractive therapeutic vista for managing diabetic symptomatology compared with insulin monotherapy.
F. Leptin's role in immune modulation
Leptin presents a pivotal role in the modulation of immune function. In normal subjects, leptin's role is associated with a balance between the number of T helper (Th)1 cells and Th2/Treg (regulatory T cells), which are able to suppress immune and autoimmune responses (71). On one hand, leptin contributes to protection from infectious diseases whereas on the other hand to loss of tolerance and autoimmunity. Studies in mice have shown that effects of leptin on the immune system are implemented via central or peripheral pathways (72, 73). Leptin plays a role in the cells belonging to both the innate and the adaptive immune responses. In fact, as a cytokine, leptin affects thymic homeostasis and promotes Th1-cell immune responses by increasing interferon-γ, tumor necrosis factor (TNF)-α, and IgG2a production from B cells (74). Leptin is involved in all processes of NK cell development, differentiation, proliferation, activation and cytotoxicity (75). Leptin constitutes a negative signal for the expansion of naturally occurring human Foxp3+CD4+CD25− regulatory T cells (Treg), a cellular subset suppressing autoreactive responses mediated by CD4+CD25− T cells (76). Hypo- and aleptinemia results in impaired Th1 response and induction of Treg cells; reducing thus the immunocompetence and autoimmunity in mice and humans and increasing susceptibility to infections such as tuberculosis (TB), pneumonia, candidiasis, and bacterial and viral diarrhea (77–79). Conversely, hyperleptinemia and leptin resistance are associated with a low proportion and proliferation of Treg cells, an expansion of Th1 cells and increased secretion of proinflammatory cytokines that sustain and enhance the development of immunoinflammatory responses and autoimmune diseases in subjects with predisposition to autoimmunity (72).
III. Leptin Signaling
A. Leptin and JAK2/STAT3/STAT5 signaling
It has been shown that ObRb activates a cascade of several signal transduction pathways, including Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) (58) (Table 2 and Figure 1). The STAT family comprises Src homology-2 (SH2) domain-containing transcription factors situated in the cytoplasm in quiescent cells. STAT activation results in homo- or heterodimerization, nuclear translocation, and transcriptional activation. STAT3 is a transcription factor that is encoded by the STAT3 gene in humans (80). It mediates the expression of a variety of genes in response to cell stimuli and thus plays a key role in many cellular processes such as cell growth and apoptosis (80, 81). Constitutive STAT3 activation is associated with various human cancers and indicates poor prognosis, suggesting that STAT3 has antiapoptotic and proliferative effects (82–84). In contrast, loss-of-function mutations in the STAT3 gene result in hyper-IgE syndrome, associated with recurrent infections as well as disordered bone and tooth development (85, 86).
Table 2.
Main Signaling Pathways, Main Sites of Action, and Main Effects of Activation by Leptina
| Signaling Pathway | Primary Site of Action | Effects |
|---|---|---|
| JAK2/STAT3 | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite, energy balance, and body weight |
| Regulates neuroendocrine function | ||
| Regulates cell proliferation | ||
| Regulates cell growth and apoptosis | ||
| Regulates bone and tooth development | ||
| STAT5 | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates energy balance and body weight |
| Regulates oncogenesis | ||
| MAPK | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite and body weight |
| Regulates fatty acid oxidation | ||
| Regulates cell hypertrophy | ||
| Regulates cell proliferation and differentiation | ||
| AMPK | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite, energy balance, and body weight |
| Regulates gluconeogenesis and energy homeostasis | ||
| Regulates fatty acid oxidation | ||
| Regulates insulin resistance | ||
| Regulates cell proliferation and survival | ||
| Regulates oncogenesis | ||
| PI3K/Akt/mTOR | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite, fuel balance, and body weight |
| Regulates insulin/leptin resistance | ||
| Regulates sympathetic outflow | ||
| Regulates neuroendocrine function | ||
| Regulates adiposity and glucose homeostasis | ||
| Regulates cell proliferation | ||
| FoxO1 | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite and body weight |
| Regulates energy expenditure | ||
| Regulates cell proliferation and differentiation | ||
| Regulates insulin signaling | ||
| SHP2/MAPK | Hypothalamus, adipocytes, PBMCs, cancer cells, muscle cells, liver cells, etc | Regulates appetite and body weight |
| Regulates STAT3 signaling with SOCS3 | ||
| Regulates mitosis, proliferation, and differentiation | ||
| Regulates cell apoptosis and survival | ||
| Regulates neuroendocrine function |
The JAK2/STAT3 pathway is the first identified signaling mechanism associated with the leptin receptor and plays a pivotal role in energy homeostasis and neuroendocrine function (80, 81, 84) (Table 2 and Figure 1). It has been well established that leptin stimulates ObRb dimerization, which results in JAK2 activation, autophosphorylation, and phosphorylation of Tyr985, Tyr1077, and Tyr1138, which act as docking sites for key downstream signaling molecules (59). Also, in response to leptin, STAT3 binds to phospho-Tyr1138, allowing JAK2 to phosphorylate and stimulate STAT3. Leptin binds to its ObRb receptor and activates the JAK2/STAT3 pathway in the ARC of the hypothalamus, inducing the transcription of the anorexigenic POMC and suppressing the orexigenic AgRP/NPY (65). Moreover, leptin administration induces phosphorylation of JAK2/STAT3 in several cell lines including keratinocytes, endometrial cancer cells, murine adipocytes, and mouse adipose tissue (87). We have recently demonstrated for the first time that leptin activates STAT3 signaling in mouse GT1-7 hypothalamic, C2C12 muscle, and AML12 liver cell lines (19). Also, we have demonstrated that leptin activates STAT3 signaling in human adipose tissue (hAT) in vivo and ex vivo and in human primary adipocytes in vitro (20, 21). Conversely, disrupting the ability of the leptin receptor to activate the STAT3 pathway in mice leads to severe obesity and several neuroendocrine abnormalities including decreased linear growth and infertility (88).
Leptin may also activate phosphorylation of ObRb on Tyr1077, which binds to STAT5 and stimulates STAT5 phosphorylation (32) (Figure 1). STAT5 deletion in the brain causes hyperphagia and obesity to a lesser extent than STAT3 deletion, suggesting that the ObRb/JAK2/STAT5 pathway may also play a role in the regulation of body weight and energy balance by leptin (70) (Table 2). It is important to study in depth the effects of leptin on human hypothalamic or other CNS cells; leptin-increased STAT3/STAT5 signaling in several tissues and cells may have important clinical implications including oncogenesis.
B. Leptin and AMPK signaling
AMPK is an evolutionarily conserved serine/threonine protein kinase central to the regulation of cell growth, proliferation, differentiation, motility, and survival (71) (Table 2 and Figure 1). AMPK is considered a cellular energy sensor that is stimulated by an increase in the intracellular AMP to ATP ratio (71). In its classical role as an intracellular metabolic stress-sensing kinase, AMPK switches on fatty acid oxidation and glucose uptake while switching off hepatic gluconeogenesis. It also exerts a broader role in metabolism by controlling appetite (89, 90). AMPK is also involved in cellular energy homeostasis through AMPK phosphorylation and inhibition of ACC, thus stimulating fatty acid oxidation (91–93).
Leptin's actions in peripheral tissues are partly due to its effects on AMPK, which contribute to leptin's effects on fatty acid oxidation and glucose uptake (94). In mouse skeletal muscle, leptin activates AMPK, which leads to phosphorylation and inhibition of ACC and subsequent stimulation of fatty acid oxidation. There appear to be 2 mechanisms through which leptin activates AMPK in muscle (95, 96). One pathway involves the hypothalamus including the α-adrenergic pathway, resulting in a prolonged effect, and as shown in our previous ex vivo and in vitro experiments (84), the second mechanism encompasses leptin's direct effect on skeletal muscle. The ability of leptin to stimulate fatty acid oxidation in skeletal muscle, which plays a pivotal role in the pathophysiology of insulin resistance (97, 98), may prevent lipotoxicity and subsequent insulin resistance and may underlie leptin's ability to improve insulin resistance and metabolic syndrome in hypoleptinemic humans (99, 100), thus supporting the potential use of leptin in treating lipodystrophy and, possibly, obesity and type 2 diabetes.
In contrast to its actions in muscle, leptin has been shown to exert an inhibitory effect on AMPK in the hypothalamus that results in stimulation of the hypothalamic ACC and subsequent reduction of food intake and weight gain (86). Leptin's hypothalamic effects on AMPK are in part mediated by the MC4R pathway, which promotes anorexia (86). Moreover, the Ca2+/calmodulin-dependent protein kinase (CaMKK2) is an upstream activator of AMPK in the hypothalamus, whereas CAMKK2 inhibition reduces appetite and body weight (88). These observations indicate that the hypothalamic CaMKK2/AMPK/ACC pathway may also mediate leptin's anorexigenic action. Further in vivo studies in humans are needed to elucidate whether leptin administration may alter in vivo signaling by altering a third intermediate factor that possibly could not be studied in vitro.
C. Leptin and PI3K/Akt/mTOR signaling
Phosphatidylinositol-3 kinase (PI3K) has been linked to an extraordinarily diverse group of cellular functions, including cell growth, proliferation, differentiation, motility, survival, and intracellular trafficking (101–103) (Table 2 and Figure 1). These functions are related to the ability of class I PI3Ks, which are responsible for the production of phosphatidylinositol 3-phosphate, phosphatidylinositol (3,4)-bisphosphate, and phosphatidylinositol (3,4,5)-trisphosphate, to modulate protein kinase B, suggesting that PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway play an important role in diabetes mellitus (103, 104). Leptin activates ribosomal S6 kinase, an important physiological substrate of mTOR kinase in the hypothalamus (92). The PI3K/Akt/mTOR signaling pathway is required for an extremely diverse array of cellular activities including proliferation and survival (105–107).
Leptin increases cell proliferation in mouse GT1-7 hypothalamic, C2C12 muscle, and AML12 liver cell lines through mTOR and Akt signaling pathways (19). Moreover, in vitro and in vivo studies have suggested that leptin stimulates cell proliferation through PI3K signaling in myocytes (19, 108) and hepatocytes (109). Leptin may also stimulate this pathway in human cancer cells (110, 111) and in human adipose (21), liver (112), and muscle tissues (19, 113). Similar to the JAK2/STAT3 pathway, the PI3K pathway is also important in central leptin action. This pathway activates POMC-expressing neurons by activating ATP-sensitive potassium channels and voltage-gated calcium channels (114). Also, similar to insulin, the appetite-suppressing effects of leptin are attenuated by intracerebroventricular (icv) administration of PI3K inhibitors (116, 286). Interestingly, the insulin-PI3K pathway hyperpolarizes POMC neurons, which makes them less sensitive to leptin, and may be one common mechanism for both leptin and insulin resistance in obesity (117, 118). Chronic activation of the PI3K pathway in POMC neurons may cause leptin resistance and hyperphagia in mice with POMC neuron-specific deletion of phosphatase and tensin homolog (PTEN) (105). Hence, activation of the PI3K signaling pathway in neuronal cells may influence energy homeostasis and neuroendocrine function, whereas activation of this pathway in the peripheral tissues may mediate leptin's effect on insulin resistance.
D. Leptin and FoxO1 signaling
Forkhead box protein O1 (FoxO1) belongs to the forkhead family of transcription factors that are characterized by a distinct forkhead domain (119) (Table 2 and Figure 1). The specific function of this transcription factor has not yet been determined; however, it may contribute to myogenic growth and differentiation (119). FoxO1 is a downstream effector of insulin signaling with an important role in the regulation of metabolism in various organs including liver (120), pancreas (121), muscle (122), adipose tissue (122), and hypothalamus (123). Also, FoxO1, as a transcription factor, is one of the substrates for Akt phosphorylation resulting in cytoplasmic shuttling from the nucleus, thereby inactivating FoxO1 (124, 125). FoxO1, which stimulates the expression of AgRP and NPY and inhibits POMC expression, is an important downstream mediator of the PI3K pathway (286). It has been demonstrated that the activation of hypothalamic FoxO1 is inhibited by leptin via the PI3K pathway (123). In contrast, overexpression of constitutively active FoxO1 in the mediobasal hypothalamus of rats by adenoviral microinjection leads to a loss of the inhibitory effect of leptin on feeding and results in body weight gain (126). Moreover, hypothalamus-specific FoxO1 knock-in mice have increased food intake and decreased energy expenditure (127).
E. Leptin and SHP2/MAPK signaling
MAPKs are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens, osmotic stress, heat-shock, and proinflammatory cytokines) and regulate various cellular activities, such as gene expression, mitosis, differentiation, proliferation, and cell survival/apoptosis (19, 21, 128, 129). MAPKs encompass a number of signaling molecules that include ERK1/2, p38 and c-Jun N-terminal kinase (JNK) (21). ERK1/2 represents the major MAPK involved in leptin's central effects contributing to the regulation of food intake, energy expenditure, and body weight (3).
Leptin stimulates ERK1/2 activation via SH2-containing protein tyrosine phosphatase 2 (SHP2) (Table 2 and Figure 1). SHP2 is a protein tyrosine phosphatase (PTP) that contains 2 SH2 domains located in its NH2 terminus as well as a COOH-terminal PTP domain (130). This specific structure suggests that SHP2 is involved in regulating signals initiated by receptor tyrosine kinases (130). These phosphotyrosines act as docking sites for recruitment of SH2-containing proteins, including SHP2, to activate downstream signaling cascades (131). SHP2 promotes ERK1/2 activation in response to insulin and epidermal growth factor binding to their receptors (132, 133).
Leptin induces phosphorylation of the Tyr985 amino acid residue of the ObRb after JAK2 activation, thereby creating a binding site for the carboxyl-terminal SH2 domain of SHP2 (134). SHP2 itself becomes phosphorylated, recruits the adaptor protein growth factor receptor-bound protein-2 and induces JAK2-dependent activation of ERK1/2 (135). Leptin also activates the MAPK pathway independently of SHP2, probably via receptor activation leading to direct binding of growth factor receptor-bound protein-2 to JAK2 (136). Recently, it has been shown that young mice homozygous for a mutation at the site of the leptin receptor phosphorylation were slightly leaner than wild-type mice, although they still developed adult-onset or DIO (137). These phenotypes probably do not reflect the effect of this mutation on leptin-induced ERK activation but are consistent with the role of the mutation site as a binding site for the suppressor of cytokine signaling 3 (SOCS3), which is a negative regulator of leptin receptor signaling (137). In vitro studies have also demonstrated that ERK is required for the phosphorylation of S6 by the S6 kinase, which enhances cap-dependent translation and protein synthesis (138).
Other members of the MAPK family, p38 and JNK, are also activated by leptin in several cell types and present mainly peripheral actions (139–141), although the associated pathways have not been well characterized. In rat cardiomyocytes, leptin administration stimulates p38, which results in an increase in fatty acid oxidation, whereas inhibition of p38 activation prevents leptin-induced fatty acid oxidation (134). Similar to p38, JNK is not activated in response to leptin treatment in neuronal cells (135, 136) but confers an oncogenic potential to leptin's action after its activation by promoting cancer cell survival and invasion (137).
F. Leptin and JAK2-independent signaling
You et al (137) observed that leptin may stimulate the MAPK and STAT3 pathways in cultured cells that are genetically JAK2 deficient; however, the JAK2-independent signaling and its pathophysiological importance have not been verified in animals or humans. The src tyrosine kinase family members appear to be responsible for mediating leptin JAK2-independent signaling pathway (138). The JAK2-dependent and independent signaling pathways may synergistically mediate responses to leptin (286). Because in vivo leptin actions may differ from in vitro actions, studies of in vivo leptin signaling in humans are needed to study the JAK2-independent leptin signaling and implications.
IV. Leptin and Insulin Signaling
Insulin is a central hormone regulating carbohydrate and fat metabolism in the body (142). Insulin causes cells in the liver, muscle, and fat tissues to take up glucose from the blood, storing it as glycogen in the liver and muscle (142–144). Insulin binds to the extracellular portion of the α-subunits of the insulin receptor (143, 144). This, in turn, causes a conformational change in the insulin receptor that activates the kinase domain residing on the intracellular portion of the β-subunits (143, 144). The activated kinase domain autophosphorylates tyrosine residues on the C terminus of the receptor as well as tyrosine residues in the insulin-receptor substrate (IRS)-1 protein (144). The actions of insulin include 1) increasing or decreasing cellular intake of certain substances, most prominently increasing glucose uptake in muscle and adipose tissue (comprising about two-thirds of total body cells) (142, 144), 2) increasing DNA replication and protein synthesis via control of amino acid uptake (145), and 3) modification of the activity of numerous enzymes (146).
