Abstract
Estrogens play a fundamental role in the physiology of the reproductive, cardiovascular, skeletal, and central nervous systems. In this report, we review the literature in both rodents and humans on the role of estrogens and their receptors in the control of energy homeostasis and glucose metabolism in health and metabolic diseases. Estrogen actions in hypothalamic nuclei differentially control food intake, energy expenditure, and white adipose tissue distribution. Estrogen actions in skeletal muscle, liver, adipose tissue, and immune cells are involved in insulin sensitivity as well as prevention of lipid accumulation and inflammation. Estrogen actions in pancreatic islet β-cells also regulate insulin secretion, nutrient homeostasis, and survival. Estrogen deficiency promotes metabolic dysfunction predisposing to obesity, the metabolic syndrome, and type 2 diabetes. We also discuss the effect of selective estrogen receptor modulators on metabolic disorders.
Contribution of Sex Hormones to Metabolic Diseases
Origin of Circulating and Tissue Estrogens in Males and Females
Mechanisms of Estrogen Receptor (ER) Action
Evolutionary Importance of ER in Energy Metabolism
-
ER and Control of Energy Intake and Expenditure
Estrogen action in the hypothalamus in relation to energy balance
ERα in the ARC and control of food intake
ERα in the ventromedial hypothalamus and control of energy expenditure
ERα in the brainstem and control of food intake
Estrogen interaction with leptin
Estrogen interaction with neuropeptide-1
-
ER and Regulation of Adipose Tissue Distribution
Intra-abdominal adipose tissue and the metabolic syndrome
Subcutaneous adipose tissue and lipid storage
ERα and adipose tissue distribution
ER and adipose tissue lipid metabolism
-
ER and Insulin Sensitivity
Estrogens and insulin sensitivity
ERα in relation to skeletal muscle glucose transporter GLUT4
ERα in relation to skeletal muscle fatty acid metabolism and inflammation
ERs and insulin sensitivity in the liver
ERα and Functioning of Macrophages and Immune Cells
ER in Relation to Pancreatic β-Cell Function
Estrogen Sulfotransferase and Metabolism
-
Estrogen Therapy and Metabolism
Relation of route of estrogen administration and metabolism
Effect of selective estrogen receptor modulators and aromatase inhibitors on metabolism
Conclusions and Perspectives
I. Contribution of Sex Hormones to Metabolic Diseases
In 1941, estrogen products were approved by the US Food and Drug Administration as a hormone supplement to treat postmenopausal symptoms. In the following decades, exogenous estrogen acquired the reputation as an antidote to a variety of health-related consequences of aging in a number of different tissues. In 1995, approximately 38% of postmenopausal women in the United States used hormone replacement therapy (HRT), consisting of estrogen with or without progestin, to treat symptoms of menopause and to prevent chronic conditions such as cardiovascular disease, osteoporosis, and Alzheimer's disease (1). The widespread enthusiasm for estrogen replacement therapy experienced its first hesitation in the 1970s when it was linked to uterine cancer. This led to the addition of progesterone for treatment among women with an intact uterus (2, 3). It was not until the Women's Health Initiative (WHI) was abruptly halted in 2002 as a result of a link between HRT and increased risk of coronary heart disease events, stroke, and breast cancer that the health benefits of HRT were seriously questioned (4). The WHI was a large clinical trial in postmenopausal women that tested whether HRT could prevent age-related health problems like cardiovascular disease and osteoporosis. Notably, this ambitious study focused on clinical events and did not consider outcomes associated with symptom relief among participants. Results of the WHI led many women and their physicians to overestimate the individual-level risk associated with HRT use. However, the overall conclusions from the WHI do not apply to most menopausal women who initiate HRT in their 50s. In fact, current scientific evidence suggests that among symptomatic menopausal women younger than age 60 or within 10 years of menopause, the benefits of HRT outweigh the risks (5). As a result of dramatic increases in life expectancy in developed countries, many women will spend the second half of their lives in a state of estrogen deficiency. Apart from degenerative diseases of the cardiovascular, skeletal, and central nervous systems, estrogen deficiency enhances metabolic dysfunction predisposing to obesity, the metabolic syndrome, type 2 diabetes, and certain cancers (eg, breast and colon, and hepatocellular carcinoma) (6, 7). Thus, the contribution of estrogen deficiency in the pathobiology of multiple chronic diseases in women is emerging as a new therapeutic challenge of the 21st century. To address this growing problem, improved understanding of how estrogens contribute to energy balance and glucose homeostasis promises to yield novel therapeutic applications for an increasingly large segment of the female population. Here, we review evidence in rodents and humans on the role of estrogens and their receptors in regulating metabolic homeostasis in health and disease.
II. Origin of Circulating and Tissue Estrogens in Males and Females
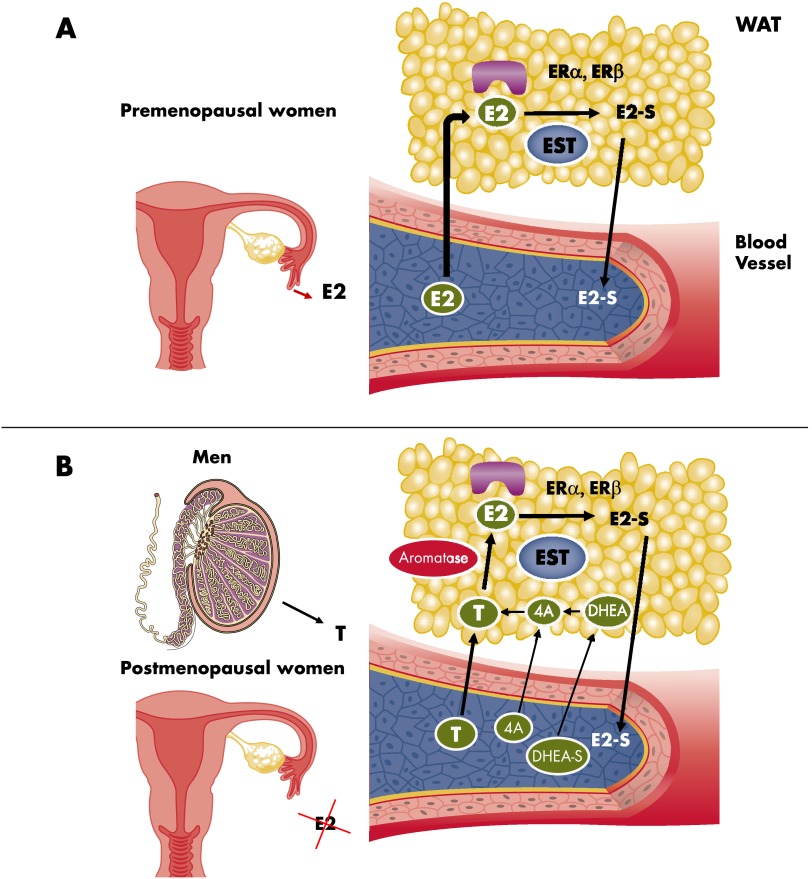
In healthy premenopausal women, 17β-estradiol (E2), the main circulating estrogen, is produced by the ovaries after aromatization of androstenedione to estrone (E1) and subsequent conversion of E1 to E2. Among women with normal menstrual cycles, E2 functions as a circulating hormone that acts on distant target tissues (Figure 1A). In postmenopausal women, however, when the ovaries fail to produce E2 and in men—who have naturally low levels of circulating E2—E2 does not function as a circulating hormone; rather, it is synthesized in extragonadal sites such as breast, brain, muscle, bone, and adipose tissue where it acts locally as a paracrine or intracrine factor (8). Therefore, among both postmenopausal women and men, the determinant of E2 action is not circulating estrogens; rather, E2 function depends on estrogen biosynthesis from a circulating source of androgens (Figure 1B). Consequently, in these individuals, a major driver of E2 action is the aromatization of androgens to estrogens (8). Thus, tissue metabolism or inactivation of E2 is also an essential parameter controlling cellular estrogenic action (9). Tissue estrogen sulfotransferase (EST) is a critical mediator of estrogen action (Figure 1, A and B). EST is a cytosolic enzyme that provides a molecular switch in target cells that inhibits estrogen activity by conjugating a sulfonate group to estrogens, thereby preventing binding to estrogen receptors and enhancing urinary excretion of the hormone (10).
Figure 1.
Origin of circulating and tissue estrogens. A, In healthy premenopausal women, estrogen (E2) is produced by the ovaries and functions as a circulating hormone that acts on distant target tissues. Here WAT is represented. B, In postmenopausal women and in men, E2 does not function as a circulating hormone; rather, it is synthesized in extragonadal sites from circulating androgenic precursors such as T, androstenedione (4A), or dehydroepiandrosterone (DHEA). Cellular estrogenic action depends on: 1) the ER signaling and sensitivity; 2) the activity of enzymes like aromatase involved in the biosynthesis of E2 from androgenic precursors; and 3) the inactivation of E2 in E2 sulfate (E2-S) by the estrogen sulfotransferase (EST).
III. Mechanisms of Estrogen Receptor (ER) Action
Early studies of the reproductive actions of estrogens led to the establishment of a paradigm in which classical nuclear ERs acted as ligand-activated transcription factors (11). ER modulation of gene transcription is a highly dynamic process. The ER exists in 2 main forms, ERα and ERβ, each of which has multiple isoforms and exhibit distinct tissue expression patterns and functions (12). The classical “genomic” mechanism of ER action typically occurs within hours, leading to activation or repression of target genes. In this classic signaling pathway, ligand-activated ER dissociates from its chaperone heat-shock protein and binds as a dimer either directly to an estrogen response element (ERE) in target gene promoters or indirectly to activator protein 1 or specificity protein 1 response elements through protein tethering to DNA (13). After binding, these ER dimers interact with cofactors (coactivators or cosuppressors) to regulate gene expression. Importantly, ERs acting on different response elements exert varying activity profiles. For example, when tethered via activator protein 1 sites, ERα exhibits E2-dependent transcription activation, whereas E2 bound to ERβ has no effect on transcription (14). In addition, depending on the identity and concentration of the ligand, ERα-ERα homodimers, ERβ-ERβ homodimers, or ERα-ERβ heterodimers are formed, with ERα playing a dominant role in heterodimer formation (15). There is overlap in binding sites for E2-liganded ERα and ERβ when these receptors are present alone in cells. However, when both ERs are present, few sites are shared between ERα and ERβ. Each ER restricts the binding site occupancy of the other, with ERα again being dominant (16). Furthermore, ligand-activated ER promotes transcription in a cyclic fashion. The ER transcription complex appears to cycle on and off target promoters as long as E2 is present. The regular cycling of the ER transcription complex may represent a mechanism that permits ongoing assessment of, and adaptation to, the external environment (17).
Although reproductive functions are mostly mediated via classical nuclear ER acting as ligand-activated transcription factors, a large component of ER actions related to energy metabolism also involves extranuclear ERs, indirectly modulating gene expression or acting independently of nuclear events (18). E2 can activate rapid signals that act within seconds or minutes via extranuclear and membrane-associated forms of ERs (19). ERα and ERβ are localized to caveolae where they congregate with other signaling molecules, thereby facilitating interaction and rapid intracellular signaling. These signal proteins include G proteins, growth factor receptors, tyrosine kinases (Src), linker proteins (MNAR), and orphan G protein-coupled receptors. This multiprotein complex provides the necessary interactions for membrane ER to activate growth factor receptors and G proteins. In turn, these E2-induced rapid signals modify protein function via phosphorylation. However, they can also modulate gene expression and thus the production of proteins. In the cardiovascular system, bone, the nervous system, and pancreatic β-cells, membrane and extranuclear pools of ER activate protein kinases that phosphorylate transcription factors. This promotes the nuclear translocation of these ERs and subsequent activation of target genes (19, 20). In breast cancer cells, extranuclear ER pools collaborate with intranuclear ER signaling to regulate cellular proliferation, migration, and invasion. For example, E2 activation of an extranuclear ERα activates ERK2, which is then recruited by nuclear ERα to ER binding sites throughout the genome of breast cancer cells (22). Although the G protein-coupled ER (GPER; also called GPR30) responds to E2, its function as an ER remains controversial. Multiple groups have described collaboration between membrane-localized ERα and GPR30, presumably at the membrane of several E2-sensitive cell lines. Recently, it has been proposed that GPER induces the expression of ERα36, a transcriptionally inactive and truncated version of the classical long isoform of ERα, ERα66 (23). The function of ERα36 is unknown.
IV. Evolutionary Importance of ER in Energy Metabolism
The study of phylogeny yields important insight into the potential role of ERs in energy metabolism. Although important to behavior, reproduction, bone, and immunity in higher order mammals, the ancestral ER existed in invertebrates lacking sexual reproductive capabilities, suggesting that it may have played an important role in energy metabolism and survival. All members of the steroid receptor family descend from a single ancestral receptor (AncSR1), which separated from the rest of the nuclear receptor super family early in animal evolution, perhaps 500 million years ago (24, 25). There is evidence to support the paradigm that AncSR1 had intrinsic functions similar to those of modern-day vertebrate ER. AncSR1 is present in annelids and mollusks, thus demonstrating that estrogen signaling via the ER is as ancient as the ancestral bilaterian animal. It is believed that AncSR1 was an estrogen-responsive, ERE-binding transcriptional activator. The ligand binding domain was hormone-activated and specifically responsive to estrogens (26). It is unknown, however, whether estrogens were synthesized before the appearance of AncSR1. If this was the case, it was a receptor for estrogens. Conversely, if estrogen synthesis is not as old as AncSR1, then AncSR1 sensitivity to estrogens developed later and may represent an example of evolution by molecular exploitation (27, 28). In that latter scenario, the ligand could have been a hormone structurally similar to E2. AncSR1 would then have been a sensor for environmental estrogens, or phytoestrogens (26, 29). Interestingly, the receptors for T, progesterone, gluco- and mineralocorticoids evolved later (26). The androgen receptor interaction with T, which is similar to the progesterone receptor interaction with progesterone, evolved when duplicated receptors diverged and recruited steroids that were originally biochemical intermediates and allosteric regulators. Thus, T was produced as a precursor in E2 biosynthesis long before AR evolved affinity for this steroid. The observation that ancestral ER was present before sexual reproduction and before the evolution of receptors for stress hormones including glucocorticoid and mineralocorticoid receptors suggests an important role for ER in cellular energy metabolism.
V. ER and Control of Energy Intake and Expenditure
A. Estrogen action in the hypothalamus in relation to energy balance
As women enter menopause, there is a decline in circulating estrogens. This is accompanied by alterations in energy homeostasis that result in increases in intraabdominal body fat (6). In animals, ovariectomy (OVX) leads to increased adiposity (30–32) that is prevented by estrogen replacement (33). Although OVX induces a transient increase in food intake in rodents and E2 replacement decreases food intake (34, 99), hyperphagia does not fully account for changes in metabolism and development of obesity after OVX (32). Indeed, reduced E2 synthesis resulting from aromatase inactivation promotes obesity in the absence of hyperphagia or reduced energy expenditure in mice of both sexes. However, the animals exhibit reduced spontaneous physical activity and lean body mass (36). Similarly, ERα deficiency in both male and female mice causes increased body weight and adiposity predominately through reduced energy expenditure and slight increases in food intake (37, 38). However, it should be noted that these 2 models—ERα and aromatase deficiency—also exhibit a marked increase in serum T levels that acts as a confounding factor. Although endogenous E2 favors body weight homeostasis by increasing energy expenditure (39), exogenous estrogens may promote energy balance by influencing both energy intake and energy expenditure. Thus, global loss of ERα action results in a decrease in energy expenditure, whereas increased ERα (and ERβ) signaling resulting from elevating serum E2 concentrations suppresses energy intake and increases energy expenditure. These topics will be discussed below.
