Abstract
Background
Hemophagocytic Lymphohistiocytosis (HLH) is characterized by uncontrolled inflammation that is generally fatal without immune modulating chemotherapy. At Texas Children's Hospital, we have observed significant central nervous system (CNS) toxicity in several patients treated for HLH according to the Histiocyte Society protocol HLH-2004 in which cyclosporine is given early in the treatment regimen.
Methods
Patients diagnosed with HLH at Texas Children's Hospital between April 2004 and October 2007 were identified and charts were reviewed. A reference group of patients treated between August 2001 and March 2004, prior to the introduction of HLH-2004, was also evaluated.
Results
Five of 17 patients in the study group developed severe neurotoxicity. Four had new onset seizures associated with significant MRI abnormalities, while the fifth died of intracerebral hemorrhage. Timing of the development of neurologic side effects ranged from Day 5 to Week 6 of therapy. Cyclosporine levels were outside the therapeutic range (200-300 ng/ml) prior to the onset of symptoms in two of the five patients. Systolic blood pressures for all five patients were greater than the ninety-fifth percentile for age on at least one measurement within twenty-four hours of the onset of neurologic symptoms. MRI scans obtained within twenty-four hours of seizure activity in four patients were consistent with posterior reversible encephalopathy syndrome (PRES). By comparison only one patient in the reference group (n=15) had neurotoxicity (PRES).
Conclusions
Patients being treated for HLH appear to be at risk for neurotoxicity, particularly PRES. Elevated blood pressure, worsening renal and liver function, increased cyclosporine levels, and CNS involvement of HLH may be triggers for the neurotoxic side effects of treatment. Patients being treated on HLH-2004 require close monitoring of their neurologic status and modifiable risk factors such as hypertension should managed aggressively. If larger studies validate our observations, it will be important to determine if up-front cyclosporine in HLH protocols confers a survival benefit that outweighs the potential risk of increased neurotoxicity.
Keywords: Hemophagocytic Lymphohystiocytosis, neurotoxicity, PRES
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a clinical syndrome caused by uncontrolled activation of cytotoxic T-cells and antigen-presenting cells. Initial signs and symptoms of HLH may mimic more common conditions including bacterial sepsis, viral infections, auto-immune disease, hepatitis, and encephalitis. Early clinical signs include fever, hepatomegaly, splenomegaly, neurologic abnormalities, rash, and lymphadenopathy [1]. In severe cases, patients may present with multiple organ system failure.
The current Histiocyte Society treatment protocol, HLH-2004, defines HLH in patients that meet at least five of the following criteria: fever, splenomegaly, cytopenias in at least two cell lines, hypertriglyceridemia and/or hypofibrinogenemia, hemophagocytosis in tissue biopsy, low or absent NK-cell activity, elevated ferritin concentration > 500 ng/dl, or elevated soluble CD25 (soluble IL-2R) > 2400 ng/dl.[2] Proven mutation in genes encoding perforin-1, MUNC13-4 or syntaxin11 are diagnostic, and family history is highly supportive.
Central nervous system (CNS) involvement is common in HLH and is highly variable in its presentation and associated imaging findings. Henter and Nennesmo reported the neurologic findings in a series of 23 HLH patients including irritability, seizures, cranial nerve palsies, ataxia, nystagmus, delayed psychomotor development, meningeal signs and evidence of increased intracranial pressure.[3] Haddad et al reported a series of 34 patients in which they observed a variety of neurologic symptoms including hypotonia or hypertonia, meningismus, seizures, coma with opisthotonos, and abnormal cardiac or respiratory rates.[4] Brain MRIs done in their patients showed peri-cerebral diffuse subdural dilation and large confluent hyperintense T2-weighted signal in the cerebrum, or necrotic areas. At our own institution we reviewed the brain CTs and MRIs of 18 patients. The most frequent findings were periventricular white-matter signal abnormalities with cerebral volume loss and enlargement of ventricles and extra-axial fluid spaces, and multiple enhancing bilateral hemispheric lesions with edema.[5]
Prior to treatment with immune modulating chemotherapy, survival for patients with HLH was less than 10%.[6] In the last Histiocyte Society treatment protocol, HLH-94, there was very little difference in survival between patients presumed to have familial HLH and patients thought to have “acquired” disease with overall survival 55%.[7] Therefore, the new protocol, HLH-2004, recommends chemotherapy for all patients who meet clinical criteria for HLH. HLH-2004 prescribes immediate treatment with etoposide, dexamethasone, and cyclosporine. Due to some early deaths observed in patients on HLH-94, initiation of cyclosporine was moved from after Week 8 to Day 1 to provide more intense up-front immune suppression. While timely administration of chemotherapy is essential to survival of patients with HLH, we have observed neurologic complications that may be attributable to therapy and not disease in several patients treated on Histiocyte Society protocols with early administration of cyclosporine. In this study we review the experiences of patients diagnosed with HLH treated at Texas Children's Hospital over a 2.5 year period.