Evidence from in vivo and in vitro studies supports the hypothesis that leptin and insulin signaling networks may overlap on several levels. Intravenous infusion of leptin in mice has several effects on insulin-regulated processes, including increased glucose turnover, increased glucose uptake in skeletal muscle and brown adipose tissue, and decreased hepatic glycogen content (147–149). In vivo leptin administration has also been reported to stimulate insulin's inhibitory effects on hepatic glucose output, while antagonizing insulin's effect on glucokinase and phosphoenolpyruvate carboxykinase (PEPCK) expression, 2 key metabolic enzymes (150–152). In HepG2 human hepatoma cells, leptin has been shown to antagonize insulin-induced down-regulation of PEPCK expression, decrease insulin-stimulated tyrosine phosphorylation of IRS-1, and enhance IRS-1-associated PI3K activity (153). Also, in C2C12 muscle cells, leptin stimulates a non-IRS-1–associated PI3K and mimics insulin action on glucose transport and glycogen synthesis. In contrast, in OB-RL-transfected HepG2 cells, leptin treatment resulted in the recruitment of p85 to IRS-2 but did not modulate the response to insulin (154). Interestingly, in Fao cells, leptin alone had no effects on the insulin signaling pathway, but leptin pretreatment transiently enhanced insulin-induced tyrosine phosphorylation and PI3K binding to IRS-1, while inhibiting these effects on IRS-2 (155). Also, this study demonstrated that treatment by leptin alone induces serine phosphorylation of Akt and glycogen synthase kinase 3 but to a lesser extent than treatment by insulin alone, and that the combination of these hormones did not result in an additive effect (155). These observations suggest complex interactions between the leptin and insulin signaling pathways that can potentially lead to differential modification of the metabolic and mitotic effects of insulin exerted through IRS-1 and IRS-2 and the downstream kinases that are activated. Together, these data point toward cell- and/or tissue-specific interactions between leptin and insulin signaling that are quite diverse and complex. Although the details of this interaction remain to be elucidated, we propose that leptin may contribute to some of the alterations in the metabolic and mitotic effects of insulin action that are involved in the development of insulin resistance, a characteristic manifestation of type 2 diabetes.
An example of the overlap between leptin and insulin resistance has been reported elsewhere (156). The JAK2/STAT3 pathway leads to increased transcription and expression of SOCS3. In fact, SOCS3 acts as a feedback inhibitor by attenuating ObR signaling, thereby playing a key role in leptin resistance, whereas it also attenuates insulin signaling, thereby playing a key role in insulin resistance (157). Apart from leptin, SOCS3 may be induced by other adipocytokines, including resistin (158), TNF-α (159), and IL-6 (160), which may act as amplifiers of SOCS3 expression, thereby further increasing both leptin resistance and insulin resistance.
In summary, it has been shown that a complex network of interacting signaling pathways appears to regulate food intake, energy balance, and body weight (161), suggesting that, in addition to signaling in the CNS, leptin exerts its metabolic effects by acting directly in peripheral tissues. The relative importance of this signaling for regulating adiposity and glucose homeostasis in the periphery remains to be explored. Furthermore, other important challenges include 1) the identification of different signaling pathways downstream of leptin that are integrated in the regulation of body weight, 2) the leptin-insulin cross talk in the CNS, 3) the elucidation of the molecular pathways responsible for leptin resistance/tolerance in obesity, and 4) the elucidation of the underlying mechanisms responsible for the overlapping leptin and insulin resistance occurring in the brain in pathological states such as obesity and diabetes that are associated with insulin resistance in the periphery.
V. Leptin Resistance or Tolerance
The poor efficacy of endogenous leptin to induce weight reduction in obese individuals has given rise to the term of functional leptin resistance, which is based on the concept of insulin resistance in diabetes type 2, where reduced cellular and metabolic insulin responsiveness coexist with insulin hypersecretion. The lack of a precise definition of the term leptin resistance in a universal and quantifiable manner led the National Institutes of Health to conduct a workshop, Toward a Clinical Definition of Leptin Resistance (162). Leptin resistance or, as in our opinion is more correctly stated as tolerance, could be generally defined as the failure of endogenous or exogenous leptin to promote anticipated salutary metabolic outcomes, such as suppression of appetite and weight gain and stimulation of energy expenditure, although leptin's inability to induce desired responses in specific situations results from multiple molecular, neural, environmental, and behavioral mechanisms (162).
The main physiological cause for leptin hypersecretion is diet-induced expansion of adipocytes. However, in DIO, the physiological function of progressive hyperleptinemia remains to be fully defined. It has been shown, however, that lipotoxicity in nonadipose tissues of congenitally aleptinemic obese rodents is far greater than in hyperleptinemic DIO rodents, leading to ectopic lipid deposition in liver, pancreatic islets, and heart and skeletal muscle and causing severe organ dysfunction and cell death with a disease cluster similar to metabolic syndrome (52, 163, 164). Increasing leptin levels in this condition appear to prevent lipotoxicity by minimizing ectopic lipid accumulation into nonadipocytes via leptin-induced fatty acid oxidation. In obesity, an expanding adipose compartment may initially improve whole-body insulin sensitivity, at least temporarily (165, 166). However, later in life, as energy storage capacity in adipocytes is exceeded during the period between the onset of overweight/obesity and the start of the metabolic syndrome, which is considered to be the result of failure to prevent organ damage inflicted by ectopic fatty acids and metabolic trauma (163, 167), leptin resistance develops.
The onset of leptin resistance varies among ObRb-expressing neurons. Diet-induced leptin resistance appears to manifest first in the ARC of the hypothalamus and later in other hypothalamic areas such as the VMH and dorsomedial hypothalamus (153). Interestingly, the impairment in the ObRb/PI3K signaling pathway precedes that of the ObRb/JAK2/STAT3 pathway (154).
First, leptin resistance was thought to be due to leptin receptor mutations or mutations of other genes downstream of leptin, such as POMC and MC4R, which also produce an obese phenotype with associated neuroendocrine dysfunction (3). The instance of genetic mutation abrogating leptin receptor function is similar to classical hormone resistance/insensitivity syndromes (type A insulin resistance, GH insensitivity, etc,), in which genetic alterations of the hormone receptor prevent hormone action. Nonetheless, only a few cases of obesity in humans are due to monogenic syndromes; instead, most cases are multifactorial (155). Hence, the underlying mechanisms responsible for leptin resistance are apparently multiple and probably include the following (Table 3): 1) impaired leptin receptor signaling (11, 127, 156–161, 163–167), 2) defective leptin transport across the BBB (168–172), 3) impaired ObRb trafficking (173, 174), 4) endoplasmic reticulum (ER) stress (21, 175–177), 5) saturable nature of leptin signaling pathways (19, 20, 178, 179), and 6) impaired leptin-induced neural plasticity/circuitry (180–183).
Table 3.
Potential Mechanisms of Underlying Leptin Resistance or Tolerance
| Leptin Resistance or Tolerance | |
|---|---|
| Gene mutations | Leptin receptor |
| Molecules downstream of leptin receptor (POMC, MC4R) | |
| Impaired leptin signaling | SH2B1 ↓ |
| SOCS3, PTP1B, SHP2 ↑ | |
| Impaired leptin neural circuitry | Altered synaptic plasticity |
| Axon guidance | |
| Impaired BBB transport | ObRe–ObRa Antagonism |
| Triglycerides ↑ | |
| Impaired ObRb trafficking | Bardet-Biedl syndrome protein ↓ |
| ER stress | UPR response → impaired STAT3 phosphorylation |
| Saturable nature of leptin pathways | At a free leptin level ≥40–50 ng/ml |
Leptin resistance occurs rarely due to monogenic mutations of leptin receptor or other genes downstream of leptin (POMC and MC4R). Leptin tolerance is usually multifactorial due to 1) impaired activation (down-regulation of SH2B1) or inhibition (up-regulation of SOCS3 or PTP1B) of leptin signaling; 2) impaired leptin-induced neural plasticity/circuitry, given that defects in MC4R and BDNF receptor signaling in the hypothalamus may modulate synaptic plasticity and axon guidance; 3) impaired leptin transport across the BBB because soluble leptin receptor isoform (ObRe) may antagonize the action of ObRa (which is abundantly expressed in brain microvessels constituting the BBB), thereby resulting in leptin's endocytosis, whereas hypertriglyceridemia may also impair leptin transport; 4) impaired ObRb trafficking, as has been reported in relation to Bardet-Biedl syndrome proteins (responsible for promoting ObRb trafficking to the plasma membrane) impairment; 5) ER stress, which promotes the UPR, thereby inhibiting STAT3 phosphorylation; and 6) saturable nature of leptin signaling pathways.
A. Impaired activation of leptin receptor signaling/inhibitors of leptin signaling
Leptin signaling is controlled by both positive (SH2B adaptor protein 1, SH2B1) and negative (SOCS3 and PTP1B) regulators. SH2B1 is an SH2 and pleckstrin homology domain-containing adaptor implicated in cell signal transduction and represents an important positive regulator of leptin sensitivity (115). SH2B1 binds to Tyr813 and enhances JAK2 stimulation, promoting leptin signaling pathways downstream of JAK2 (111). Moreover, SH2B1 binds directly to IRS-1 and IRS-2, inhibiting tyrosine dephosphorylation of IRS-1/2 and increasing PI3K pathway activation (115).
A large contributor to leptin resistance is the defect in negative feedback mechanisms such as SOCS3 and PTP1B, which dampen leptin receptor signaling and prevent overactivation of leptin signaling pathways (168, 169) (Figure 1). The JAK/STAT pathway is negatively regulated by members of the SOCS family, and leptin signaling via Tyr1138 and STAT3 induces SOCS3 expression (169, 170), which in turn switches off leptin signaling by binding to tyrosine residue Tyr985 and inhibiting phosphorylation/activation of JAK2 (171). Therefore, SOCS3 represents a pivotal feedback mechanism preventing the overactivation of leptin-signaling pathways. Overexpression of SOCS3 has been proposed as one of the main mechanisms for the onset of leptin resistance (169). Indeed, hypothalamic SOCS3 expression is significantly increased in several leptin-resistant animal models (169). Immunohistochemical studies suggest that the ARC is selectively leptin resistant in DIO mice, and this may be caused by elevated SOCS3 in this hypothalamic nucleus (172). Mutation of Tyr985 to leucine (l/l) increases leptin sensitivity in female mice (173). However, in contrast to female l/l mice, both male and female Tyr985 mice showed increased susceptibility to high-fat diet (HFD)-induced obesity, supporting a positive action of signaling via Tyr985 in attenuating diet-induced impairment of energy metabolism. Also, because PTP1B is increased in leptin-resistant animals, a role for PTP1B in the onset of leptin resistance has been suggested (174). PTP1B is a ubiquitously expressed tyrosine phosphatase implicated in the negative regulation of leptin and insulin receptor signaling (156, 157, 163) (Figure 1). It has been shown that PTP1B deficiency in LepRb-expressing neurons results in reduced body weight and adiposity compared with wild-type controls and likely underlies the improved metabolic phenotype of global and brain-specific PTP1B-deficient models, suggesting that PTP1B may, independent of leptin signaling, contribute to the regulation of energy balance in mice (184). PTP1B represents another signaling molecule that may inhibit leptin signaling via JAK2 dephosphorylation (164). PTP1B-knockout mice are lean and have increased leptin sensitivity (164). Overexpression of the endogenous leptin enhancer SH2B1 has been shown to counteract PTP1B-mediated inhibition of leptin signaling in cultured cells (175). PTP1B expression is increased by HFD feeding and inflammation (176), but it is still unclear how PTP1B expression and activity are regulated after leptin stimulation or in case of DIO.
SHP2 has also recently been shown to play a critical role in leptin signaling by functioning in the hypothalamic control of energy balance and metabolism (177) (Table 2 and Figure 1). SHP2 appears to down-regulate the ObRb-JAK2/STAT3 pathway while promoting ERK1/2 activation by leptin (177, 178). It has been shown that a prominent phenotype of the mutant (CaMKIIα-Cre:Shp2flox/flox or CaSKO) mice was the development of early-onset obesity, with increased serum levels of leptin, insulin, glucose, and triglycerides (178). This study demonstrated that SHP2 down-regulates JAK2/STAT3 activation by leptin in the hypothalamus but that JAK2/STAT3 down-regulation is offset by the dominant SHP2 promotion of the leptin-stimulated ERK1/2 pathway, leading to induction rather than suppression of leptin resistance upon SHP2 deletion in the brain. These results suggest that a primary function of SHP2 in postmitotic forebrain neurons is to control energy balance and metabolism, representing a critical signaling component of leptin receptor ObRb in the hypothalamus (178). Hence, pharmaceutical augmentation of SHP2 activity in the brain might prove to be an efficient therapeutic strategy for alleviation of leptin resistance in obese patients.
B. Impaired leptin transport across the BBB
Leptin enters the brain by a saturable transport system (179). To reach the ObRb-expressing neurons in the brain, leptin has to cross the BBB (180). Leptin levels in the cerebrospinal fluid are decreased in cases of obesity, suggesting that defective leptin transport may contribute to leptin resistance (180). The short isoform of the leptin receptor, ObRa, abundantly expressed in brain microvessels constituting the BBB, plays a key role in leptin transport across the BBB (181). The soluble leptin receptor isoform ObRe has been shown to antagonize ObRa action and leptin transport through inhibition of leptin surface binding and endocytosis (171). However, a distinct subpopulation of ARC neurons, which can be labeled by BBB-impermeable fluorescent tracers, has been shown to respond more rapidly and sensitively to circulating leptin compared with other hypothalamic ObRb neurons. These neurons might therefore make direct contact with the blood circulation by projections through the BBB (179, 180). In addition to hyperleptinemia, hypertriglyceridemia may also impair leptin transport (171). The exact clinical significance of these findings and/or any differential effects of icv leptin administration in humans remain to be elucidated.
Further studies are needed to clarify the contribution of impaired leptin transport to the pathogenesis of leptin resistance. Because leptin does not appear to be able to cross the BBB easily, it may be possible to develop leptin agonists that cross the BBB more freely or, possibly, antagonists acting only peripherally. Such agents could possibly have beneficial effects on immune responses in autoimmune disease and/or on cardiovascular disease, without causing excess weight gain as would be expected from blocking central leptin action (182).
C. Impaired ObRb trafficking
Few ObRbs are located on the plasma membrane mediating leptin effects; most ObRbs are present in the trans-Golgi network and in small vesicles (173). It has been shown that specific proteins, named Bardet-Biedl syndrome proteins, are responsible in promoting ObRb trafficking to the plasma membrane (173). In mouse models, deletion of Bardet-Biedl syndrome proteins results in leptin resistance and obesity (174). Nonetheless, the importance of these proteins in humans with obesity has not been determined yet.
D. ER stress
ER homeostasis is regulated by balancing ER loading of nascent proteins with ER ability to fold these proteins. An imbalance may result in an overload of unfolded/misfolded proteins in the ER lumen, inducing ER stress (175). ER stress promotes the unfolded protein response (UPR) involving the activation of several UPR intracellular signaling pathways (176).
ER stress has recently been shown to play a role in the development of leptin resistance. Obese, diabetic humans and animals present higher levels of ER stress in the liver, adipose tissue, and pancreatic β-cells (175), suggesting that ER capacity is directly related to leptin sensitivity (183, 184). Hence, ER stress reversal via treatment with chemical chaperones that decrease ER stress (176) could be a strategy to sensitize obese mice, and by extension humans, to leptin. Studies have shown that reducing ER function in mice creates ER stress, leading to blocked leptin action and hence leptin resistance, with a significant augmentation of obesity on a HFD (183). This suggests that ER stress inhibits STAT3 phosphorylation at an upstream step and provides a potential mechanism by which increased ER stress antagonizes STAT3-mediated leptin signaling. We have demonstrated that pretreatment with the ER stress inducers, tunicamycin and dithiothreitol, completely blocks leptin-stimulated STAT3 activation in human primary adipocytes and human peripheral blood mononuclear cells (PBMCs) in vitro, suggesting that increased ER stress in obese people may induce leptin resistance (21). ER stress may also act via other pathways. For example, overnutrition atypically activates the inhibitor of nuclear factor κB (NF-κB) kinase subunit β (IKKβ)/NF-κB, at least in part through elevated ER stress in the hypothalamus (185). Although forced activation of hypothalamic IKKβ/NF-κB interrupts central leptin signaling and actions, suppression of IKKβ significantly protects against obesity and leptin resistance (185). Because in vivo leptin actions may differ from in vitro actions, studies of in vivo leptin signaling in humans are needed to explore this hypothesis. Also, the cross talk between leptin and UPR signaling pathways needs to be fully clarified.
E. Saturable nature of leptin signaling pathways
To study leptin signaling in humans, we have recently performed for the first time in vivo experiments involving metreleptin administration in humans in doses that result in an increase of circulating levels of free leptin up to a peak level of 40 to 50 ng/mL (20). In long-term leptin administration trials in humans, we found that circulating free leptin levels did not result in weight loss, which indicates that free leptin levels in or above the ∼40- to 50-ng/mL range may be clinically ineffective (20). Also, we observed that in vivo metreleptin administration (0.01 mg/kg for 30 minutes) induces phospho-STAT3 in both hAT and hPBMCs, but this signaling is saturable at a level of ∼50 ng/mL metreleptin (20). These levels are higher than the putative threshold for saturating the BBB leptin transport system, which may explain the absence of clinical effect of high leptin levels in obese humans despite the dramatic effects seen when replacing leptin in subjects with very low concentrations of leptin (178, 179).
Consistent with this result, we have also observed in hAT ex vivo and human primary adipocytes in vitro and in mouse hypothalamic, muscle, and liver cell lines in vitro that leptin signaling pathways were saturable at a level of ∼50 ng/mL (19), suggesting that no additional signaling effect may be observed at higher doses. Overall, we suggest that the saturable nature of leptin signaling pathways may play a key role in the development of leptin tolerance in obese humans.