The hypothalamus is one area in the central nervous system that controls food intake, energy expenditure, and body weight homeostasis. Dramatic changes in all of these characteristics can be induced by lesioning specific hypothalamic nuclei, such as the ventromedial hypothalamus (VMH) (40, 41) or the lateral hypothalamic area (42, 43). ERα is abundantly expressed in the rodent brain in the ventrolateral portion of the ventromedial nucleus (VMN), the arcuate nucleus (ARC), the medial preoptic area, and the paraventricular nuclei (44–50). ERβ is found in the same hypothalamic nuclei, but ERβ expression is significantly lower relative to ERα.
The effects of E2 on energy balance are primarily mediated by ERα. Female mice with mutations in the ERα gene are obese (37, 51), and deletion of ERα in mice blocks the antiobesity effects of estrogen replacement (33). ERβ is less effective in mediating the physiological antiobesity effects of estrogen because deletion of ERβ, unlike that of ERα, does not promote obesity (51) or any metabolic consequences associated with obesity. This distinction is consistent with pharmacological studies showing that, whereas the selective ERα agonist propylpyrazole triol (PPT) suppresses food intake in ovariectomized mice, the selective ERβ agonist diarylpropionitrile failed to influence feeding behavior at any dose (52, 53). However, E2 also suppresses food intake through ERβ because the anorectic effect of intracerebroventricular injection of E2 is blocked by coadministration of ERβ antisense oligodeoxynucleotides in female rats (54). In addition, administration of an ERβ-selective agonist to high fat diet (HFD)-fed female mice increased expression of the thermogenic uncoupling protein-1 in brown adipose tissue, thereby reducing obesity (55). Thus, under specific circumstances, activation of ERβ can suppress food intake and increase energy expenditure. By contrast, the role of GPER in body weight regulation still requires validation. In 1 study of female mice lacking GPER, the obesity phenotype emerged in only 1 of 4 GPER mutant mouse lines (56).
Although the signaling mechanisms of ER actions in hypothalamic neurons are not fully understood, available evidence suggests that it primarily involves extranuclear ER. Indeed, E2 triggers a rapid increase in excitatory inputs to pro-opiomelanocortin (POMC) neurons in the ARC in vivo, an observation that is consistent with rapid, extranuclear or membrane-initiated actions (34). Thus, E2 can suppress neuropeptide Y (NPY) in clonal hypothalamic neurons via a membrane form of ERα (57). In addition, an E2-responsive, Gq-coupled membrane receptor is involved in mediating the anorectic effects of E2 on food intake and body temperature in hypoestrogenic female rodents (58, 59).
B. ERα in the ARC and control of food intake
ERα is expressed prominently on POMC neurons within the ARC. These neurons modulate food intake, energy expenditure, and reproduction (60). POMC neurons also secrete αMSH, which acts in the paraventricular nuclei and lateral hypothalamus on melanocortin 3 and melanocortin 4 (MC3/MC4) receptors. This produces a pronounced catabolic effect by reducing food intake and increasing energy expenditure (61–64). ARC POMC ERα mRNA levels fluctuate over the course of the estrous cycle, with the most dramatic increase on the day of proestrus, when E2 concentration is highest (65–68). Conversely, reduced POMC levels are observed in ERα knockout (KO) mice (69). Using electron microcopy, Horvath and co-workers (34) reported that the number of excitatory synaptic inputs to ARC POMC neurons increases as mice enter proestrus. Furthermore, central E2 administration rapidly increases the excitatory synapses on POMC neurons, an effect that is also reflected by increased miniature excitatory postsynaptic currents recorded from POMC-green fluorescent protein neurons (34). These synaptological rearrangements in POMC neurons are in tight parallel with the effects of estrogens on food intake, energy expenditure, and body weight (34). These effects of E2 are mediated via MC4 receptor because E2 is unable to induce anorexia when the MC4 receptor antagonists Shu 9119 or agouti-related peptide (AgRP) are applied concomitantly with E2 administration in rats (70). E2 administration to hypothalamic slices also increases excitability of POMC neurons by rapidly uncoupling GABAB receptors from their G protein-gated inwardly rectifying K+ channels (71). Therefore, estrogens directly act on POMC neurons and regulate their cellular activity. Recent findings provide additional support for the importance of ERα in POMC neurons and the suppression of food intake. Indeed, deletion of ERα in POMC neurons in mice leads to hyperphagia without directly influencing energy expenditure or adipose tissue distribution (60).
C. ERα in the ventromedial hypothalamus and control of energy expenditure
The VMN of the hypothalamus (also known as the VMH) has long been considered an important feature of the neural circuits that are responsible for homeostatic regulation of body weight and food intake. As noted above, numerous studies have shown that lesions of the VMN produce obesity due to both increased food intake and decreased energy expenditure (72). However, these findings have been called into question because of the aggressive methodologies that have been used to lesion the VMN. These approaches likely damaged the surrounding regions (such as the ARC) as well as neuronal fibers passing through the VMN (73). Recent genetic studies in mice have circumvented these issues by noninvasive selective deletion of genes within VMH neurons. These studies were based on the concept that the transcription factor steroidogenic factor-1 (SF1) is expressed exclusively in the VMN neurons in the brain (74). Deletion of SF1 in mice disrupts the VMN structure (75) and leads to obesity (76). Estrogens have been shown to directly alter the electrophysiological properties of VMN neurons (77). Mice with small hairpin RNA-mediated ERα gene silencing as well as transgenic mice in which ERα has been selectively deleted from SF1-containing neurons of the VMH, develop reduced sensitivity to E2-induced weight loss, increased visceral fat deposition, and reductions in energy expenditure. All of these results occur without an impact on food intake (60, 78), supporting the notion that ERα signaling in VMN neurons plays an important role in regulating physical activity, thermogenesis, and fat distribution. The different actions of ERα signaling in hypothalamic neurons on food intake and energy expenditure are summarized in Figure 2.
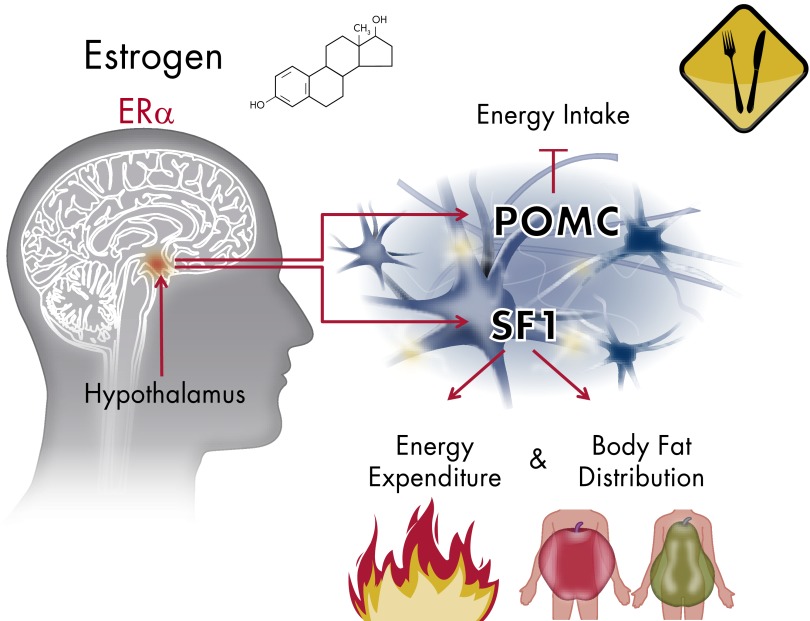
Figure 2.
Summary of hypothalamic ERα actions regulating energy balance. In the hypothalamus, estrogen action through ERα in ARC POMC neurons suppresses food intake. On the other end, estrogen actions through ERα in VMN SF1 neurons stimulate physical activity and energy expenditure and regulate body fat distribution.
D. ERα in the brainstem and control of food intake
ERα is also expressed in the brainstem, including the nucleus tractus solitarius (NTS), and dorsal medial vagus (47, 50, 79). Geary and co-workers (80, 81) showed that E2 replacement in wild-type mice suppresses food intake, potentiates cholecystokinin (CCK)-induced satiety, and is accompanied by increased activity of NTS neurons. CCK is synthesized and released from cells of the upper intestine and acts on abdominal CCK-A receptors. CCK plays a variety of roles in the digestive process, including slowing gastric emptying and intestinal motility (82). CCK exerts its satiety action primarily by activating subdiaphragmatic vagal afferent neurons (83). E2 increases the potency of CCK by increasing the sensitivity of vagal CCK-A receptors, although it does not increase CCK secretion or the number of CCK-A receptors (84–87). Interestingly, these responses are all absent in mice lacking ERα (81). Furthermore, it has been shown that administration of E2 directly to the NTS potentiates CCK-induced satiety signals (88). Collectively, these findings suggest that ERα in the brainstem, and specifically the NTS, is an additional site mediating the anorexigenic effects of estrogens.
E. Estrogen interaction with leptin
First described in 1994 (89), leptin has proven to be a powerful catabolic signal to the brain that inhibits food intake and increases energy expenditure. These effects are mediated via the long form of the leptin receptor (leprb) (63, 90, 91). Leprb are localized in several brain areas including the VMN and the ARC, and leprb are colocalized with several neuropeptides involved in regulating food intake and reproduction (92–94). It has been reported that leprb expression in the ARC is colocalized with ERα (95). Furthermore, estrogens have been reported to down-regulate expression of leprb mRNA in the ARC (96), possibly via an ERE on the leptin receptor gene (97). The extensive hypothalamic colocalization of these 2 receptors suggests a closely coupled interaction between these peripheral signals in the regulation of energy homeostasis. Estrogens may be modulators of leptin's catabolic action in the brain. In fact, higher levels of E2 have been associated with increased central leptin sensitivity in rodents (98–100). Although circulating leptin levels do not change appreciably during the estrous cycle, ARC leprb expression is highest during estrous and metestrous and is inversely correlated with NPY mRNA expression (96). OVX reduces sensitivity to central leptin relative to females with intact uteri, a deficiency that can be restored by E2 replacement (100). Analogously, exogenous E2 administration to male rats increases sensitivity to central leptin (100). Differences in central leptin sensitivity caused by the presence or absence of estrogens may occur downstream of leprb transcription and translation. As a result, there may be a threshold beyond which estrogens enhance central sensitivity to leptin. This “leptino-mimetic” function of E2 is best observed in hypogonadal leptin-deficient (ob/ob) and leptin-resistant (db/db) mice of both sexes. In these models, E2 decreases food intake and increases energy expenditure, thereby resulting in reduced body weight (34).
F. Estrogen interaction with neuropeptide-1 (NPY)
NPY is an anabolic peptide. Central administration of NPY results in substantial increases in food intake and decreases energy expenditure and fat oxidation (101–104). ARC neurons coexpress NPY mRNA and leprb protein. Leptin administration decreases, whereas leptin deficiency or leptin resistance increases NPY (and AgRP) mRNA, indicating that leptin is a critical determinant of ARC NPY function (105). NPY neurons in the hypothalamus not only affect feeding, but also influence reproduction. Therefore, E2 can modulate both of these neuroendocrine systems by regulating NPY gene expression. E2 stimulates NPY and NPY Y1 receptor expression (106) and NPY release (107). In an ex vivo hypothalamic neuronal cell line, N-38, E2 affected expression of NPY in a biphasic manner corresponding to changes in the ERα:ERβ ratio. When the ERα:ERβ ratio was high, NPY transcription was repressed; conversely, when the ratio was low, NPY transcription was stimulated (108). Additionally, NPY/AgRP neurons are required to mediate the anorexigenic effects of E2. Xu and co-workers (109) showed that hypothalamic expression of NPY and AgRP is tightly regulated across the estrus cycle, with the lowest levels during estrus, which coincides with the E2 peak and feeding nadir in wild-type mice. Furthermore, E2 administration suppresses fasting-induced c-Fos activation in NPY/AgRP neurons and blunts the refeeding response (109). Importantly, the cyclic changes in food intake and E2-induced anorexia are blunted in mice with degenerated NPY/AgRP neurons (109). These data indicate that neurons coexpressing NPY and AgRP are functionally required for the cyclic changes in feeding throughout the estrous cycle and that NPY/AgRP neurons are essential mediators of the anorexigenic function of E2. Surprisingly, ERα was absent from NPY/AgRP neurons in the mouse hypothalamus (109), suggesting that E2 may regulate these neurons indirectly via ERα action in presynaptic neurons. However, the precise ERα-expressing presynaptic neurons that act on NPY/AgRP remain to be identified.
VI. ER and Regulation of Adipose Tissue Distribution
Sexual dimorphisms in body fat distribution are well-described. Men, on average, have less total body fat but more central/intra-abdominal (described further in Section VI.A.) adipose tissue, whereas women tend to have more total fat that favors gluteal/femoral and sc depots (110–114). After menopause, fat distribution shifts in women to a phenotype more similar to that of men (115, 116); however, estrogen replacement therapy prevents the male-patterned accumulation of intra-abdominal adipose (117–119). Additionally, estrogen treatment of male-to-female transsexuals increases the amount of sc adipose tissue accrual relative to intra-abdominal adipose tissue (120). Therefore, estrogens have been proposed as regulators of fat distribution.
A. Intra-abdominal adipose tissue and the metabolic syndrome
Excess accumulation of adipose tissue in the central region of the body (intra-abdominal, “android,” or male-pattern obesity) (121) correlates with increased risk of, and mortality from, disorders including type 2 diabetes, hyperlipidemia, hypertension, and atherosclerosis. Intra-abdominal adipose tissue is thought to be metabolically and functionally different from sc adipose tissue. Indeed, intra-abdominal adipose tissue has more capillaries and efferent sympathetic axons per unit volume than sc fat, and unlike sc fat, it drains into the hepatic portal vein (121). In humans, surgical removal of intra-abdominal adipose tissue by omentectomy during bariatric surgery has been shown to either decrease insulin and glucose levels (122) or to have no effect on components of the metabolic syndrome and insulin resistance (123, 124). However, in male rodents, surgical removal of visceral adipose tissue prevents insulin resistance and glucose intolerance (125). In contrast, in male rodents, surgical removal of sc fat tissue of equal weight has no appreciable impact on the same parameters (125). Teleologically, males may have preferentially deposited adipose tissue in the intra-abdominal depot because this depot is rapidly mobilized, providing an energy source for immediate protection against predators and hunting.
B. Subcutaneous adipose tissue and lipid storage
Subcutaneous adipose tissue deposition is not associated with the metabolic disturbances due to its enhanced ability to expand, allowing for storage of excess caloric intake through angiogenesis and reduced hypoxia and fibrosis (121). Females have more sc adipose tissue on average, than males. Subcutaneous adipose tissue is dispersed in a broad area under the skin, is relatively poorly innervated and vascularized, and has a larger average cell diameter than intra-abdominal adipose tissue (121). Subcutaneous adipose tissue permits efficient storage of maximal calories per unit volume of tissue. Lipid deposition into sc adipose tissue may provide an evolutionary advantage for females because it provides protection from fluctuations in caloric supply, thereby maintaining reproductive capacity. Importantly, females mobilize adipose tissue stored in the sc depot to augment the caloric demands associated with breast feeding. Additionally, “gynoid” (or female-pattern sc) fat distribution is poorly correlated with risk for metabolic disorders (126–130). In fact, transplantation of sc fat from donor mice into visceral regions of recipient mice decreases fat mass and improves glucose homeostasis, demonstrating that sc fat produces substances that can act systemically to improve glucose metabolism (131).
