Abstract
Objectives
The role of antioxidants in survival of cancer patients is controversial. No data on the relationships between antioxidant intake and survival of glioma patients is available. Our objective was to examine such association in a large series of cases.
Methods
The study population includes 814 glioblastoma multiforme cases that were newly diagnosed, histologically confirmed, aged 20 or older, and residing in the San Francisco Bay Area at diagnosis. Cases were identified via the regional cancer registry’s rapid case ascertainment system during 1991–1994 (series I), 1997–2000 (series II), and 2001–2004 (series III). Daily dietary antioxidant intake at diagnosis was assessed via food frequency questionnaire and was expressed as Total Antioxidant Index calculated based on Trolox equivalent per gram of food. In addition, the study collected information on supplements/vitamin intake.
Results
Overall, our results indicated no consistent, significant association of survival with dietary antioxidant intake or its combination with vitamin supplements. However, in series III, we observed a significant association between higher antioxidant index and better survival: HR= 0.58 (95% CI: 0.46, 0.74) for each unit of antioxidant index on a log-scale.
Conclusions
Although it is possible that this is a chance finding, the association between dietary antioxidants and survival in the most recently recruited patients warrants further investigations.
Keywords: Epidemiology, Glioblastoma, Survival, Antioxidants
Introduction
Glioblastoma multiforme (GM) is one of the most rapidly fatal cancers [1]. With limited improvements in survival with current standard treatments, even small improvements due to lifestyle factors could be a significant contribution. However, the research on the role of the lifestyle factors for survival after glioma diagnosis is very limited. To the best of our knowledge, this manuscript is the first to attempt to analyze the relationship between dietary factors and survival of glioma patients.
Our analysis focuses on antioxidant intake among a large group of GM patients, because earlier studies demonstrated that glioma risk may be reduced by higher dietary intake of antioxidants [2–6]. Furthermore, although there is extensive debate and clinical trial evidence regarding the influence of antioxidant supplementation during radiation and chemotherapy for other cancer sites [7], only one previous study considered such factors with respect to glioblastoma [8]. Lissoni et al. showed improved survival for GM patients receiving melatonin during radiation therapy [8]. Two opposing hypotheses about the effects of antioxidants on survival of glioma patients can be formulated [7]: (a) antioxidants may make the glioma more resistant to tumor killing by radiation or chemotherapy [7] possibly leading to poorer patient survival; or (b) antioxidants may reduce treatment toxicity by protecting normal tissues from oxidative damage possibly leading to better survival [9]. Supporting the first hypothesis, studies showed that glioma cells have amplified antioxidant defense (i.e. increased expression of antioxidant enzymes) [10–13], which is responsible for their resistance to radiation [14–16] and is associated with shorter survival [17, 18]. On the other hand, a recent animal study suggests that aggressiveness of glioma can be reduced by dietary antioxidants [19].
To assess dietary intake of antioxidants, we calculated the Total Antioxidant Index. This methodology is based on the concept that diet presents a complicated mixture of single antioxidants. Thus, comparison of individual diets with respect to their antioxidant capacity requires a unifying scale. Trolox equivalent assay provides a standardized and validated measurement of antioxidant capacity for each food [20–24]. We used the Trolox equivalent to calculate the Total Antioxidant Index in our analysis.
To explore evidence for or against a beneficial effect of antioxidants on survival of glioma patients, we examined the associations between total dietary antioxidant index and survival of 814 GM patients from the population-based San Francisco Bay Area Adult Glioma Study.
Materials and Methods
The University of California Committee on Human Research approved the methods (approval number H6539-04956) and subjects signed consent forms for the study. The San Francisco Bay Area Adult Glioma Study is a population-based cases-control study; its population, 1991–2004, is described in detail elsewhere [2, 25–30]. Newly diagnosed, histologically confirmed GM cases aged 20 years or older and residing in the San Francisco Bay Area at diagnosis were identified using the regional cancer registry’s rapid case ascertainment system during three ascertainment periods: August 1991 to April 1994 (series I), May 1997 to August 2000 (series II) and November 2001 to September 2004 (series III). The present analysis is restricted to GM patients.
