Abstract
The emission of a specific blend of volatiles in response to Mythimna separata (herbivore-induced plant volatiles, HIPVs) plays a great ecological role by priming neighbouring plants. Maize plants placed downwind of infested, conspecific plants showed reduced larval development not only immediately after exposure to HIPVs but also when receiver plants were tested after a time lag of up to 5 days, compared to those exposed to volatiles from uninfested plants and tested after the same time lag. The molecular basis of this plant memory was, in part, the similarly recalled expression of a Bowman-Birk type trypsin inhibitor (TI) gene, in a jasmonic acid induction-independent manner. Moreover, in the promoter region of TI, a suite of methylation sites was found to be demethylated by the HIPV treatment. These findings provide an innovative mechanism for the epigenetic basis of the memory of HIPV-mediated habituation for plant defence.
In response to herbivory, plants start to defend themselves against herbivores1. Herbivore-induced plant volatiles (HIPVs), including a wide array of low molecular weight terpenes and green leaf volatiles, function as airborne signals within and between plants2,3,4,5,6,7,8. Such signals allow plants experiencing them, but themselves not under attack, to tailor their defences to their current and expected risk of herbivory. In some cases, these receiver plants do not show immediate changes in their level of defences, but respond more strongly and more rapidly when damaged by herbivores than non-receiver plants7,9,10,11,12,13,14. Such readying of a defence response, ‘priming', is induced when volatiles emitted from clipped sagebrush (Artimisia tridentata) affect neighbouring Nicotiana attenuata plants by accelerating their production of trypsin proteinase inhibitors only after Manduca sexta larvae start to attack them12,15. Priming also occurs in maize plants exposed to HIPVs emitted by other maize plants infested with the generalist herbivore Spodoptera littoralis11 or the specialist Mythimna separata14,16. Exposure of maize plants to S. littoralis-induced volatiles does not activate genes that are responsive to wounding, jasmonic acid (JA) or caterpillar regurgitant, but primes the expression of these genes post-herbivory11. Exposure to the volatiles also enhances the emission in receiver plants of volatiles that can attract natural enemies of herbivores11,14. These natural enemies can help plant indirect defences.
Field studies conducted by Richard Karban's group show the defensive consequences of responding to volatile cues emitted by experimentally damaged neighbours. Resistance was induced about four months after the volatile treatment was applied in May17,18. These findings prompted us to investigate how long receiver plants are able to store information conveyed by volatiles and how such information is recalled. Since HIPVs are emitted by damaged plants for only a few days after the onset of damage19, neighbouring plants can only receive volatile signals when emitter plants are damaged, or slightly later. Moreover, plants cannot be aware of how much later the herbivores will arrive. This issue is relevant to considerations of the ecological consequences for both emitters and receivers. For instance, plants should rapidly cease releasing HIPVs that attract parasitoids after the herbivore has left or become invulnerable20. If they do not, the cues will provide unreliable information and the parasitoids will be unable to track their hosts. Similarly, in the case of plant communications, receivers must pay costs for priming their defences to their current and expected risks. The appropriate time scale and the mode of sustaining priming should be linked to the cost performance of receiver plants.
In order to better understand the above issues, we assessed the memory abilities of maize plants placed downwind of conspecific plants infested with the specialist herbivore M. separata in controlled assay conditions. In response to M. separata attack, receiver maize plants emit high levels of HIPVs16. These include terpenoids [myrecene, (E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene, linalool, (E)-β-caryophyllene, (E)-α-bergamotene, (E)-β-farnesene and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene], green leaf volatiles [(Z)-3-hexen-1-yl acetate] and indole. Our results revealed the appropriate time period and possible mechanisms underlying the plants' memory of HIPV stimuli for priming defences against herbivores. To reveal the possible mechanisms, we briefly focused on DNA methylation, a conserved epigenetic mechanism involved in many important biological processes, including gene regulation of stress responses in plants21.
Results
Appropriate time lag for HIPVs to impact the defence ability of receiver maize plants against herbivores
Larvae of the common armyworm (M. separata) were applied onto HIPV-receiver maize plants after the receiver plants had been maintained post-volatile exposure for 0, 1, 5 or 10 days (Fig. 1). Larvae on HIPV-receivers had lower weight gain than did those on CV-receivers for the first three of these periods of post-exposure maintenance (Student's t-test, P < 0.05). However, larvae on HIPV-receiver plants maintained for 10 days after exposure grew similarly to those on CV-receivers.