Methods
Patient Selection
We performed a retrospective chart review of patients being treated for HLH at Texas Children's Hospital from April 2004 to October 2007, and identified five patients who developed severe neurotoxicity. Patients were treated according to HLH-94 (1/5) or HLH-2004 guidelines (4/5) though the patient treated according HLH-94 had early initiation of cyclosporine therapy. The patients treated according to HLH-2004 had cyclosporine started at the time of diagnosis. The patient treated according to HLH-94 guidelines had cyclosporine started on D#22 of therapy. This is earlier than the prescribed by the HLH-94 protocol guidelines in which cyclosporine is not started until the ninth week of therapy. This change was made at the discretion of the treating physician. Demographic, clinical, and laboratory data were collected with a standardized data collection form. We also analyzed the charts of a reference group of patients treated for HLH between August 2001 and March 2004 (prior to the introduction of HLH-2004 and the use of upfront cyclosporine therapy), and identified one patient who developed neurotoxicity.
Pharmacokinetic Modeling
Two of the five patients presented had documented elevation of cyclosporine levels near the time of neurologic side effects. For one of these patients, pharmacokinetic modeling was done to more closely evaluate the exposure to cyclosporine with MW/PHARM (MediWare, Groningen, the Netherlands) using a Bayesian approach. A two-compartment model was assumed. The Bayesian priors for the pharmacokinetic parameters (volume of distribution, rate constants, etc) were based on historical data from the Cincinnati Children's Medical Center Therapeutic Drug Monitoring Service. PK parameters and concentration time curves were then estimated using a Bayesian feedback method with MW/PHARM.
Statistical Considerations
Fisher's Exact tests were done to determine if there were significant differences between the study group and the reference group in the fraction of patients with neurotoxicity. The quoted P-value is a one-sided.
Results
Five patients who experienced severe neurologic toxicity while being treated for HLH are described. Table I summarizes the clinical and laboratory data at the time of their diagnoses. All five met criteria for the diagnosis of HLH according to the Histiocyte Society protocols. Two of the five patients had subtle abnormal MRI findings consistent with HLH CNS involvement at the time of diagnosis. All five patients had some degree of liver dysfunction as indicated by either elevation of the liver enzymes or abnormalities in their coagulations tests. None of the five patients had abnormal creatinine levels.
Table I. Characteristics at Presentation.