F. Impaired leptin neural circuitry
It has been shown that leptin-deficient (ob/ob) mice differ from wild-type mice in terms of numbers of excitatory and inhibitory synapses and in terms of postsynaptic currents onto NPY and POMC neurons (181). Hence, when leptin was delivered systemically to ob/ob mice, the synaptic density rapidly normalized, an effect detectable within 6 hours, several hours before leptin's effect on food intake (181). This study suggested that leptin-mediated plasticity in the ob/ob hypothalamus may underlie some of the hormone's behavioral effects. Also, it has been reported that leptin changes brain structure, neuron excitability, and synaptic plasticity and regulates feeding circuits, suggesting that leptin may play a role in the development of the CNS (182). Indeed, leptin has been proposed to be a crucial regulator of both synaptic plasticity and axon guidance within the hypothalamus, suggesting fresh links between nutrition and neurodevelopment mediated by leptin, with potentially important implications for the physiology of energy balance and body weight homeostasis (183). Leptin's anorexigenic and metabolic effects are mediated by a sophisticated network of neurons in the hypothalamus as well as other areas in the brain. Leptin resistance, hyperphagia and obesity are characteristic manifestations after defects in MC4R and BDNF receptor signaling in the paraventricular hypothalamus and the VMH, respectively (180). Therefore, leptin sensitivity may be impaired by genetic or environmental factors that modulate leptin neural circuitry. More studies are required to study in depth the leptin neurocircuity by analyzing its anatomic, biochemical, and electrical components.
VI. Effects of Leptin on Glucose Metabolism in Animals and Humans
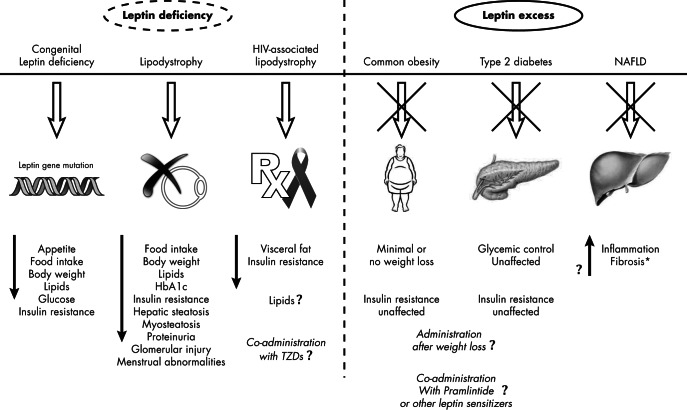
Leptin replacement is a promising therapeutic approach in several disease states characterized by aleptinemia or hypoleptinemia, including congenital complete leptin deficiency, and states of energy deprivation, comprising anorexia nervosa or milder forms of hypothalamic amenorrhea, as well as syndromes of insulin resistance observed in conditions such as congenital or acquired lipodystrophy. Conversely, states of energy excess such as garden-variety obesity and diabetes type 2, which represent the vast majority of the obese and diabetic population, are associated with hyperleptinemia and are characterized by the absence of clinical effects of leptin, reflecting leptin tolerance or leptin resistance. Table 4 displays leptin administration effects in different disease states based on initial leptinemia. If the leptin resistance or tolerance obstacle is overcome, leptin treatment would become an effective therapeutic approach against common obesity and diabetes type 2.
Table 4.
Effects of Leptin Administration in Different Disease State on the Basis of Circulating Leptin Levels at Baselinea
| Disease States Associated With Different Initial Leptin Levels | Therapeutic Approach | Main Results |
|---|---|---|
| Disease states associated with undetectable initial leptin levels (<0.05 ng/mL) | ||
| Congenital leptin deficiency | Leptin | Significantly reduced food intake and body weight and improved metabolic derangements |
| Disease states associated with very low initial leptinemia (<5 ng/mL) | ||
| Congenital lipodystrophy syndromes | Leptin | Significantly improved insulin sensitivity and dyslipidemia |
| HALS | Leptin | Significantly decreased central fat mass and improved insulin sensitivity but not dyslipidemia |
| HA | Leptin | Recuperation of menstruation; correction in the gonadal, thyroid, GH, and adrenal axes; improvements in bone metabolism and skeletal health |
| Diabetes mellitus type 1 | Leptin and reduced insulin | Improved insulin sensitivity, glycemic control, and dyslipidemia in 2 patients |
| Disease states associated with moderately low initial leptinemia (∼5 ng/mL) | ||
| Lipodystrophy-associated diabetes type 2 | Leptin | Ameliorated lipidemic profile but not glucose abnormalities |
| Disease states associated with initial hyperleptinemia (>15 ng/mL) | ||
| Garden-variety obesity | Leptin monotherapy | No effect on weight reduction and glycemic control |
| Garden-variety obesity | Leptin and pramlintide | Moderately diminished body weight |
| Obesity and diabetes type 2 | Leptin | Did not alter body weight, marginal or no amelioration in glycemic control |
A. States of leptin deficiency
1. Congenital leptin deficiency
Mice homozygous for a nonsense mutation in the ob gene, the ob/ob mice, have been studied for a long time and represent a commonly used model of obesity. The first demonstration of an effect of leptin on glucose homeostasis was reported in 1995 when these mice were treated with ip injections of leptin (2, 5, 6). Administration of leptin effectively decreased serum glucose and insulin concentrations in a dose-dependent manner, even at the lowest dose where no significant body weight loss was observed. Similarly, a single icv injection of leptin improved glucose tolerance as assessed by an ip glucose tolerance test (GTT) (186). In contrast, db/db mice, which have a mutation in the leptin receptor gene resulting in expression of a truncated and dysfunctional receptor, show no reduction in body weight or improvement in metabolic abnormalities in response to treatment with leptin (187). These observations collectively suggest that leptin may play an important role in glucose metabolism and that leptin administration may correct the metabolic disturbance in leptin-deficient states.
Congenital leptin deficiency is a rare autosomal recessive disorder, caused by mutations in the gene encoding for leptin. In 1997, 2 severely obese children who were members of the same highly consanguineous pedigree of Pakistani origin were reported to exhibit nearly undetectable concentrations of serum leptin (188). They had a homozygous frame-shift mutation involving the deletion of a single nucleotide in codon 133 of the leptin gene. Clinically, they exhibited profound obesity, hyperphagia, hyperinsulinemia, and hyperlipidemia as well as reproductive and immune dysfunction (189–191). Leptin replacement in 1 of these congenital leptin-deficient children decreased appetite, food intake, body weight, and serum insulin and cholesterol concentrations (192), and similar effects have been noted with leptin replacement in other unrelated individuals with leptin deficiency (193, 194). Since then, several other Pakistani children with leptin deficiency have been identified (195). We have studied another group of 4 adult patients of Turkish origin who carry a homozygous missense mutation (78, 191, 196, 197). Hyperinsulinemia was a common feature in these patients, and 1 of them had diabetes mellitus (196). Leptin replacement dramatically reduced body weight and food intake in all patients. Leptin effectively decreased fasting and postprandial glycemia and normalized glycated hemoglobin (HbA1C) levels within 2 months of treatment in the diabetic patient (191). The other patients, who had normal glucose and insulin levels at baseline, maintained normal fasting glucose levels, but their insulin and C-peptide levels decreased significantly to less than half of their baseline values (191). Among them, only 1 adult male patient was submitted to meal tolerance tests before and after 1 week, 18 months, and 24 months of leptin replacement (198). The authors used deconvolution analysis of C-peptide kinetics, modified insulin kinetics modeling, and minimal glucose modeling to determine pancreatic insulin secretion, hepatic insulin extraction, and peripheral insulin sensitivity. After 1 week, leptin replacement increased hepatic insulin extraction by 2.4-fold (resulting in a 1.8-fold decrease of posthepatic insulin delivery), without significant changes in insulin secretion, and also increased insulin sensitivity by 10%. After 24 months, leptin replacement increased insulin sensitivity by at least 10-fold, with additional decreases in insulin secretion and hepatic insulin extraction (198).
In 1998, Clément et al (199) reported a homozygous mutation in the human leptin receptor gene that results in a truncated leptin receptor lacking both the transmembrane and the intracellular domains. Patients homozygous for this mutation showed early-onset morbid obesity, whereas their serum leptin concentrations were much higher than normal. These patients presented normal fasting glycemia and normal glucose tolerance despite their extreme obesity (199). More recently, Farooqi et al (200) sequenced the leptin receptor gene from subjects with early, severe obesity to determine the prevalence of pathologic mutations. Of the 300 subjects, 8 (3%) had nonsense or missense leptin receptor gene mutations; 7 were homozygous and 1 was heterozygous. Most subjects with the mutation presented normal glucose concentrations, although the 2 oldest adults had type 2 diabetes managed with oral hypoglycemic medications. All subjects had hyperinsulinemia to a degree consistent with their obesity (200). These data demonstrate that, in humans, several phenotypic features seen in subjects with leptin receptor mutations are not as severe as those in subjects with leptin deficiency. This is different from the situation observed in mice, where mice homozygous for a mutation in the leptin receptor gene (the db/db mouse) develop more evident diabetes than obesity when compared with ob/ob mice (201). The discrepancy between humans and mice is not yet explained, although mouse studies have shown that genetic background is an important modifier of the effects of leptin deficiency on insulin resistance and diabetes (202).
Leptin is currently available for life-long treatment of subjects with congenital leptin deficiency through a manufacturer-sponsored expanded-access program (203, 204). Figure 2 depicts the effect of recombinant leptin administration in congenital leptin deficiency.
Figure 2.
The effect of recombinant leptin administration in leptin deficiency or leptin excess states. Recombinant leptin may have beneficial effects when administered in leptin-deficiency states, but minimal benefit and/or mostly adverse effects when administered in leptin-excess states. In congenital leptin deficiency and congenital lipodystrophy, recombinant leptin administration results in metabolic and neuroendocrine improvement; improvement seems to be more limited in HIV-associated acquired lipodystrophy syndrome, in which coadministration of pioglitazone, which acts by up-regulating endogenous adiponectin, may increase the metabolic benefit. On the other hand, recombinant leptin administration in type 2 diabetes mellitus and/or common obesity and normal or high circulating leptin levels has minimal or null metabolic effect, whereas it increases circulating leptin-binding protein and antileptin antibodies. Coadministration of pramlintide, an amylin analog targeting leptin tolerance, or leptin administration after weight loss, when leptin's declined levels increase appetite, might prove to be of some benefit. Similarly, leptin up-regulation in animal models with NAFLD resulted in increased inflammation and fibrosis, thereby progression to NASH. Data on adverse effects, including renal impairrment or T-cell lymphoma, observed mainly in a minority of leptin-deficiency patients, are currently inconclusive. Data on carcinogenic effect of leptin, mainly observed in hyperleptinemic individuals, are also inconclusive but may be tissue-specific; however, hypoleptinemia itself may be also implicated in carcinogenesis in hypoleptinemic individuals, and thus this aspect of leptin physiology remains to be fully elucidated. *, Only experimental data.
2. Lipodystrophy
Lipodystrophy is a group of clinically heterogeneous acquired or inherited disorders characterized by complete or partial absence of sc fat (lipoatrophy) that can occur in conjunction with the pathological accumulation of adipose tissue (lipohypertrophy) in other distinct regions of the body (10, 204). Although congenital lipodystrophies are exceedingly rare, acquired lipodystrophies (especially those associated with HIV infection and its treatment) are more common. Interestingly, lipodystrophic patients exhibit insulin resistance, hyperglycemia, dyslipidemia, and hepatic steatosis (205). The severity of these metabolic derangements typically correlates with the degree of adipose tissue loss.
Several mouse models of lipodystrophy exist, and the metabolic profiles of these models are similar to those of human lipodystrophy (206). Among them, the lipodystrophic adipocyte fatty acid binding protein 2 sterol regulatory element-binding protein 1c (aP2-SREBP-1c) transgenic mice exhibit many typical features of human generalized lipodystrophy (207). These animals have marked insulin resistance, hyperglycemia, and dyslipidemia and very low serum concentrations of leptin due to the lack of sc adipose tissue. When these mice were treated with leptin, their metabolic abnormalities and diabetic phenotypes were almost normalized (207). Leptin treatment was associated with reduced food intake and weight loss, but inducing weight loss by restricting access to food did not lead to changes in insulin or glucose concentrations in transgenic mice, suggesting that the beneficial effects of leptin are separate from its effects on body weight (207). The icv administration of leptin was as effective as peripheral administration (208).
Another mouse model of lipodystrophy is the lipoatrophic A-ZIP/F-1 mouse model (209), which has a more severe phenotype with almost complete lack of adipose tissue, insulin resistance and diabetes, and low leptin levels. In this mouse model, administration of a high dose of leptin (30 μg/d) led to only moderate improvements in glucose and insulin concentrations (209). In contrast, transgenic overexpression of leptin in these mice leads to a lipoatrophic mouse model that has elevated leptin concentrations (LepTg/+:A-ZIPTg/+). These mice have significantly fewer metabolic abnormalities when compared with the low-leptin A-ZIP/F-1 mice (210). The somewhat discrepant findings may be related to the difference in the achieved leptin concentrations with exogenous administration versus transgenic overexpression of leptin. In contrast to the former study in which plasma leptin concentration increased to 5 ng/mL (209), plasma leptin concentrations in the latter study were markedly elevated to 50 ng/mL (210). Collectively, these animal experiments demonstrated that exogenous leptin administration may overcome the diabetic abnormalities characteristic of lipodystrophy, and thus, these studies prompted therapeutic trials of leptin replacement in patients with lipodystrophy.
In humans, congenital lipodystrophy syndromes are rare; there have been fewer than 1000 described cases in the literature (203, 211). Lipodystrophic subjects have partial or total leptin deficiency (222). Leptin replacement dramatically improves dyslipidemia and insulin sensitivity and reduced HbA1C levels and intrahepatic fat content in various types of human lipodystrophy, notably those associated with severe hypoleptinemia (fasting serum leptin less than 4–5 ng/mL in our laboratory) (213–216, 218–220, 319). Fasting blood glucose and HbA1C levels decreased markedly even in patients who are not fully responsive to other antihyperglycemic medications or high doses of insulin. Javor et al (216) also reported that 15 patients with lipoatrophic diabetes were able to decrease or even discontinue diabetic therapy and maintain normoglycemia after 12 months of leptin replacement. Petersen et al (214) performed a hyperinsulinemic-euglycemic clamp in 3 patients with lipoatrophic diabetes and showed remarkable improvement in insulin sensitivity after leptin treatment. This beneficial effect was seen both with hepatic insulin sensitivity and with whole-body insulin sensitivity. In conjunction with this, there was an 86% reduction in intrahepatic triglyceride content and a 33% reduction in muscle triglyceride content (214). Chong et al (218) recently published an open-label, uncontrolled prospective study in 48 patients with various acquired and inherited forms of lipodystrophy. Leptin replacement effectively decreased serum triglyceride concentrations by 59% and HbA1C levels by 1.5 percentage points within 1 year in these patients. The benefits of leptin replacement were sustained for up to 8 years of follow-up (218). Oral and Chan (220) collected several small, nonrandomized, open-label trials in a composite study reporting on a total of more than 100 patients with severe lipodystrophy. Based on these studies, they reported that leptin treatment resulted in improvement in several metabolic parameters, including glycemic control, insulin sensitivity, plasma triglycerides, caloric intake, liver volume and lipid content, and intramyocellular lipid content. These beneficial responses were durable after 3 years of therapy (221).
However, lipodystrophy comprises a heterogeneous group of disorders with diverse circulating levels of leptin (222). Indeed, most patients with lipodystrophy may exhibit moderate hypoleptinemia (fasting serum leptin ∼5 ng/mL). Recent data have shown that although leptin treatment is effective in ameliorating lipid profiles, notably hypertriglyceridemia, it does not restore metabolic homeostasis in lipodystrophic subjects with moderate hypoleptinemia (223), hence questioning the antidiabetic potential of leptin in lipodystrophic patients. Therefore, lipodystrophic subjects with moderate hypoleptinemia are expected to respond poorly to leptin's effect on glycemic control but may benefit from its hypolipidemic effect. This limitation of leptin treatment in the context of lipodystrophy associated with moderately low levels of circulating leptin highlights the need of developing better therapeutic approaches including combination therapy to treat metabolic derangements in these patients.
Leptin administration in replacement doses constitutes an important step forward in the understanding and treatment of various lipodystrophic syndromes, conditions characterized by a hypoleptinemic state. Particularly, recombinant methionyl leptin administration in patients with lipodystrophy exhibiting severe hypoleptinemia ameliorated hyperinsulinemia, insulin resistance, hyperglycemia, hypertriglyceridemia, and neuroendocrine abnormalities without significant adverse effects and provided the rationale for metreleptin treatment in these patients. It is important to underscore that the antidiabetic effect of leptin observed in these patients was achieved even after discontinuation of previously administered antidiabetic treatment, therefore excluding the possibility of a synergistic positive effect of antidiabetic medication and leptin on glucose and lipid abnormalities (213). However, there are several caveats. Existing studies are all open-label and uncontrolled; hence, a placebo-controlled trial is warranted, although the small number of patients and the lack of firm diagnostic criteria present hurdles to this endeavor (203). Well-designed, probably crossover, randomized, placebo-controlled trials would be needed to accurately assess and quantify efficacy as well as to allow for a comparison with the current standard of care. Furthermore, the side-effect profile of leptin has not yet been fully characterized. Deterioration of renal function, development of lymphomas, and other side effects have been reported in lipodystrophic subjects (223–225), but because these studies were not placebo controlled, it remains unknown whether the observed side effects are leptin-specific or random and/or represent the natural history of the underlying disease. Thus, although the optimal treatment regimen for this condition has not yet been fully determined, metreleptin is currently available for these subjects as part of an expanded access program, while an application for approval for this indication is under review by the FDA. Figure 2 depicts the effect of recombinant leptin administration in lipodystrophy.