C. ERα and adipose tissue distribution
Estrogens are produced in the adipocytes (via aromatization from androgenic precursors) and increase in proportion to total body adiposity (132, 133). ERα is expressed in adipose tissue (134, 135). Mice of both sexes with a targeted deletion of the ERα gene (ERαKO) have increased adiposity, with a near doubling of visceral adipose depots relative to their age-matched wild-type counterparts (37). However, inguinal adipose depots also increased in ERβKO mice, suggesting that ERα elimination may not target only intra-abdominal depots. Thus, body fat distribution results should be interpreted with caution in rodents. Still, these data are in contrast to those of the ERβKO mouse, which is not obese (136), indicating that ERα is more important than ERβ in preventing adipose tissue deposition. Reduced ERα expression and impaired ERα function have been linked with increased prevalence of certain aspects of the metabolic syndrome in both male and female humans and rodents (137–141), and certain polymorphisms of the human ERα gene are associated with abnormal adiposity (138, 142). In a cross-sectional study, over 2000 middle-aged, premenopausal Japanese women with the ERα gene polymorphism had increased fat mass and increased waist-hip ratios (an index of intra-abdominal adiposity) relative to those with the normal genotype (142–144). The polymorphism did not affect adiposity in postmenopausal women. Thus, polymorphisms of the human ERα gene may predispose to increased intra-abdominal adiposity and its related health risks.
D. ER and adipose tissue lipid metabolism
E2 also suppresses white adipose tissue (WAT) accumulation by decreasing fatty acid and triglyceride synthesis and lipogenesis. Greenberg and co-workers (145) showed that administration of E2 reduces adipocyte size in ovariectomized female mice by reducing fatty acid uptake (down-regulation of lipoprotein lipase) and lipogenesis (down-regulation of acetyl-coenzyme A carboxylate and fatty acid synthase [FAS]). These changes increase catecholamine-stimulated lipolysis and increase lipid-oxidative pathways in muscle (145). In postmenopausal women, estrogen therapy decreased the expression of genes involved in lipogenesis including acetyl-coenzyme A (CoA) carboxylate-α and -β, sterol regulatory element-binding protein 1c (SREBP-1c), stearoyl-CoA desaturase, lipoprotein lipase (LPL), FAS, fatty acid desaturase, and peroxisome proliferator-activated receptor (PPAR)-γ (146, 147). E2 suppresses lipogenic genes and triglyceride accumulation in WAT and liver in HFD-fed (148) and leptin-resistant female mice (149). Surprisingly, this effect was not reproduced by the ERα agonist, PPT (150). However, this effect was reproduced by a novel ERβ agonist (55).
LPL is a key regulating enzyme for energy metabolism that breaks down plasma triglycerides into free fatty acids and glycerol. The activity of this enzyme is mainly regulated at the transcriptional and translational levels (151). E2 is a major suppressor of fasting LPL activity in adipose tissue in women (152, 153), and evidence suggests that E2 also represses LPL gene expression at the transcriptional level via an ERE on the LPL promoter (154). Additionally, Lipin 1 (LPIN1) is a gene involved with lipid homeostasis and metabolism (155). Enhanced LPIN1 expression promotes obesity (156, 157) and is markedly down-regulated by E2 (158). The E2 regulation of LPIN1 may provide a mechanistic link between estrogens, lipid metabolism, and lipid signaling. LPL activity and LPIN1 mRNA are 2 examples of how estrogen may directly regulate the equilibrium between lipogenesis and lipolysis in adipose tissue, thereby reducing adipocyte hypertrophy and ectopic lipid accumulation.
Extensive evidence demonstrates that E2 has direct effects on cultured adipocytes with the overall effect of inhibiting lipogenesis and adipogenesis (159). Thus, the E2 effects described above could result from ER action in peripheral tissues. Nonetheless, the exact contribution of in vivo E2 antilipogenic effects resulting from direct ER action in WAT or from central ER actions that indirectly affect adipose and liver are still unknown. Although the overall effect of E2 is to decrease lipid deposition in WAT, E2 favors sc WAT accumulation via central (100) and peripheral mechanisms in both sexes (159).
ERβ has direct antilipogenic and antiadipogenic effects in adipocytes. ERβ deficiency favors WAT accumulation in female mice during high fat feeding by increasing PPARγ signaling in WAT, thereby demonstrating that ERβ acts directly on adipocytes in vivo and is a negative regulator of PPARγ (160). In addition, ERβ selective ligands show PPARγ antagonistic actions in adipocytes that are mediated through a mechanism involving competition between ERβ and PPARγ for PPARγ coactivator 1α (55).
VII. ER and Insulin Sensitivity
A. Estrogens and insulin sensitivity
Insulin resistance is a central disorder in the pathogenesis of type 2 diabetes. It is also a defining feature of the metabolic syndrome. Compared with age-matched men, premenopausal women with a normal menstrual history have enhanced insulin sensitivity normalized to lean mass. This is a likely contributor to the reduced incidence of type 2 diabetes observed in premenopausal women (161, 162). Indeed, although a 40–50% reduction in insulin-mediated glucose disposal is consistently observed in male mice after high fat feeding (163, 164), E2-replete females, humans, and rodents are typically protected against a HFD- and acute fatty acid-induced insulin resistance (165–168). After menopause or OVX, a precipitous decline in insulin sensitivity parallels an increase in fat mass and elevations in circulating inflammatory markers, low-density lipoprotein (LDL), triglycerides, and fatty acids (6, 169, 170). Ovariectomized mice and rats are insulin resistant, have impaired exercise-stimulated glucose disposal into muscle (171), and are more susceptible to the deleterious effects of HFD or lipid oversupply. Restoration of E2 at physiological concentrations maintains insulin action and glucose tolerance (172).
Although chronic administration of E2 improves insulin sensitivity in rodents, the acute action of E2 on promotion of insulin-stimulated glucose uptake into muscle remains disputed despite consistent observations of E2-induced activation of Akt and AMP-activated protein kinase (AMPK) (173, 174). Furthermore, although iv conjugated estrogens and E2 administered to postmenopausal women or ovariectomized rats elicit significant increases in glucose disposal during hyperinsulinemic-euglycemic clamp studies (175, 176), ex vivo treatment of skeletal muscle with E2 failed to yield similar increases in insulin-stimulated glucose disposal (173). This finding is also in contrast to in vitro studies showing enhanced insulin action after short-term E2 treatment of myotubes from postmenopausal women and age-matched men (177). Thus, a basic question remains concerning which tissues of E2 action confer protection against insulin resistance.
Similar to findings for ovarian failure in women and female rodents, a reduction in circulating estrogens that results in inactivation of mutation of Cyp19 (aromatase) in men or experimental deletion in Cyp19 in male mice confers an obesity-insulin resistant phenotype (36, 137, 178–184). Both physiological and genetic evidence argue that E2 and ER favor insulin sensitivity in rodents and humans of both sexes when E2 concentrations stay within a tight physiological concentration. In contrast, replacement or augmentation of E2 to supraphysiological levels or overstimulation of ER are thought to induce insulin resistance secondary to hyperinsulinemia (liver) or a reduction in total glucose transporter 4 (GLUT4) expression (muscle) (185, 186). Furthermore, 2 studies have reported prospectively that higher plasma levels of E2 in postmenopausal women were related to increased risk of type 2 diabetes (187, 188). Clearly, additional studies in rodents and humans using a dose-response strategy are necessary to better understand the interplay of steroid hormones including E2, T, and progesterone on regulation of metabolism and insulin action in glucoregulatory tissues.
Although several laboratories have characterized the whole-body ERαKO mouse, many questions still remain, including the tissues responsible for conferring the severe insulin-resistant obesity phenotype. Does obesity arise from loss of ERα within adipocytes in particular, or can it be driven as a secondary phenotype resulting from a loss of ERα in brain, skeletal muscle, liver, or even in selective immune cells? Does loss of ERα from myocytes drive skeletal muscle insulin resistance, or does this ERαKO phenotype arise from increased adiposity and altered adipokine/cytokine secretion? To address these specific questions, several laboratories have embarked on phenotypic evaluation of mice harboring tissue selective deletion of ERα using the Cre-lox approach. Findings from these studies are just now emerging.
B. ERα in relation to skeletal muscle glucose transporter GLUT4
Although 2 forms of the ER receptor are expressed in many glucoregulatory tissues, ERα is found in much higher abundance than ERβ; ERβ transcription levels are nearly undetectable in human and rodent muscle, as well as human myotubes in culture (177, 189, 190). Furthermore, homozygous ERβ deletion failed to produce insulin resistance (51), a finding that contrasts with marked skeletal muscle insulin resistance observed in ERαKO animals (192, 193).
The mechanistic function of ERα in the maintenance of skeletal muscle insulin action remains unknown. Although early reports suggested a role for ERα in the regulation of GLUT4 expression in skeletal muscle, recent conflicting evidence calls this explanation into question concerning the insulin resistance phenotype observed in ERαKO mice. Findings reported by Bryzgalova et al (194), suggesting reduced total GLUT4 levels in muscle as an underlying cause of the ERαKO insulin resistance phenotype, were not supported by data from Ribas et al (192). Furthermore, despite maintenance of GLUT4 mRNA and protein, Ribas et al (192) reported more dramatic skeletal muscle insulin resistance in ERαKO mice than Bryzgalova et al (194). Hevener and colleagues (195) suggest that the skeletal muscle insulin resistance observed in ERαKO mice is due predominantly to the direct effects of ERα deletion and proinflammatory signaling on proximal insulin action. Indeed, in muscle from muscle-specific ERαKO mice, primary myotubes, and myotubes with ERα knockdown, no alteration in GLUT4 mRNA or protein was observed despite reduced insulin-stimulated glucose disposal (195). Furthermore, additional studies by Barros et al (186, 196) assessing GLUT4 expression in response to OVX and E2 supplementation are in conflict with other studies of similar design (177, 190, 197–199). Given the lack of consensus ERE in the GLUT4 promoter (200) and the absence of confirmatory findings in cellular reporter and chromatin immunoprecipitation assays, the issue of ERα regulation of GLUT4 expression requires further investigation. Indeed, GLUT4 is regulated by several redundant transcriptional pathways (201, 202). Given that total GLUT4 transcription and protein are not reduced in humans or rodents in the context of insulin resistance, obesity or type 2 diabetes, or between men and women (203, 204), it is likely that in the absence of ERα, other transcription factors compensate to maintain GLUT4 levels (205–210).
This is not to suggest that ERα activation cannot promote total GLUT4 up-regulation. This could occur after endurance exercise training where transcript and protein levels of both are simultaneously elevated in trained human and mice compared to their sedentary counterparts (189, 203, 211–214). Myocyte enhancer factor 2 (MEF2) expression and a functional MEF2 element in the GLUT4 promoter are critical for GLUT4 gene expression (215). Furthermore, reciprocal regulation between ERα and MEF2 can be observed in cardiomyocytes via ERα interaction with class II histone deacetylase (216). Despite complex transcriptional signal integration in the regulation of GLUT4 expression (201, 202, 217–220), it is conceivable that elevated ERα action could promote increased GLUT4 transcription via heightened protein tethering with MEF2 on the GLUT4 promoter or by indirect action via AMPK (173, 221). It is important to note that transcriptional activity of the GLUT4 promoter is quite low under basal conditions, and other ovarian hormones such as progesterone play an antagonistic role in regulation of GLUT4 expression (171). These issues, as well as the dose of E2 administration during interventional studies, the age, and the hormone status of the human subjects and rodents used are important considerations when interpreting the literature. Given the different roles that muscle and adipose tissue play in controlling whole-body metabolism, it is likely that the interplay of transcriptional regulators of GLUT4 varies markedly among tissues. Although the data suggest a potential role for ERα as an enhancer of GLUT4 transcription in muscle under certain conditions, they do not support direct regulation of GLUT4 expression under basal conditions. Collectively, work by Hevener and colleagues (195) suggests that the skeletal muscle insulin resistance observed in whole-body ERαKO mice and animals with a muscle-specific deletion of ERα results predominantly from impaired insulin signal transduction, not a deficiency in total availability of GLUT4.
A role for ERα in the regulation of proximal insulin signal transduction has been suggested because E2 administration to insulin-resistant rodents increases insulin receptor substrate-1 abundance, insulin-stimulated tyrosine phosphorylation, and Akt phosphorylation (173, 193, 222). Akt serves many functions in myocytes, including ERα-induced regulation of myogenic differentiation (223), suppression of muscle-atrophy ubiquitin ligases via FOXO1 inhibition (224), and induction of genes associated with myocellular proliferation (223, 225–228). E2 activation of phosphatidylinositol-3 kinase and suppression of the tumor suppressor and phosphatidylinositol-3 kinase inhibitory protein, PTEN, is also well-established (229–233) in breast cancer cell lines, endothelial cells, and cortical neurons, suggesting a potential effect of E2 in modulating insulin-stimulated effects on these critical signaling molecules. However, studies on these interactions are limited in skeletal muscle. Additionally, E2 acting via ERα is also shown to promote phosphorylation of p38 MAPK (234, 235), a signaling molecule associated with insulin-stimulated GLUT4 intrinsic activity and glucose uptake (236–238). ERα activation of Akt and MAPK pathways is also thought to underlie E2-mediated protection of muscle against age-induced sarcopenia (239–245), exercise-induced muscle damage (227, 241, 246, 247), and myocyte apoptosis in response to a variety of cellular perturbations (248–251). Thus, ERα stimulation of muscle growth and ERα action on insulin signal transduction may underlie the protective effects of E2 on muscle insulin sensitivity.
It is possible that ERβ, despite low expression in muscle and other glucoregulatory tissues, promotes insulin resistance, particularly in the context of diminished ERα action. Indeed, OVX in hyperestrogenic female ERα-deficient mice—which suppresses E2 action through the remaining ERβ—improves glucose tolerance and insulin sensitivity (252). Furthermore, administration of an ERβ-selective agonist in male E2-deficient aromatase-deficient mice decreased total skeletal muscle GLUT4 expression, a finding that could promote insulin resistance (196). Conversely, administration of tamoxifen—in this context, an ERβ antagonist—to male ERα-deficient mice increased GLUT4 expression and improved insulin sensitivity (253). Finally, indirect evidence indicates that ERβ deficiency protects against diet-induced insulin resistance in male mice by increasing PPARγ signaling and lipid deposition in adipose tissue, thereby improving skeletal muscle insulin action by reducing ectopic lipid accumulation in muscle (160).
C. ERα in relation to skeletal muscle fatty acid metabolism and inflammation
Cycling women are protected against acute lipid-induced insulin resistance relative to estrogen-deficient women and men (165, 254). Compared to age-matched men, muscle from premenopausal women has enhanced insulin sensitivity despite 47% higher triglyceride content (204). This is consistent with a reduced respiratory quotient and greater reliance on fatty acid oxidation as a fuel source in women (255). These data highlight interesting similarities between E2-replete women and exercise-trained subjects: elevated muscle ERα expression (189, 213, 214), heightened insulin sensitivity (208), elevated muscle lipid tolerance (256), and enhanced oxidative capacity (257, 258). Consistent with the reported effects of E2 on metabolism, estrogen supplementation enhances lipid oxidation in vivo in men during acute endurance exercise (259), as well as palmitate oxidation in cultured myotubes obtained from male subjects (177). The effect of E2 on increased expression of fatty acid transport protein FAT/CD36 and fatty acid binding protein, as well as transcription factors and key enzymes that regulate oxidative metabolism (197, 203, 260), likely underlie these observations in males. In addition, exercise and E2 rapidly stimulate AMPK phosphorylation in both muscle and myotubes in culture (173, 261). It should be noted that AMPK is considered a central regulator of many cellular processes, including cell growth, mitochondrial biogenesis, and oxidative metabolism (262, 263). Comparable to the effects of E2, the ERα-selective agonist PPT stimulates AMPK phosphorylation in muscle from ovariectomized rats (174). By contrast, diminished estrogen action resulting from OVX or whole-body ERα deletion is associated with reduced skeletal muscle levels of phosphorylated AMPK (192, 264). Muscle PPARα, PPARδ, and UCP2 expression are also reduced in whole-body ERαKO mice suggesting that E2 acting via ERα is essential for regulation of coordinated oxidative metabolism. However, emerging data suggest that these effects on expression of muscle PPAR family members in the ERαKO mouse may be mediated by indirect action resulting from ERα deletion in other metabolic tissues. These may include the central nervous system, adipose, or liver. While the impairment in muscle fatty oxidation is sustained in the muscle-specific ERαKO mice (MERKO), no alteration in basal p-AMPK, PPARα, PPARδ, or UCP2 was observed (195). Despite these differences, skeletal muscle insulin resistance and bioactive lipid accumulation were surprisingly similar between ERαKO and MERKO. Triacylglycerol, diacylglycerol and ceramides were all substantially elevated in muscle from female mice lacking ERα globally or specifically in muscle. Consistent with these observations, oxygen consumption rates in C2C12 myotubes with ERα knockdown were reduced significantly in this model. In addition, mitochondria from muscle cells depleted of ERα produced high levels of radical oxygen species that promote oxidative stress. Collectively, these data support the notion that ERα is critical in the regulation of oxidative metabolism in skeletal muscle by mechanisms including regulation of: 1) fatty acid transport into the cell; 2) activation of intermediary signaling critical for shifting substrate metabolism and 3) transcriptional regulators of fatty acid metabolism and mitochondrial function. Thus, skeletal muscle ERα may be a critical regulator of adiposity via indirect action as MERKO mice recapitulated the obesity phenotype observed in the whole-body ERαKO mice.