Figure 1. The appropriate time lag for priming the ability of receiver plants to defend themselves against herbivores.
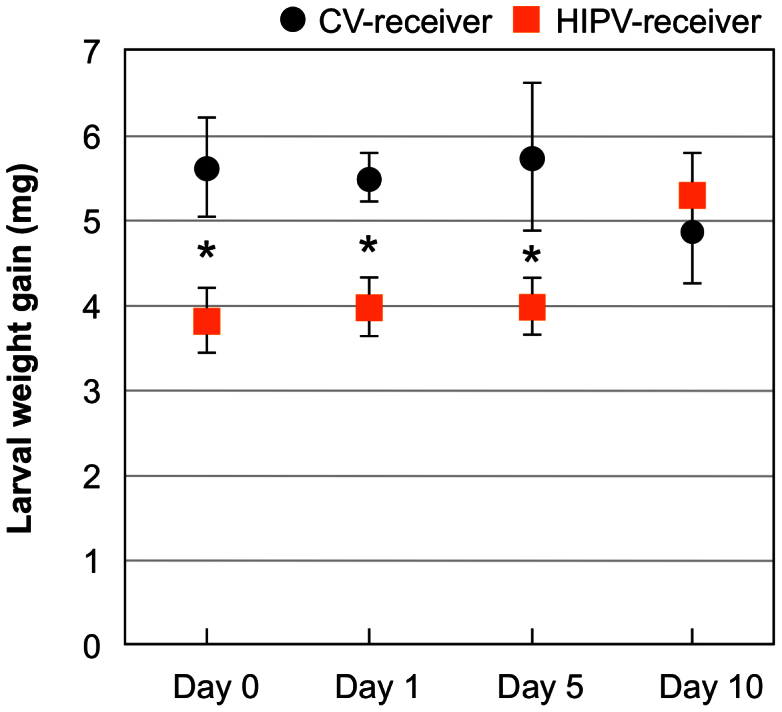
Maize plants were exposed to HIPVs and control volatiles (CVs) emitted from M. separata-infested and uninfested conspecific plants for three days in an open-flow tunnel. A second-instar larva was put onto HIPV- and CV-receiver plants immediately after exposure (Day 0), or after receiver plants had been maintained for 1, 5 or 10 days. The net body weight that M. separata larvae gained during 3 days after they had been applied was determined. Data represent the mean ± standard errors (n = 17–22). Asterisks indicate significant differences between HIPV- and CV-receivers based on a Student's t-test (P < 0.05).
Appropriate time lag for priming defence responses
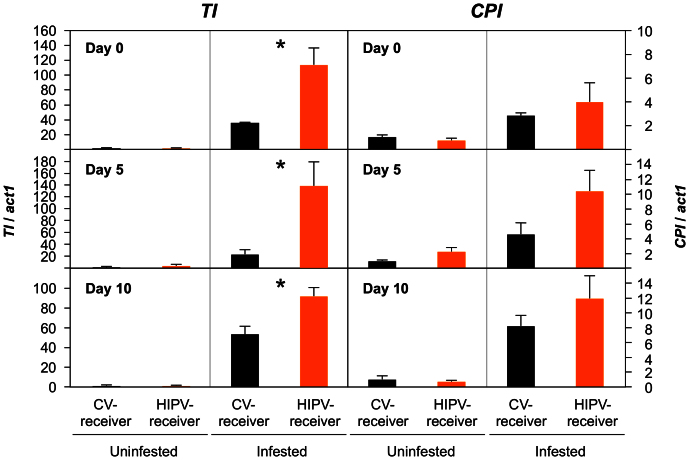
We analysed the transcription level of defence genes for Bowman-Birk type trypsin inhibitor (TI) and cysteine protease inhibitor (CPI) in leaves of HIPV-receiver and CV-receiver plants. These plants were maintained for 0, 5 or 10 days after exposure and then challenged with M. separata larvae or left unchallenged for an additional day (Fig. 2). TI expression was higher in infested CV-receivers compared with that in uninfested CV-receivers. The expression was 36 times higher in infested compared to uninfested plants maintained for no additional days, 23 times higher in those maintained for five days and 53 times in those maintained for 10 days. Moreover, upon infestation, the induction was increased 3.2 and 6.0 times in HIPV-receiver leaves compared with those in CV-receiver leaves after 0 and 5 days of post-exposure maintenance (Tukey-Kramer HSD test, P < 0.05). There was only very slightly increased expression (1.9 times), however, in HIPV-receiver plants maintained for 10 days when compared with those in CV-receiver plants maintained for the same period of time. Expression of CPI, the other defence gene examined, was very weakly increased by herbivore feeding to similar levels between HIPV- and CV-receiver leaves. This was in line with observation of the similarly increased accumulations of JA between HIPV-receiver and CV-receiver leaves in response to herbivory (Tukey-Kramer HSD test, P > 0.05; Fig. 3).