3. HIV-associated lipodystrophy syndrome
Currently, the most prevalent type of lipodystrophy is an acquired form of the syndrome that occurs in HIV-infected individuals treated with highly active antiretroviral therapy (HAART) (224). HIV-associated lipodystrophy syndrome (HALS) affects an estimated 15% to 36% of HIV-infected patients and is associated with an increased risk of insulin resistance, diabetes mellitus, dyslipidemia, and cardiovascular disease (225). HALS has been associated with dyslipidemia including elevated serum low-density lipoprotein (LDL) cholesterol and triglyceride (TG) levels and lower high-density lipoprotein (HDL) cholesterol (226). HALS patients usually exhibit sc fat loss and increased abdominal fat. The impact of lipodystrophy on the psychosocial health in HIV-positive individuals, ranging from somatic discomfort to low self-esteem, stigmatization, and depression can be significant (227). The exact physiological mechanisms that lead to HALS are still not fully defined, but it has been suggested that protease inhibitors interfere with peroxisome proliferator-activated receptor-γ (PPARγ) action leading to an impairment of normal metabolic processes in the adipocyte (228). Nucleoside analogs are also associated with HALS, which is thought to be secondary to mitochondrial injury. There may also be direct effects of the HIV itself (224, 228).
In patients with HIV who are on HAART, leptin levels are mostly related to their overall fat mass, as also seen in individuals without HIV infection (229–231). Adiponectin levels, which are also low in patients with HIV, are mainly related to abnormal fat distribution rather than overall fat mass (232). Approximately 20% to 30% of individuals who have HALS manifest hypoadiponectinemia and hypoleptinemia, but these manifestations are less dramatic than those seen in individuals with congenital lipodystrophies (231). Nevertheless, both lower adiponectin and leptin levels have been associated with worse insulin resistance (231). Hence, we and others have investigated the role of leptin replacement in these patients. Nonetheless, generally it will be fair to expect a less dramatic response to leptin treatment in individuals suffering from HALS given that these patients do not manifest an absolute leptin deficiency comparable to congenital lipodystrophy (203).
In our initial randomized, placebo-controlled, crossover 2-month interventional study, we found that leptin in physiological doses improved metabolic disturbances in these patients (233). Leptin treatment was associated with a 15% decrease in central fat mass as well as significant improvements in insulin sensitivity and fasting insulin and glucose levels, albeit having no effects on LDL and TG (233). These results were confirmed by a longer independent study of open-label leptin treatment for 6 months (234). Leptin treatment was associated with a 32% decrease in visceral fat as well as significant improvements in fasting insulin, glucose levels, and lipid parameters including a decrease in LDL and non-HDL cholesterol and an increase in HDL by the sixth month (234). This study showed that whole-body insulin sensitivity was markedly improved and found that the leptin-induced increase in insulin sensitivity was especially pronounced in the liver but not skeletal muscle (234). These observations suggest that the benefits of leptin replacement are sustained, and even increased, for as long as treatment continues in patients with HALS. Although there are no head to head comparisons, studies performed to date indicate that leptin's ability to improve insulin sensitivity is comparable to or better than that of other available medications, including metformin and thiazolidinediones (235, 236). However, the more pronounced effect of leptin on lipid metabolism in the study by Mulligan et al (234) needs to be interpreted cautiously given that this study was nonrandomized and not placebo-controlled and its study participants continued to use their lipid-lowering regimens during leptin treatment. Recent results from a small randomized, double-blinded, placebo-controlled clinical trial indicate that leptin administration improves glycemia and non-HDL cholesterol levels to some extent but cannot ameliorate lipid kinetics in adults with HIV-associated dyslipidemic lipodystrophy, providing a mechanistic reasoning behind the inconsistent effect of leptin on lipid profiles (237). Specifically, metreleptin administration to hypoleptinemic patients with HIV-associated dyslipidemic lipodystrophy at doses that significantly elevate plasma leptin levels does not ameliorate the markedly abnormal fasting lipid kinetic defects characteristic of this condition, ie, accelerated lipolysis and adipocyte and hepatic reesterification, with blunted oxidative disposal of free fatty acids (237). Conversely, recombinant human GH and human GHRH, which present beneficial effects on lipid metabolism, have been shown to improve visceral fat, TG, and cholesterol levels but not glucose parameters in patients with HALS, presenting also an unclear impact on cardiovascular endpoint (238, 239).
Similar to leptin, hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and adipose tissue redistribution in patients with HALS (240–242). Adiponectin administration has been demonstrated in animals to improve insulin sensitivity and dyslipidemia (243, 244). Although adiponectin or leptin alone only partly alleviate insulin resistance in mouse models of lipodystrophy, the combined administration of both hormones fully normalizes insulin sensitivity (244). Given that no synthetic form of adiponectin is yet available for human use, animal studies as well as studies on medications that indirectly increase endogenous levels of adiponectin such as thiazolidinediones, have highlighted adiponectin's therapeutic potential (245). We have found that pioglitazone, a thiazolidinedione that increases adiponectin levels, may have beneficial effects on HALS-associated insulin resistance. Recently, we performed a randomized, double-blinded pilot study on the combined administration of leptin and pioglitazone for 3 months in 9 HALS patients (246). Compared with pioglitazone alone, combined treatment reduced fasting serum insulin concentrations, increased adiponectin concentrations, improved insulin resistance (homeostasis model assessment [HOMA]), and attenuated postprandial glycemia in response to a mixed meal (246). These preliminary findings indicate that this combination could further improve glycemic control and needs to be replicated in larger and longer-term studies. If confirmed and proven to be superior to the current standard of care, the treatment of HALS in the future may involve leptin replacement in conjunction with pioglitazone and/or adiponectin. Figure 2 depicts the effect of recombinant leptin administration in HALS.
4. Hypothalamic amenorrhea
Hypothalamic amenorrhea (HA) is a disorder characterized by the cessation of menstrual cycles with anovulatory infertility and moderate to severe hypoleptinemia, usually caused by reduced food intake or chronic energy deficiency secondary to strenuous exercise (204). Leptin administration to lean women who are affected by this disorder and undertake strenuous exercise resulted in recuperation of reproductive outcomes, correction in the gonadal, thyroid, GH, and adrenal axes, and improvements in markers of bone formation (247–249). Nonetheless, larger clinical trials are needed to confirm the previous studies.
B. States of leptin excess
1. Common obese state
In contrast to the few obese subjects with congenital leptin deficiency, most obese individuals exhibit greater leptin concentrations than lean subjects due to their greater amount of fat mass and are unresponsive to exogenous leptin in terms of anorectic effects, body weight reduction, and glycemic control, due to leptin resistance as discussed in Introduction (22, 23) (Table 5). These results reduced expectations from leptin-based antiobesity therapeutic approaches.
Table 5.
Disease States Associated With Leptin Deficiency and Leptin Resistancea
| Disease State | Estimated Prevalence | Clinical Characteristics |
|---|---|---|
| Fat loss associated with leptin deficiency | ||
| Congenital generalized lipoatrophy | Rare | Generalized fat wasting, impaired glucose tolerance, insulin resistance or type 2 diabetes, dyslipidemia, hepatic steatosis, acanthosis nigricans |
| HAART-induced lipoatrophy | 15%–36% of HIV-infected patients | Fat wasting of the face, arms, legs, and buttocks; impaired glucose tolerance, insulin resistance, or type 2 diabetes; hypertriglyceridemia, hepatic steatosis |
| HA (functional) | 3%–8.5% in women aged 13–44 y | Strenuous exercise, psychogenic stress, energy deficit, osteoporosis, neuroendocrine dysfunction with decreased GnRH pulsatility and estradiol levels, decreased thyroid and IGF-I levels, and increased GH levels |
| Up to 69% of trained female athletes | ||
| Anorexia nervosa | 1%–3% of college-age girls | Disturbed body image, severe restriction of food intake, body weight loss, neuroendocrine dysfunction |
| Obesity as a manifestation of leptin deficiency | ||
| Complete congenital leptin deficiency | Rare | Hyperphagia, early-onset morbid obesity hypogonadotropic hypogonadism, advanced bone age, hyperinsulinemia and type 2 diabetes, immune dysfunction |
| Heterozygous leptin deficiency | ≤5%–6% in obese subjects | Garden-variety obesity with hypoleptinemia relative to fat mass, normal neuroendocrine function |
| Obesity associated with leptin resistance (involving leptin and molecular signaling pathways downstream of the leptin receptor) | ||
| Leptin receptor gene mutation | Rare | Phenotype similar to congenital leptin deficiency, hyperphagia but less remarkable hyperinsulinemia, hypogonadotropic hypogonadism, mild growth retardation, hypothalamic hypothyroidism, abnormal GH secretion |
| POMC mutations | Rare | Hyperphagia, early-onset obesity, ACTH deficiency with adrenal insufficiency/crisis, lack of MSH function at MC1Rs resulting in pale skin and red hair |
| Prohormone convertase deficiency | Rare | Hyperphagia, early-onset obesity, hypogonadotropic hypogonadism, abnormal glucose homeostasis, hypoinsulinemia, hypocortisolemia |
| MC4R mutations | 5%–8% of childhood obesity | Hyperphagia, early-onset obesity, increased fat and lean body mass, increased linear growth and bone density, severe hyperinsulinemia |
| Melanin-concentrating hormone receptor-1 mutations | Rare | Dysregulation of energy homeostasis with onset in childhood |
| Neurotropin receptor tropomyosin-related kinase B mutations | Rare | BDNF deficiency resulting in severe obesity, hyperphagia, developmental delay, and cognitive dysfunction |
| Mutations of other molecules downstream of leptin receptor | Rare | Obesity with onset in childhood |
| Mechanism to be discovered | >90% of obese individuals | Garden-variety obesity |
Despite their shortcomings, mouse models with HFD-induced obesity may provide important insights into understanding the effects of leptin on glucose metabolism in obese humans because these mouse models are the closest to human obesity. Chronic infusion of leptin in C57BL/6J mice on HFD did not improve the glucose response to insulin during an insulin tolerance test, even though the body weight of mice effectively decreased compared with control mice (250). Moderate hyperleptinemia achieved by adenovirus gene therapy somewhat improved glucose tolerance in animals that had 4-fold greater leptin concentrations compared with control rats (251). Therefore, serum concentrations of leptin may be an important factor responsible for the restoration of aberrant glucose metabolism in HFD-induced obese animal models. Leptin administration in other mouse models of obesity has shown mixed results. Harris et al (252) found that leptin treatment improved insulin resistance and hyperglycemia in some strains of db/db mice, but the effects depended on sex and mode of administration (chronic or bolus ip administration). Other authors found no benefits of leptin administration in db/db mice (5). Leptin treatment in a mouse model of obesity that is deficient in brown adipose tissue resulted in no improvement in glucose metabolism (253).
Subsequently, Heymsfield et al (22) conducted a randomized, double-blind, placebo-controlled trial to determine the relationship between increasing doses of exogenous leptin administration and weight loss in both lean and obese humans. The results of this trial indicated that daily administration of recombinant human methionyl leptin induced dose-dependent but modest weight loss in most but not all subjects. Only at pharmacological doses did leptin induce some consistent degree of weight loss, but even then, the effect was relatively mild (average weight loss was 7.1 kg after 24 weeks in the group receiving the highest dose of leptin). In addition, these investigators performed a standard oral GTT at baseline and after 24 weeks and found no indication that leptin administration affected glycemic control (22). Another study also demonstrated that weekly injection of 60 mg of pegylated recombinant human leptin did not lead to clinically significant weight loss after 8 weeks of treatment, despite serum leptin concentrations being as high as 3000 ng/mL, ie, 100-fold higher than physiological levels. Insulin sensitivity, assessed with the HOMA score, was also not significantly affected by leptin treatment (254). This has been confirmed in a recent study using the hyperinsulinemic-euglycemic clamp, where leptin treatment for 14 days did not have any effect on insulin sensitivity (281). These results were confirmed by others and illustrate that leptin administration is not an effective treatment for metabolic disturbances in common obesity, at least not unless leptin resistance can be overcome (256, 257). Nevertheless, in a manner equivalent to administration of insulin for the control of glycemia in type 2 diabetes with insulin resistance, the possibility exists that leptin administration might be able to overcome leptin resistance.
In leptin-based antiobesity approaches, another important issue to consider is that leptin levels drop dramatically with weight loss and remain low for up to 1 year after weight loss (258–260). This has been proposed to be associated with activation of mechanisms inducing weight regain, such as increased food intake and reduced energy expenditure (259) as seen in the case of lean subjects (261). Hence, studies have looked at the role of leptin replacement to physiological levels after weight loss. Small studies have shown an improvement in weight loss maintenance as well as a reversal of changes in thyroid hormones, energy expenditure, and neural activity centers involved in the regulatory, emotional, and cognitive control of food intake (259, 262). However, the fact that these studies were small and of sequential study design indicates that future randomized and controlled studies are required to define this potential use of leptin in more detail. This may be related to saturability of the leptin pathways at higher leptin concentrations, leading to absent response to increases beyond the saturation level but marked responses after weight loss when leptin levels are low (20). In this respect, studies of leptin administration in lean women showed a further reduction in fat mass without a reduction in lean body mass, indicating that lean subjects with low physiological leptin levels at baseline are sensitive to exogenously administered leptin, which decreases primarily fat mass while sparing lean mass and increasing bone mass (12).
Recent clinical data have shown that combination treatment with leptin and pramlintide, an amylin analog, modestly decreased body weight (−12%) in leptin-resistant obese individuals more than leptin or amylin alone, but the effect of the combination was rather additive not synergistic as had previously been suggested by experiments in rodents (264, 283). However, due to the relatively small period of treatment (20 weeks), it remains to be seen whether a longer-term clinical trial would result in further reduction or maintenance of body weight or may cause unaticipated beneficial or adverse effects. To address these issues, longer-term clinical trials have been initiated, and results are awaited with interest.
In summary, leptin monotherapy is not an effective therapeutic approach for obesity in most obese subjects (representing ∼90% of the obese population) with hyperleptinemia (circulating leptin levels above 15 ng/mL). However, the efficacy of leptin monotherapy or combination therapy in obese subjects exhibiting hypoleptinemia or normoleptinemia remains unknown. Figure 2 depicts the effect of recombinant leptin administration in the common obese state.
2. Diabetes mellitus
a. Type 1 diabetes mellitus.
Many patients with type 1 diabetes have low levels of leptin (265), and this has also been seen in animal models of type 1 diabetes mellitus (266). A decrease in leptin secretion may be related to a reduced adipose mass and reduced assimilation and storage of energy substrates in adipose tissue in insulin deficiency (266).
Hence, several studies have examined the effects of leptin administration on glucose metabolism in insulin-deficient animal models of diabetes mellitus. Subcutaneous infusion of leptin restored euglycemia and normalized peripheral insulin sensitivity in streptozotocin (STZ)-induced type 1 diabetic animals (267, 272). Central leptin infusion also restored euglycemia in STZ-induced diabetic animals (269–271). Similar effects of leptin were also demonstrated in other studies by using various animal models of type 1 diabetes (58, 272–274). Pair-feeding studies indicate that decreased food intake does not account by itself for the restoration of normoglycemia in leptin-treated type 1 diabetic animals (273). These effects of leptin were achieved through an insulinlike or insulin-sensitizing but insulin-independent mechanism mediated by the CNS (68). It has been suggested that leptin may act via activating intracellular signaling pathways that overlap with those of insulin and/or by other mechanisms (mainly suppression of glucagon) that remain to be fully elucidated. Indeed, leptin monotherapy or combination with low-dose insulin reversed the lethal catabolic state via suppression of hyperglucagonemia in nonobese diabetic mice with uncontrolled type 1 diabetes (58, 67). Moreover, leptin normalized HbA1C levels with far less glucose variability compared with insulin monotherapy (58). This recent promising work from the Unger laboratory (54, 55) suggests that leptin administration in humans may present many advantages over insulin monotherapy for the treatment of diabetes type 1, characterized as a condition with relative leptin deficiency. In humans, significant reductions in circulating leptin levels have been documented in the newly diagnosed untreated human type 1 diabetic patients, suggesting that reduced leptin secretion might be a common feature in insulin-deficient diabetes (265). Insulin deficiency in type 1 diabetes leads to a state of increased lipolysis from adipocytes elevating circulating levels of free fatty acids and ketonemia. These metabolic products may reduce adipocyte's ability to secrete leptin, which signals an energy deficit. In view of its beneficial effects in animals, it has been suggested that leptin replacement therapy may prove to be a useful adjunct therapy to restore euglycemia in type 1 diabetes (277). Indeed, 1 year of leptin therapy was effective in improving glycemic control and lipid profiles in 2 patients with type 1 diabetes and acquired generalized lipodystrophy (276). In these individuals, leptin therapy improved insulin sensitivity, although insulin was not completely discontinued but was significantly reduced (276). Leptin's main therapeutic benefits in patients with type 1 diabetes may include its slow-acting glycemia-lowering effect preferred over the fast-acting glycemia-lowering effect that can trigger hypoglycemic events, its antisteatotic and antilipotoxic action in peripheral tissues preferred over the steatosis promoted by insulin therapy that may cause cardiovascular disease, its suppressive effect on glucagon that could reduce the need for insulin, and its ability to restore insulin secretion in β-islets by reversing lipotoxicity (277). Nonetheless, larger clinical trials are needed to be performed to determine leptin's safety and efficacy in reducing insulin requirements, hyperglycemia, glycemic fluctuations, and dyslipidemia in subjects with diabetes type 1.
b. Type 2 diabetes mellitus.