| Data | Units | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 |
|---|---|---|---|---|---|---|
| Age | yrs | 6 | 16 | 9 | 15 | 7 |
| Ethnicity | NA | Hispanic | Caucasian | Hispanic | Pakistani | Anglo |
| Signs & Symptoms | NA | Fever, organomegaly, liver failure | Fever, hepatomegaly, adenopathy | Fever, adenopathy, h/o osteomyelitis | Fever, splenomegaly, adenopathy | Fever, bilateral knee swelling |
| Hgb | g/dl | 8.3 | 9.4 | 9.6 | 7.6 | 8.5 |
| WBC | 103/ml | 2.8 | 1.2 | 61.9 | 0.47 | 7.5 |
| Platelets | 103/ml | 54 | 22 | 116 | 91 | 87 |
| Triglycerides | mg/dl | 366 | 178 | 413 | 284 | 200 |
| Fibrinogen | mg/dl | 88 | 184 | 541 | 243 | 287 |
| NK-Activity | Lytic Units | NA | Decreased | Normal | Absent | Normal |
| Ferritin | ng/ml | 63,919 | 9,640 | 7,718 | 1,542 | 30,486 |
| Soluble CD-25 | pg/ml | NA | 17,697 | 79,060 | 4,470 | 9,572 |
| Hemophagocytosis | NA | Bone Marrow - positive | Bone Marrow - positive | Liver - positive | Bone Marrow - negative | Bone Marrow - positive |
| Blood Pressure | mm Hg | 100/50 | 92/51 | 94/49 | 78/32 | 99/44 |
| AST/ALT | U/L | 4736/1406 | 761/430 | 77/22 | 570/102 | 319/226 |
| PT/PTT | sec | 24.1/80.8 | 18.2/55.8 | 15.7/41.3 | 16.3/64.4 | 16.7/40.1 |
| Serum Cr | mg/dl | 0.6 | 0.9 | 0.8 | 0.7 | 0.5 |
| CNS Disease at Diagnosis | NA | Unknown | Positive – based on MRI | Positive – based on MRI | Negative | Negative |
| Baseline MRI | NA | NA | 4 mm left parietal lesion | Scattered small white matter lesions in frontal lobes | Cerebral volume loss | Cerebral volume loss |
| Baseline Lumbar Puncture | Cells/mm3 | NA | 0 WBC 10 RBC | 0 WBC 157 RBC | 10 WBC 15 RBC | NA |
Table II summarizes the clinical and laboratory data at the time each patient experienced neurotoxicity. Of note, all five patients had hypertension, 2/5 had elevated cyclosporine levels near the time of symptoms, 4/5 had some degree of liver dysfunction as indicated by either elevation of the liver enzymes or abnormalities in their coagulation tests, and 2/5 had acute renal failure. All five patients had some prodromal mental status changes (either somnolence, decreased speech, or delirium).
Table II. Characteristics at Onset of Toxicity.
| Data | Units | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 |
|---|---|---|---|---|---|---|
| Time from Diagnosis | Days | 6 | 41 (5 days from disease reactivation) | 42 | 5 | 25 |
| Duration of Cyclosporine Therapy | Days | 6 | 41 | 20 | 5 | 25 |
| Prodromal Symptoms | NA | AMS* | AMS, somnolence, delirium | Tremors, AMS, somnolence, decreased speech | AMS, somnolence, decreased speech | AMS, somnolence |
| Blood Pressure | mm Hg | 148/75 | 145/90 | 145/62 | 155/90 | 175/106 |
| Ferritin | ng/ml | 40,312 | 603 | 2,755 | 10,297 | 529 |
| AST/ALT | U/L | 808/315 | 459/132 | 27/31 | 848/165 | 42/40 |
| PT/PTT | sec | 14.9/31.8 | 21.2/64.8 | 13.8/34.1 | 15.0/32.1 | 13/20.7 |
| Serum Cr | mg/dl | 4.0 | 4.2 | 0.6 | 0.8 | 0.4 |
| Cyclosporine Trough | ng/dl | 532 | 384 | 204 | 269 | 224 |
AMS = Altered Mental Status
Table III summarizes the outcome data. Four of the five patients had new onset seizures and MRI abnormalities consistent with PRES while the fifth had a fatal intracerebral hemorrhage. Overall, two patients (including the one with the intracerebral hemorrhage) died of disease or complications, one had permanent sensorineural hearing loss and two had complete neurological recovery.
Table III. Summary of Clinical Outcomes.
| Outcome | Incidence |
|---|---|
| Seizures | 4/5 |
| Radiographic Evidence of PRES | 4/5 |
| Intracranial Hemorrhage | 1/5 |
| Sensorineural Hearing Loss | 1/5 |
| Full Neurological Recovery | 2/5 |
| Death | 2/5 |
MRI studies obtained within twenty-four hours of seizure activity in four patients revealed significant symmetric areas of increased signal in the cerebellum and subcortical white matter on the fluid-attenuated inversion recovery (FLAIR) sequences. Two patients also showed areas of increased signal in the corpus collosum and basal ganglia. No diffusion restriction or enhancement was seen, suggesting transient vasogenic edema consistent with PRES. Follow-up MRIs were obtained at varying intervals in all four patients and showed resolution of the abnormal imaging findings.
