Abstract
A number of cyclic and linear peptides containing various combinations of amino acids were evaluated for their Src kinase inhibitory potency. Among all the peptides, cyclic decapeptide C[RW]5 containing alternative arginine (R) and tryptophan (W) residues was found to be the most potent Src kinase inhibitor. C[RW]5 showed higher inhibitory activity (IC50 = 2.8 µM) than C[KW]5, L(KW)5, C[RW]4, and C[RW]3 with IC50 values of 46.9, 69.1, 21.5, and 25.0 µM, respectively, as determined in a fluorescence intensity-based assay. Thus, the cyclic nature, the presence of arginine, ring size, and the number of amino acids in the structure of the peptide were found to be critical in Src kinase inhibitory potency. The IC50 value of C[RW]5 was found to be 0.8 µM in a radioactive assay using [γ-32P]-ATP and polyE4Y as the substrate. C[RW]5 was a non-competitive Src kinase inhibitor, showing approximately 4-fold more selectivity towards Src than Abl.
Protein tyrosine kinases (PTKs) are enzymes that catalyze the transfer of the γ-phosphate group from ATP to the hydroxyl group in tyrosine residues in proteins. Src family kinases (SFKs) including Src, Yes, Fyn, Fgr, Hck, Lck, Blk, Lyn, and Frk are non-receptor tyrosine kinases that play critical roles in several cellular signaling pathways. SFKs contain five distinct functional domains from the N- to C-terminals, including fatty acid acylation, Src homology 3 (SH3), Src homology 2 (SH2), kinase (containing ATP and substrate binding sites), and a C-terminal regulatory domain.1 Src contributes a significant role in cell proliferation, differentiation, and motility in many normal cell lines.2 Overexpression of Src kinase is observed in different types of cancer cells.3 Thus, the design of potent Src kinase inhibitors has become a subject of major interest in drug discovery.4, 5 Most of the attention has been on designing Src kinase inhibitors through blocking Src-dependent substrate phosphorylation. The kinase domain inhibitors are designed to inhibit the binding of ATP (ATP binding site inhibitors)6, 7 or the protein substrate (substrate binding site inhibitors).8–10
Peptide-based inhibitors with enhanced specificity can be designed and synthesized to target conserved regions of PTKs, such as substrate binding site, SH3 domain, and the SH2 domain. The sequence of amino acids in the structure of the peptide can mimic the PTK substrates that target non-conserved regions of PTKs. Thus, peptides can be designed to be more selective compared to small molecule inhibitors targeting conserved ATP binding site of PTKs. Furthermore, the diversity of amino acids in the peptide sequence can be used to generate diversified structures.
In general, linear peptides adopt highly flexible conformations in a solution that could lead to weak interactions with the desired target. The optimized linear peptides usually show PTK inhibitory potency in the micromolar range.11 Moreover, linear peptides have limited stability and low bioavailability because of proteolytic degradation by natural cellular protease. Thus, further structural modification is required to enhance the stability, potency, and selectivity of the linear peptides.
Cyclization strategy has been shown to be effective in developing more potent and stable peptidic and peptidomimetic agents.12–15 Cyclization of peptides has been commonly used to reduce the conformational freedom of the peptides. Introducing conformational constraints in peptides make them more stable towards proteases. In addition, cyclization often results in higher receptor binding affinity towards specific receptors possibly by decreasing unfavorable entropic effects.16 For example, G7-18NATE peptide (cyclo-[CH2COWFEGYDNTFPC]-amide) is a non-phosphorylated tyrosine-containing peptide that was developed to target specifically the SH2 domain of the growth factor receptor bound protein 7 (Grb7).17 We have previously reported the synthesis of a number of cyclic peptides, including analogues of Ac-CIYKYY and GpYEEI as Src kinase inhibitors and Src SH2 domain binding ligands, respectively. Conformational constrained peptides of CIYKYY significantly improved the inhibitory potency of the peptides against active Src kinase when compared with the corresponding linear peptide.18 Conformationally constrained analogs of GpYEEI also showed significantly higher binding affinity towards the Src SH2 domain.16
However, a detailed mechanistic study of the role of hydrophobicity, charge, and amino acid sequence on their Src inhibitory potency of cyclic peptides remained elusive. In continuation of our efforts to develop new cyclic peptide scaffolds as Src kinase inhibitors,11, 19 herein we report the design and evaluation of cyclic peptides containing charged and hydrophobic residues for potential applications as a new class of Src kinase inhibitors. The hydrophobic residues such as aromatic side chains of phenylalanine and tryptophan were expected to occupy and/or interact with a large hydrophobic pocket19 in the ATP binding site. Furthermore, the indole ring of tryptophan could mimic the adenine base of ATP in binding to the nucleotide binding site.