The use of leptin in type 2 diabetes has generated interest in view of the potential insulin-independent activation of intracellular pathways involved in glucose metabolism. Hence, several studies have investigated the use of leptin in animal models of type 2 diabetes mellitus, and more recently, we and others published human studies (in this Section). Despite results from several animal studies demonstrating that leptin ameliorated insulin resistance, glycemic control, and lipid abnormalities in mice with diabetes type 2, the results of 2 clinical trials have shown that leptin treatment is largely ineffective in improving insulin resistance and diabetes in obese subjects with type 2 diabetes (20, 281). Given the fact that a nonnegligible proportion of subjects with diabetes type 2 are not obese, it will be important to evaluate the antidiabetic effect of leptin in those subjects who exhibit normal or low levels of leptin.
Toyoshima et al (278) investigated the use of leptin in the MKR mouse, a diabetic mouse model with normal leptin levels and defects in IGF-I and insulin receptor signaling. They found an improvement in diabetes that was independent of the reduced food intake and was associated with reduced lipid stores in liver and muscle (278). Similarly, Kusakabe et al (279) generated a mouse model mimicking a combination of human type 1 and type 2 diabetes using low-dose STZ and HFD and examined the effect of leptin treatment. These animals exhibited hyperglycemia and hyperleptinemia and only mildly elevated serum insulin levels, suggestive of both insulin deficiency and insulin resistance. After leptin infusion, food intake and body weight decreased, glucose tolerance improved, and serum insulin levels decreased during an ip GTT. Pair feeding showed that the improved glycemic control was independent of the reduction in food intake (279). Finally, our group has investigated leptin therapy in a mouse model deficient in brown fat, which is hyperleptinemic and very sensitive to DIO (253). In these mice, treatment with ip leptin was not associated with any changes in insulin or glucose concentrations (253).
Human studies have also evaluated the use of leptin in obese diabetic patients. Recently, we conducted a double-blinded, placebo-controlled, randomized clinical trial to evaluate the effects of leptin in obese type 2 diabetic patients (20). Seventy-one obese subjects with diet-controlled type 2 diabetes mellitus were recruited and randomized to receive either metreleptin (20 mg/d) or placebo for 16 weeks. Metreleptin administration did not alter body weight or circulating inflammatory markers, but marginally reduced HbA1C (8.01 ± 0.93 to 7.96 ± 1.12, P = .03) (20). Furthermore, we have found that levels of leptin-binding protein and antibodies against metreleptin increased in response to metreleptin treatment, limiting circulating free leptin to ∼50 ng/mL despite mean total leptin levels of ∼1000 ng/mL in these subjects. Although these levels are higher than the putative threshold for saturating the BBB leptin transport system (280), circulating free leptin levels did not correlate with weight loss, indicating clinical ineffectiveness of free leptin levels in the 40- to 50-ng/mL range. Mittendorfer et al (281) also performed a study to evaluate the effects of exogenous leptin in obese type 2 diabetic patients. They conducted a randomized, placebo-controlled trial in obese subjects with newly diagnosed type 2 diabetes assigned to placebo or low-dose (30 mg/d) or high-dose (80 mg/d) metreleptin for 14 days and performed a hyperinsulinemic-euglycemic clamp in conjunction with stable isotope-labeled tracer infusions to evaluate multiorgan insulin sensitivity before and after leptin treatment. There were no significant differences between groups in body weight and body composition. Insulin sensitivity of the liver (suppression of glucose production), skeletal muscle (stimulation of glucose uptake), and adipose tissue (suppression of lipolysis) and basal substrate kinetics were not affected by leptin treatment, even though serum leptin concentrations increased 150-fold compared with baseline in the high-dose group (281). This is very different from leptin replacement in lipodystrophic patients, where the obtained leptin levels were near-physiological (214).
These findings provide more evidence of leptin resistance in the population of obese diabetic subjects. Because most patients with type 2 diabetes are overweight/obese and present hyperleptinemia with leptin resistance, leptin treatment is considered ineffective (20, 281). Targeting the molecular mechanisms of leptin resistance will be important in the treatment for obesity and diabetes type 2. For instance, it has been suggested that the combination of leptin treatment with leptin sensitizers could be a potentially feasible way to overcome leptin resistance. Amylin has been suggested to be one such sensitizer on the basis of animal experiments (282). Amylin may act with leptin to induce fat-specific weight reduction (274), and the amylin analog pramlintide has been used in conjunction with leptin in clinical trials presenting modest effects (277) and potential safety concerns (276). In a phase II clinical trial, the combination of an amylin analog and recombinant leptin produced significantly more weight loss than either treatment alone in obese and overweight subjects (283). This effect was accompanied by trends for improved metabolic parameters such as total cholesterol (−9%), LDL cholesterol (−8%), glycemia (−4 mg/dl), insulinemia (−22%), and insulin resistance assessed by HOMA (−25%) (283). However, these results seem to be additive rather than synergistic, which suggests that amylin may have independent effects on weight loss but does not likely improve leptin sensitivity. We have recently shown that leptin administration does not influence circulating amylin levels (284), and subsequently, we have performed signaling studies in human adipose tissue ex vivo and human primary adipocytes and peripheral blood mononuclear cells in vitro (21). We demonstrated that leptin and amylin alone and in combination activate STAT3, AMPK, Akt, and ERK1/2 signaling pathways (21). These data indicate that leptin and amylin activate overlapping intracellular signaling pathways in humans and have additive, but not synergistic, effects (21).
However, leptin resistance or tolerance may or may not represent an insurmountable obstacle regarding the antidiabetic actions of leptin. Leptin resistance could selectively affect leptin signaling pathways related to appetite and body weight reduction and not those related to glycemic control. Although the ObRb/JAK2/STAT3 pathway is important for leptin-mediated reduction of appetite and body weight, it plays a minor role in leptin's ability to improve euglycemia (285). Moreover, pharmacological inhibition of the hypothalamic PI3K signaling pathway impairs the insulin-sensitizing effects of leptin (286). Research aiming at identifying molecular mechanisms and medications that could up-regulate insulin-sensitizing signaling pathways downstream of leptin is awaited. Data from rodents have also indicated that low doses of leptin can ameliorate hyperglycemia without affecting food intake or body weight, underscoring that the pathways underlying the glucose-lowering actions of leptin are more sensitive than the ones responsible for its weight-reducing effects. Future clinical trials are needed to uncover the clear antidiabetic action of leptin in nonobese patients with diabetes type 2 who exhibit normo- or hypoleptinemia due to low adipose tissue mass in contrast to previous studies examining obese patients with type 2 diabetes. Results of larger studies on combination treatments or the development of specific leptin sensitizers that would enhance leptin's efficacy are awaited with great interest. Figure 2 depicts the effect of recombinant leptin administration in type 2 diabetes.
3. Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD), which is considered to be the hepatic manifestation of insulin resistance syndrome, has emerged as a significant public health problem with worldwide distribution. The prevalence of NAFLD in the general population is 10% to 46% in the United States and 6% to 35% in the rest of the world, with a median of approximately 20% (287). The incidence of NAFLD in both adults and children is rising, in conjunction with the growing epidemics of obesity and type 2 diabetes mellitus. The histologic spectrum of NAFLD encompasses a wide spectrum of liver damage ranging from nonalcoholic simple steatosis (SS) to nonalcoholic steatohepatitis (NASH) and NASH-related cirrhosis with its complications. SS progresses to NASH in about 15% to 30% and in cirrhosis in less than 5% of cases, whereas NASH progresses to cirrhosis in 10% to 15% of cases over 10 years and in 25% to 30% of cases in the presence of advanced fibrosis (288).
As described in the section of leptin signaling (Section III), under normal conditions, leptin suppresses hepatic glucose production and de novo lipogenesis, whereas it induces fatty acid oxidation in hepatocytes, thereby providing an antisteatotic and insulin-sensitizing effect. On the other hand, hyperleptinemia has been proposed to play a role in the pathogenesis of NAFLD (156, 289). There is evidence that leptin acts as a proinflammatory and profibrogenic cytokine, thereby affecting liver fibrosis, a key feature of NASH. Xenobiotics-induced liver fibrosis was extremely diminished in ob/ob mice and Zucker (fa/fa) rats (290). Activated hepatic stellate cells (HSCs) express ObRb and possibly other ObR isoforms, whose activation leads to increased expression of proinflammatory and proangiogenic cytokines and growth factors, such as angiopoietin-1 and vascular endothelial growth factor, which affect hepatic inflammation and fibrosis (291). Moreover, leptin was shown to up-regulate collagen α1 in HSCs through the sequence p38 MAPK activation and SREPB-1c down-regulation (292). Furthermore, leptin may induce hepatic fibrogenesis by stimulating the production of tissue inhibitor of metalloproteinase-1 (293) and repressing matrix metalloproteinase-1 gene expression (294) in activated HSCs. Both these processes are mediated by the JAK/STAT and the JAK-mediated H2O2-dependant MAPK pathways. Inflammatory, fibrogenic, and proliferative leptin actions on HSCs may also be mediated via nicotinamide adenine dinucleotide phosphate oxidase, which acts downstream of JAK activation, but independently of STAT3; inhibition of nicotinamide adenine dinucleotide phosphate oxidase in HSCs resulted in a reduction of leptin-mediated up-regulation of fibrogenic markers (collagen α1 and α-smooth muscle actin) and of inflammatory mediators (monocyte chemotactic protein-1 and macrophage inflammatory proteins 1 and 2) and in a reduction of leptin-mediated HSC proliferation (295). Additionally, leptin facilitates proliferation and prevents apoptosis of HSCs (296), and it modulates all features of the activated phenotype of HSCs in a profibrogenic manner (myofibroblastic phenotype) (297). Once activated, HSCs contribute to further leptin expression (298), thereby possibly establishing a vicious cycle (156).
Data regarding the role of leptin on hepatic fibrosis via an effect on Kupffer or sinusoidal endothelial cells are currently limited. Leptin may promote hepatic fibrogenesis through up-regulation of TGF-β and connective tissue growth factor in isolated Kupffer cells; based on this finding, Kupffer cells-HSCs cross talk has been proposed for hepatic fibrosis (299). Leptin has also been reported to affect hepatic fibrosis through up-regulation of TGF-β in sinusoidal endothelial cells (290).
More recently, an up-regulation of CD14 (an endotoxin receptor recognizing bacterial lipopolysaccharide) in Kupffer cells was followed by hyperreactivity toward low-dose lipopolysaccharide in HFD steatotic mice but not in chow-fed control mice, which resulted in NASH progression (300). When recombinant leptin was administered in chow-fed mice, an up-regulation of CD14 in Kupffer cell was similarly observed resulting in hyperreactivity toward lipopolysaccharide, thereby inducing hepatic inflammation and fibrosis without steatosis. On the contrary, recombinant leptin administration to ob/ob mice did not up-regulate CD14 or induce inflammation and fibrosis, despite severe steatosis (300); these results demonstrated that leptin deficiency, apparently due to lack of its lipolytic effects, may lead to hepatic steatosis, but it is protective toward the progression of SS to NASH, whereas excess of this proinflammatory adipocytokine may favor hepatic inflammation and fibrosis.
In line with the aforementioned data, it was previously speculated that leptin under normal conditions may prevent liver steatosis, whereas its effect on quiescent HSCs and Kupffer and sinusoidal cells is minimal. However, prolonged hyperleptinemia, as observed in leptin tolerance states (ie, common obesity), may ultimately result in overexpression of SOCS3 and in activation of HSCs and Kupffer and sinusoidal cells. Overexpressed SOCS3 may aggravate both leptin tolerance and insulin resistance in hepatocytes and outweigh the primarily beneficial effect of leptin on hepatocytes. Activated HSCs and Kupffer and sinusoidal cells, which seem to display minimal or no leptin tolerance, may trigger the proinflammatory and profibrogenic cascade (156).
Although evidence for the inflammatory and fibrogenic role of leptin in NAFLD from in vivo and animal models is strong, relevant data from clinical studies in NAFLD patients are based mainly on cross-sectional studies, which cannot establish a causative relationship. Most authors have reported higher leptin levels in NAFLD (301, 302) or NASH (303, 304) patients than controls, in parallel with higher insulin resistance. However, other authors have reported similar leptin levels between NASH patients and controls (305, 306). Regarding liver histology, some authors have also reported that leptin levels were positively associated with hepatic fibrosis (303, 307, 308) or inflammation (303, 308). However, other authors reported no association between serum leptin levels and hepatic fibrosis or inflammation (304, 306).
Soluble leptin receptor (sOB-R) has been reported to be lower in the NAFLD patients than controls, as well as negatively associated with circulating leptin in one study, which might be attributed to counteracting sOB-R down-regulation (301). However, other authors have reported higher sOB-R levels in morbidly obese patients with diabetes, which are positively associated with the stage of hepatic fibrosis (309), whereas others found no association of sOB-R levels with hepatic histology in children with NAFLD (308). More studies are needed to elucidate the interaction of leptin with its soluble receptor and its role, if any, in the pathogenesis of NAFLD. The cross-sectional nature of the aforementioned clinical studies together with the heterogeneity within them render the drawing of any conclusion risky. Larger studies with better characterized and/or homogenous populations and carefully matched controls are of importance.
Prospective data regarding circulating leptin levels in NAFLD are limited. In a 3-year prospective study with paired biopsies, serum leptin levels were decreased more in patients with stable or improved disease compared with those with disease worsening; however, leptin could not independently predict disease or fibrosis progression (310). In a 7-year prospective study, leptin levels were higher in patients with than without NAFLD at baseline; however, leptin change was similar in those with disease remission or sustaining disease (311). The limitation of this study was its noninvasive nature (NAFLD was not biopsy-proven). Further prospective studies with paired biopsies and long-term follow-up are needed.
The effect of various therapeutic interventions on circulating leptin levels in patients with NAFLD are summarized in Table 6. Briefly, leptin levels remained essentially unaffected after treatment with pioglitazone (312), rosiglitazone (312), atorvastatin (313), ezetimibe (314), cysteamine (315), and ursodeoxycholic acid with or without vitamin E (316) but increased after short-term melatonin treatment (317) or decreased equally after metformin and lifestyle modifications or only lifestyle modifications (57).
Table 6.
Effect of Various Therapeutic Interventions on Circulating Leptin Levels in Patients With NAFLD
| Ref. | Intervention | Duration | Circulating Leptin Levels |
|---|---|---|---|
| 317 | Melatonin 10 mg/d | 28 d | Increased |
| 275 | Rosiglitazone 4 mg /d | 6 mo | Unchanged |
| 315 | Enteric-coated cysteamine 600–2000 mg/d | 6 mo | Unchanged |
| 314 | Ezetimibe 10 mg/d | 2 y | Unchanged |
| 316 | Ursodeoxycholic acid 12–15 mg/kg/d ± Vitamin E 800 IU/d | 2 y | Unchanged |
| 313 | Atorvastatin 10 mg/d | 2 y | Unchanged |
| 57 | Metformin 1700 mg/d ± diet/exercise | 6 mo | Decreased in either group |
| 312 | Pioglitazone 30 mg/d | 1 y | Unchanged |
Data are presented in publication date order.
Importantly, no study to date has investigated or is planned to investigate the effects of recombinant leptin administration on NAFLD patients with normal or high leptin levels. On the other hand, metreleptin administered in 10 NAFLD patients (8 with NASH) with lipodystrophy for a mean duration of 6.6 months resulted in improvement of hepatic steatosis and ballooning but not portal inflammation or fibrosis (63). These results are similar to Imajo et al (300) experimental model, indicating that recombinant leptin administration in NAFLD patients and lipodistrophy may improve steatosis but not fibrosis. Furthermore, there are 2 ongoing clinical trials having as primary endpoint the effect of recombinant leptin administration in hepatic histology in NAFLD patients with low leptin levels. In one of them (single-group, prospective, open-label), metreleptin (0.1 mg/kg/d once a day sc) in 10 adult male patients with NASH and low circulating leptin levels, but not lipodystrophy, was to be administered for 1 year (NCT00596934). In the other study, (single-group, prospective, open-label), metreleptin (0.1 mg/kg/d once a day sc) was planned to be administered for 1 year in 20 male or female patients with SS or NASH and lipodystrophy (NCT01679197).
Despite the lack of direct clinical evidence, leptin administration in NAFLD patients with hyperleptinemia might have adverse effect on liver fibrosis, thereby possibly worsening the prognosis of liver disease. If the results of the Imajo et al study (300) were translated into human pathophysiology, they might have 2 main implications: 1) leptin excess, which is usually observed in common forms of obesity, may be contributing toward NASH progression and 2) recombinant leptin administration for severe obesity may adversely affect the liver of obese individuals, because it may promote proinflammatory responses, whereas it has no lipolytic effects above a certain level due to its permissive role in controlling obesity (20). Considering that the prevalence of NAFLD in obese individuals is 50% to 95%, this becomes of crucial importance. Figure 2 depicts the effect of recombinant leptin administration in NAFLD.
C. Benefits, potential adverse effects, and challenges in relation to leptin replacement therapy
The benefits and potential adverse effects of recombinant leptin administration in leptin deficiency or leptin excess states are summarized in Table 3. Generally, leptin replacement therapy using an analog of leptin, metreleptin, also known as recombinant methionyl human leptin, has been proven effective for the treatment of congenital leptin deficiency, congenital and non-HIV–related acquired generalized lipodystrophy associated with severe hypoleptinemia, and HA (219, 220).