The Total Antioxidant Index expressed as Trolox equivalents per gram of food was calculated using data from several references [20, 24, 31] as described previously by Tedeschi-Blok et al. [2]. Briefly, food-frequency questions were based on the US National Cancer Institutes' (Block's) Health Habits and History Questionnaire; these questions were modified to emphasize antioxidant, nitrite, and nitrate–containing foods. For cases, all questions referred to the year prior to glioma diagnosis, whereas for controls – to the year prior to interview. Total daily intake of foods containing antioxidants was calculated based on food frequency dietary questionnaires which included a total of 35 items of fruits, vegetables, juices and teas in series II or III questionnaires, but only 22 of these 35 food items in series I. Total calorie intake was calculated by summation of calorie intake for individual foods using available on line (http://www.nal.usda.gov/fnic/foodcomp/Data/SR13/sr13.html) the USDA Nutrient Database for Standard Reference (1999) to convert each food intake in gram into calorie content. Questions about vitamin supplement use were asked in all three series of the study.
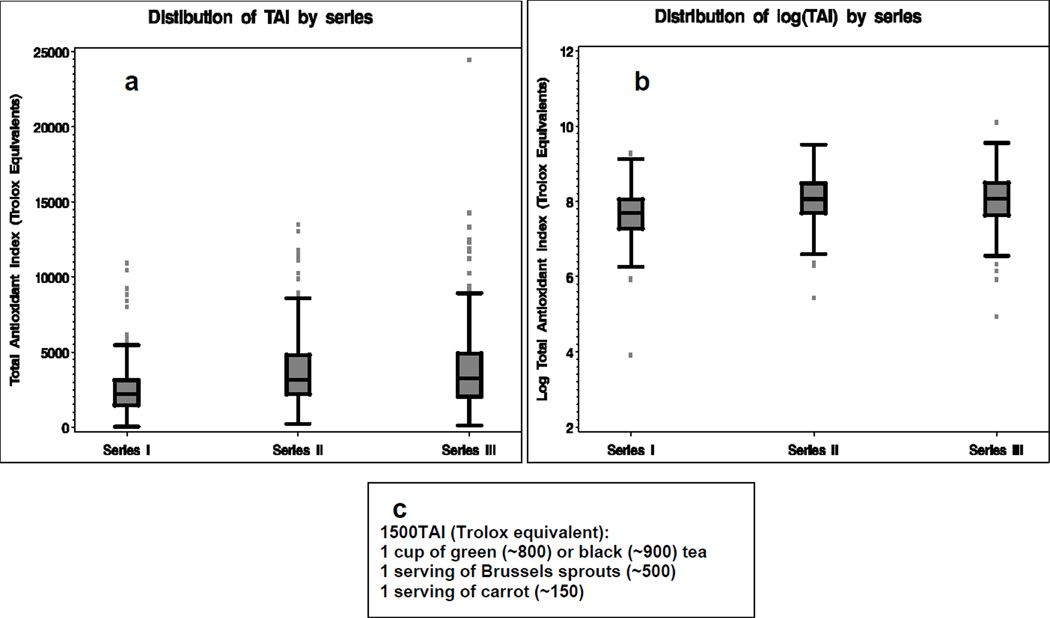
Statistical analysis was performed in SAS v9.1.3 (SAS Institute, Cary, NC). Kaplan-Meier methods were used to compute median survival. Other descriptive statistics were generated using standard SAS procedures such as “proc freq”, “proc means”, and ‘proc univariate”. Cox proportional hazard models estimated relative hazard and the 95% confidence intervals associated with the Total Antioxidant Index, either expressed in the original units or in log-transformed units (Models 1 and 2, respectively). We also categorized the Total Antioxidant Index in four equally-spaced categories with the increment between the categories of 1500 units (Model 3). The increment of the Total Antioxidant Index equal to 1500 units seems reasonable, because such interval includes different combinations of foods and drinks that can be either incorporated or omitted in the individual diet. One such example is illustrated in Figure 1C. For Model 3, we calculated p-value for trend using median for each category as data point. Also, we created combined categories for antioxidant index and vitamin/supplement intake with four combinations of low and high Total Antioxidant Index (cut point at the median) and regular vitamin/supplement use (yes/no) (Model 4). Models 1, 2, and 3 adjusted for age (years), gender, ethnicity (white/other), series, treatments (resection, radiation, temodar, and other chemotherapy), total calorie intake, proxy responder (yes/no), and regular vitamin/supplement use (yes/no). Model 4 adjusted for all these factors except vitamin supplement use. We also restricted models to series III patients who received what has become the current standard of care treatment, that is, any type of resection plus radiation therapy with temodar.