The mechanistic link between accumulation of lipid intermediates, activation of inflammatory signaling cascades, and impaired insulin action is evident in both myocytes and rodent muscle. Indeed, these factors are observed concurrently in obese, type 2 diabetic subjects (265–268), as well as in muscle from whole-body and muscle-specific ERαKO mice (192). E2 treatment reduces HFD-induced insulin resistance in skeletal muscle by 50% during hyperinsulinemic euglycemic clamp in an ERα-dependent manner (193). Bioactive lipid intermediates including diacylglycerol and ceramides are believed to activate stress kinases including inhibitor of nuclear factor κβ kinase, c-Jun-N-terminal kinase, and certain novel protein kinase Cs (266, 269–271). Indeed, muscle from normal chow fed whole-body ERαKO mice showed heightened inflammatory signaling reflected by markedly increased c-Jun-N-terminal kinase phosphorylation and TNFα transcript (192). In addition to the marked increase in bioactive lipid intermediates observed in ERαKO muscle, production of radical oxygen species as well as the possible ERα derepression of selective inflammatory targets are likely mediators of elevated muscle inflammation.
Indeed, markers of inflammation and oxidative stress are both elevated in rodent models of diabetes and in patients with type 2 diabetes (272, 273). Myotubes and skeletal muscle with ERα deletion exhibit a marked reduction in Gpx3 expression, a primary antioxidant enzyme that scavenges hydrogen peroxide (190, 192). In contrast, E2 replacement in OVX animals leads to a substantial increase in Gpx3 expression in skeletal muscle (190). Muscle Gpx3 expression levels are elevated in healthy females compared to males (274), and they are reduced in T2DM patients compared to healthy subjects (275). In addition, they are associated with insulin resistance and the metabolic syndrome (275) and have been now identified as a causal candidate for obesity (276). Additional work studying the direct role of estrogen action in the regulation of antioxidant enzymes appears warranted. Although reductions in mitochondrial number and function have been implicated in the pathobiology of insulin resistance (277–280), and indeed gender dimorphisms in mitochondrial biology have been described (281), whether E2/ERα preserves insulin action by maintenance of mitochondrial integrity is still unknown. The consequences of ERα deletion in skeletal muscle on whole-body metabolic dysfunction are summarized in Figure 3.
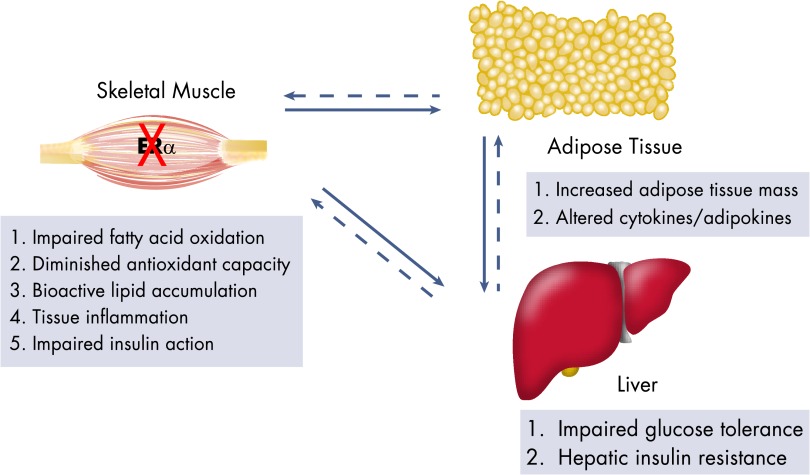
Figure 3.
Consequences of ERα deletion in skeletal muscle on metabolic dysfunction. Skeletal muscle-specific ERα deletion impairs muscle metabolism, leading to increased accumulation of bioactive lipids and muscle inflammation, which impairs muscle insulin action. Because skeletal muscle is a primary tissue responsible for fatty acid oxidation, impaired substrate oxidation promotes increased accumulation of fat in adipose tissue, which alters the secretory signature of the tissue inducing secondary phenotypes in muscle and liver. Thus, muscle ERα deletion precipitates impaired glucose tolerance and mild hepatic insulin resistance. The arrows represent the effect of skeletal muscle, adipose, and liver described above on each other.
D. ERs and insulin sensitivity in the liver
The predominant role of hepatic insulin resistance as distinct from skeletal muscle insulin resistance in hyperglycemia and type 2 diabetes has been demonstrated by experiments of conditional knockout of insulin receptors in these tissues (282). However, the direct role of the liver in insulin resistance induced by E2 deficiency or E2 resistance is still unclear. In one study, female mice globally lacking ERα did not exhibit insulin resistance in skeletal muscle, but they did display decreased insulin suppression of hepatic glucose production (HGP) during a euglycemic, hyperinsulinemic clamp (194). These data suggest that ERα deficiency leads to hepatic insulin resistance. However, Ribas et al (38) reported that conscious, ERα-deficient female mice exhibit minor alterations in liver insulin sensitivity during euglycemic, hyperinsulinemic clamp conditions. Thus, the possibility exists that anesthesia contributed to the increased HGP phenotype observed in anesthetized ERα-deficient mice (194). E2 suppresses lipogenic genes, triglyceride accumulation, and liver steatosis in HFD-fed (148) and leptin-resistant female mice (149). Surprisingly, this effect is not reproduced by the ERα agonist PPT (150). However, this effect is reproduced by a novel ERβ agonist (55). Nonetheless, E2 and PPT treatments improve insulin resistance in female mice fed a HFD (148, 193) and in obese female mice with genetic leptin resistance (149) through a pathway that is at least partially dependent on ERα (150, 193). However, the direct role of ERα in liver is unclear. Together, these data suggest that ERα (and possibly ERβ) activation is protective against hepatic insulin resistance by preventing ectopic lipid accumulation in the liver (lipotoxicity). Yet, the involvement of ERα in hepatocytes is still unknown.
In summary, ERα deficiency impairs lipid homeostasis in both skeletal muscle and liver of rodents, thereby decreasing the ability of insulin to suppress HGP and to promote skeletal muscle glucose utilization. As a result, activation of ERα during a HFD and genetic leptin resistance improves insulin resistance induced by ectopic lipid accumulation in skeletal muscle (148–150, 193). Nonetheless, the effect of ERα in mediating insulin sensitivity via central mechanisms remains to be determined.
VIII. ERα and Functioning of Macrophages and Immune Cells
Estrogens affect many immune and inflammatory conditions including autoimmune diseases (283–288), and they also influence immunomodulatory responses to parasitic and bacterial infections (289–294). After OVX, immune cell infiltration and increased tissue inflammation (TNFa, iNOS, and CD11c) are associated with an approximately 4-fold increase in perigonadal and inguinal fat. The T-cell marker CD3 and the Th1 cytokine interferon-γ are elevated in perigonadal fat from ovariectomized female mice (39), suggesting that the absence of E2 promotes immune cell inflammation. Indeed, circulating levels of proinflammatory cytokines are elevated in women after natural or surgical menopause (169).
ERα is expressed in macrophages and other immune cells that are known to exert dramatic effects on glucose homeostasis. Macrophages are elemental players in innate and adaptive immunity; over the past decade their roles in modulating whole-body metabolism and insulin sensitivity have been topics of increasing interest (295, 296). Hevener and colleagues (297) investigated the impact of ERα expression on macrophage function to determine whether hematopoietic or myeloid-specific ERα deletion manifests obesity-induced insulin resistance in mice. Indeed, altered plasma adipokine and cytokine levels, glucose intolerance, insulin resistance, and increased adipose tissue mass were observed in animals with a hematopoietic or myeloid-specific deletion of Esr1. A similar obese phenotype with increased atherosclerotic lesions was observed in LDL receptor-KO mice transplanted with ERαKO bone marrow. In isolated macrophages, ERα is necessary for repression of inflammation, maintenance of oxidative metabolism, IL-4-mediated induction of alternative activation, full phagocytic capacity in response to lipopolysaccharide, and oxidized LDL-induced expression of apolipoprotein E and ATP-binding cassette transporter. In line with these findings, prior work showed that E2 heightens the inflammatory response to ip injection of thioglycollate or lipopolysaccharide (291), and that ERα is critical not only in mediating these actions but also for reducing bacterial burden through phagocytosis.
Bone marrow-derived macrophages lacking ERα secrete factors that induce skeletal muscle and adipocyte insulin resistance in culture (297). A major limitation in the field is the failure to identify these proinflammatory, insulin resistance-producing substances. Additional metabolomic, lipidomic, and proteomic analyses will be necessary to move the field forward because it is likely that these macrophage-secreted factors include a combination of cytokines, a variety of lipid species, and radical oxygen/nitrogen species that act on adjacent cells.
Taken together, these data suggest that ERα expression in immune cells is critical for mediating a variety of cellular responses that are necessary for normal innate as well as adaptive immunity. When E2 levels are low or ERα action is compromised, disease susceptibility increases because the functionality of critical immune cell types becomes compromised and the essential phenotypic repertoire is diminished. Although a few direct ERα targets in myeloid cells have been identified, the impact of sex steroids on immunometabolism requires further examination that focuses on the intricate and diverse signaling by ERα as well as the complex nature and crosstalk among cells.
IX. ER in Relation to Pancreatic β-Cell Function
The role of estrogens and ER in β-cell function and in protection of β-cell mass has been reviewed recently (20). In this review, we focus on the most recent developments concerning these issues. In rodent models, treatment with E2 protects pancreatic β-cells against various injuries associated with both T1DM and T2DM. These include oxidative stress, amyloid polypeptide toxicity, lipotoxicity, and apoptosis (20). Three ERs—ERα, ERβ, and GPER—have been identified in rodent and human β-cells. Unlike the classical nuclear ERs that act as ligand-activated transcription factors in breast or uterine cells, β-cell ERs reside mainly in extranuclear locations. They exert their effect via cytosolic interactions with kinases such as Src, ERK, and AMPK or via transcription factors of the STAT family (20, 298–301). Activation of ERα enhances glucose-stimulated insulin biosynthesis (298, 302) through a pathway involving Src, ERK, and stimulation of the nuclear translocation and binding to the insulin promoter of NeuroD1, an insulinotropic transcription factor (298). This action may assist the islets in adapting to the increased metabolic demand of pregnancy by enhancing insulin biosynthesis. Activation of ERα reduces islet excess de novo synthesis of fatty acids and lipogenesis and accumulation of toxic lipid intermediates (299–301). This antilipogenic action involves at least 2 pathways. First, an extranuclear ERα activates and promotes the nuclear translocation of signal transducer and activator of transcription 3 (STAT3), which leads to inhibition of the master regulator of lipogenesis, the liver X receptor (LXR)β, and its transcriptional targets, the SREBP-1c and the carbohydrate response element binding protein. Suppression of LXRβ and SREBP-1c mRNA may be mediated by a pool of ERα—that is associated with the plasma membrane—and activates Src leading to STAT3 activation (301). In β-cells, chronic LXR activation leads to excess lipogenesis, which in turn is associated with lipotoxicity and apoptosis (304). Thus, ER suppression of LXR mRNAs in β-cells may account for the inhibition of lipogenesis and prevention of islet lipotoxicity (301). In the second pathway, activation of ERα induces AMPK to suppress SREBP-1c gene and protein expression (301). Together, ERα extranuclear actions in β-cells via STAT3 and AMPK lead to decreased expression and activity of the master effector of fatty acid synthesis under conditions of glucose surplus—FAS— converting malonyl-CoA into saturated long-chain fatty acid that can then undergo β-oxidation or esterification to monoacylglycerol, diacylglycerol, and triglyceride (299). Activation of ERα also promotes β-cell survival from most proapoptotic stimuli associated with diabetes (305–307). This antiapoptotic mechanism involves a combination of rapid actions that are independent of nuclear events and that potentially lead to alteration in protein phosphorylation (306, 307) and a more classical genomic mechanism inducing an anti-inflammatory cascade via expression of the orphan receptor, liver receptor homolog-1 (308). Activation of ERβ seems to predominantly enhance glucose-stimulated insulin secretion (309, 310) via a membrane pathway that leads to activation of the atrial natriuretic factor receptor and closure of ATP-sensitive potassium channels (309). Activation of GPER, however, protects β-cells from lipid accumulation (311) and promotes their survival (306, 312, 313). Activation of GPER also enhances glucose-stimulated insulin secretion (312, 314) via activation of the epidermal growth factor receptor and ERK (314), although it has no effect on insulin biosynthesis (303). However, it has been proposed that GPER induces expression of ERα36, a short isoform of the classical long isoform of ERα, ERα66 (23). Both ERα66 and ERα36 are expressed in β-cells (299). Thus, it is unclear whether GPER-mediated effects in β-cells are due to intrinsic GPER actions, or whether GPER is interacting with ERα36 at the membrane level. Importantly, the beneficial effects of ER ligands on β-cell survival, function, and β-cell nutrient homeostasis that are described above are all observed in human β-cells (20, 299, 306, 313, 315).
Perhaps the most translational prospect of E2 therapy for β-cell protection involves pancreatic islet transplantation. Fertile women with type 1 diabetes exhibit E2 deficiency relative to healthy women (316). Therefore, type 1 diabetes women undergoing islet transplantation may have lost part of their endogenous E2-related islet protections and could benefit from short-term E2 supplementation. To explore this hypothesis, Mauvais-Jarvis and co-workers (317) used a type 1 diabetes model with xenotransplantation of a marginal dose of human islets in nude mice rendered insulin-deficient by streptozotocin (STZ). In this model, a transient 4-week E2 treatment protected functional β-cell mass and enhanced islet revascularization and engraftment. These results suggest that transient E2 treatment in women could provide an immediate therapeutic alternative to improve pancreatic islet transplantation and also achieve insulin independence with fewer islets. This therapeutic approach could be developed long before other surrogate islet β-cell sources or β-cell regeneration therapy can be developed and therefore warrants further investigation.
From a therapeutic point of view, the risk of hormone-dependent cancer precludes the use of general estrogen therapy as a chronic treatment for β-cell failure in diabetes. To preferentially target E2 to the β-cells without the undesirable effect of general estrogen therapy, DiMarchi and co-workers (318) created novel fusion peptides combining glucagon-like peptide-1 (GLP-1) and E2 in a single molecule. By combining the pharmacologies of GLP-1 and E2, these investigators envisioned synergistic actions on β-cell function and survival using the combined insulinotropic and antiapoptotic activities on pancreatic β-cells that express ER and GLP-1 receptor (GLP-1R). Two conjugates were synthesized with E2 stably linked to GLP-1 to avoid E2 release in the circulation and to maximize E2 delivery at target cells: a GLP-1 agonist stably linked to E2 (aGLP1-E2), and an inactive GLP-1 stably linked to E2 (iGLP1-E2). This second conjugate binds GLP-1R normally, but it is pharmacologically incapable of activating GLP-1R signaling and is used to direct E2 to β-cells. Tiano et al (319) tested the efficiency of GLP1-E2 conjugates in preventing insulin-deficient diabetes in a model of β-cell destruction induced by multiple low-dose injections of STZ. They observed that the iGLP1-E2 conjugate prevented STZ-induced insulin-deficient diabetes, thereby demonstrating that, in vivo, the inactive GLP-1 was able to bind the GLP-1R and to direct E2 to β-cells for protection. Most importantly, the aGLP1-E2 conjugate was more potent than both the GLP-1 agonist and the iGLP1-E2 individually in preventing STZ-induced diabetes. All conjugates were devoid of E2 gynecological effects compared to general E2 therapy (319). These observations provide proof of concept that combining GLP-1 and E2 in a single molecule results in synergies for protection of β-cell function without the side effects associated with general estrogen therapy.