Figure 2. The appropriate time lag for priming the expression of defence genes in receiver plants.
Relative transcript levels of genes for Bowman-Birk type trypsin inhibitor (TI) and cysteine protease inhibitor (CPI) were determined in leaves of HIPV-receiver and CV-receiver plants and the plants then maintained for 0, 5 or 10 days. They were then either treated by the addition of four M. separata larvae for an additional day or maintained for an additional day without a larva. Transcript levels of genes were normalized to those of act1. Data represent the mean + standard errors (n = 4–5). Asterisks indicate significant differences between HIPV-receivers and CV-receivers based on a Tukey-Kramer HSD test (P < 0.05).
Figure 3. The appropriate time lag for priming induced accumulation of JA in receiver plants.
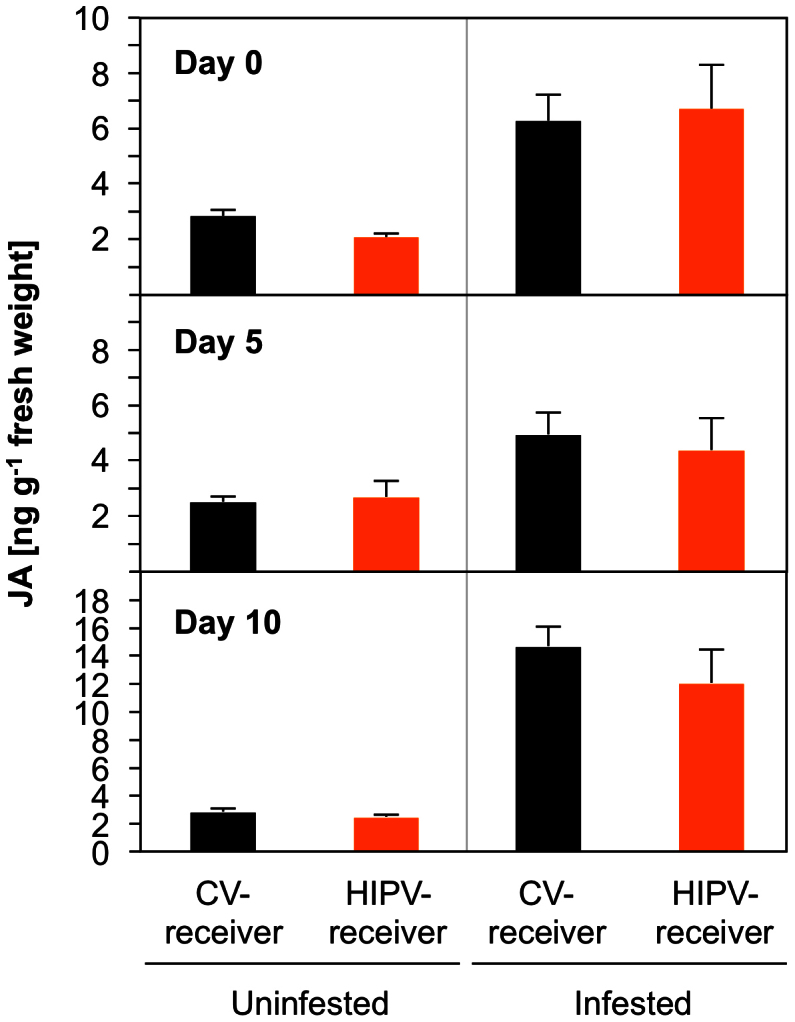
Endogenous JA levels were determined in leaves of HIPV-receiver and CV-receiver plants. They were then either treated by the addition of four M. separata larvae for an additional day or maintained for an additional day without a larva. Data represent the mean + standard errors (n = 4–5). There were no significant differences between HIPV-receivers and CV-receivers based on a Tukey-Kramer HSD test (P > 0.05).