Figure 1.
Distribution of Total Antioxidant Index (TAI): a – original scale, b – log-transformed scale, c – an example of foods corresponding to 1500 TAI.
Results
The data collection for this study has been conducted longer than a decade – from 1991 to 2004. The basic demographic characteristics (age at diagnosis, proportions of males and white patients) of GM patients did not change substantially during that time period (Table 1). Temodar in combination with resection and radiation became standard of care during series III subject recruitment, with concomitant improved survival and a reduced proportion of interviews with proxies as a result of improved patient survival. Besides current standard of treatment, among 363 GM patients Series III, 74 (20%) had different combination of radiation and either temodar or other chemotherapy (no resection); 14 (4%) had resection, radiation and chemotherapy other than temodar; and 107 (29%) had combination of missing data with different types of treatment.
Table 1.
Characteristics of population based glioblastoma multiforme cases from three recruitment series of the San Francisco Bay Area Adult Glioma Study, 1991–2004
| All | Series I (1991–1994) |
Series II (1997–2000) |
Series III (2001–2004) |
|
|---|---|---|---|---|
| Number of GM cases/ censored (non-deceased) patients | 814/37 | 257/2 | 228/1 | 329/34 |
| Median survival, days (95% CI) | 256 (236,280) | 207 (176,240) | 241 (212,280) | 327 (287,385) |
| Proxy for patient responded to questionnaire | 353 (43%) | 151 (59%) | 94 (41%) | 108 (33%) |
| Demographics | ||||
| Age at diagnosis, yearsa | 61 (14) | 61 (14) | 61 (13) | 60 (14) |
| Males | 57% | 55% | 58% | 58% |
| White | 82% | 84% | 84% | 78% |
| Treatment | ||||
| Resection (partial or total) | 73 % | 69 % | 76% | 73% |
| Radiation therapy | 84% | 79% | 87% | 85% |
| Temodar | 24% | 0 | 2% | 63% |
| Other Chemotherapy | 12% | 11% | 23% | 6% |
| Current standard of care (resection/radiation/temodar) | 17% | 0 | 2% | 41% |
| Antioxidant intake | ||||
| TAI (Trolox equivalent)b | 3371 (2327) | 2495 (1575) | 3787 (2399) | 3769 (2572) |
| Vitamin/Supplement use | 56% | 57% | 52% | 58% |
| Total calorie intake | 1901 (979) | 1674 (896) | 2023 (1064) | 1995 (952) |
Descriptive statistics for the continuous variables is presented by mean values and standard deviation in parenthesis.
TAI- Total antioxidant index.