X. Estrogen Sulfotransferase and Metabolism
As discussed in Section II, EST terminates estrogen activity and represents an important parameter of estrogen output upstream of ER. With regard to metabolism, EST is highly expressed in WAT of male mice, but it is not detectable in WAT of normal cycling female mice (320). EST is induced by T in both sexes (320). Transgenic female mice overexpressing EST in adipose tissue at the level of males—which inactivates estrogen in this tissue—have altered adipocyte differentiation and smaller WAT depots in sc and visceral areas. This phenotype is associated with WAT insulin resistance (321). EST is also expressed in human sc adipose tissue. EST expression correlates with expression of proinflammatory factors in patients with metabolic syndrome, suggesting that excess estrogen inactivation in WAT may predispose to metabolic dysfunction (322). In addition, EST is highly expressed in the liver of insulin-resistant and diabetic db/db mice, again suggesting that estrogen inactivation in the liver may play a role in insulin resistance (323). In fact, eliminating EST in female mouse models of insulin resistance and type 2 diabetes by gene targeting improves glucose homeostasis (324). Loss of liver EST expression in these mice restores hepatic estrogen action, thus ameliorating hepatic insulin resistance. However, the antidiabetic effect of EST elimination is not observed in ob/ob male mice. In fact, the loss of EST in these mice yields a different phenotype of WAT inflammation, which aggravates the diabetic phenotype by provoking pancreatic islet inflammation and β-cell failure (324). EST elimination does not result in β-cell failure via a direct islet effect because EST is not expressed in islets (324), and estrogen action in islets is beneficial to islet function and survival. Rather, β-cell failure is associated with WAT macrophage infiltration and WAT inflammation followed by islet macrophage infiltration and β-cell apoptosis. An obvious explanation for these observations is that EST expression is high in male WAT (320) to protect from excessive estrogen actions. It follows that EST suppression produces WAT estrogen excess leading to inflammation. Consistent with this possibility is the observation that E2 treatment leading to high blood concentrations produces WAT inflammation even in females (193).
The tissue-specific effects of estrogen with regard to glucose metabolism and energy homeostasis in health and disease are summarized in Figure 4.
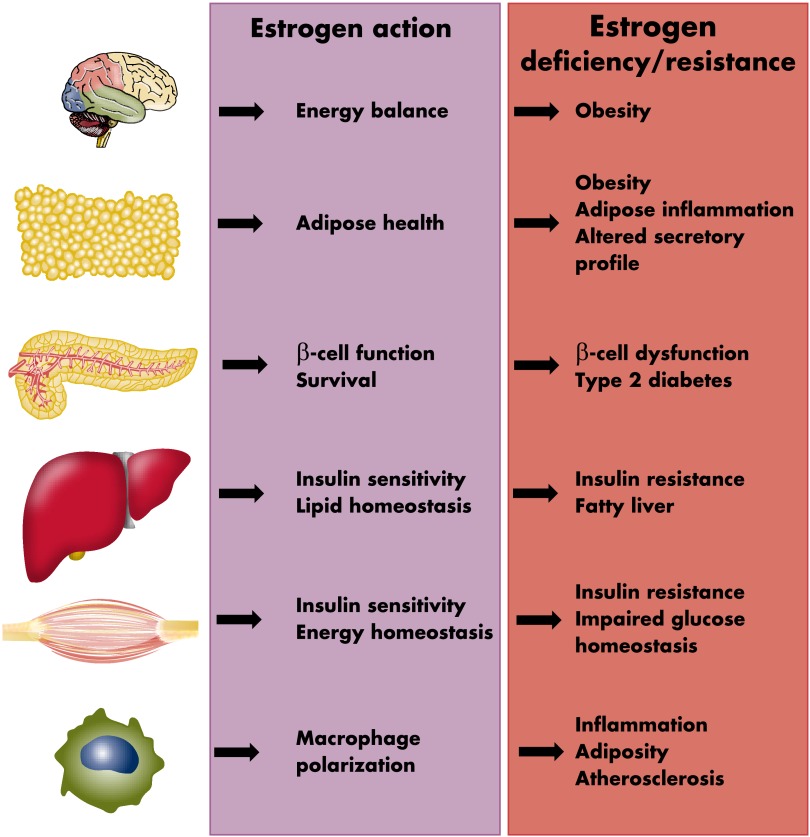
Figure 4.
Summary of estrogen actions in glucose homeostasis and energy metabolism in physiology and menopause. Estrogen actions in the brain, adipose, pancreatic islets, skeletal muscle, liver, and macrophages synergize to promote glucose and lipid homeostasis. Estrogen deficiency or resistance in these tissues all contribute to metabolic dysfunction predisposing to the metabolic syndrome, type 2 diabetes, and obesity.
XI. Estrogen Therapy and Metabolism
A. Relation of route of estrogen administration and metabolism
The estrogen type and route of administration appears to affect clinical outcomes. Major placebo-controlled trials like the WHI used only conjugated equine estrogen (CEE) and progestin. However, the physiological form of estrogen is E2, and it is available is some oral preparations as well as all patches, creams, and gels for transdermal or percutaneous absorption. In contrast to orally administered HRT, transdermal delivery avoids first pass liver metabolism, thereby resulting in more stable serum levels without supraphysiological liver exposure (325). Treatment with transdermal E2 results in higher E2 levels than corresponding doses of CEE, which results in higher levels of E1 and E1 sulfate (326). Percutaneous E2 administration in menopausal women is a safe and effective approach to delivering the hormone into the circulation, thereby mimicking the physiological condition (327) without the metabolic complications of oral CEE therapy (328). There are reports suggesting that oral E2 may exacerbate insulin resistance and adipocytokine parameters, worsening cardiovascular risk (329). Transdermal E2, however, has minimal effects on insulin resistance and results in higher adiponectin. This suggests that transdermal E2 may be a preferable treatment compared to oral CEE for obese women with metabolic syndrome. In addition, oral estrogen is associated with increased proinflammatory factors (matrix metallopeptidase 9), a side effect that is not observed with transdermal administration (330). A meta-analysis of over 100 randomized trials in postmenopausal women has recently analyzed the effect of HRT on components of the metabolic syndrome (331). The authors concluded that in women without diabetes, both oral and transdermal estrogen, with or without progestin, increase lean body mass, reduce abdominal fat, improve insulin resistance, decrease LDL/high-density lipoprotein-cholesterol ratio, and decrease blood pressure (331). In women with diabetes, both oral and transdermal estrogen, with or without progestin, reduce fasting glucose, improve insulin resistance, and decrease LDL/high-density lipoprotein-cholesterol ratio (331). Estrogen beneficial effects on metabolism were dose dependent and were reduced by the addition of a progestin (331). These estrogen beneficial effects on clinical features of the metabolic syndrome probably account for the reduction in mortality and cardiovascular events observed when HRT is initiated in younger women (331–333). Oral estrogen therapy and, particularly, CEE result in a stronger beneficial metabolic effect. The stronger effect of oral therapy on blood glucose could be the result of first pass liver metabolism leading to a better suppression of hepatic insulin resistance as discussed previously in rodent models (194). However, oral estrogen therapy is also associated with an increase in triglyceride, as well as inflammatory markers such as C-reactive protein and coagulation inhibitors like protein S (331). This would be expected to increase cardiovascular and thrombotic risks and could account for the increase in cardiovascular events observed when HRT is initiated in older women (4, 334–336). In contrast, transdermal estrogen has no adverse effect on triglyceride or inflammatory and coagulation factors (331). Further studies are warranted to determine whether transdermal estrogen therapy can safely reduce cardiovascular morbidity in postmenopausal women.
B. Effect of selective estrogen receptor modulators and aromatase inhibitors on metabolism
Selective estrogen receptor modulators (SERMs) act as ER agonists or antagonists in a tissue-specific fashion. Tamoxifen, for example, acts as an ERα antagonist in breast and as an ERα agonist in bone. As a result, tamoxifen is used for treatment of breast cancer while simultaneously preventing osteoporosis. However, tamoxifen treatment is associated with an increased risk of developing fatty liver (steatosis) (337–339), with approximately 43% of breast cancer patients treated with tamoxifen developing hepatic steatosis (339). The exact mechanism by which this occurs is still unclear. In rats, tamoxifen increases liver fatty acid and triglyceride content, despite decreased de novo lipogenesis associated with increased (340) or normal (341) fatty acid oxidation. Indeed, tamoxifen treatment predominantly down-regulates FAS expression and activity in liver (340), classical features of ERα agonistic action (299). The first hypothesis to explain tamoxifen-induced hepatic steatosis is that, in response to a continued supply of exogenous free fatty acid, the suppression of liver fatty acid oxidation may drive steatosis (340). A second hypothesis is that tamoxifen may increase hepatic triglyceride levels as a result of the combination of increased biosynthesis and unchanged β-oxidation (341). However, a recent study also showed that tamoxifen could induce triglyceride accumulation in mouse liver via activation of fatty acid synthesis (342). Accordingly, it is still unclear whether fatty acids used for triglyceride synthesis come from an exogenous source or de novo synthesis. In the hypothalamus, tamoxifen appears to act as an ER agonist. In rodents, tamoxifen inhibits hypothalamic FAS expression and malonyl-CoA accumulation in the VMN (343). This is associated with a potent anorectic effect. In fact, reanalysis of a primary breast cancer prevention study showed that obese women treated with tamoxifen gained less body weight over a 6-year period than obese controls (343). However, in cultured β-cells, tamoxifen reverses E2 antiapoptotic protection (305). In addition, treatment with tamoxifen reverses E2 protection from STZ-induced β-cell destruction in female mice, which leads to insulin-deficient diabetes (305). Thus, tamoxifen acts as an ERα antagonist in β-cells with regard to antiapoptotic protection. In fact, tamoxifen therapy in a case-control study breast cancer survivor was associated with a 24% increased risk of developing diabetes (344).
Raloxifene is approved by the FDA for the treatment of postmenopausal osteoporosis because of its ER-agonist activity in bone (345). In OVX mice, raloxifene reversed OVX-induced increases in food intake, body weight, fat mass, and hyperleptinemia to an extent similar to that of E2. This suggests that in rodents, raloxifene acts as an ER agonist in hypothalamic neurons and fat (346). In postmenopausal women, raloxifene prevents the shift from android to gynoid fat distribution, increases in abdominal adiposity (347), as well as total increases in adiposity (348). The absence of significant effect on body weight in women (349) could be due to the concomitant increase in lean mass and water after raloxifene treatment (350). In cultured INS-1 insulin-secreting cells, raloxifene acts as an ER agonist and prevents lipid accumulation both alone and in the presence of E2 (311). Nonetheless, in healthy and diabetic postmenopausal women, short-term raloxifene treatment did not impact fasting glucose, glucose tolerance, or indices of β-cell function and sensitivity (351, 352). However, it decreased hepatic insulin extraction and, as a result, increased insulin half-life (352). Additionally, in the Multiple Outcomes of Raloxifene Evaluation trial, 3-year raloxifene treatment of postmenopausal women with or without type 2 diabetes reduced total cholesterol and LDL-cholesterol but had no effect on glycemic control compared to placebo (349). Thus, there is no argument for an effect of raloxifene in improving glucose homeostasis in postmenopausal women with type 2 diabetes. In fact, women with a previous history of hypertriglyceridemia who receive oral estrogen therapy are at risk for clinically relevant progression in this existing risk factor during raloxifene therapy (353).
A novel approach to postmenopausal therapy is the tissue-selective estrogen complex (TSEC) or the pairing of a SERM with estrogens. The goals of TSEC are: 1) to provide the benefits of estrogens by treating hot flashes and vulvar–vaginal atrophy; 2) preventing menopausal osteoporosis events; and 3) protecting the endometrium and breast from estrogen stimulation (354, 355). In preclinical studies, the TSEC partnering bazedoxifene with CEE seemed to provide these benefits. Thus far, the effect of TSEC partnering bazedoxifene on glucose and energy metabolism is unknown.
Sullivan et al (356) looked at the effect of a novel SERM (GSK232802A) on body weight, food intake, physical activity, and metabolic rate in an OVX nonhuman primate model. They observed that GSK232802A produced a 5% decrease in weight and reduced adiposity by suppressing food intake and increasing activity. These results occurred without changes in energy expenditure, suggesting that GSK232802A treatment may counteract postmenopausal obesity (356).
Aromatase inhibitors (AIs; anastrozole, exemestane, and letrozole) are used as adjuvant therapy of postmenopausal breast cancer survivors (357). Because AIs inhibit aromatase, postmenopausal women taking AIs experience a further decrease in circulating estrogens and a theoretical increase in androgens (357). A prolonged period of profound estrogen suppression and (relative) hyperandrogenemia could induce alterations in body composition and favor adiposity and type 2 diabetes. A recent study reported that postmenopausal women with breast cancer using AIs over 24 months demonstrated increased lean body mass with stable body fat mass (21). However, they also exhibited increased free serum T concentration and decreased SHBG concentrations. Considering that hyperandrogenemia (7) and decreased SHBG (35, 191) predispose to type 2 diabetes in women, further studies are needed to evaluate the long-term metabolic impact of AIs in postmenopausal women.
XII. Conclusions and Perspectives
Novel molecular targets have emerged that offer possibilities for pharmacological intervention to combat diabetes and obesity. The inherent beauty of targeting estrogen action is the depth of knowledge of E2 and SERM biological and clinical efficacy and toxicity that has accumulated from decades of in vivo studies in preclinical models and in humans. E2 promotes energy homeostasis, improves body fat distribution, ameliorates insulin resistance (or enhances insulin sensitivity), improves β-cell function, and reduces inflammation. The challenge with estrogens, however, is their relatively narrow therapeutic index when administered as a long-term medication. Thus, translation of basic advances in diabetes and obesity treatment described in this review, although successful in rodents, is problematic when extended to clinical practice. However, 10 years after the WHI inappropriately concluded that the risks of hormone therapy outweighed its benefits—and overstated the risks of breast cancer, coronary heart disease, stroke, and pulmonary embolism with estrogen-progestin treatment—a position statement issued by the North American Menopause Society has stated that HRT has a role in short-term treatment of menopausal symptoms (303). Thus, at least during this interval, the metabolic dysfunction caused by E2 deficiency can be addressed.
Considering the dramatic rise in obesity and metabolic syndrome over the past decade, important clinical questions have emerged. Chief among these is how can we enhance the metabolic actions of E2 in chronic therapies aimed at prevention and treatment of metabolic diseases? Obviously, one solution is to target only the ER involved in energy balance and glucose homeostasis and to develop estrogen-like drugs that only initiate cellular events that produce metabolic benefit without unwanted side effects. This could be achieved via a tissue-targeted fusion protein approach with GLP-1-mediated delivery of E2 to tissues with high density of GLP-1Rs—to minimize gynecological effects—through the use of fusion peptides (318, 319) or through novel SERMs that will retain the beneficial metabolic effects of E2 in desired tissues while antagonizing ERs in breast and uterus.