Metreleptin offers metabolic benefits such as improvements in insulin sensitivity, glucose tolerance, fasting glucose, glycosylated hemoglobin, hypertrigyceridemia, and liver function tests (219, 220). Patients with partial lipodystrophy, including those with PPARγ mutations (216), also gain metabolic benefits from leptin administration. In these subjects, leptin administration has been extensively studied in the context of open-label clinical trials (221). HIV patients with HALS also exhibit significant improvements in their metabolic profile that are comparable to other treatment options such as metformin and thiazolidinediones (234). Leptin replacement therapy provides benefits beyond metabolic improvements: it ameliorates glomerular injury in generalized lipodystrophy (223), it has immunomodulatory effects in hypoleptinemic patients with severe lipodystrophy (222), it normalizes menstrual abnormalities in young women with lipodystrophy and polycystic ovary syndrome (224), and it improves the thyroid, GH, and adrenal axis abnormalities seen in women with HA (224).
Although open-label trials have shown benefits of metreleptin use, larger, randomized, placebo-controlled clinical trials are needed to conclusively demonstrate its efficacy and safety, particularly because severe adverse effects have been noted, including deterioration of renal function and proteinuric nephropathy (224) and occurrence of T-cell lymphomas (223) in a small number of patients with lipodystrophy. It is important to underscore that the open-label nature of the study design did not allow the investigators to establish or refute a causal link between metreleptin treatment and reported adverse effects. Similar adverse effects would not be entirely unexpected on the basis of the natural history of the disease, particularly in patients suffering from acquired lipodystrophy, which is traditionally thought to be due to autoimmune factors. Approximately 25% of cases with acquired generalized lipodystrophy are caused by autoimmune disease such as juvenile-onset dermatomyositis, rheumatoid arthritis, systemic lupus erythematosus, and Sjögren syndrome (219). Given the immunomodulatory effect of metreleptin, which clearly regulates the Th1/Th2 imbalance in leptin-deficient individuals (224), large, crossover, randomized, placebo-controlled clinical trials of sufficient duration are needed to study efficacy and adverse effects more tightly. Potential disadvantages of leptin therapy may include increased immune system function and inflammation and the generation of neutralizing autoantibodies against exogenous leptin (115, 190, 212, 219). Moreover, it has been reported that leptin accelerates autoimmune diabetes in the nonobese diabetic mouse model of diabetes type 1, probably by inducing proinflammatory cell responses (217).
It remains unknown whether similar adverse effects in response to leptin administration could potentially also be observed in disease states accompanied by hyperleptinemia due to leptin resistance such as garden-variety obesity. It also remains unknown whether similar side effects could be seen in response to higher than normal leptin levels, which are needed to effectively lower glycemia in conditions such as type 1 diabetes. In these conditions, potential side effects of leptin may become really prevalent due to the increased prevalence of the underlying disease. Common adverse events seen particularly in combination treatment with pramlintide/metreleptin in obese subjects included nausea and injection site erythema and were mild to moderate and decreasing over time (263). It has been proposed that leptin could possibly cause hypertension, impair endothelial function, promote platelet aggregation leading to thrombosis, increase immune function, foster inflammation and angiogenesis, and worsen diabetic complications and other concomitant diseases on the basis of preclinical studies, but no such side effects have been seen in the clinic (283). We have failed to observe effects in hypertension (255, 283), angiogenesis (263), or inflammation (20). Although it cannot be excluded that prolonged administration of leptin may cause hypertension, particularly in predisposed obese diabetic individuals in contrast to obese hypotensive subjects who present with leptin deficiency (255), we and others have not observed such side effects in our trials (20, 268). Moreover, leptin's action in suppressing glucagon may place patients suffering from type 1 diabetes at increased risk for severe hypoglycemic episodes by impairing the counterregulatory response necessary to restore glycemia (283). Until now, these particular side effects have not been reported in leptin-deficient lipodystrophic or other patients treated with replacement doses of leptin. Other probable adverse effects may theoretically include sleep disorders, an early onset of puberty in children, and an increased risk of unplanned pregnancy, but again, they have not yet been observed in the clinic, whereas the potential teratogenic effects of leptin treatment need to be explored.
It is of paramount importance to discuss the potential carcinogenic adverse effects of leptin administration in patients with garden-variety obesity. It has been proposed that leptin may exert neoplastic effects via 2 mechanisms. First, leptin can act directly on cancer cells by stimulating receptor-mediated signaling pathways leading to tumor cell growth, migration, and invasion (193). Notably, this activity of leptin is commonly reinforced through entangled cross talk with multiple oncogenes, cytokines, and growth factors. Second, leptin may act indirectly by reducing tissue sensitivity to insulin, causing hyperinsulinemia and by regulating inflammatory responses with overproduction of cytokines such as IL-6, IL-12, and TNF-α (284) and influencing tumor angiogenesis, but we have not observed such effects of leptin in vivo (285). Leptin has also been proposed to play a role in hormone-dependent malignancies, such as breast and endometrial cancers, by activating the enzyme aromatase, an enzyme responsible for the transformation of androgens to estrogens (81). Expression of leptin receptors has been reported in numerous cancer cell types including breast, colon, prostate, and thyroid (80). Leptin, through its receptor ObRb, may modulate growth and proliferation of cancer cells via activation of various growth and survival signaling pathways including canonical (JAK2/STAT3, PI3K/Akt, and MAPK/ERK1/2) and noncanonical (protein kinase C, JNK, and p38 MAPK) signaling pathways (80). For example, leptin has been demonstrated in vitro to stimulate JNK in human breast cancer cells in both a time- and a dose-dependent manner, with greater phosphorylated JNK levels after long-term exposure (80). In breast cancer, JNK activation by leptin leads to an up-regulation of matrix metalloproteinase-2 activity, which promotes cancer cell invasion (80). Leptin exerts proliferative and antiapoptotic effects on human colon cancer cells, which are mediated in part though JNK activation (80). It should be noted, however, that most in vitro studies have used extremely high leptin levels.
However, in contrast to many in vitro studies, epidemiological studies report inconsistent associations between serum leptin levels and risk of several malignancies. For example, in the case of colorectal cancer (CC), an obesity-associated cancer, some prospective studies found a positive association between serum leptin levels and CC risk (291), whereas others did not show any relationship. Moreover, some studies revealed that even hypoleptinemia was associated with CC risk, independently from BMI (293). Our group, which has focused on adipokines and cancer, has recently shown that hypoleptinemia, and not hyperleptinemia, was linked to pancreatic cancer independently from BMI and weight loss, to B cell chronic lymphocytic leukemia, and to low-risk myelodysplastic syndrome after adjustment for BMI and other risk factors (297) but these associations may reflect reverse causality. In addition, increasing serum leptin levels did not appear to increase substantially the risk of premenopausal breast cancer in situ, invasive pre- and postmenopausal breast cancer, endometrial cancer, prostate cancer, multiple myeloma, melanoma, and lung cancer (318–321). Thus, the possible association of leptin and malignancies in vitro appears to be not supported by published studies in humans. A possible association with leptin needs to be studied further with larger prospective, longitudinal, and mechanistic studies, which are needed to prove causality, provide further insights into both the mechanisms underlying the actions of this hormone and its potential role in cancer, and analyze its action in tumor progression if leptin is prescribed as an alternative or adjunct approach in patients with diabetes and malignancies in the future.
In conclusion, future larger trials using metreleptin are needed to systematically determine 1) the diagnostic criteria to be used as indicating hypoleptinemia and thus serve as inclusion criteria in ongoing and future trials, 2) the appropriate initial metreleptin dose, 3) how high the dose of metreleptin should be raised (based upon which algorithm) for the best metabolic response to be achieved or what dose would be needed to safely break through a possible resistance to metreleptin in certain individuals, 4) how patients on metreleptin should be monitored, and 5) whether the concomitant use of other leptin-sensitizing treatments could be useful therapeutic options in the treatment of obesity or to prevent metabolic adaptation induced by caloric restriction and to promote weight maintenance. Garden-variety obesity is a chronic disease with currently limited successful long-term therapy options, apart from bariatric surgery, which is both costly and risky. Combination therapy for obesity represents an encouraging step forward in the pharmacologic armamentarium. Finally, large randomized leptin administration studies are needed to obtain accurate information on potential adverse effects and long-term outcomes.
VII. Future Directions
Leptin is an adipocyte-secreted hormone that interacts with several organs that are involved in the regulation of energy homeostasis and metabolism. Although great progress has been made in terms of understanding leptin's pathophysiological role, several fundamental questions, particularly regarding the effects of leptin on glucose metabolism, still remain to be answered. Much progress has been made with leptin treatment in patients with leptin deficiency and lipodystrophy, and such treatment does correct metabolic abnormalities. Results from these studies suggest that leptin could prove to be a potential antidiabetic agent in leptin-deficient states. However, formal double-blinded placebo-controlled phase III clinical trials are required to accurately determine placebo-subtracted efficacy and to assess comparative efficacy in relation to other effective therapies, and dose-finding studies are necessary to help guide appropriate treatment for these individuals. It is also unclear whether all patients with lipodystrophy would benefit from leptin therapy or whether only those with the most profound leptin deficiency are more likely to benefit. In contrast, the vast majority of obese individuals are hyperleptinemic and resistant or tolerant to exogenous leptin. Therefore, it is questionable whether leptin alone can be used to favorably modify glucose metabolism in garden-variety obesity and/or diabetes. Hence, findings from ongoing and upcoming studies combining leptin with other antiobesity medications or sensitizers will be of particular interest.
Acknowledgments
The Mantzoros laboratory is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 58785, 79929, 81913, and AG032030 and a VA Merit award (Grant 10684957). The Mantzoros Laboratory is also supported by a discretionary grant from Beth Israel Deaconess Medical Center.
All authors contributed to writing the manuscript. All authors read and approved the final manuscript.
Disclosure Summary: All authors state that there is no conflict of interest related to this paper.
Footnotes
- ACC
- acetyl-coenzyme A carboxylase
- AgRP
- agouti-related protein
- ARC
- arcuate nucleus
- AMPK
- AMP-activated protein kinase
- BDNF
- brain-derived neurotrophic factor
- BBB
- blood-brain barrier
- BMI
- body mass index
- CaMKK2
- Ca2+/calmodulin-dependent protein kinase
- CC
- colorectal cancer
- CNS
- central nervous system
- CoA
- coenzyme A
- DIO
- diet-induced obesity
- ER
- endoplasmic reticulum
- FoxO1
- Forkhead box O1
- GTT
- glucose tolerance test
- HALS
- HIV-associated lipodystrophy syndrome
- hAT
- human adipose tissue
- HbA1C
- glycated hemoglobin
- HAART
- highly active antiretroviral therapy
- HFD
- high-fat diet
- HOMA
- homeostatic model assessment
- HA
- hypothalamic amenorrhea
- HSC
- hepatic stellate cell
- icv
- intracerebroventricular
- IKKβ
- inhibitor of nuclear factor-κB kinase subunit β
- IRS
- insulin receptor substrate
- JAK2
- Janus kinase 2
- JNK
- c-Jun N-terminal kinase
- LDL
- low-density lipoprotein
- MC3R
- melanocortin-3 receptor
- mTOR
- mammalian target of rapamycin
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NF-κB
- nuclear factor-κB
- NPY
- neuropeptide Y
- ObR
- obese receptor
- P13K
- phosphatidylinositol 3-kinase
- PBMC
- peripheral blood mononuclear cell
- POMC
- proopiomelanocortin
- PPARγ
- peroxisome proliferator-activator receptor-γ
- PTP
- protein tyrosine phosphatase
- SH2
- Src homology-2
- SH2B1
- SH2B adaptor protein 1
- SHP2
- SH2-containing protein tyrosine phosphatase 2
- sOB-R
- soluble leptin receptor
- SOCS3
- suppressor of cytokine signaling 3
- SS
- simple steatosis
- STAT3
- signal transducer and activator of transcription 3
- STZ
- streptozotocin
- TG
- triglyceride
- Th
- T helper
- UPR
- unfolded protein response
- VMH
- ventromedial hypothalamus.
References
- 1. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. [DOI] [PubMed] [Google Scholar]
- 2. Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. [DOI] [PubMed] [Google Scholar]
- 3. Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol. 2010;31:377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366:74–85. [DOI] [PubMed] [Google Scholar]
- 5. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. [DOI] [PubMed] [Google Scholar]
- 6. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. [DOI] [PubMed] [Google Scholar]
- 7. Chan JL, Moschos SJ, Bullen J, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-α receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90:1625–1631. [DOI] [PubMed] [Google Scholar]
- 8. Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45:1638–1643. [DOI] [PubMed] [Google Scholar]
- 9. Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51:1337–1345. [DOI] [PubMed] [Google Scholar]
- 10. Blüher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology: emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. [DOI] [PubMed] [Google Scholar]
- 12. Brinkoetter M, Magkos F, Vamvini M, Mantzoros CS. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab. 2011;301:E99–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bluher S, Mantzoros CS. The role of leptin in regulating neuroendocrine function in humans. J Nutr. 2004;134:2469S–2474S. [DOI] [PubMed] [Google Scholar]
- 15. Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–2112. [DOI] [PubMed] [Google Scholar]
- 16. Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4:87–92. [DOI] [PubMed] [Google Scholar]
- 17. Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000;900:174–183. [DOI] [PubMed] [Google Scholar]
- 18. Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671–680. [DOI] [PubMed] [Google Scholar]
- 19. Moon HS, Chamberland JP, Mantzoros CS. Amylin and leptin activate overlapping signalling pathways in an additive manner in mouse GT1-7 hypothalamic, C(2)C (12) muscle and AML12 liver cell lines. Diabetologia. 2012;55:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moon HS, Chamberland JP, Diakopoulos KN, et al. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2011;34:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. [DOI] [PubMed] [Google Scholar]
- 23. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. [DOI] [PubMed] [Google Scholar]
- 24. de Luis DA, Perez Castrillón JL, Dueñas A. Leptin and obesity. Minerva Medica. 2009;100:229–236. [PubMed] [Google Scholar]
- 25. Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond). 2008;32:S98–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Minocci A, Savia G, Lucantoni R, et al. A Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24:1139. [DOI] [PubMed] [Google Scholar]
- 27. Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. [DOI] [PubMed] [Google Scholar]
- 28. Müller TD, Föcker M, Holtkamp K, Herpertz-Dahlmann B, Hebebrand J. Leptin-mediated neuroendocrine alterations in anorexia nervosa: somatic and behavioral implications. Child Adolesc Psychiatr Clin North Am. 2009;18:117–129. [DOI] [PubMed] [Google Scholar]
- 29. Leshan RL, Björnholm M, Münzberg H, Myers MG., Jr 2006. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring). 14:208S–212S. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–256. [DOI] [PubMed] [Google Scholar]
- 31. Peelman F, Couturier C, Dam J, Zabeau L, Tavernier J, Jockers R. Techniques: new pharmacological perspectives for the leptin receptor. Trends Pharmacol Sci. 2006;27:218–225. [DOI] [PubMed] [Google Scholar]
- 32. Walker CD, Naef L, d'Asti E, et al. Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring: a potential role for leptin. Ann N Y Acad Sci. 2008;1144:189–202. [DOI] [PubMed] [Google Scholar]
- 33. Cohen MM., Jr 2006. Role of leptin in regulating appetite, neuroendocrine function, and bone remodeling. Am J Med Genet A. 140:515–524. [DOI] [PubMed] [Google Scholar]
- 34. Frühbeck G. 2006. Intracellular signalling pathways activated by leptin. Biochem J. 393(Pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457–465. [DOI] [PubMed] [Google Scholar]
- 36. Mencarelli A, Distrutti E, Renga B, et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS One. 2011;6:e229–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee Y, Yu X, Gonzales F, et al. PPARα is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc Natl Acad Sci U S A. 2002;99:11848–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. [DOI] [PubMed] [Google Scholar]
- 39. Muhlhausler BS, Morrison JL, McMillen IC. Rosiglitazone increases the expression of peroxisome proliferator-activated receptor-γ target genes in adipose tissue, liver, and skeletal muscle in the sheep fetus in late gestation. Endocrinology. 2009;150:4287–4294. [DOI] [PubMed] [Google Scholar]
- 40. Prieur X, Tung YC, Griffin JL, Farooqi IS, O'Rahilly S, Coll AP. Leptin regulates peripheral lipid metabolism primarily through central effects on food intake. Endocrinology. 2008;149:5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coya R, Martul P, Algorta J, Aniel-Quiroga MA, Busturia MA, Señarís R. Progesterone and human placental lactogen inhibit leptin secretion on cultured trophoblast cells from human placentas at term. Gynecol Endocrinol. 2005;21:27–32. [DOI] [PubMed] [Google Scholar]
- 42. Seufert J. Leptin effects on pancreatic β-cell gene expression and function. Diabetes. 2004;53 Suppl 1:S152–S158. [DOI] [PubMed] [Google Scholar]