The lower antioxidant index and total calorie values for series I patients (Table 1, Fig. 1) is due to there being fewer food items on the questionnaire for that period. However, most frequently consumed foods containing antioxidants were the same in all three series. We calculated percent of GM cases that consumed antioxidant-containing foods at least once a week. Among foods with the antioxidant score > 10 Trolox equivalent units per serving, the most frequently consumed foods were black tea (30–45% in three series) and broccoli (48–60%). Among foods with the antioxidant score 4–10 Trolox equivalent units per serving, the most frequently consumed foods were green salad (81–89%) and potatos (63–74%). Among foods with the antioxidant score <4 Trolox equivalent units per serving, the most frequently consumed foods were tomatos (67–82%) and bananas (72–74% for Series II and III only). The distribution of the antioxidant index is consistently skewed in all three series with a long tail at the high end (Fig.1A), whereas the distribution of log-transformed antioxidant index is symmetrical (Fig. 1B).
As expected, older patients consistently had worse survival (hazard ratio estimates per year of age ranged from 1.02 to 1.03, p<0.05), while those with any type of resection and radiation treatment had better survival (hazard ratios were between 0.3–0.7, p<0.05, for resection and between 0.1 and 0.3, p<0.05, for radiation therapy).
The main analysis is presented by results from Models 1 and 2 (Table 2). These models combined for series I-III showed no association between survival and the antioxidant index (Table 2, Models 1 and 2). These results did not change when we broadly stratified the analysis by age (with median age of 62 as a cut point), by gender, or by race (restricted models to whites only). Interestingly, the same models with minimum covariates (only age and gender) showed a weak association with dietary antioxidant intake: for all series, HR=0.96 (95% CI: 0.93, 0.99) in Model 1 and HR=0.88 (95% CI: 0.79, 0.97) in Model 2. Since Total Antioxidant Index was higher in series II and III, we also combined series II and III: no association was detected by Model 1 (HR=0.95, 95% CI: 0.91, 1.0), but a weak inverse association was detected by Model 2 (HR=0.78, 95% CI: 0.66, 0.92). In series III, there was a weak but statistically significant association of better survival with increased antioxidant intake (Table 2, Models 1 and 2). When models for series III were restricted to those who received the current standard of care (resection/radiation/temodar), the association of survival with the antioxidant index moved towards the null: the reduction in the inverse association was 43% and 49% in models 1 and 2, respectively. Limiting Models 1 and 2 to self-report (excluding proxy-responders) did not change the HR estimates substantially for all series and for series I and II; for series III, this restriction resulted in approximately 40–45% attenuation of the association.
Table 2.
Association between total antioxidant index (TAI) and survival in population-based glioblastoma multiforme patients from the San Francisco Bay Area, 1991–2004
| Model: TAI variable | Hazard Ratio (95% Confidence Interval Limits) |
|||
|---|---|---|---|---|
| All | Series I (1991–1994) |
Series II (1997–1999) |
Series III (2001–2004) |
|
| 1:TAI (Trolox equivalent)a | 0.98 (0.94, 1.02) | 1.07 (0.98, 1.16) | 1.01 (0.95,1.07) | 0.88 (0.82, 0.94) |
| 2: Log (TAI) | 1.01 (0.90, 1.13) | 1.30 (1.07, 1.58) | 1.06 (0.83, 1.35) | 0.58 (0.46, 0.74) |
| 3:TAI categories:b TAI < 1500 | reference level | reference level | reference level | reference level |
| 1500 ≤ TAI ≤ 2999 | 1.06(0.84, 1.33) | 1.29 (0.94, 1.79) | 1.07(0.63, 1.81) | 0.54 (0.34, 0.85) |
| 3000 ≤ TAI ≤ 4499 | 1.13 (0.87, 1.45) | 1.51 (1.00, 2.27) | 1.32 (0.76, 2.27) | 0.48 (0.30, 0.77) |
| TAI ≥ 4500 | 0.99 (0.75, 1.31) | 1.39 (0.80, 2.40) | 1.22 (0.69, 2.14) | 0.42 (0.26, 0.69) |
| 4: TAI and vitamin/supplement intake: | ||||
| Low TAI (no regular vitamin/supplements)c | reference level | reference level | reference level | reference level |
| Low TAI + regular vitamin/supplements | 0.90 (0.673, 1.10) | 0.97 (0.70, 1.34) | 0.64 (0.42, 0.99) | 0.94 (0.63, 1.39) |
| High TAI (no regular vitamin/supplements) | 0.99 (0.79, 1.24) | 1.20 (0.77, 1.86) | 1.07 (0.71, 1.60) | 0.70 (0.47, 1.04) |
| High TAI + regular vitamin/supplement | 0.94 (0.77, 1.16) | 1.01 (0.76, 1.59) | 0.94 (0.64, 1.37) | 0.66 (0.46, 0.96) |
TAI scaled such that HR represents a 1000 unit change.