Epigenetic modifications of the promoter region of TI
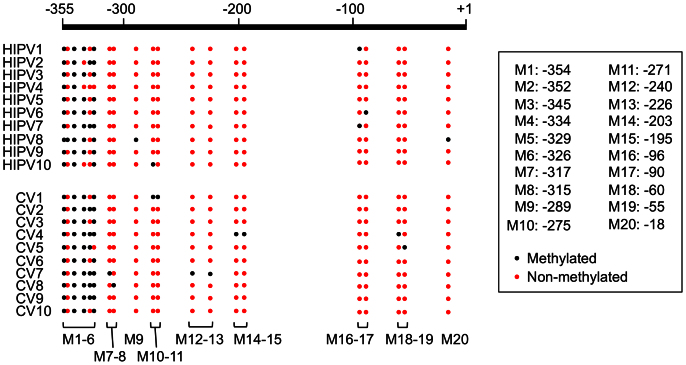
To identify the mechanism of plant memory of HIPVs we assessed the methylation of appropriate defence genes. Such epigenetic modification of DNA is one of the most reliable strategies for priming defence induction22, especially because, in our case, JA (primary activator of herbivore-responsive genes23) is unlikely to be a possible effector (see Fig. 3). We therefore assessed cytosine (Cyt) methylation in the promoter region upstream of the predicted transcription start site of the TI gene in HIPV- or CV-receiver leaves. Twenty independent sample sequences from HIPV-receiver and CV-receiver leaves (10 sequences each) showed two constantly methylated Cyt (positions M1 (−354) and M3 (−345); Fig. 4), and a single or two methylated Cyt in one of the 20 sequences at positions M2 and M7-20. Intriguingly, at positions M4 and M5, larger numbers of non-methylated Cyt were detected in HIPV-receivers compared to CV-receivers (3 and 0 at M4 in HIPV-receivers and CV-receivers; and 8 and 4 at M5 in HIPV-receivers and CV-receivers, respectively).
Figure 4. DNA methylation analysis of the TI gene.
Distribution of DNA methylation in the promoter region upstream of the predicted transcription start site (355 bp) in the HIPV-receivers (HIPV1 to 10) and CV-receivers (CV1 to 10) (ten independent sequences each). Black and red circles in the map indicate methylated and non-methylated Cyt, respectively.
Discussion
The priming effect of HIPVs on resistance against herbivores is memorised and stored by plants. The plant then recalls this memory when it is fed on by herbivores. The memory lasts for at least 5 days after exposure to HIPVs. The machinery of this memory might involve epigenetic regulation of DNA.
Genes for defensive compounds such as protease inhibitors play an essential role against herbivores24,25. In the leaves of receiver maize plants, TI expression was not promoted directly by HIPVs but rather was recalled by the plant when it was later fed on. Moreover, the priming of TI expression was only 1.9 fold (HIPV-receivers vs CV-receivers) after 10 days of post-exposure maintenance, in contrast to the stronger priming at 0 day (3.2 fold) and 5 days (6.0 fold) of maintenance. These minor impacts might not have been sufficient for certain anti-herbivore phenotypes at 10 days. Altogether, we conclude that exposure to HIPVs enables priming of the expression of defensive genes, including TI, and that the HIPV information is stored for days, until the moment when recalled by the plant under the stimulus of herbivory.
Generally, since JA-mediated defence signalling is predominantly activated when plants are attacked by chewing herbivores26, it might be expected that induced JA formation would be relevant to the HIPV-primed defence responses, as in (Z)-3-hexen-1-yl acetate-primed poplar receiver plants10. Our results were inconsistent with that possibility, however, as we observed that both priming of TI expression and memorizing of HIPV information in receiver leaves were independent of JA induction (Fig. 3). Nonetheless, we are unable to exclude the possibility that, rather than formation of JA, its signalling mode may be involved. Moreover, it cannot be doubted that jasmonates (JA-related compounds) are a master switch for eliciting transcription of herbivore-responsive genes23.