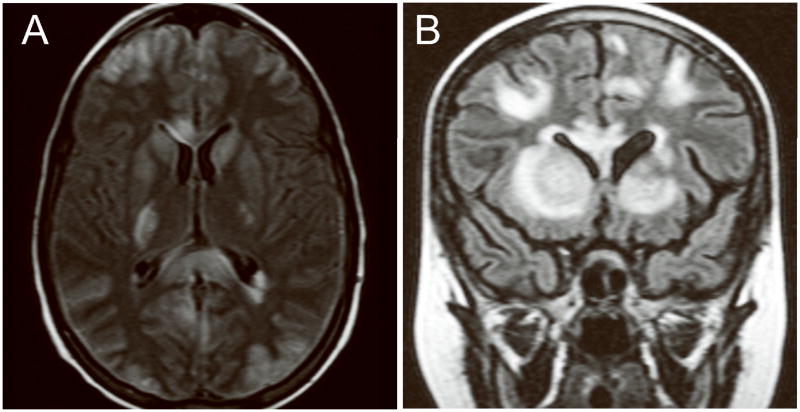
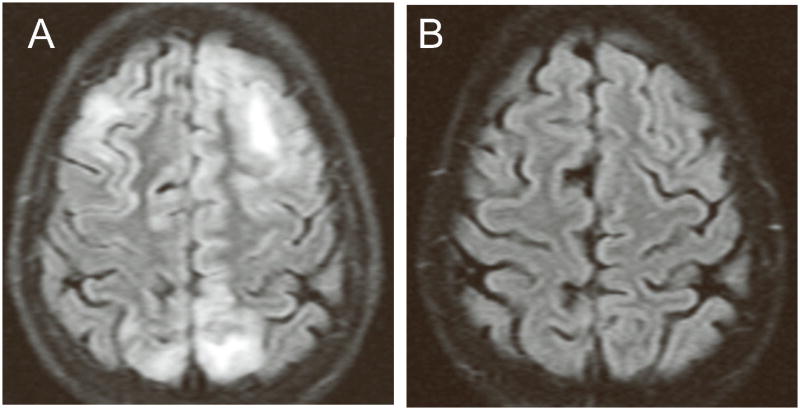
Figures 1A and 1B show the axial and coronal MRI images from Patient 2 and Patient 3, respectively, and illustrate the areas of increased FLAIR signal in the basal ganglia and sub-cortical white matter. Figures 2A and 2B show the imaging results for Patient 4. Figure 2A is from the FLAIR sequence of an MRI one day after she developed seizures. Figure 2B, obtained 12 days later, shows complete resolution of the abnormal signal increase.
Figure 1.
MRI images illustrative of PRES. A: Axial images from Patient 2 showing increased FLAIR signal in the basal ganglia and sub-cortical white matter. B: Coronal images from Patient 3 also showing FLAIR signal abnormalities in the basal ganglia and sub-cortical white matter.
Figures 2.
MRI images illustrating the rapid resolution of the imaging abnormalities associated with PRES. A: FLAIR sequence of an MRI one day after Patient 4 developed seizures showing increased signal in the sub-cortical white matter. B: Follow-up images obtained 12 days later showing complete resolution of the abnormal signal increase.
Table IV compares the study group (treated after April 2004) to the reference group (treated between August 2001 and March 2004). As the shows the study group had a higher incidence of neurotoxicity. However, the differences between the two groups did not quite reach statistical significance (p= 0.11) with this small sample size. Table III also shows that the characteristics of these two groups in terms of age and gender were somewhat different. The impact of these differences is not known.