With regard to obesity, future research should focus on identifying critical brain sites where ERs regulate body weight homeostasis and delineate the intracellular signaling pathways that are required for the actions of estrogens. Additionally, understanding the functional role and molecular mechanisms of ER action in islet cells, skeletal muscle, liver, and adipose tissue may reveal new pharmacological targets for the beneficial actions of estrogens.
A major limitation in our understanding and interpretation of E2 action is the lack of information pertaining to the tissue-specific effects of E2 in metabolism. There is also a lack of data regarding the contribution of extranuclear vs nuclear ER actions, as well as ligand- vs nonligand-mediated functions of its receptors in these tissue-specific actions of E2. Furthermore, little is known regarding the mechanisms by which ER mediate membrane-initiated signaling events independently of nuclear events, or how they activate and repress target genes in various tissues. Clarification of these signaling pathways and the tissue specificity with which these pathways are engaged will be critical in moving the field forward and will lay the foundation for improved targeted therapies.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK069362, HD044405, and DK074970, to F.M.-J.; DK073689, DK088220, and DK088761, to D.J.C.; DK078760, DK089109, and DK063491, to A.L.H.), the Juvenile Diabetes Research Foundation (1-2006-837, to F.M.-J.), the March of Dimes (6-FY07-678, to F.M.-J.), and by Northwestern University Institute for Women's Health Research Pioneer Award (to F.M.-J.).
Disclosure Summary: F.M.-J. received research support from Pfizer Inc. D.J.C. and A.L.H. have nothing to declare.
Footnotes
- AgRP
- Agouti-related peptide
- AI
- aromatase inhibitor
- AMPK
- AMP-activated protein kinase
- ARC
- arcuate nucleus
- CCK
- cholecystokinin
- CEE
- conjugated equine estrogen
- CoA
- coenzyme A
- E1
- estrone
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen response element
- EST
- estrogen sulfotransferase
- FAS
- fatty acid synthase
- GLP-1
- glucagon-like peptide-1
- GLP-1R
- GLP-1 receptor
- GLUT4
- glucose transporter 4
- GPER
- G protein-coupled ER
- HFD
- high fat diet
- HGP
- hepatic glucose production
- HRT
- hormone replacement therapy
- KO
- knockout
- LDL
- low-density lipoprotein
- leprb
- leptin receptor
- LPL
- lipoprotein lipase
- LXR
- liver X receptor
- MC4
- melanocortin 4
- MEF2
- myocyte enhancer factor 2
- MERKO
- muscle-specific ERαKO (mice)
- NPY
- neuropeptide Y
- NTS
- nucleus tractus solitarius
- OVX
- ovariectomy
- POMC
- pro-opiomelanocortin
- PPAR
- peroxisome proliferator-activated receptor
- PPT
- propylpyrazole triol
- SERM
- selective estrogen receptor modulator
- SF1
- steroidogenic factor-1
- SREBP-1c
- sterol regulatory element-binding protein 1c
- STAT3
- signal transducer and activator of transcription 3
- STZ
- streptozotocin
- TSEC
- tissue-selective estrogen complex
- VMH
- ventromedial hypothalamus
- VMN
- ventromedial nucleus
- WAT
- white adipose tissue.
References
- 1. Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Intern Med. 1999;130:545–553. [DOI] [PubMed] [Google Scholar]
- 2. Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–1170. [DOI] [PubMed] [Google Scholar]
- 3. Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. [DOI] [PubMed] [Google Scholar]
- 4. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 5. Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 suppl 1):s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. [DOI] [PubMed] [Google Scholar]
- 7. Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2011;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpson ER, Misso M, Hewitt KN, et al. Estrogen—the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. [DOI] [PubMed] [Google Scholar]
- 9. Mauvais-Jarvis F. Estrogen sulfotransferase: intracrinology meets metabolic diseases. Diabetes. 2012;61:1353–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strott CA. Steroid sulfotransferases. Endocr Rev. 1996;17:670–697. [DOI] [PubMed] [Google Scholar]
- 11. O'Malley BW. Mechanisms of action of steroid hormones. N Engl J Med. 1971;284:370–377. [DOI] [PubMed] [Google Scholar]
- 12. Nilsson S, Makela S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 13. Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol. 2008;41:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. [DOI] [PubMed] [Google Scholar]
- 15. Powell E, Xu W. Intermolecular interactions identify ligand-selective activity of estrogen receptor α/β dimers. Proc Natl Acad Sci USA. 2008;105:19012–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS. Genome-wide dynamics of chromatin binding of estrogen receptors α and β: mutual restriction and competitive site selection. Mol Endocrinol. 2010;24:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. [DOI] [PubMed] [Google Scholar]
- 18. Liu S, Mauvais-Jarvis F. Minireview: estrogenic protection of β-cell failure in metabolic diseases. Endocrinology. 2010;151:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. [DOI] [PubMed] [Google Scholar]
- 20. Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. [DOI] [PubMed] [Google Scholar]
- 21. van Londen GJ, Perera S, Vujevich K, et al. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat. 2011;125:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol. 2011;31:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang L, Zhang X, Xie Y, et al. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laudet V. Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol. 1997;19:207–226. [DOI] [PubMed] [Google Scholar]
- 25. Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. [DOI] [PubMed] [Google Scholar]
- 26. Eick GN, Thornton JW. Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol. 2011;334:31–38. [DOI] [PubMed] [Google Scholar]
- 27. Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. [DOI] [PubMed] [Google Scholar]
- 28. Carroll SM, Bridgham JT, Thornton JW. Evolution of hormone signaling in elasmobranchs by exploitation of promiscuous receptors. Mol Biol Evol. 2008;25:2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Markov GV, Tavares R, Dauphin-Villemant C, Demeneix BA, Baker ME, Laudet V. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc Natl Acad Sci USA. 2009;106:11913–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. [DOI] [PubMed] [Google Scholar]
- 31. Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. [DOI] [PubMed] [Google Scholar]
- 32. Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131:2351–2357. [DOI] [PubMed] [Google Scholar]
- 33. Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142:4751–4757. [DOI] [PubMed] [Google Scholar]
- 34. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. [DOI] [PubMed] [Google Scholar]
- 35. Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers NH, Perfield JW, II, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. [DOI] [PubMed] [Google Scholar]
- 41. Louis-Sylvestre J. Neuroendocrinology of hyperphagias and obesities. Reprod Nutr Dev. 1980;20:1545–1562. [DOI] [PubMed] [Google Scholar]
- 42. Danguir J, Nicolaidis S. Cortical activity and sleep in the rat lateral hypothalamic syndrome. Brain Res. 1980;185:305–321. [DOI] [PubMed] [Google Scholar]
- 43. Milam KM, Stern JS, Storlien LH, Keesey RE. Effect of lateral hypothalamic lesions on regulation of body weight and adiposity in rats. Am J Physiol. 1980;239:R337–R343. [DOI] [PubMed] [Google Scholar]
- 44. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. [DOI] [PubMed] [Google Scholar]
- 45. Simonian SX, Herbison AE. Differential expression of estrogen receptor α and β immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J Neuroendocrinol. 1997;9:803–806. [DOI] [PubMed] [Google Scholar]
- 46. Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78:215–228. [DOI] [PubMed] [Google Scholar]
- 47. Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. [DOI] [PubMed] [Google Scholar]
- 48. Wilkinson HA, Dahllund J, Liu H, et al. Identification and characterization of a functionally distinct form of human estrogen receptor β. Endocrinology. 2002;143:1558–1561. [DOI] [PubMed] [Google Scholar]
- 49. Shima N, Yamaguchi Y, Yuri K. Distribution of estrogen receptor β mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat Sci Int. 2003;78:85–97. [DOI] [PubMed] [Google Scholar]
- 50. Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. [DOI] [PubMed] [Google Scholar]
- 51. Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-α- deficient male mice. Biochem Biophys Res Commun. 2000;278:640–645. [DOI] [PubMed] [Google Scholar]
- 52. Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. [DOI] [PubMed] [Google Scholar]
- 53. Santollo J, Wiley MD, Eckel LA. Acute activation of ER α decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–R2201. [DOI] [PubMed] [Google Scholar]
- 54. Liang YQ, Akishita M, Kim S, et al. Estrogen receptor β is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–1109. [DOI] [PubMed] [Google Scholar]
- 55. Yepuru M, Eswaraka J, Kearbey JD, et al. Estrogen receptor-β selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–31303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Langer G, Bader B, Meoli L, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. [DOI] [PubMed] [Google Scholar]
- 57. Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int J Obes (Lond). 2011;35:198–207. [DOI] [PubMed] [Google Scholar]
- 58. Qiu J, Bosch MA, Tobias SC, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roepke TA, Bosch MA, Rick EA, et al. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. [DOI] [PubMed] [Google Scholar]
- 62. Elias CF, Aschkenasi C, Lee C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. [DOI] [PubMed] [Google Scholar]
- 63. Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. [DOI] [PubMed] [Google Scholar]
- 64. Elias CF, Kelly JF, Lee CE, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 65. Wise PM, Scarbrough K, Weiland NG, Larson GH. Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: a possible role in cyclic luteinizing hormone secretion. Mol Endocrinol. 1990;4:886–892. [DOI] [PubMed] [Google Scholar]
- 66. Bohler HC, Jr, Tracer H, Merriam GR, Petersen SL. Changes in proopiomelanocortin messenger ribonucleic acid levels in the rostral periarcuate region of the female rat during the estrous cycle. Endocrinology. 1991;128:1265–1269. [DOI] [PubMed] [Google Scholar]
- 67. Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology. 1999;70:377–383. [DOI] [PubMed] [Google Scholar]
- 68. Slamberová R, Hnatczuk OC, Vathy I. Expression of proopiomelanocortin and proenkephalin mRNA in sexually dimorphic brain regions are altered in adult male and female rats treated prenatally with morphine. J Pept Res. 2004;63:399–408. [DOI] [PubMed] [Google Scholar]
- 69. Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A. Ablation of estrogen receptor α (ERα) prevents upregulation of POMC by leptin and insulin. Biochem Biophys Res Commun. 2008;371:320–323. [DOI] [PubMed] [Google Scholar]
- 70. Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–1700. [DOI] [PubMed] [Google Scholar]
- 71. Malyala A, Zhang C, Bryant DN, Kelly MJ, Ronnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. [DOI] [PubMed] [Google Scholar]
- 72. King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. [DOI] [PubMed] [Google Scholar]
- 73. Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–490. [DOI] [PubMed] [Google Scholar]
- 74. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. [DOI] [PubMed] [Google Scholar]
- 75. Dellovade TL, Young M, Ross EP, et al. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. [DOI] [PubMed] [Google Scholar]
- 76. Majdic G, Young M, Gomez-Sanchez E, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. [DOI] [PubMed] [Google Scholar]
- 77. Minami T, Oomura Y, Nabekura J, Fukuda A. 17 β-Estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. [DOI] [PubMed] [Google Scholar]
- 78. Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors α and β in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006;1123:89–100. [DOI] [PubMed] [Google Scholar]
- 80. Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. [DOI] [PubMed] [Google Scholar]
- 81. Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. [DOI] [PubMed] [Google Scholar]
- 82. Moran TH. Gut peptides in the control of food intake. Int J Obes (Lond). 2009;33(suppl 1):S7–S10. [DOI] [PubMed] [Google Scholar]
- 83. Degen L, Matzinger D, Drewe J, Beglinger C. The effect of cholecystokinin in controlling appetite and food intake in humans. Peptides. 2001;22:1265–1269. [DOI] [PubMed] [Google Scholar]
- 84. Geary N, Smith GP, Corp ES. The increased satiating potency of CCK-8 by estradiol is not mediated by upregulation of NTS CCK receptors. Brain Res. 1996;719:179–186. [DOI] [PubMed] [Google Scholar]
- 85. Linden A, Uvnas-Moberg K, Forsberg G, Bednar I, Sodersten P. Involvement of cholecystokinin in food intake. III. Oestradiol potentiates the inhibitory effect of cholecystokinin octapeptide on food intake in ovariectomized rats. J Neuroendocrinol. 1990;2:797–801. [DOI] [PubMed] [Google Scholar]
- 86. Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–1238. [DOI] [PubMed] [Google Scholar]
- 87. Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides. 1999;20:445–450. [DOI] [PubMed] [Google Scholar]
- 88. Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 90. Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 91. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Dijk G, Thiele TE, Donahey JC, et al. Central infusion of leptin and GLP-1 (7–36) amide differentially stimulate c-Fos-like immunoreactivity in the rat brain. Am J Physiol. 1996;271:R1096–R1100. [DOI] [PubMed] [Google Scholar]
- 93. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 95. Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812:256–259. [DOI] [PubMed] [Google Scholar]
- 96. Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69:417–423. [DOI] [PubMed] [Google Scholar]
- 97. Lindell K, Bennett PA, Itoh Y, Robinson IC, Carlsson LM, Carlsson B. Leptin receptor 5′untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Mol Cell Endocrinol. 2001;172:37–45. [DOI] [PubMed] [Google Scholar]
- 98. Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. [DOI] [PubMed] [Google Scholar]
- 99. Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. [DOI] [PubMed] [Google Scholar]
- 100. Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. [DOI] [PubMed] [Google Scholar]
- 101. Chavez M, van Dijk G, Arkies BJ, Woods SC. 1995 ventricular insulin infusion attenuates NPY-induced feeding at the level of the paraventricular nucleus. Obes Res. 1995;3:335s [Google Scholar]
- 102. Levin BE. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol. 1999;276:R382–R387. [DOI] [PubMed] [Google Scholar]
- 103. Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(suppl 5):S63–S67. [DOI] [PubMed] [Google Scholar]
- 104. Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. [DOI] [PubMed] [Google Scholar]
- 105. Baskin DG, Schwartz MW, Seeley RJ, et al. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and colocalization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47:353–362. [DOI] [PubMed] [Google Scholar]
- 106. Hill JW, Urban JH, Xu M, Levine JE. Estrogen induces neuropeptide Y (NPY) Y1 receptor gene expression and responsiveness to NPY in gonadotrope-enriched pituitary cell cultures. Endocrinology. 2004;145:2283–2290. [DOI] [PubMed] [Google Scholar]
- 107. Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide-Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134:2367–2370. [DOI] [PubMed] [Google Scholar]
- 108. Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. [DOI] [PubMed] [Google Scholar]
- 109. Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci USA. 2009;106:15932–15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev. 1993;14:72–93. [DOI] [PubMed] [Google Scholar]
- 111. Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. [DOI] [PubMed] [Google Scholar]
- 112. Bjorntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992;24:15–18. [DOI] [PubMed] [Google Scholar]
- 113. Bjorntorp P. Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol. 1992;20(suppl 8):S26–S28. [PubMed] [Google Scholar]
- 114. Bjørntorp P. 1992. Hormonal effects on fat distribution and its relationship to health risk factors. Acta Paediatr Suppl 383:59–60; discussion 61. [PubMed] [Google Scholar]
- 115. Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women's Midlife Health Project. Climacteric. 2004;7:375–389. [DOI] [PubMed] [Google Scholar]
- 116. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gambacciani M, Ciaponi M, Cappagli B, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82:414–417. [DOI] [PubMed] [Google Scholar]
- 118. Haarbo J, Hansen BF, Christiansen C. Hormone replacement therapy prevents coronary artery disease in ovariectomized cholesterol-fed rabbits. APMIS. 1991;99:721–727. [DOI] [PubMed] [Google Scholar]
- 119. Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323–1326. [DOI] [PubMed] [Google Scholar]
- 120. Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276:E317–E325. [DOI] [PubMed] [Google Scholar]
- 121. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- 122. Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. [DOI] [PubMed] [Google Scholar]