In Models 1–3 – covariates included age, gender, ethnicity, series, proxy-reporting, treatment (resection, radiation, temodar, and other chemotherapy), total calorie intake, and regular vitamin/supplement use (yes/no);
In Model 4 - covariates included all the covariates used in Models 1–3 except regular vitamin/supplement use (yes/no), which was part of the main exposure.
1500 TAI units correspond approximately to 1 cup of green (~800) or black (~900) tea plus 1 serving of Brussels sprouts (~500) plus 1 serving of carrots (~150).
High TAI is above median, low TAI is equal or below median.
Dose-response analysis using categories (Table 2, Model 3) showed no dose-response trend overall and in series I and II: p-values for trend were 0.83 for all series, and 0.11 and 0.37 for series I and II, respectively. In series III, this analysis showed noticeable improvement in survival associated with the increase of 1500 units in Total Antioxidant Index; however, further increase in dietary antioxidant intake was associated with smaller improvements in survival (p value for trend was 0.01). Examination of antioxidant intake from diet and from vitamins/supplements did not indicate a complementary effect in series I and II (Table 2, Model 4). However, there was a suggestion of complementary effect of the antioxidants from different sources in series III (Table 2, Model 4).
Discussion
The role of antioxidants in survival of cancer patients is controversial, with some studies suggesting associations with better survival and others suggesting associations with worse survival [7]. However, these studies did not include GM patients. This analysis included a large number of GM patients recruited over a fourteen-year period (1991–2004) in three series, in which the standard of care for these patients changed. Our GM patients were similar to GM patients from other studies, with the expected associations of survival with age, resection, radiation treatment, and temodar therapy. Such similarity with the other populations of GM patients and the population-based study design suggest these results are generalizable to other GM patients. Overall, our results indicated no consistent, statistically significant association of survival with dietary antioxidant intake or the combination of dietary antioxidant intake and vitamin supplements. Thus, the combined analysis does not support an association of antioxidant intake and survival. However, in series III, we did observe a weak but statistically significant association between higher antioxidant index and better survival. This association tended to be more pronounced when antioxidant index was analyzed in log-scale or categories. Taking into account that both modeling techniques reduce the influence of high-end values, such tendency suggests that a potential effect of dietary antioxidants might be beneficial only to a certain degree, with such effect fading at very high values. The association of antioxidant intake with better survival in series III remained but became non-significant and moved towards the null when analyses were restricted to patients on current standard therapy.
It would be logical to examine the association between antioxidants and survival in patients who received treatment that generates ROS. Certainly, radiation treatment involves generation of ROS. However, restriction of the models to patients who received radiation is not meaningful, because 85% of patients received radiation leaving only a small group for comparison. Temodar works through conversion at physiologic pH to the active metabolite 5-(3-methyl)-1-triazen-1-yl-imidazole-4-carboxamide (MTIC) [32], a reactive DNA-methylating compound. The connection between oxidative environments and the mechanism of Temodar’s action has not been studied.