Table IV. Comparison of Neurotoxicity in Study Group to Reference Group.
| Group | Study | Reference |
|---|---|---|
| Treatment Dates | 4/2004-10/2007 | 8/2001-3/2004 |
| Treatment Protocol | HLH-2004 or HLH-94 with early introduction of cyclosporine | HLH-94 |
| Number of Patients | 17 | 15 |
| Male/Female | 5/12 | 9/6 |
|
Age (yrs) Range Median |
0.1-16 4.9 |
0.2-12 1.6 |
|
CNS Status at Diagnosis (CNS Dz +/-) |
4/13 | 3/12 |
|
Survival (Alive/Deceased) |
7/10 | 8/7 |
|
Neurotoxicity (Present/Absent) |
5/12 | 1/14 |
Pharmacokinetic Modeling
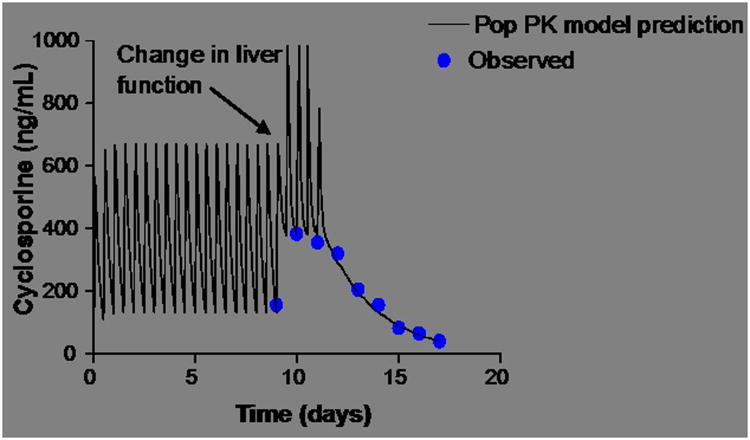
Pharmacokinetic modeling was used to analyze the significant change in cyclosporine concentration experienced by Patient #2. In this patient the cyclosporine concentration increased from 156 ng/mL to 384 ng/mL within 24 hours. Figure 3 shows the concentration time profile. As illustrated in the figure, the half-life of the drug increased from approximately 5 hours to 42 hours. None of the concomitant medications the patient was receiving have any documented interactions with cyclosporine that could explain such a change. Since the metabolism of cyclosporine occurs in the liver and is mediated by CYP3A4, the most likely explanation appears to be HLH related liver injury.
Figure 3.
Effect of hepatic dysfunction on cyclosporine exposure for Patient 2. Pop PK modeling with MW/PHARM (MediWare, Groningen, the Netherlands). As shown by the data, the half-life of the drug increased from approximately 5 hours to 42 hours.
Discussion
At our institution, the increase in neurotoxicity during HLH treatment has coincided with the introduction of cyclosporine earlier into the HLH treatment regimen. Cyclosporine, a calcinuerin inhibitor used for immunosuppression, clearly has an important role in the treatment of HLH. However, its use is not without the risk of side effects. It is estimated that 10-28% of patients who receive cyclosporine experience some neurotoxic side effect.[8] The neurotoxic effects of cyclosporine are usually mild but encompass a wide-rage of symptoms including mild upper extremity tremors, mental status changes, seizures, and sensory losses including deafness.[9-12] Episodes of acute intracranial hemorrhage have also been reported.[9,13,14]
One of the neurotoxicities associated with cyclosporine and other calcineurin inhibitors is posterior reversible encephalopathy syndrome (PRES), a poorly understood clinical syndrome. Features associated with this syndrome include headaches, vomiting, confusion, seizures, cortical blindness and other visual abnormalities and motor signs.[15] PRES has been reported in association with a variety of conditions including sepsis, hypertension, lupus, renal disease, and sickle cell disease as well as with numerous medications.[16-19]. PRES is also a well documented side effect of the immunosuppressive therapy used after both solid organ and bone marrow transplants.[20,21]
Estimates of the overall incidence of PRES vary widely. In the largest review of PRES in solid organ transplant patients (over 4000 patients included) the incidence of was 0.49%.[20] In a series of bone marrow transplant patients the overall incidence was 1.6% but higher in matched-unrelated, mismatched-related, and cord-blood transplants with incidences ranging from 3.5% to 7.1%.[21] The incidence of PRES in HLH patients is unkown, but case reports of the syndrome have been reported.[22]