Epigenetic mechanisms, such as DNA methylation, can cause stable alterations in gene activity without changes in the underlying DNA sequences. In both animals and plants, Cyt is primarily methylated in the CG dinucleotide context27. However, methylated Cyt in DNA in plants has been found in all three Cyt contexts, namely, at CG sites, CHG sites (where H is A, C or T) and CHH sites28,29. Especially, methylation in promoter regions of genes leads to a profound reduction of transcripts, but methylation in gene bodies exhibits a parabolic relationship with transcription29. We examined methylation sites in the promoter region of the TI gene, and observed that some Cyt sites are preferentially demethylated by HIPV treatment. Unfortunately, because genetic tools such as mutant plants inheriting a loss of function, for instance, in DNA methyltransferases, are not available in maize, it remains to be clarified whether these demethylated sites are actually involved in the priming machinery for TI expression. However, epigenetic modification now appears to be a widespread phenomenon with strong potential to adapt to biotic and abiotic stresses, which require a complex orchestration of the transcriptional output of the genome in many plant taxa21. Therefore, undoubtedly, our findings indicate the participation of epigenetic regulation in plant communication. Epigenetic regulation, including DNA methylation, is distinct from metabolic changes such as JA induction, and therefore it can be beneficial for receiver plants by minimizing their costs of memorizing volatile information.
Methods
Plants and herbivores
Maize (Zea mays L. cv. Royal Dent) plants were grown in a greenhouse. Each individual plant was grown in a plastic pot in a growth chamber at 25°C, L:D 16:8 (natural + supplemental light). M. separata was transferred to our laboratory in 2001 from a culture reared at the National Institute of Sericultural and Entomological Science in Tsukuba, Ibaraki, Japan. The insects were reared on artificial diet (Insecta LFS, Nihon-Nosan Kogyo Co. Ltd., Yokohama, Japan) in the laboratory at 25°C, L:D 16:8.
Inter-plant communication assays
Air-flow experiments were conducted in polypropylene open-flow tunnels (0.4 m wide, 0.8 m long and 0.4 m high). All the tunnels were open at both ends and a fan at one end produced a continuous air flow (0.3 m s−1) from emitter to receiver plants through the tunnel. Uninfested maize plants (HIPV-receiver) were placed downwind of emitter plants treated with four third instar larvae of M. separata for three days. Uninfested plants placed downwind of uninfested conspecific plants served as controls (CV-receiver). Eight maize emitter plants (two-week-old) and 4–8 maize receiver plants (one-week-old) were placed 0.3 m apart. We used this short separation because inter-plant communication only occurs over short distances14,30,31. During treatments, the temperature was maintained at 25°C, L:D 16:8. The light period was from 07:00 to 23:00. One second-instar M. separata larva in the insect performance assay and four third-instar larvae in tests of gene expression or JA analysis, respectively, were put on receiver maize plants immediately after exposure to volatiles from the emitters (Day 0). Otherwise, the receiver plants were then maintained for 1, 5 or 10 days and used for insect treatment.
Performance of M. separata larvae
One second-instar M. separata larva was released onto a receiver plant in a pot. All larvae weighed between 2 and 4 mg. Each plant with larva was maintained in a climate-controlled room at 25°C. L:D 16:8. The larvae were collected and weighed after 3 days.
Quantitative reverse transcription (RT)-PCR
Total RNA was isolated from leaf tissues using a Qiagen RNeasy Plant Mini Kit and an RNase-Free DNase Set (Qiagen, Hilden, Germany) following the manufacturer's protocol. First-strand cDNA was synthesized using a PrimeScript RT reagent Kit (Takara, Japan), and 0.5 μg of total RNA at 37°C for 15 min. Real-time PCR was performed on an Applied Biosystems 7300 Real-Time PCR System (Foster City, CA, USA) using Power SYBR Green Master Mix (Applied Biosystems), cDNA (1 μl from 10 μl of each RT product pool), and 300 nM primers. The following protocol was followed: initial polymerase activation 2 min at 50°C, and 10 min at 95°C, 40 cycles of 15 s at 95°C, and 60 s at 60°C. PCR conditions were chosen by comparing threshold values in a dilution series of the RT product, followed by non-RT template control and non-template control for each primer pair. Relative RNA levels were calibrated and normalized with the level of act1 (NM_001155179) mRNA. The primers used were as follows: act1 (5′-AGGCCACGTACAACTCCATC-3′ and 5′-CCACCGATCCAGACACTGTA-3′), TI (NM_001154840) (5′-GCTACCTGTCTGACCCGCCG-3′ and 5′-GAGGATGTCGGCGCAGCGGT-3′) and CPI (BT055357) (5′-CGGTTAGGTGTGGATTGAAGA-3′ and 5′-GCCATGCTCCCTTGTGTAAT-3′).