- 43. Gallardo N, Bonzón-Kulichenko E, Fernández-Agulló T, et al. Tissue-specific effects of central leptin on the expression of genes involved in lipid metabolism in liver and white adipose tissue. Endocrinology. 2007;148:5604–5610. [DOI] [PubMed] [Google Scholar]
- 44. Sakoguchi T, Horiuchi M, Asakawa A, Ushikai M, Yoshida G, Fujimiya M, Kato I, Nakazato M, Takeuchi T, Saheki T, Inui A. Failure of the feeding response to fasting in carnitine-deficient juvenile visceral steatosis (JVS) mice: involvement of defective acyl-ghrelin secretion and enhanced corticotropin-releasing factor signaling in the hypothalamus. Biochim Biophys Acta. 2009;1792:1087–1093. [DOI] [PubMed] [Google Scholar]
- 45. Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipids Res. 2010;51:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uotani S, Bjørbaek C, Tornøe J, Flier JS. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48:279–286. [DOI] [PubMed] [Google Scholar]
- 47. Lee Y, Hirose H, Ohneda M, Johnson J, McGarry JD, Unger RH. β-Cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-β-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. [DOI] [PubMed] [Google Scholar]
- 49. Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci U S A. 1999;96:2327–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koyama K, Chen G, Lee Y, Unger RH. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol Endocrinol Metab. 1997;273:E708–E713. [DOI] [PubMed] [Google Scholar]
- 51. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced β-cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of β-cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci U S A. 1998;95:9558–9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores β cell function of Zucker diabetic fatty rats. J Biol Chem. 1998;273:3547–3550. [DOI] [PubMed] [Google Scholar]
- 54. Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. [DOI] [PubMed] [Google Scholar]
- 55. Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Unger R, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985;28:119–121. [DOI] [PubMed] [Google Scholar]
- 57. Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol. 2009;46:113–118. [DOI] [PubMed] [Google Scholar]
- 58. Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107:4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic β-cell leptin signaling. Endocrinology. 2010;151:4178–4186. [DOI] [PubMed] [Google Scholar]
- 60. Maedler K, Schulthess FT, Bielman C, et al. Glucose and leptin induce apoptosis in human β-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J. 2008;22:1905–1913. [DOI] [PubMed] [Google Scholar]
- 61. do Prado WL, de Piano A, Lazaretti-Castro M, et al. Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab. 2009;27:613–619. [DOI] [PubMed] [Google Scholar]
- 62. Miras M, Ochetti M, Martín S, et al. Serum levels of adiponectin and leptin in children born small for gestational age: relation to insulin sensitivity parameters. J Pediatr Endocrinol Metab. 2010;23:463–471. [DOI] [PubMed] [Google Scholar]
- 63. Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. [DOI] [PubMed] [Google Scholar]
- 64. Berglund ED, Vianna CR, Donato J, Jr, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107:16009–16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105:14070–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391–17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kalra SP. Central leptin gene therapy ameliorates diabetes type 1 and 2 through two independent hypothalamic relays; a benefit beyond weight and appetite regulation. Peptides. 2009;30:1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;15;174:3137–3142. [DOI] [PubMed] [Google Scholar]
- 72. Carbone F, LA Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka M, Suganami T, Kim-Saijo M, et al. Role of central leptin signaling in the starvation-induced alteration of B-cell development. J Neurosci. 2011;31:8373–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–900. [DOI] [PubMed] [Google Scholar]
- 75. Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. [DOI] [PubMed] [Google Scholar]
- 76. De Rosa V, Procaccini C, Calì G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. [DOI] [PubMed] [Google Scholar]
- 77. Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–4024. [DOI] [PubMed] [Google Scholar]
- 78. Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. [DOI] [PubMed] [Google Scholar]
- 79. Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. [DOI] [PubMed] [Google Scholar]
- 80. McCormack D, Schneider J, McDonald D, McFadden D. The antiproliferative effects of pterostilbene on breast cancer in vitro are via inhibition of constitutive and leptin-induced Janus kinase/signal transducer and activator of transcription activation. Am J Surg. 2011;202:541–544. [DOI] [PubMed] [Google Scholar]
- 81. Feng Y, Shao R, Weijdegård B, et al. Effects of androgen and leptin on behavioral and cellular responses in female rats. Horm Behav. 2011;60:427–438. [DOI] [PubMed] [Google Scholar]
- 82. Gu FM, Li QL, Gao Q, et al. Sorafenib inhibits growth and metastasis of hepatocellular carcinoma by blocking STAT3. World J Gastroenterol. 2011;17:3922–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Okitsu K, Kanda T, Imazeki F, et al. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer. 2010;1:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang H, Zhang D, Luan X, Xie G, Pan X. Inhibition of the signal transducers and activators of transcription (STAT) 3 signalling pathway by AG490 in laryngeal carcinoma cells. J Int Med Res. 2010;38:1673–1681. [DOI] [PubMed] [Google Scholar]
- 85. Saito M, Nagasawa M, Takada H, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heimall J, Davis J, Shaw PA, et al. Paucity of genotype-phenotype correlations in STAT3 mutation positive Hyper IgE Syndrome (HIES). Clin Immunol. 2011;139:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buettner C, Muse ED, Cheng A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gao Q, Wolfgang MJ, Neschen S, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dzamko N, van Denderen BJ, Hevener AL, et al. AMPK β1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dzamko NL, Steinberg GR. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol (Oxf). 2009;196:115–127. [DOI] [PubMed] [Google Scholar]
- 91. Motawi TM, Hashem RM, Rashed LA, El-Razek SM. Comparative study between the effect of the peroxisome proliferator activated receptor-α ligands fenofibrate and n-3 polyunsaturated fatty acids on activation of 5′-AMP-activated protein kinase-α1 in high-fat fed rats. J Pharm Pharmacol. 2009;61:1339–1346. [DOI] [PubMed] [Google Scholar]
- 92. Kohan AB, Talukdar I, Walsh CM, Salati LM. A role for AMPK in the inhibition of glucose-6-phosphate dehydrogenase by polyunsaturated fatty acids. Biochem Biophys Res Commun. 2009;388:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. [DOI] [PubMed] [Google Scholar]
- 94. Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor α gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the α2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kus V, Prazak T, Brauner P, et al. Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am J Physiol Endocrinol Metab. 2008;295:E356–E367. [DOI] [PubMed] [Google Scholar]
- 96. Tanaka T, Masuzaki H, Yasue S, et al. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab. 2007;5:395–402. [DOI] [PubMed] [Google Scholar]
- 97. Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: Is it a consequence of AMPK downregulation? Cell Cycle. 2011;10:3447–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grisouard J, Dembinski K, Mayer D, Keller U, Müller B, Christ-Crain M. Targeting AMP-activated protein kinase in adipocytes to modulate obesity-related adipokine production associated with insulin resistance and breast cancer cell proliferation. Diabetol Metab Syndr. 2011;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. [DOI] [PubMed] [Google Scholar]
- 100. Nascimento AF, Luvizotto RA, Leopoldo AS, et al. Long-term high-fat diet-induced obesity decreases the cardiac leptin receptor without apparent lipotoxicity. Life Sci. 2011;88:1031–1038. [DOI] [PubMed] [Google Scholar]
- 101. Gunn RM, Hailes HC. Insights into the PI3-K-PKB-mTOR signalling pathway from small molecules. J Chem Biol. 2008;1:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cho JE, Kim YS, Park S, Cho SN, Lee H. Mycobacterium tuberculosis-induced expression of leukotactin-1 is mediated by the PI3-K/PDK1/Akt signaling pathway. Mol Cells. 2010;29:35–39. [DOI] [PubMed] [Google Scholar]
- 103. Zhang Z, Song T, Jin Y, et al. Epidermal growth factor receptor regulates MT1-MMP and MMP-2 synthesis in SiHa cells via both PI3-K/AKT and MAPK/ERK pathways. Int J Gynecol Cancer. 2009;19:998–1003. [DOI] [PubMed] [Google Scholar]
- 104. Filiputti E, Rafacho A, Araújo EP, et al. Augmentation of insulin secretion by leucine supplementation in malnourished rats: possible involvement of the phosphatidylinositol 3-phosphate kinase/mammalian target protein of rapamycin pathway. Metab Clin Exp. 2010;59:635–644. [DOI] [PubMed] [Google Scholar]
- 105. Cui W, Li W, Han R, et al. PI3-K/Akt and ERK pathways activated by VEGF play opposite roles in MPP(+)-induced neuronal apoptosis. Neurochem Int. 2011;59:945–953. [DOI] [PubMed] [Google Scholar]
- 106. Que J, Lian Q, El Oakley RM, Lim B, Lim SK. PI3 K/Akt/mTOR-mediated translational control regulates proliferation and differentiation of lineage-restricted RoSH stem cell lines. J Mol Signal. 2007;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang Y, Yan W, Lu X, et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the av?3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol. 2011;90:642–648. [DOI] [PubMed] [Google Scholar]
- 108. Rodríguez A, Gómez-Ambrosi J, Catalán V, Fortuño A, Frühbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediators Inflamm. 2010;1054–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab. 2011;96:E586–E597. [DOI] [PubMed] [Google Scholar]
- 110. Cheng SP, Yin PH, Hsu YC, et al. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26:1265–1271. [DOI] [PubMed] [Google Scholar]
- 111. Samuel-Mendelsohn S, Inbar M, Weiss-Messer E, Niv-Spector L, Gertler A, Barkey RJ. Leptin signaling and apoptotic effects in human prostate cancer cell lines. Prostate. 2011;71:929–945. [DOI] [PubMed] [Google Scholar]
- 112. Barazzoni R, Zanetti M, Bosutti A, et al. Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle–tissue-specific impact on tissue triglyceride content and AKT activation. Endocrinology. 2005;146:2098–2106. [DOI] [PubMed] [Google Scholar]
- 113. Fang X, Fetros J, Dadson KE, Xu A, Sweeney G. Leptin prevents the metabolic effects of adiponectin in L6 myotubes. Diabetologia. 2009;52:2190–2200. [DOI] [PubMed] [Google Scholar]
- 114. Ernst MB, Wunderlich CM, Hess S, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shetty GK, Matarese G, Magkos F, et al. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol. 2011;165:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mirshamsi S, Laidlaw HA, Ning K, et al. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hill JW, Elias CF, Fukuda M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Al-Qassab H, Smith MA, Irvine EE, et al. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 2010;57:939–946. [DOI] [PubMed] [Google Scholar]
- 120. Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between β-cell proliferation and cell size. Diabetes. 2011;60:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cypess AM, Zhang H, Schulz TJ, et al. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology. 2011;152:3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Coope A, Milanski M, Araújo EP, et al. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582:1471–1476. [DOI] [PubMed] [Google Scholar]
- 124. Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Iskandar K, Cao Y, Hayashi Y, et al. PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. Am J Physiol Endocrinol Metab. 2010;298:E787–E798. [DOI] [PubMed] [Google Scholar]
- 126. Sasaki T, Kim HJ, Kobayashi M, et al. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556–2566. [DOI] [PubMed] [Google Scholar]
- 127. Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4:e8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fernández-Riejos P, Goberna R, Sánchez-Margalet V. Leptin promotes cell survival and activates Jurkat T lymphocytes by stimulation of mitogen-activated protein kinase. Clin Exp Immunol. 2008;151:505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol. 2009;221:490–497. [DOI] [PubMed] [Google Scholar]
- 130. Löthgren A, McCartney M, Rupp Thuresson E, James SR. A model of activation of the protein tyrosine phosphatase SHP-2 by the human leptin receptor. Biochim Biophys Acta. 2001;1545:20–29. [DOI] [PubMed] [Google Scholar]
- 131. Chen W, Garcia JG, Jacobson JR. Integrin β4 attenuates SHP-2 and MAPK signaling and reduces human lung endothelial inflammatory responses. J Cell Biochem. 2010;110:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Carver KC, Piazza TM, Schuler LA. Prolactin enhances insulin-like growth factor I receptor phosphorylation by decreasing its association with the tyrosine phosphatase SHP-2 in MCF-7 breast cancer cells. J Biol Chem. 2010;285:8003–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kolli S, Zito CI, Mossink MH, Wiemer EA, Bennett AM. The major vault protein is a novel substrate for the tyrosine phosphatase SHP-2 and scaffold protein in epidermal growth factor signaling. J Biol Chem. 2004;279:29374–29385. [DOI] [PubMed] [Google Scholar]
- 134. Bjørbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. [DOI] [PubMed] [Google Scholar]
- 135. Anhê GF, Caperuto LC, Pereira-Da-Silva M, et al. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J Neurochem. 2004;90:559–566. [DOI] [PubMed] [Google Scholar]
- 136. Banno R, Zimmer D, De Jonghe BC, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. You J, Yu Y, Jiang L, et al. Signaling through Tyr985 of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol Cell Biol. 2010;30:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chung JH, Choi HJ, Kim SY, et al. Proteomic and biochemical analyses reveal the activation of unfolded protein response, ERK-1/2 and ribosomal protein S6 signaling in experimental autoimmune myocarditis rat model. BMC Genomics. 2011;12:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ben-Eliezer M, Phillip M, Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32:235–244. [DOI] [PubMed] [Google Scholar]
- 140. Katiyar SK, Meeran SM. Obesity increases the risk of UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling. Free Radic Biol Med. 2007;42:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. [DOI] [PubMed] [Google Scholar]
- 142. Muretta JM, Mastick CC. How insulin regulates glucose transport in adipocytes. Vitam Horm. 2009;80:245–286. [DOI] [PubMed] [Google Scholar]
- 143. Nakayama M. Insulin as a key autoantigen in the development of type 1 diabetes. Diabetes Metab Res Rev. 2011;27:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Colberg SR. Physical activity, insulin action, and diabetes prevention and control. Curr Diabetes Rev. 2007;3:176–184. [DOI] [PubMed] [Google Scholar]
- 145. Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML. Regulation of pancreatic islet cell survival and replication by γ-aminobutyric acid. Diabetologia. 2007;50:764–773. [DOI] [PubMed] [Google Scholar]
- 146. Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121:3598–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Keung W, Palaniyappan A, Lopaschuk GD. Chronic central leptin decreases food intake and improves glucose tolerance in diet-induced obese mice independent of hypothalamic malonyl CoA levels and skeletal muscle insulin sensitivity. Endocrinology. 2011;152:4127–4137. [DOI] [PubMed] [Google Scholar]
- 148. Brøns C, Jacobsen S, Hiscock N, et al. Effects of high-fat overfeeding on mitochondrial function, glucose and fat metabolism, and adipokine levels in low birth weight subjects. Am J Physiol Endocrinol Metab. 2012;302:E43–E51. [DOI] [PubMed] [Google Scholar]
- 149. Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Levi J, Gray SL, Speck M, et al. Acute disruption of leptin signaling in vivo leads to increased insulin levels and insulin resistance. Endocrinology. 2011;152:3385–3395. [DOI] [PubMed] [Google Scholar]
- 151. Takahashi A, Shimano H, Nakagawa Y, et al. Transgenic mice overexpressing SREBP-1a under the control of the PEPCK promoter exhibit insulin resistance, but not diabetes. Biochim Biophys Acta. 2005;1740:427–433. [DOI] [PubMed] [Google Scholar]
- 152. Slosberg ED, Desai UJ, Fanelli B, et al. Treatment of type 2 diabetes by adenoviral-mediated overexpression of the glucokinase regulatory protein. Diabetes. 2001;50:1813–1820. [DOI] [PubMed] [Google Scholar]
- 153. Kaser S, Föger B, Ebenbichler CF, et al. Influence of leptin and insulin on lipid transfer proteins in human hepatoma cell line, HepG2. Int J Obes Relat Metab Disord. 2001;25:1633–1639. [DOI] [PubMed] [Google Scholar]
- 154. Maroni P, Bendinelli P, Piccoletti R. Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int. 2005;29:542–550. [DOI] [PubMed] [Google Scholar]
- 155. Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci U S A. 2000;97:2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Polyzos SA, Kountouras J, Zavos C, Deretzi G. The potential adverse role of leptin resistance in nonalcoholic fatty liver disease: a hypothesis based on critical review of the literature. J Clin Gastroenterol. 2011;45:50. [DOI] [PubMed] [Google Scholar]
- 157. Lebrun P, Van Obberghen E. SOCS proteins causing trouble in insulin action. Acta Physiol. 2008;192:29–36. [DOI] [PubMed] [Google Scholar]
- 158. Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wang P, Li N, Li JS, Li WQ. The role of endotoxin, TNF-α, and IL-6 in inducing the state of growth hormone insensitivity. World J Gastroenterol. 2002;8:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. [DOI] [PubMed] [Google Scholar]
- 161. Bjørbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. [DOI] [PubMed] [Google Scholar]
- 162. Myers MG, Jr, Heymsfield SB, Haft C, et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lee Y, Naseem RH, Duplomb L, et al. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci U S A. 2004;101:13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lee Y, Wang MY, Kakuma T, et al. Liporegulation in diet-induced obesity: the antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276:5629–5635. [DOI] [PubMed] [Google Scholar]
- 165. Oral EA, Burant C. Leptin and insulin resistance: good, bad, or still unclear? Am J Physiol Endocrinol Metab. 2009;296:E394–E395. [DOI] [PubMed] [Google Scholar]
- 166. Paz-Filho G, Esposito K, Hurwitz B, et al. Changes in insulin sensitivity during leptin replacement therapy in leptin-deficient patients. Am J Physiol Endocrinol Metab. 2008;295:E1401–E1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Unger RH. Hyperleptinemia protecting the heart from lipid overload. Hypertension. 2005;45:1031–1034. [DOI] [PubMed] [Google Scholar]
- 168. Waelput W, Verhee A, Broekaert D, et al. Identification and expression analysis of leptin-regulated immediate early response and late target genes. Biochem J. 2000;348(Pt 1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 169. Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. [DOI] [PubMed] [Google Scholar]