- 123. Herrera MF, Pantoja JP, Velazquez-Fernandez D, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010;33:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. [DOI] [PubMed] [Google Scholar]
- 126. Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed). 1984;289:1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Donahue RP, Orchard TJ, Becker DJ, Kuller LH, Drash AL. Sex differences in the coronary heart disease risk profile: a possible role for insulin. The Beaver County Study. Am J Epidemiol. 1987;125:650–657. [DOI] [PubMed] [Google Scholar]
- 128. Donahue RP, Abbott RD. Central obesity and coronary heart disease in men. Lancet. 1987;2:1215. [DOI] [PubMed] [Google Scholar]
- 129. Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. [DOI] [PubMed] [Google Scholar]
- 130. Bjorntorp P. The android woman—a risky condition. J Intern Med. 1996;239:105–110. [DOI] [PubMed] [Google Scholar]
- 131. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. [DOI] [PubMed] [Google Scholar]
- 133. Tchernof A, Despres JP, Dupont A, et al. Relation of steroid hormones to glucose tolerance and plasma insulin levels in men. Importance of visceral adipose tissue. Diabetes Care. 1995;18:292–299. [DOI] [PubMed] [Google Scholar]
- 134. Mizutani T, Nishikawa Y, Adachi H, et al. Identification of estrogen receptor in human adipose tissue and adipocytes. J Clin Endocrinol Metab. 1994;78:950–954. [DOI] [PubMed] [Google Scholar]
- 135. Price TM, O'Brien SN. Determination of estrogen receptor messenger ribonucleic acid (mRNA) and cytochrome P450 aromatase mRNA levels in adipocytes and adipose stromal cells by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab. 1993;77:1041–1045. [DOI] [PubMed] [Google Scholar]
- 136. Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96:12887–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. [DOI] [PubMed] [Google Scholar]
- 138. Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor α gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27:1020–1027. [DOI] [PubMed] [Google Scholar]
- 139. Nilsson M, Dahlman I, Ryden M, et al. Oestrogen receptor α gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond). 2007;31:900–907. [DOI] [PubMed] [Google Scholar]
- 140. Deng HW, Li J, Li JL, et al. Association of estrogen receptor-α genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85:2748–2751. [DOI] [PubMed] [Google Scholar]
- 141. Casazza K, Page GP, Fernandez JR. The association between the rs2234693 and rs9340799 estrogen receptor α gene polymorphisms and risk factors for cardiovascular disease: a review. Biol Res Nurs. 2010;12:84–97. [DOI] [PubMed] [Google Scholar]
- 142. Yamada Y, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms of the estrogen receptor α gene with bone mineral density of the femoral neck in elderly Japanese women. J Mol Med. 2002;80:452–460. [DOI] [PubMed] [Google Scholar]
- 143. Okura T, Koda M, Ando F, Niino N, Shimokata H. Relationships of resting energy expenditure with body fat distribution and abdominal fatness in Japanese population. J Physiol Anthropol Appl Human Sci. 2003;22:47–52. [DOI] [PubMed] [Google Scholar]
- 144. Okura T, Koda M, Ando F, Niino N, Tanaka M, Shimokata H. Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum Genet. 2003;113:432–436. [DOI] [PubMed] [Google Scholar]
- 145. D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. [DOI] [PubMed] [Google Scholar]
- 146. Lundholm L, Zang H, Hirschberg AL, Gustafsson JA, Arner P, Dahlman-Wright K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil Steril. 2008;90:44–48. [DOI] [PubMed] [Google Scholar]
- 147. Price TM, O'Brien SN, Welter BH, George R, Anandjiwala J, Kilgore M. Estrogen regulation of adipose tissue lipoprotein lipase—possible mechanism of body fat distribution. Am J Obstet Gynecol. 1998;178:101–107. [DOI] [PubMed] [Google Scholar]
- 148. Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–E912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gao H, Bryzgalova G, Hedman E, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20:1287–1299. [DOI] [PubMed] [Google Scholar]
- 150. Lundholm L, Bryzgalova G, Gao H, et al. The estrogen receptor α-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199:275–286. [DOI] [PubMed] [Google Scholar]
- 151. Semenkovich CF, Wims M, Noe L, Etienne J, Chan L. Insulin regulation of lipoprotein lipase activity in 3T3–L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem. 1989;264:9030–9038. [PubMed] [Google Scholar]
- 152. Iverius PH, Brunzell JD. Relationship between lipoprotein lipase activity and plasma sex steroid level in obese women. J Clin Invest. 1988;82:1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Urabe M, Yamamoto T, Kashiwagi T, et al. Effect of estrogen replacement therapy on hepatic triglyceride lipase, lipoprotein lipase and lipids including apolipoprotein E in climacteric and elderly women. Endocr J. 1996;43:737–742. [DOI] [PubMed] [Google Scholar]
- 154. Homma H, Kurachi H, Nishio Y, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J Biol Chem. 2000;275:11404–11411. [DOI] [PubMed] [Google Scholar]
- 155. Reue K, Dwyer JR. Lipin proteins and metabolic homeostasis. J Lipid Res. 2009;50 suppl:S109–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Miranda M, Chacon MR, Gomez J, et al. Human subcutaneous adipose tissue LPIN1 expression in obesity, type 2 diabetes mellitus, and human immunodeficiency virus–associated lipodystrophy syndrome. Metabolism. 2007;56:1518–1526. [DOI] [PubMed] [Google Scholar]
- 157. van Harmelen V, Ryden M, Sjolin E, Hoffstedt J. A role of lipin in human obesity and insulin resistance: relation to adipocyte glucose transport and GLUT4 expression. J Lipid Res. 2007;48:201–206. [DOI] [PubMed] [Google Scholar]
- 158. Gonzalez CR, Novelle MG, Caminos JE, et al. Regulation of lipin1 by nutritional status, adiponectin, sex and pituitary function in rat white adipose tissue. Physiol Behav. 2012;105:777–783. [DOI] [PubMed] [Google Scholar]
- 159. Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood). 2004;229:1127–1135. [DOI] [PubMed] [Google Scholar]
- 160. Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor β (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet. 2008;4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism. 1984;33:1011–1015. [DOI] [PubMed] [Google Scholar]
- 163. Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPAR γ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes. 2001;50:1344–1350. [DOI] [PubMed] [Google Scholar]
- 166. Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes. 2002;51:1907–1912. [DOI] [PubMed] [Google Scholar]
- 167. Djouadi F, Weinheimer CJ, Saffitz JE, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J Clin Invest. 1998;102:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. [DOI] [PubMed] [Google Scholar]
- 170. Sites CK, Toth MJ, Cushman M, et al. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril. 2002;77:128–135. [DOI] [PubMed] [Google Scholar]
- 171. Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282:E1139–E1146. [DOI] [PubMed] [Google Scholar]
- 172. Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. [DOI] [PubMed] [Google Scholar]
- 173. Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gorres BK, Bomhoff GL, Morris JK, Geiger PC. In vivo stimulation of oestrogen receptor α increases insulin-stimulated skeletal muscle glucose uptake. J Physiol. 2011;589:2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285:E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Alonso A, Gonzalez-Pardo H, Garrido P, et al. Acute effects of 17 β-estradiol and genistein on insulin sensitivity and spatial memory in aged ovariectomized female rats. Age (Dordr). 2010;32:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Salehzadeh F, Rune A, Osler M, Al-Khalili L. Testosterone or 17β-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol. 2011;210:219–229. [DOI] [PubMed] [Google Scholar]
- 178. Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet Med. 2007;24:1491–1495. [DOI] [PubMed] [Google Scholar]
- 179. Guercio G, Di Palma MI, Pepe C, et al. Metformin, estrogen replacement therapy and gonadotropin inhibition fail to improve insulin sensitivity in a girl with aromatase deficiency. Horm Res. 2009;72:370–376. [DOI] [PubMed] [Google Scholar]
- 180. Takeda K, Toda K, Saibara T, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol. 2003;176:237–246. [DOI] [PubMed] [Google Scholar]
- 181. Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89:61–70. [DOI] [PubMed] [Google Scholar]
- 182. Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf). 2007;67:218–224. [DOI] [PubMed] [Google Scholar]
- 183. Jones ME, McInnes KJ, Boon WC, Simpson ER. Estrogen and adiposity—utilizing models of aromatase deficiency to explore the relationship. J Steroid Biochem Mol Biol. 2007;106:3–7. [DOI] [PubMed] [Google Scholar]
- 184. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–3698. [DOI] [PubMed] [Google Scholar]
- 185. Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304:63–68. [DOI] [PubMed] [Google Scholar]
- 186. Barros RP, Morani A, Moriscot A, Machado UF. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol Cell Endocrinol. 2008;295:24–31. [DOI] [PubMed] [Google Scholar]
- 187. Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. [DOI] [PubMed] [Google Scholar]
- 188. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wiik A, Gustafsson T, Esbjornsson M, et al. Expression of oestrogen receptor α and β is higher in skeletal muscle of highly endurance-trained than of moderately active men. Acta Physiol Scand. 2005;184:105–112. [DOI] [PubMed] [Google Scholar]
- 190. Baltgalvis KA, Greising SM, Warren GL, Lowe DA. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5:e10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Perry JR, Weedon MN, Langenberg C, et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet. 2010;19:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. [DOI] [PubMed] [Google Scholar]
- 194. Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. [DOI] [PubMed] [Google Scholar]
- 195. Ribas V, Drew BG, Soleymani T, Daraei P, Hevener A. Skeletal muscle specific ERα deletion is causal for the metabolic syndrome. Endocr Rev. 2010;31:S5. [Google Scholar]
- 196. Barros RP, Machado UF, Warner M, Gustafsson J-Å. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA. 2006;103:1605–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17β-Estradiol upregulates the expression of peroxisome proliferator-activated receptor α and lipid oxidative genes in skeletal muscle. J Mol Endocrinol. 2003;31:37–45. [DOI] [PubMed] [Google Scholar]
- 198. Alonso A, Ordonez P, Fernandez R, et al. 17β-Estradiol treatment is unable to reproduce p85 α redistribution associated with gestational insulin resistance in rats. J Steroid Biochem Mol Biol. 2009;116:160–170. [DOI] [PubMed] [Google Scholar]
- 199. Hansen PA, McCarthy TJ, Pasia EN, Spina RJ, Gulve EA. Effects of ovariectomy and exercise training on muscle GLUT-4 content and glucose metabolism in rats. J Appl Physiol. 1996;80:1605–1611. [DOI] [PubMed] [Google Scholar]
- 200. Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. [DOI] [PubMed] [Google Scholar]
- 201. Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol. 2009;587:4319–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zorzano A, Palacin M, Guma A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183:43–58. [DOI] [PubMed] [Google Scholar]
- 203. Fu MH, Maher AC, Hamadeh MJ, Ye C, Tarnopolsky MA. Exercise, sex, menstrual cycle phase, and 17β-estradiol influence metabolism-related genes in human skeletal muscle. Physiol Genomics. 2009;40:34–47. [DOI] [PubMed] [Google Scholar]
- 204. Hoeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol. 2009;107:824–831. [DOI] [PubMed] [Google Scholar]
- 205. Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992;41:465–475. [DOI] [PubMed] [Google Scholar]
- 207. Banks EA, Brozinick JT, Jr, Yaspelkis BB, III, Kang HY, Ivy JL. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol. 1992;263:E1010–E1015. [DOI] [PubMed] [Google Scholar]
- 208. Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, III, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol. 1993;265:E419–E427. [DOI] [PubMed] [Google Scholar]
- 209. Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, III, Ivy JL. Glucose uptake and GLUT-4 protein distribution in skeletal muscle of the obese Zucker rat. Am J Physiol. 1994;267:R236–R243. [DOI] [PubMed] [Google Scholar]
- 210. Hevener AL, Reichart D, Olefsky J. Exercise and thiazolidinedione therapy normalize insulin action in the obese Zucker fatty rat. Diabetes. 2000;49:2154–2159. [DOI] [PubMed] [Google Scholar]
- 211. Dela F, Ploug T, Handberg A, et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. [DOI] [PubMed] [Google Scholar]
- 212. Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes. 1990;39:1425–1429. [DOI] [PubMed] [Google Scholar]
- 213. Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor α expression in different rat skeletal muscle type. Acta Physiol Scand. 2002;175:211–217. [DOI] [PubMed] [Google Scholar]
- 214. Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor α transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174:283–289. [DOI] [PubMed] [Google Scholar]
- 215. Mora S, Pessin JE. The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. J Biol Chem. 2000;275:16323–16328. [DOI] [PubMed] [Google Scholar]
- 216. van Rooij E, Fielitz J, Sutherland LB, et al. Myocyte enhancer factor 2 and class II histone deacetylases control a gender-specific pathway of cardioprotection mediated by the estrogen receptor. Circ Res. 2010;106:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Moreno H, Serrano AL, Santalucia T, et al. Differential regulation of the muscle-specific GLUT4 enhancer in regenerating and adult skeletal muscle. J Biol Chem. 2003;278:40557–40564. [DOI] [PubMed] [Google Scholar]
- 218. Gan Z, Burkart-Hartman EM, Han DH, et al. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011;25:2619–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Oshel KM, Knight JB, Cao KT, Thai MV, Olson AL. Identification of a 30-base pair regulatory element and novel DNA binding protein that regulates the human GLUT4 promoter in transgenic mice. J Biol Chem. 2000;275:23666–23673. [DOI] [PubMed] [Google Scholar]
- 220. Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab. 2008;295:E698–E704. [DOI] [PubMed] [Google Scholar]
- 221. Gong H, Xie J, Zhang N, Yao L, Zhang Y. MEF2A binding to the Glut4 promoter occurs via an AMPKα2-dependent mechanism. Med Sci Sports Exerc. 2011;43:1441–1450. [DOI] [PubMed] [Google Scholar]
- 222. Ordonez P, Moreno M, Alonso A, Llaneza P, Diaz F, Gonzalez C. 17β-Estradiol and/or progesterone protect from insulin resistance in STZ-induced diabetic rats. J Steroid Biochem Mol Biol. 2008;111:287–294. [DOI] [PubMed] [Google Scholar]
- 223. Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17β-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. Am J Physiol Cell Physiol. 2009;297:C1249–C1262. [DOI] [PubMed] [Google Scholar]
- 224. Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. [DOI] [PubMed] [Google Scholar]
- 225. Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf). 2008;194:81–93. [DOI] [PubMed] [Google Scholar]
- 226. Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104:347–353. [DOI] [PubMed] [Google Scholar]
- 227. Thomas A, Bunyan K, Tiidus PM. Oestrogen receptor-α activation augments post-exercise myoblast proliferation. Acta Physiol (Oxf). 2010;198:81–89. [DOI] [PubMed] [Google Scholar]
- 228. Kamanga-Sollo E, White ME, Hathaway MR, Weber WJ, Dayton WR. Effect of estradiol-17β on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 2010;39:54–62. [DOI] [PubMed] [Google Scholar]
- 229. Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17β-estradiol through activation of estrogen receptor-α, but not estrogen receptor-β, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336:1221–1226. [DOI] [PubMed] [Google Scholar]
- 230. Noh EM, Lee YR, Chay KO, et al. Estrogen receptor α induces down-regulation of PTEN through PI3-kinase activation in breast cancer cells. Mol Med Report. 2011;4:215–219. [DOI] [PubMed] [Google Scholar]
- 231. Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Simoncini T, Fornari L, Mannella P, et al. Novel non-transcriptional mechanisms for estrogen receptor signaling in the cardiovascular system. Interaction of estrogen receptor α with phosphatidylinositol 3-OH kinase. Steroids. 2002;67:935–939. [DOI] [PubMed] [Google Scholar]
- 233. Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Ronda AC, Vasconsuelo A, Boland R. Extracellular-regulated kinase and p38 mitogen-activated protein kinases are involved in the antiapoptotic action of 17β-estradiol in skeletal muscle cells. J Endocrinol. 2010;206:235–246. [DOI] [PubMed] [Google Scholar]
- 235. Ronda AC, Buitrago C, Boland R. Role of estrogen receptors, PKC and Src in ERK2 and p38 MAPK signaling triggered by 17β-estradiol in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;122:287–294. [DOI] [PubMed] [Google Scholar]
- 236. Niu W, Huang C, Nawaz Z, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis. J Biol Chem. 2003;278:17953–17962. [DOI] [PubMed] [Google Scholar]
- 237. Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–578. [DOI] [PubMed] [Google Scholar]
- 238. Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3–L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–10078. [DOI] [PubMed] [Google Scholar]