Extraction and analysis of JA
Leaves (0.5 mg) were ground in liquid nitrogen. Ethyl acetate (2.5 ml), spiked with 10 ng of internal standard (d2-JA), was added to each sample and then the mixture was homogenized using a mortar and pestle. After centrifugation at 800 g for five minutes at 4°C, supernatants were transferred to recovery flasks. Each pellet was re-extracted with 2.5 ml of ethyl acetate and centrifuged, supernatants were combined and then evaporated to dryness under vacuum. The residue was resuspended in 1.25 ml of 70% methanol/water (v/v) and centrifuged to clarify phases, and the supernatants were analysed using a liquid chromatography-tandem mass spectrometry (LC/MS/MS) system as described in Ozawa et al. (2009)32.
Bisulfite sequencing of TI gene
Genomic DNA was isolated from leaf tissue using a NucleoSpin Plant II Kit (MACHEREY-NAGEL, Düren, Germany) following the manufacturer's protocol. The genomic DNA was bisulfate treated and purified using a MethylEasy Xceed DNA Bisulphite Modification Kit (Takara, Otsu, Japan) following the manufacturer's protocol. Two micrograms of the bisulfite-treated DNA solution were added to a first-round PCR mixture containing 1× PCR buffer, 2.5 mM MgCl2, 0.3 mM dNTPs, 0.4 μM of each primer (5′-TTTTTATAGAGAGTATTATTAGATGGTTTA-3′ and 5′-ACCATCATAAAAAACATCTAACCACA-3′), and 0.625 U Takara EpiTaq HS enzyme (Takara) in a final volume of 25 μl. PCR conditions were as follows: 10 s at 98°C, 38 cycles of 10 s at 98°C, 60 s at 55°C, and 90 s at 72°C, and additional extension of five minutes at 72°C. Further amplification was accomplished by nested PCR using a primer set (5′-GATGGTTTATTTTAAGTGTTTGG-3′ and 5′-AAAAACCTCATACAAAACACAAATC-3′). The cDNA was subcloned into the pGEM-T Easy Vector System (Promega, Madison, WI, USA) and sequenced using an ABI 3130xl Genetic Analyzer (Life Technologies, Foster City, CA, USA), following the manufacturer's protocols. The sequences obtained were aligned with the reference sequence in MaizeSequence.org (http://www.maizesequence.org/index.html).
Author Contributions
M.A., K.S., A.R., G.A. designed the study; M.A., K.S., A.R., G.A. performed the experiments and data analyses; G.A. wrote the manuscript.
Acknowledgments
This work was financially supported in part by Global COE Program A06 of Kyoto University; the Core-to-Core Program from the Japan Society for the Promotion of Science (JSPS) (No.20004); and a Grant-in-Aid for Scientific Research from JSPS (No. 24770019) to GA.