- 170. Dunn SL, Björnholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr(1138) of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. [DOI] [PubMed] [Google Scholar]
- 171. Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. [DOI] [PubMed] [Google Scholar]
- 172. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. [DOI] [PubMed] [Google Scholar]
- 173. Björnholm M, Münzberg H, Leshan RL, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Morrison CD, White CL, Wang Z, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104. [DOI] [PubMed] [Google Scholar]
- 176. Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Feng GS. Shp2 as a therapeutic target for leptin resistance and obesity. Expert Opin Ther Targets. 2006;10:135–142. [DOI] [PubMed] [Google Scholar]
- 178. Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101:16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. [DOI] [PubMed] [Google Scholar]
- 180. El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hileman SM, Pierroz DD, Masuzaki H, et al. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–783. [DOI] [PubMed] [Google Scholar]
- 182. Gertler A. Development of leptin antagonists and their potential use in experimental biology and medicine. Trends Endocrinol Metab. 2006;17:372–378. [DOI] [PubMed] [Google Scholar]
- 183. Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. [DOI] [PubMed] [Google Scholar]
- 184. Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. [DOI] [PubMed] [Google Scholar]
- 185. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Koch C, Augustine RA, Steger J, et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chua SC, Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. [DOI] [PubMed] [Google Scholar]
- 188. Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. [DOI] [PubMed] [Google Scholar]
- 189. Farooqi IS, Keogh JM, Kamath S, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. [DOI] [PubMed] [Google Scholar]
- 190. Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101:4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. [DOI] [PubMed] [Google Scholar]
- 193. Gibson WT, Farooqi IS, Moreau M, et al. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89:4821–4826. [DOI] [PubMed] [Google Scholar]
- 194. Paz-Filho G, Mastronardi C, Delibasi T, Wong ML, Licinio J. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arq Bras Endocrinol Metab. 2010;54:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Fatima W, Shahid A, Imran M, et al. Leptin deficiency and leptin gene mutations in obese children from Pakistan. Int J Pediatr Obes. 2011;419–427. [DOI] [PubMed] [Google Scholar]
- 196. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. [DOI] [PubMed] [Google Scholar]
- 197. Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–3291. [DOI] [PubMed] [Google Scholar]
- 198. Andreev VP, Paz-Filho G, Wong ML, Licinio J. Deconvolution of insulin secretion, insulin hepatic extraction post-hepatic delivery rates and sensitivity during 24-hour standardized meals: time course of glucose homeostasis in leptin replacement treatment. Horm Metab Res. 2009;41:142–151. [DOI] [PubMed] [Google Scholar]
- 199. Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. [DOI] [PubMed] [Google Scholar]
- 200. Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hunt CE, Lindsey JR, Walkley SU. Animal models of diabetes and obesity, including the PBB/Ld mouse. Fed Proc. 1976;35:1206–1217. [PubMed] [Google Scholar]
- 202. Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. [DOI] [PubMed] [Google Scholar]
- 203. Mantzoros CS. W(h)ither metreleptin for lipodystrophy and the metabolic syndrome? Endocr Pract. 2010;29:1–18. [DOI] [PubMed] [Google Scholar]
- 204. Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Garg A, Agarwal AK. Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta. 2009;1791:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Reue K, Péterfy M. Mouse models of lipodystrophy. Curr Atheroscler Rep. 2000;2:390–396. [DOI] [PubMed] [Google Scholar]
- 207. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. [DOI] [PubMed] [Google Scholar]
- 208. Asilmaz E, Cohen P, Miyazaki M, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gavrilova O, Marcus-Samuels B, Leon LR, Vinson C, Reitman ML. Leptin and diabetes in lipoatrophic mice. Nature. 2000;403:850. [DOI] [PubMed] [Google Scholar]
- 210. Ebihara K, Ogawa Y, Masuzaki H. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50:1440–1448. [DOI] [PubMed] [Google Scholar]
- 211. Chan JL, Oral EA. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract. 2010;16:310–323. [DOI] [PubMed] [Google Scholar]
- 212. Vadacca M, Margiotta DP, Navarini L, Afeltra A. Leptin in immuno-rheumatological diseases. Cell Mol Immunol. 2011;8:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. [DOI] [PubMed] [Google Scholar]
- 214. Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ebihara K, Masuzaki H, Nakao K. Long-term leptin-replacement therapy for lipoatrophic diabetes. N Engl J Med. 2004;351:615–616. [DOI] [PubMed] [Google Scholar]
- 216. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. [DOI] [PubMed] [Google Scholar]
- 217. Matarese G, Sanna V, Lechler RI, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. [DOI] [PubMed] [Google Scholar]
- 218. Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. [DOI] [PubMed] [Google Scholar]
- 219. Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92:532–541. [DOI] [PubMed] [Google Scholar]
- 220. Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16:324–333. [DOI] [PubMed] [Google Scholar]
- 221. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. [DOI] [PubMed] [Google Scholar]
- 223. Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Leow MK, Addy CL, Mantzoros CS. Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88:1961–1976. [DOI] [PubMed] [Google Scholar]
- 225. Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40:1837–1845. [DOI] [PubMed] [Google Scholar]
- 226. Magkos F, Mantzoros CS. Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment. Metab Clin Exp. 2011;60:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Power R, Tate H, McGill S, Taylor C. A qualitative study of the psychosocial implications of lipodystrophy syndrome on HIV positive individuals. Sex Transm Infect. 2003;79:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:448–462. [DOI] [PubMed] [Google Scholar]
- 229. Brennan AM, Lee JH, Tsiodras S, et al. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metab Clin Exp. 1997;46:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Nagy GS, Tsiodras S, Martin LD, et al. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis. 2003;36:795–802. [DOI] [PubMed] [Google Scholar]
- 232. Addy CL, Gavrila A, Tsiodras S, Brodovicz K, Karchmer AW, Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88:627–636. [DOI] [PubMed] [Google Scholar]
- 233. Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91:2605–2611. [DOI] [PubMed] [Google Scholar]
- 234. Mulligan K, Khatami H, Schwarz JM, et al. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA. 2000;284:472–477. [DOI] [PubMed] [Google Scholar]
- 236. Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med. 2004;140:786–794. [DOI] [PubMed] [Google Scholar]
- 237. Sekhar RV, Jahoor F, Iyer D, et al. Leptin replacement therapy does not improve the abnormal lipid kinetics of hypoleptinemic patients with HIV-associated lipodystrophy syndrome. Metabolism. 2012;61:1395–1403 [DOI] [PubMed] [Google Scholar]
- 238. Falutz J, Allas S, Blot K, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359–2370. [DOI] [PubMed] [Google Scholar]
- 239. Falutz J, Mamputu JC, Potvin D, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95:4291–4304. [DOI] [PubMed] [Google Scholar]
- 240. Tong Q, Sankalé JL, Hadigan CM. Regulation of adiponectin in human immunodeficiency virus-infected patients: relationship to body composition and metabolic indices. J Clin Endocrinol Metab. 2003;88:1559–1564. [DOI] [PubMed] [Google Scholar]
- 241. Vigouroux C, Maachi M, Nguyên TH. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. 2003;17:1503–1511. [DOI] [PubMed] [Google Scholar]
- 242. Mynarcik DC, Combs T, McNurlan MA, Scherer PE, Komaroff E, Gelato MC. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J Acquir Immune Defic Syndr. 2002;31:514–520. [DOI] [PubMed] [Google Scholar]
- 243. Duntas LH, Popovic V, Panotopoulos G. Adiponectin: novelties in metabolism and hormonal regulation. Nutr Neurosci. 2004;7:195–200. [DOI] [PubMed] [Google Scholar]
- 244. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. [DOI] [PubMed] [Google Scholar]
- 245. Gavrila A, Hsu W, Tsiodras S, et al. Improvement in highly active antiretroviral therapy-induced metabolic syndrome by treatment with pioglitazone but not with fenofibrate: a 2 × 2 factorial, randomized, double-blinded, placebo-controlled trial. Clin Infect Dis. 2005;40:745–749. [DOI] [PubMed] [Google Scholar]
- 246. Magkos F, Brennan A, Sweeney L, et al. Leptin replacement improves postprandial glycemia and insulin sensitivity in human immunodeficiency virus-infected lipoatrophic men treated with pioglitazone: a pilot study. Metab Clin Exp. 2011;60:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108:6585–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Sienkiewicz E, Magkos F, Aronis KN, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211–1221. [DOI] [PubMed] [Google Scholar]
- 249. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. [DOI] [PubMed] [Google Scholar]
- 250. Harris RB, Mitchell TD, Hebert S. Leptin-induced changes in body composition in high fat-fed mice. Exp Biol Med (Maywood). 2003;228:24–32. [DOI] [PubMed] [Google Scholar]
- 251. Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab. 2000;278:563–569. [DOI] [PubMed] [Google Scholar]
- 252. Harris RB, Mitchell TD, Yan X, Simpson JS, Redmann SM., Jr Metabolic responses to leptin in obese db/db mice are strain dependent. Am J Physiol Regul Integr Comp Physiol. 2001;281:R115–R132. [DOI] [PubMed] [Google Scholar]
- 253. Mantzoros CS, Frederich RC, Qu D, Lowell BB, Maratos-Flier E, Flier JS. Severe leptin resistance in brown fat-deficient uncoupling protein promoter-driven diphtheria toxin A mice despite suppression of hypothalamic neuropeptide Y and circulating corticosterone concentrations. Diabetes. 1998;47:230–238. [DOI] [PubMed] [Google Scholar]
- 254. Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. [DOI] [PubMed] [Google Scholar]
- 255. Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res. 2004;59:225–244. [DOI] [PubMed] [Google Scholar]
- 256. Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–509. [DOI] [PubMed] [Google Scholar]
- 257. Zelissen PM, Stenlof K, Lean ME, et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2005;7:755–761. [DOI] [PubMed] [Google Scholar]
- 258. Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;1597–1604. [DOI] [PubMed] [Google Scholar]
- 259. Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Ahima R, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. [DOI] [PubMed] [Google Scholar]
- 261. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. [DOI] [PubMed] [Google Scholar]
- 263. Aronis KN, Diakopoulos KN, Fiorenza CG, Chamberland JP, Mantzoros CS. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia. 2011;54:2358–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105:7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Kiess W, Anil M, Blum WF, et al. Serum leptin levels in children and adolescents with insulin-dependent diabetes mellitus in relation to metabolic control and body mass index. Eur J Endocrinol. 1998;138:501–509. [DOI] [PubMed] [Google Scholar]
- 266. Havel PJ, Uriu-Hare JY, Liu T, et al. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:1482–1491. [DOI] [PubMed] [Google Scholar]
- 267. Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. [DOI] [PubMed] [Google Scholar]
- 268. Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf). 2007;66:49–57. [DOI] [PubMed] [Google Scholar]
- 269. Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:1084–1091. [DOI] [PubMed] [Google Scholar]
- 270. Hidaka S, Yoshimatsu H, Kondou S, et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 2002;16:509–518. [DOI] [PubMed] [Google Scholar]
- 271. German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Denroche HC, Levi J, Wideman RD, et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. German JP, Wisse BE, Thaler JP, et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Naito M, Fujikura J, Ebihara K, et al. Therapeutic impact of leptin on diabetes, diabetic complications, and longevity in insulin-deficient diabetic mice. Diabetes. 2011;60:2265–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Saryusz-Wolska M, Szymanska-Garbacz E, Jablkowski M, et al. Rosiglitazone treatment in nondiabetic subjects with nonalcoholic fatty liver disease. Pol Arch Med Wewn. 2011;121:61–66. [PubMed] [Google Scholar]
- 276. Park JY, Chong AY, Cochran EK, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Oral EA. Leptin for type 1 diabetes: coming onto stage to be (or not?). Pediatr Diabetes. 2011;13:68–73. [DOI] [PubMed] [Google Scholar]
- 278. Toyoshima Y, Gavrilova O, Yakar S, et al. Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology. 2005;146:4024–4035. [DOI] [PubMed] [Google Scholar]
- 279. Kusakabe T, Tanioka H, Ebihara K, et al. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia. 2009;52:675–683. [DOI] [PubMed] [Google Scholar]
- 280. Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Design. 2001;7:125–133. [DOI] [PubMed] [Google Scholar]
- 281. Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Trevaskis JL, Lei C, Koda JE, Weyer C, Parkes DG, Roth JD. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity. 2010;18:21–26. [DOI] [PubMed] [Google Scholar]
- 283. Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009;17:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Hwang JJ, Chan JL, Ntali G, Malkova D, Mantzoros CS. Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide: interventional studies in humans. Diabetes Care. 2008;31:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. [DOI] [PubMed] [Google Scholar]
- 286. Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. [DOI] [PubMed] [Google Scholar]
- 287. Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. [DOI] [PubMed] [Google Scholar]
- 288. Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. [DOI] [PubMed] [Google Scholar]
- 289. Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. [DOI] [PubMed] [Google Scholar]
- 290. Ikejima K, Okumura K, Lang T, et al. The role of leptin in progression of non-alcoholic fatty liver disease. Hepatol Res. 2005;33:151–154. [DOI] [PubMed] [Google Scholar]
- 291. Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. [DOI] [PubMed] [Google Scholar]
- 292. Yan K, Deng X, Zhai X, et al. p38 mitogen-activated protein kinase and liver X receptor-α mediate the leptin effect on sterol regulatory element binding protein-1c expression in hepatic stellate cells. Mol Med. 2012;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells. J Biol Chem. 2004;279:4292–4304. [DOI] [PubMed] [Google Scholar]
- 294. Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–133. [DOI] [PubMed] [Google Scholar]
- 295. De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008;48:2016–2026. [DOI] [PubMed] [Google Scholar]
- 296. Ikejima K, Okumura K, Kon K, Takei Y, Sato N. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22:S87–S92. [DOI] [PubMed] [Google Scholar]
- 297. Dalamaga M, Crotty BH, Fargnoli J, et al. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: a case-control study in Greece. Cancer Causes Control. 2010;21:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Potter JJ, Womack L, Mezey E, Anania FA. Transdifferentiation of rat hepatic stellate cells results in leptin expression. Biochem Biophys Res Commun. 1998;244:178–182. [DOI] [PubMed] [Google Scholar]
- 299. Wang J, Leclercq I, Brymora JM, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Imajo K, Fujita K, Yoneda M, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16:44–54. [DOI] [PubMed] [Google Scholar]
- 301. Huang XD, Fan Y, Zhang H, et al. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. W J Gastroenterol. 2008;14:2888–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Wong V, Hui AY, Tsang S, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:1154–1161. [DOI] [PubMed] [Google Scholar]
- 303. Chitturi S, Farrell G, Frost L, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403–409. [DOI] [PubMed] [Google Scholar]
- 304. Muñoz LE, Cordero P, Torres L, Sauceda AY, Flores JP, Segura JJ. Adipokines in a group of mexican patients with nonalcoholic steatohepatitis. Ann Hepatol. 2009;8:123–128. [PubMed] [Google Scholar]
- 305. Argentou M, Tiniakos DG, Karanikolas M, et al. Adipokine serum levels are related to liver histology in severely obese patients undergoing bariatric surgery. Obes Surg. 2009;19:1313–1323. [DOI] [PubMed] [Google Scholar]
- 306. Chalasani N, Crabb DW, Cummings OW, et al. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am J Gastroenterol. 2003;98:2771–2776. [DOI] [PubMed] [Google Scholar]
- 307. Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943–949. [DOI] [PubMed] [Google Scholar]
- 308. Nobili V, Manco M, Ciampalini P, et al. Leptin, free leptin index, insulin resistance and liver fibrosis in children with non-alcoholic fatty liver disease. Eur J Endocrinol. 2006;155:735–743. [DOI] [PubMed] [Google Scholar]
- 309. Medici V, Ali MR, Seo S, et al. Increased soluble leptin receptor levels in morbidly obese patients with insulin resistance and nonalcoholic fatty liver disease. Obesity. 2012;18:2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. [DOI] [PubMed] [Google Scholar]
- 311. Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012 [DOI] [PubMed] [Google Scholar]
- 312. Lutchman G, Promrat K, Kleiner DE, et al. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol. 2006;4:1048–1052. [DOI] [PubMed] [Google Scholar]
- 313. Hyogo H, Tazuma S, Arihiro K, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–1718. [DOI] [PubMed] [Google Scholar]
- 314. Park H, Shima T, Yamaguchi K, et al. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101–107. [DOI] [PubMed] [Google Scholar]
- 315. Dohil R, Schmeltzer S, Cabrera B, et al. Enteric-coated cysteamine for the treatment of paediatric non[b]-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:1036–1044. [DOI] [PubMed] [Google Scholar]
- 316. Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184–1188. [DOI] [PubMed] [Google Scholar]
- 317. Gonciarz M, Bielański W, Partyka R, et al. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res. 2013;54:154–161. [DOI] [PubMed] [Google Scholar]
- 318. Hoda MR, Hamza A, Fischer K, et al. [Obesity as a risk factor for prostate cancer: role for adipocytokines and involvement of tyrosine kinase pathway]. Aktuelle Urol. 2010;41:178–183. [DOI] [PubMed] [Google Scholar]
- 319. Gogas H, Trakatelli M, Dessypris N, et al. Melanoma risk in association with serum leptin levels and lifestyle parameters: a case-control study. Ann Oncol. 2008;19:384–389. [DOI] [PubMed] [Google Scholar]
- 320. Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol Oncol. 2010;119:65–69. [DOI] [PubMed] [Google Scholar]
- 321. Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis - focus on mammary tumorigenesis. Biochimie. 2012;94:2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]