Patients with PRES typically have findings on MRI of hyperintensity of the white matter on T2-weighted images and FLAIR sequences, suggestive of edema, typically in the posterior regions of the cerebral hemispheres.[8,23] The imaging changes however, can also involve other cerebral areas, the brain stem or the cerebellum.[15] These findings are distinct from those typically associated with HLH. In contrast, frequent findings associated with HLH CNS disease include periventricular white-matter signal abnormalities, cerebral volume loss, enlargement of ventricles and extra-axial fluid spaces, necrotic areas and multiple enhancing bilateral hemispheric lesions with edema.[4,5]
Our series suggests that patients with HLH may be at high risk for PRES and other forms of neurotoxicity. In the period we reviewed 24% (4/17) of patients treated for HLH had PRES and 29% (5/17) had severe neurologic side effects. Although this is only a small series from a single institution, the rate of PRES and other severe neurologic side effects appears to be higher in HLH patients than in other groups at risk for PRES.[20,21]
HLH patients have several factors that may predispose them to neurotoxicity. HLH patients can have significant liver dysfunction which could interfere with cyclosporine metabolism. Cyclosporine is extensively metabolized by CYP3A4 in the liver. Only 0.1% of a cyclosporine dose is eliminated in the urine as unchanged drug; the remainder is eliminated as metabolites.[24] Consequently, in the case of liver dysfunction, supratherapeutic drug levels could be achieved and the risk of toxicity increased. Patient 2 in our series experienced a dramatic change in her ability to metabolize cyclosporine that was most probably disease related and likely led to her neurotoxicity. Two of the other patients we report also had significant liver dysfunction, as indicated either by elevated enzymes or abnormal coagulation, immediately prior to the onset of neurotoxicity.
Hypertension also appears to play a role in the onset of HLH treatment related neurotoxicity. The combination of cyclosporine and dexamethasone used to treat HLH dramatically increases the risk of patients becoming hypertensive. None of the five patients reported in this series had hypertension at baseline. However, at the time they experienced neurotoxicity all five patients were hypertensive (at least one blood pressure measurement greater than the 95th percentile for age within twenty-four hours of the onset of symptoms).
The blood-brain barrier in HLH patients may also be affected by the extreme pro-inflammatory state which characterizes the disease. As a result, newly diagnosed patients and patients with reactivated disease who have the greatest amount of inflammation, as reflected by elevated ferritin levels, might be at greater risk for neurotoxicity. Patients 1, 2, and 4 in our series experienced toxicity either near the time of diagnosis or at the time of reactivation.
Of note, all the patients experiencing toxicity were older than six years of age at diagnosis. This is older than the mean age in either study or reference groups of patients at our institution. This suggests that age may also be a risk factor for neurotoxicity. However, we are not aware of a mechanism that explains this finding.
The cause of neurotoxicity in patients with HLH is almost certainly multifactorial. Elevated blood pressure, liver dysfunction, and inflammation all likely play some role. The incidence of neurotoxicity in HLH for patients being treated on HLH-2004 appears to be significant. As a result, neurologic status should be closely monitored in patients being treated on HLH-2004 and modifiable risk factors such as hypertension should be managed aggressively. If larger studies validate our observations, it will be important to determine if up-front cyclosporine in HLH protocols confers a survival benefit that outweighs the risk of increased neurotoxicity.