References
- Karban R. & Baldwin I. T. Induced responses to herbivory. (The University of Chicago Press, 1997). [Google Scholar]
- Arimura G., Shiojiri K. & Karban R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 71, 1642–1649 (2010). [DOI] [PubMed] [Google Scholar]
- Heil M. & Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 25, 137–144 (2010). [DOI] [PubMed] [Google Scholar]
- Baldwin I. T. & Schultz J. C. Rapid changes in tree leaf chemistry induced by damage:. evidence for communication between plants. Science 221, 277–279 (1983). [DOI] [PubMed] [Google Scholar]
- Arimura G. et al. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406, 512–515 (2000). [DOI] [PubMed] [Google Scholar]
- Bruin J., Sabelis M. W. & Dicke M. Do plants tap SOS signals from their infested neighbours? Tree 10, 167–170 (1995). [DOI] [PubMed] [Google Scholar]
- Heil M. & Silva Bueno J. C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 104, 5467–5472 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R. Plant behaviour and communication. Ecol. Lett. 11, 727–739 (2008). [DOI] [PubMed] [Google Scholar]
- Engelberth J., Alborn H. T., Schmelz E. A. & Tumlinson J. H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 101, 1781–1785 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost C. J. et al. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 180, 722–734 (2008). [DOI] [PubMed] [Google Scholar]
- Ton J. et al. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 49, 16–26 (2006). [DOI] [PubMed] [Google Scholar]
- Kessler A., Halitschke R., Diezel C. & Baldwin I. T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148, 280–292 (2006). [DOI] [PubMed] [Google Scholar]
- Choh Y. & Takabayashi J. Herbivore-induced extrafloral nectar production in lima bean plants enhanced by previous exposure to volatiles from infested conspecifics. J. Chem. Ecol. 32, 2073–2077 (2006). [DOI] [PubMed] [Google Scholar]
- Muroi A. et al. The composite effect of transgenic plant volatiles for acquired immunity to herbivory caused by inter-plant communications. PLoS One 6, e24594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R., Baldwin I. T., Baxter K. J., Laue G. & Felton G. W. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125, 66–71 (2000). [DOI] [PubMed] [Google Scholar]
- Ramadan A., Muroi A. & Arimura G. Herbivore-induced maize volatiles serve as priming cues for resistance against post-attack by the specialist armyworm Mythimna separata. J. Plant Interact. 6, 155–158 (2011). [Google Scholar]
- Karban R. & Shiojiri K. Self-recognition affects plant communication and defense. Ecol Lett. 12, 502–506 (2009). [DOI] [PubMed] [Google Scholar]
- Karban R., Shiojiri K. & Ishizaki S. An air transfer experiment confirms the role of volatile cues in communication between plants. Am. Nat. 176, 381–384 (2010). [DOI] [PubMed] [Google Scholar]
- Arimura G., Huber D. P. W. & Bohlmann J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. Plant J. 37, 603–616 (2004). [DOI] [PubMed] [Google Scholar]
- Puente M. E., Kennedy G. G. & Gould F. The impact of herbivore-induced plant volatiles on parasitoid foraging success: a general deterministic model. J. Chem. Ecol. 34, 945–958 (2008). [DOI] [PubMed] [Google Scholar]
- Grativol C., Hemerly A. S. & Ferreira P. C. Genetic and epigenetic regulation of stress responses in natural plant populations. Biochim. biophys. Acta, in press (2011). [DOI] [PubMed] [Google Scholar]
- Holeski L. M., Jander G. & Agrawal A. A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27, 618–626 (2012). [DOI] [PubMed] [Google Scholar]
- Maffei M. E., Arimura G. & Mithofer A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 29, 1269–1368 (2012). [DOI] [PubMed] [Google Scholar]
- Royo J. et al. Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc. Natl. Acad. Sci. USA 96, 1146–1151 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U. et al. Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. J. Exp. Bot. 61, 4169–4183 (2010). [DOI] [PubMed] [Google Scholar]
- Arimura G. et al. Effects of feeding Spodoptera littoralis on Lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 146, 965–973 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll M. G. & Bestor T. H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 74, 481–514 (2005). [DOI] [PubMed] [Google Scholar]
- Furner I. J. & Matzke M. Methylation and demethylation of the Arabidopsis genome. Curr. Opin. Plant Biol. 14, 137–141 (2011). [DOI] [PubMed] [Google Scholar]
- Zemach A., McDaniel I. E., Silva P. & Zilberman D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328, 916–919 (2010). [DOI] [PubMed] [Google Scholar]
- Heil M. & Adame-Alvarez R. M. Short signalling distances make plant communication a soliloquy. Biol. Lett. 6, 843–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R., Shiojiri K., Huntzinger M. & McCall A. C. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87, 922–930 (2006). [DOI] [PubMed] [Google Scholar]
- Ozawa R. et al. Exogenous polyamines elicit herbivore-induced volatiles in lima bean leaves: involvement of calcium, H2O2 and jasmonic acid. Plant Cell Physiol. 50, 2183–2199 (2009). [DOI] [PubMed] [Google Scholar]