- 239. Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9:622–626. [DOI] [PubMed] [Google Scholar]
- 240. Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–175B. [DOI] [PubMed] [Google Scholar]
- 241. Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25:274–287. [DOI] [PubMed] [Google Scholar]
- 242. Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68:331–336. [DOI] [PubMed] [Google Scholar]
- 243. Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition—a substudy of the estrogen plus progestin trial of the Women's Health Initiative. Am J Clin Nutr. 2005;82:651–656. [DOI] [PubMed] [Google Scholar]
- 244. Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond). 2001;101:147–157. [PubMed] [Google Scholar]
- 245. Teixeira PJ, Going SB, Houtkooper LB, et al. Resistance training in postmenopausal women with and without hormone therapy. Med Sci Sports Exerc. 2003;35:555–562. [DOI] [PubMed] [Google Scholar]
- 246. Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009;107:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Sotiriadou S, Kyparos A, Albani M, et al. Soleus muscle force following downhill running in ovariectomized rats treated with estrogen. Appl Physiol Nutr Metab. 2006;31:449–459. [DOI] [PubMed] [Google Scholar]
- 248. Boland R, Vasconsuelo A, Milanesi L, Ronda AC, de Boland AR. 17β-Estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids. 2008;73:859–863. [DOI] [PubMed] [Google Scholar]
- 249. Vasconsuelo A, Milanesi L, Boland R. 17β-Estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2008;196:385–397. [DOI] [PubMed] [Google Scholar]
- 250. Wang F, He Q, Sun Y, Dai X, Yang XP. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol. 2009;297:C548–C555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Naaz A, Zakroczymski M, Heine P, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor α (ERα): a potential role for estrogen receptor β (ERβ). Horm Metab Res. 2002;34:758–763. [DOI] [PubMed] [Google Scholar]
- 253. Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297:E124–E133. [DOI] [PubMed] [Google Scholar]
- 254. Hoeg LD, Sjoberg KA, Jeppesen J, et al. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signaling. Diabetes. 2011;60:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Cortright RN, Koves TR. Sex differences in substrate metabolism and energy homeostasis. Can J Appl Physiol. 2000;25:288–311. [DOI] [PubMed] [Google Scholar]
- 256. Amati F, Dube JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Maher AC, Akhtar M, Vockley J, Tarnopolsky MA. Women have higher protein content of β-oxidation enzymes in skeletal muscle than men. PLoS One. 2010;5:e12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–E799. [DOI] [PubMed] [Google Scholar]
- 259. Hamadeh MJ, Devries MC, Tarnopolsky MA. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J Clin Endocrinol Metab. 2005;90:3592–3599. [DOI] [PubMed] [Google Scholar]
- 260. Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17β-estradiol have increased β-oxidation capacity in skeletal muscle. Physiol Genomics. 2010;42:342–347. [DOI] [PubMed] [Google Scholar]
- 261. D'Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring). 2008;16:1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kim JY, Jo KJ, Kim OS, et al. Parenteral 17β-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010;87:358–366. [DOI] [PubMed] [Google Scholar]
- 265. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51:2005–2011. [DOI] [PubMed] [Google Scholar]
- 267. Adams JM, II, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. [DOI] [PubMed] [Google Scholar]
- 268. Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. [DOI] [PubMed] [Google Scholar]
- 270. Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. [DOI] [PubMed] [Google Scholar]
- 271. Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–S46. [DOI] [PubMed] [Google Scholar]
- 272. Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond). 2008;32(suppl 7):S52–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 274. Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. [DOI] [PubMed] [Google Scholar]
- 275. Chung SS, Kim M, Youn BS, et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor γ in human skeletal muscle cells. Mol Cell Biol. 2009;29:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Yang X, Deignan JL, Qi H, et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. 2009;41:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Gomez-Perez Y, Amengual-Cladera E, Catala-Niell A, et al. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem. 2008;22:539–548. [DOI] [PubMed] [Google Scholar]
- 282. Mauvais-Jarvis F, Kulkarni RN, Kahn CR. Knockout models are useful tools to dissect the pathophysiology and genetics of insulin resistance. Clin Endocrinol (Oxf). 2002;57:1–9. [DOI] [PubMed] [Google Scholar]
- 283. Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Spence RD, Voskuhl RR. 2011 Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Gold SM, Sasidhar MV, Morales LB, et al. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor α (ERα). Lab Invest. 2009;89:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Atwater I, Gondos B, DiBartolomeo R, Bazaes R, Jovanovic L. Pregnancy hormones prevent diabetes and reduce lymphocytic infiltration of islets in the NOD mouse. Ann Clin Lab Sci. 2002;32:87–92. [PubMed] [Google Scholar]
- 287. Dulos J, Vijn P, van Doorn C, et al. Suppression of the inflammatory response in experimental arthritis is mediated via estrogen receptor α but not estrogen receptor β. Arthritis Res Ther. 2010;12:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Yang YH, Ngo D, Jones M, Simpson E, Fritzemeier KH, Morand EF. Endogenous estrogen regulation of inflammatory arthritis and cytokine expression in male mice, predominantly via estrogen receptor α. Arthritis Rheum. 2010;62:1017–1025. [DOI] [PubMed] [Google Scholar]
- 289. Klein PW, Easterbrook JD, Lalime EN, Klein SL. Estrogen and progesterone affect responses to malaria infection in female C57BL/6 mice. Gend Med. 2008;5:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Vegeto E, Cuzzocrea S, Crisafulli C, et al. Estrogen receptor-α as a drug target candidate for preventing lung inflammation. Endocrinology. 2010;151:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Calippe B, Douin-Echinard V, Delpy L, et al. 17β-Estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor α signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. [DOI] [PubMed] [Google Scholar]
- 292. Hepworth MR, Hardman MJ, Grencis RK. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur J Immunol. 2010;40:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Lezama-Davila CM, Isaac-Marquez AP, Barbi J, Cummings HE, Lu B, Satoskar AR. Role of phosphatidylinositol-3-kinase-γ (PI3Kγ)-mediated pathway in 17β-estradiol-induced killing of L. mexicana in macrophages from C57BL/6 mice. Immunol Cell Biol. 2008;86:539–543. [DOI] [PubMed] [Google Scholar]
- 294. Douin-Echinard V, Calippe B, Billon-Gales A, et al. Estradiol administration controls eosinophilia through estrogen receptor-α activation during acute peritoneal inflammation. J Leukoc Biol. 2011;90:145–154. [DOI] [PubMed] [Google Scholar]
- 295. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. [DOI] [PubMed] [Google Scholar]
- 296. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Ribas V, Drew BG, Le JA, et al. Myeloid-specific estrogen receptor α deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA. 2011;108:16457–16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Wong WP, Tiano JP, Liu S, et al. Extranuclear estrogen receptor-α stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA. 2010;107:13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Tiano JP, Delghingaro-Augusto V, Le May C, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121:3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Tiano J, Mauvais-Jarvis F. Selective estrogen receptor modulation in pancreatic β-cells and the prevention of type 2 diabetes. Islets. 2012;4:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Tiano JP, Mauvais-Jarvis F. Molecular mechanisms of estrogen receptors' suppression of lipogenesis in pancreatic β-cells. Endocrinology. 2012;153:2997–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER α. PLoS One. 2008;3:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Choe SS, Choi AH, Lee JW, et al. Chronic activation of liver X receptor induces β-cell apoptosis through hyperactivation of lipogenesis: liver X receptor-mediated lipotoxicity in pancreatic β-cells. Diabetes. 2007;56:1534–1543. [DOI] [PubMed] [Google Scholar]
- 305. Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103:9232–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Liu S, Le May C, Wong WP, et al. Importance of extranuclear estrogen receptor-α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1:273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Baquie M, St-Onge L, Kerr-Conte J, et al. The liver receptor homolog-1 (LRH-1) is expressed in human islets and protects β-cells against stress-induced apoptosis. Hum Mol Genet. 2011;20:2823–2833. [DOI] [PubMed] [Google Scholar]
- 309. Soriano S, Ropero AB, Alonso-Magdalena P, et al. Rapid regulation of K(ATP) channel activity by 17β-estradiol in pancreatic β-cells involves the estrogen receptor β and the atrial natriuretic peptide receptor. Mol Endocrinol. 2009;23:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Soriano S, Alonso-Magdalena P, Garcia-Arevalo M, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One. 2012;7:e31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Tiano J, Mauvais-Jarvis F. Selective estrogen receptor modulation in pancreatic β-cells and the prevention of type 2 diabetes. Islets. 2012;4:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320:16–24. [DOI] [PubMed] [Google Scholar]
- 313. Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology. 2011;152:2568–2579. [DOI] [PubMed] [Google Scholar]
- 314. Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology. 2011;152:3030–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74:1252–1259. [DOI] [PubMed] [Google Scholar]
- 316. Salonia A, Lanzi R, Scavini M, et al. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care. 2006;29:312–316. [DOI] [PubMed] [Google Scholar]
- 317. Liu S, Kilic G, Meyers MS, et al. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia. 2013;56:370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Finan B, Yang B, Ottaway N, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Tiano J, Finan B, DiMarchi R, Mauvais-Jarvis F. A glucagon-like peptide-1-estrogen fusion peptide shows enhanced efficacy in preventing insulin-deficient diabetes in mice. Endocr Rev. 2012;33:OR21–OR26. [Google Scholar]
- 320. Khor VK, Tong MH, Qian Y, Song WC. Gender-specific expression and mechanism of regulation of estrogen sulfotransferase in adipose tissues of the mouse. Endocrinology. 2008;149:5440–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Khor VK, Dhir R, Yin X, Ahima RS, Song WC. Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice. Am J Physiol Endocrinol Metab. 2010;299:E657–E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Ahima RS, Stanley TL, Khor VK, Zanni MV, Grinspoon SK. Estrogen sulfotransferase is expressed in subcutaneous adipose tissue of obese humans in association with TNF-α and SOCS3. J Clin Endocrinol Metab. 2011;96:E1153–E1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Leiter EH, Chapman HD. Obesity-induced diabetes (diabesity) in C57BL/KsJ mice produces aberrant trans-regulation of sex steroid sulfotransferase genes. J Clin Invest. 1994;93:2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Gao J, He J, Shi X, et al. Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes. 2012;61:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Mauvais-Jarvis P, Wepierre J, Vickers CF. 1990. Percutaneous absorption of steroids. New York: Academic Press Inc. [Google Scholar]
- 326. Good WR, John VA, Ramirez M, Higgins JE. Comparison of Alora estradiol matrix transdermal delivery system with oral conjugated equine estrogen therapy in relieving menopausal symptoms. Alora Study Group. Climacteric. 1999;2:29–36. [DOI] [PubMed] [Google Scholar]
- 327. Sitruk-Ware R, de Lignieres B, Basdevant A, Mauvais-Jarvis P. Absorption of percutaneous oestradiol in postmenopausal women. Maturitas. 1980;2:207–211. [DOI] [PubMed] [Google Scholar]
- 328. Elkik F, Gompel A, Mercier-Bodard C, et al. Effects of percutaneous estradiol and conjugated estrogens on the level of plasma proteins and triglycerides in postmenopausal women. Am J Obstet Gynecol. 1982;143:888–892. [DOI] [PubMed] [Google Scholar]
- 329. Chu MC, Cosper P, Nakhuda GS, Lobo RA. A comparison of oral and transdermal short-term estrogen therapy in postmenopausal women with metabolic syndrome. Fertil Steril. 2006;86:1669–1675. [DOI] [PubMed] [Google Scholar]
- 330. Chu MC, Cushman M, Solomon R, Lobo RA. Metabolic syndrome in postmenopausal women: the influence of oral or transdermal estradiol on inflammation and coagulation markers. Am J Obstet Gynecol. 2008;199:526.e1–526.e7. [DOI] [PubMed] [Google Scholar]
- 331. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. [DOI] [PubMed] [Google Scholar]
- 332. Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. [DOI] [PubMed] [Google Scholar]
- 333. Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med. 2009;122:1016–1022.e1. [DOI] [PubMed] [Google Scholar]
- 334. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. [DOI] [PubMed] [Google Scholar]
- 335. Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the Nurses' Health Study. A prospective, observational study. Ann Intern Med. 2001;135:1–8. [DOI] [PubMed] [Google Scholar]
- 336. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 337. Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351:725. [DOI] [PubMed] [Google Scholar]
- 338. Coskun U, Toruner FB, Gunel N. Tamoxifen therapy and hepatic steatosis. Neoplasma. 2002;49:61–64. [PubMed] [Google Scholar]
- 339. Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180:129–134. [DOI] [PubMed] [Google Scholar]
- 340. Lelliott CJ, Lopez M, Curtis RK, et al. Transcript and metabolite analysis of the effects of tamoxifen in rat liver reveals inhibition of fatty acid synthesis in the presence of hepatic steatosis. FASEB J. 2005;19:1108–1119. [DOI] [PubMed] [Google Scholar]
- 341. Gudbrandsen OA, Rost TH, Berge RK. Causes and prevention of tamoxifen-induced accumulation of triacylglycerol in rat liver. J Lipid Res. 2006;47:2223–2232. [DOI] [PubMed] [Google Scholar]
- 342. Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 2010;52:1258–1265. [DOI] [PubMed] [Google Scholar]
- 343. Lopez M, Lelliott CJ, Tovar S, et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55:1327–1336. [DOI] [PubMed] [Google Scholar]
- 344. Lipscombe LL, Fischer HD, Yun L, et al. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118:2615–2622. [DOI] [PubMed] [Google Scholar]
- 345. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. [DOI] [PubMed] [Google Scholar]
- 346. Meli R, Pacilio M, Raso GM, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–3121. [DOI] [PubMed] [Google Scholar]
- 347. Tommaselli GA, Di Carlo C, Di Spiezio Sardo A, et al. Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause. 2006;13:660–668. [DOI] [PubMed] [Google Scholar]
- 348. Francucci CM, Daniele P, Iori N, Camilletti A, Massi F, Boscaro M. Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J Endocrinol Invest. 2005;28:623–631. [DOI] [PubMed] [Google Scholar]
- 349. Barrett-Connor E, Ensrud KE, Harper K, et al. Post hoc analysis of data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial on the effects of three years of raloxifene treatment on glycemic control and cardiovascular disease risk factors in women with and without type 2 diabetes. Clin Ther. 2003;25:919–930. [DOI] [PubMed] [Google Scholar]
- 350. Jacobsen DE, Samson MM, Emmelot-Vonk MH, Verhaar HJ. Raloxifene and body composition and muscle strength in postmenopausal women: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2010;162:371–376. [DOI] [PubMed] [Google Scholar]
- 351. Andersson B, Johannsson G, Holm G, et al. Raloxifene does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes mellitus: a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:122–128. [DOI] [PubMed] [Google Scholar]
- 352. Nagamani M, Szymajda A, Sepilian V, Urban RJ, Gilkison C. Effects of raloxifene on insulin sensitivity, β-cell function, and hepatic insulin extraction in normal postmenopausal women. Fertil Steril. 2008;89:614–619. [DOI] [PubMed] [Google Scholar]
- 353. Carr MC, Knopp RH, Brunzell JD, et al. Effect of raloxifene on serum triglycerides in women with a history of hypertriglyceridemia while on oral estrogen therapy. Diabetes Care. 2005;28:1555–1561. [DOI] [PubMed] [Google Scholar]
- 354. Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084–6091. [DOI] [PubMed] [Google Scholar]
- 355. Komm BS. A new approach to menopausal therapy: the tissue selective estrogen complex. Reprod Sci. 2008;15:984–992. [DOI] [PubMed] [Google Scholar]
- 356. Sullivan EL, Shearin J, Koegler FH, Cameron JL. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab. 2012;302:E759–E767. [DOI] [PubMed] [Google Scholar]
- 357. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. [DOI] [PubMed] [Google Scholar]