References
- 1.Henter JI, Elinder G, Soder O, et al. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80(4):428–435. doi: 10.1111/j.1651-2227.1991.tb11878.x. [DOI] [PubMed] [Google Scholar]
- 2.Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367–2373. doi: 10.1182/blood-2002-01-0172. [DOI] [PubMed] [Google Scholar]
- 3.Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. 1997;130(3):358–365. doi: 10.1016/s0022-3476(97)70196-3. [DOI] [PubMed] [Google Scholar]
- 4.Haddad E, Sulis ML, Jabado N, et al. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood. 1997;89(3):794–800. [PubMed] [Google Scholar]
- 5.Fitzgerald NE, MacClain KL. Imaging characteristics of hemophagocytic lymphohistiocytosis. Pediatr Radiol. 2003;33(6):392–401. doi: 10.1007/s00247-003-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983;140(3):221–230. doi: 10.1007/BF00443367. [DOI] [PubMed] [Google Scholar]
- 7.Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 8.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5):313–326. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 9.Shah AK. Cyclosporine A neurotoxicity among bone marrow transplant recipients. Clin Neuropharmacol. 1999;22(2):67–73. doi: 10.1097/00002826-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Marioni G, Perin N, Tregnaghi A, et al. Progressive bilateral sensorineural hearing loss probably induced by chronic cyclosporin A treatment after renal transplantation for focal glomerulosclerosis. Acta Otolaryngol. 2004;124(5):603–607. doi: 10.1080/00016480410016225. [DOI] [PubMed] [Google Scholar]
- 11.Arinsoy T, Akpolat T, Ataman M, et al. Sudden hearing loss in a cyclosporin-treated renal transplantation patient. Nephron. 1993;63(1):116–117. doi: 10.1159/000187158. [DOI] [PubMed] [Google Scholar]
- 12.Min DI, Ku YM, Rayhill S, et al. Sudden hearing loss associated with tacrolimus in a kidney-pancreas allograft recipient. Pharmacotherapy. 1999;19(7):891–893. doi: 10.1592/phco.19.10.891.31562. [DOI] [PubMed] [Google Scholar]
- 13.Teksam M, Casey SO, Michel E, et al. Subarachnoid hemorrhage associated with cyclosporine A neurotoxicity in a bone-marrow transplant recipient. Neuroradiology. 2001;43(3):242–245. doi: 10.1007/s002340000475. [DOI] [PubMed] [Google Scholar]
- 14.Mori A, Tanaka J, Kobayashi S, et al. Fatal cerebral hemorrhage associated with cyclosporin-A/FK506-related encephalopathy after allogeneic bone marrow transplantation. Ann Hematol. 2000;79(10):588–592. doi: 10.1007/s002770000192. [DOI] [PubMed] [Google Scholar]
- 15.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 16.Bartynski WS, Boardman JF, Zeigler ZR, et al. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27(10):2179–2190. [PMC free article] [PubMed] [Google Scholar]
- 17.Parameswaran BK, Krishnan PR, Al Dossary J. Recurrent posterior reversible encephalopathy syndrome in a patient with sickle cell disease. Ann Saudi Med. 2007;27(3):206–211. doi: 10.5144/0256-4947.2007.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hourani R, Abboud M, Hourani M, et al. L-asparaginase-induced posterior reversible encephalopathy syndrome during acute lymphoblastic leukemia treatment in children. Neuropediatrics. 2008;39(1):46–50. doi: 10.1055/s-2008-1076740. [DOI] [PubMed] [Google Scholar]
- 19.Nagel S, Kohrmann M, Huttner HB, et al. Linezolid-induced posterior reversible leukoencephalopathy syndrome. Arch Neurol. 2007;64(5):746–748. doi: 10.1001/archneur.64.5.746. [DOI] [PubMed] [Google Scholar]
- 20.Bartynski WS, Tan HP, Boardman JF, et al. Posterior Reversible Encephalopathy Syndrome After Solid Organ Transplantation. AJNR Am J Neuroradiol. 2008 doi: 10.3174/ajnr.A0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong R, Beguelin GZ, de Lima M, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003;122(1):128–134. doi: 10.1046/j.1365-2141.2003.04447.x. [DOI] [PubMed] [Google Scholar]
- 22.Yakushijin K, Mizuno I, Sada A, et al. Cyclosporin neurotoxicity with Epstein-Barr virus-associated hemophagocytic syndrome. Haematologica. 2005;90(3):ECR11. [PubMed] [Google Scholar]
- 23.Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplantation Reviews. 2006;20(1):49–60. [Google Scholar]
- 24.Buchler M, Johnston A. Seeking optimal prescription of cyclosporine ME. Ther Drug Monit. 2005;27(1):3–6. doi: 10.1097/00007691-200502000-00002. [DOI] [PubMed] [Google Scholar]