The most puzzling finding of this analysis is inconsistency between the series. We demonstrated that difference in treatment, i.e. temodar, is not responsible for the observed inconsistency. Several factors may either obscure true associations or enhance false associations; these include the inherent misclassification problems with retrospective recall of diet and potential confounding of diet with other factors that might influence either treatment or survival. For example, retrospective recall of diet could be inaccurate and introduce a random error, which would result in attenuation of a true association. On the other hand, the accuracy of recall could introduce a systematic error. Patients in better condition may have recalled diet with better accuracy and lived longer than patients in worse condition, whose accuracy suffered and whose survival was shorter. Such systematic difference in recall could produce a spurious association. Also, this inconsistency may reflect chance findings. Nevertheless, the finding of a suggestive association between antioxidants and better GM surivival in the most recently recruited patients is important, taking into account the generally poor prognosis for GM. It may be worthwhile to obtain a more definitive answer to the question of whether dietary antioxidants improve GM survival by conducting a prospective study in a larger group of patients receiving the current standard of care treatment.
Novelty and impact: The most important role of this work is to raise a question whether dietary and other life-style risk factors can improve the survival of glioma patients. With such poor prognosis, even very modest improvement in survival is an important contribution. If published, our analysis would become the first scientific statement on this topic. Clinicians have asked us whether dietary factors or vitamin supplementation during chemotherapy might help or hinder response; but there is no published data on this topic for glioma patients to answer their questions.
Acknowledgement
This research was supported by the NIH grants CA108786-04, CA52689, CA097257, Robert J. and Helen H. Glaser Family Foundation and Elvira Olsen Family Fund.
Abbreviations
- GM
glioblastoma multiforme
- HR
hazard ratio
- TAI
Total Antioxidant Index
Reference List
- 1.Wen PY, Kesari S. Malignant Gliomas in Adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Tedeschi-Blok N, Lee M, Sison JD, Miike R, Wrensch M. Inverse association of antioxidant and phytoestrogen nutrient intake with adult glioma in the San Francisco Bay Area: a case-control study. BMC Cancer. 2006;6:148. doi: 10.1186/1471-2407-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, La VC, Negri E, et al. Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer. 1999;81:20–23. doi: 10.1002/(sici)1097-0215(19990331)81:1<20::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Ward MH, Tucker KL, et al. Diet and risk of adult glioma in eastern Nebraska, United States. Cancer Causes and Control. 2002;13:647–655. doi: 10.1023/a:1019527225197. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA) Cancer Causes and Control. 1997;8:13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzbaum JA, Fisher JL, Goodman J, Octaviano D, Cornwell DG. Hypotheses concerning roles of dietary energy, cured meat, and serum tocopherols in adult glioma development. Neuroepidemiology. 1999;18:156–166. doi: 10.1159/000026207. [DOI] [PubMed] [Google Scholar]
- 7.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should Supplemental Antioxidant Administration Be Avoided During Chemotherapy and Radiation Therapy? J Natl Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 8.Lissoni P, Meregalli S, Nosetto L, et al. Increased survival time in brain glioblastomas by a radioneuroendocrine strategy with radiotherapy plus melatonin compared to radiotherapy alone. Oncology. 1996;53:43–46. doi: 10.1159/000227533. [DOI] [PubMed] [Google Scholar]
- 9.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Yan T, Lim R, Oberley LW. Expression of superoxide dismutases, catalase, and glutathione peroxidase in glioma cells. Free Radical Biology and Medicine. 1999;27:1334–1345. doi: 10.1016/s0891-5849(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 11.Pu PY, Lan J, Shan SB, et al. Study of the antioxidant enzymes in human brain tumors. J Neurooncol. 1996;29:121–128. doi: 10.1007/BF00182134. [DOI] [PubMed] [Google Scholar]
- 12.Nordfors K, Haapasalo J, Helen P, et al. Peroxiredoxins and antioxidant enzymes in pilocytic astrocytomas. Clin Neuropathol. 2007;26:210–218. doi: 10.5414/npp26210. [DOI] [PubMed] [Google Scholar]
- 13.Khalil AA. Biomarker discovery: a proteomic approach for brain cancer profiling. Cancer Sci. 2007;98:201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preuss M, Girnun GD, Darby CJ, Khoo N, Spector AA, Robbins MEC. Role of antioxidant enzyme expression in the selective cytotoxic response of glioma cells to [gamma]-linolenic acid supplementation. Free Radical Biology and Medicine. 2000;28:1143–1156. doi: 10.1016/s0891-5849(00)00210-0. [DOI] [PubMed] [Google Scholar]
- 15.Smith PS, Zhao W, Spitz DR, Robbins ME. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radical Biology and Medicine. 2007;42:787–797. doi: 10.1016/j.freeradbiomed.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Smith-Pearson PS, Kooshki M, Spitz DR, Poole LB, Zhao W, Robbins ME. Decreasing peroxiredoxin II expression decreases glutathione, alters cell cycle distribution, and sensitizes glioma cells to ionizing radiation and H2O2. Free Radical Biology and Medicine. doi: 10.1016/j.freeradbiomed.2008.07.015. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvela S, Bragge H, Paunu N, et al. Antioxidant enzymes in oligodendroglial brain tumors: association with proliferation, apoptotic activity and survival. J Neurooncol. 2006;77:131–140. doi: 10.1007/s11060-005-9030-z. [DOI] [PubMed] [Google Scholar]
- 18.Okcu MF, Selvan M, Wang LE, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 19.Pouliquen D, Olivier C, Hervouet E, et al. Dietary prevention of malignant glioma aggressiveness, implications in oxidant stress and apoptosis. Int J Cancer. 2008;123:288–295. doi: 10.1002/ijc.23513. [DOI] [PubMed] [Google Scholar]
- 20.Cao G, Sofic E, Prior RL. Antioxidant Capacity of Tea and Common Vegetables. J Agric Food Chem. 1996;44:3426–3431. [Google Scholar]
- 21.Pellegrini N, Salvatore S, Valtuena S, et al. Development and Validation of a Food Frequency Questionnaire for the Assessment of Dietary Total Antioxidant Capacity. J Nutr. 2007;137:93–98. doi: 10.1093/jn/137.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Rautiainen S, Serafini M, Morgenstern R, Prior RL, Wolk A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr. 2008;87:1247–1253. doi: 10.1093/ajcn/87.5.1247. [DOI] [PubMed] [Google Scholar]
- 23.Serafini M, Del RD. Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Cao G, Prior RL. Total Antioxidant Capacity of Fruits. J Agric Food Chem. 1996;44:701–705. [Google Scholar]
- 25.Krishnan G, Felini M, Carozza SE, Miike R, Chew T, Wrensch M. Occupation and adult gliomas in the San Francisco Bay Area. J Occup Environ Med. 2003;45:639–647. doi: 10.1097/01.jom.0000069245.06498.48. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA) Cancer Causes and Control. 1997;8:13–24. doi: 10.1023/a:1018470802969. [DOI] [PubMed] [Google Scholar]
- 27.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–615. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 28.Wrensch M, Lee M, Miike R, et al. Familial and Personal Medical History of Cancer and Nervous System Conditions among Adults with Glioma and Controls. Am J Epidemiol. 1997;145:581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- 29.Wrensch M, Kelsey KT, Liu M, et al. Glutathione-S-Transferase Variants and Adult Glioma. Cancer Epidemiol Biomarkers Prev. 2004;13:461–467. [PubMed] [Google Scholar]
- 30.Wrensch M, McMillan A, Wiencke J, et al. Nonsynonymous coding single-nucleotide polymorphisms spanning the genome in relation to glioblastoma survival and age at diagnosis. Clin Cancer Res. 2007;13:197–205. doi: 10.1158/1078-0432.CCR-06-1199. [DOI] [PubMed] [Google Scholar]
- 31.Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans. 1996;24:790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 32.Mason WP, Cairncross JG. Drug Insight: temozolomide as a treatment for malignant glioma[mdash]impact of a recent trial. Nat Clin Pract Neuro. 2005;1:88–95. doi: 10.1038/ncpneuro0045. [DOI] [PubMed] [Google Scholar]