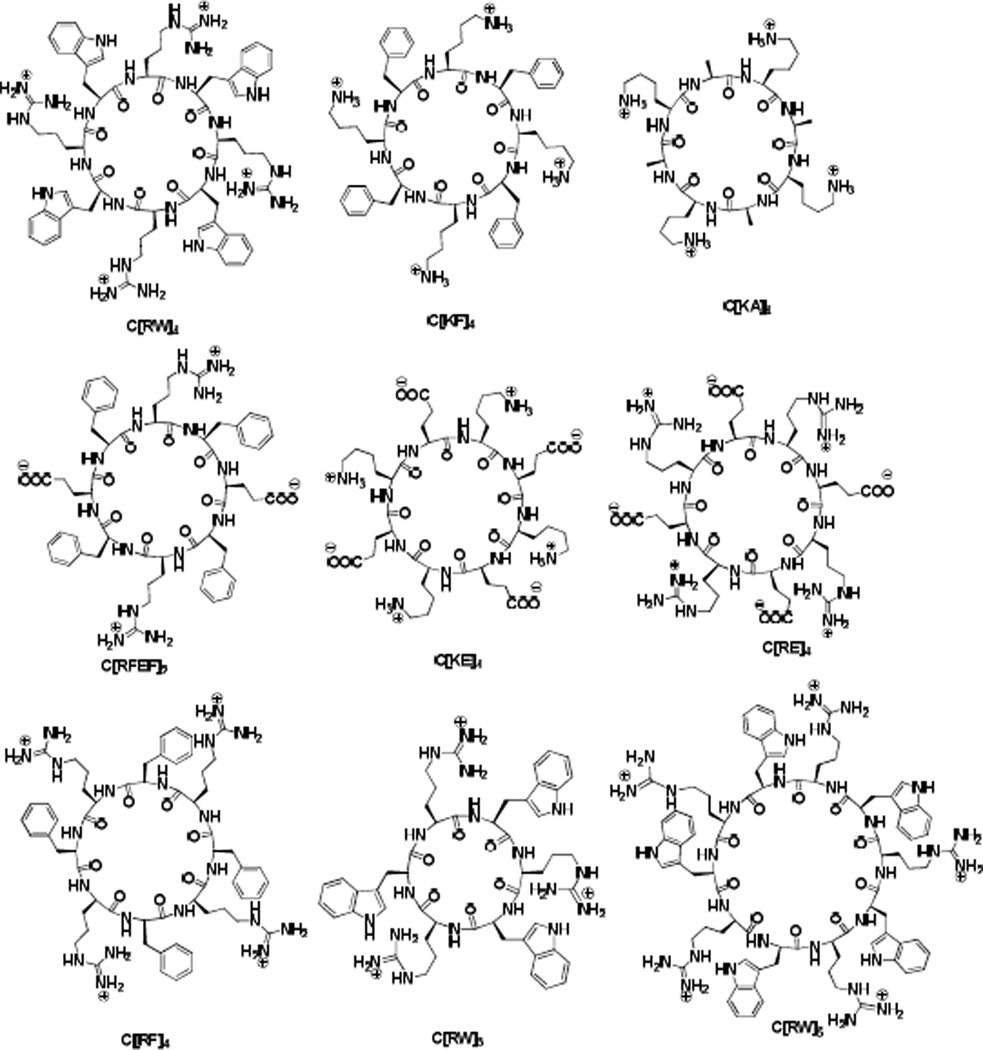
Seven cyclic octapeptides with various combination of L-amino acids, C[RW]4, C[KF]4, C[KA]4, C[RFEF]2, C[KE]4, C[RE]4, and C[RF]4 (Figure 1), were synthesized by employing 9-fluorenylmethyloxycarbonyl (Fmoc)-based chemistry according to our previously reported procedure.20 The design and selection of the cyclic peptides was based on the presence of hydrophobic (e.g., W, F, A) and charged residues (e.g., K, R, E).
Figure 1.
Chemical structures of synthesized cyclic peptides.
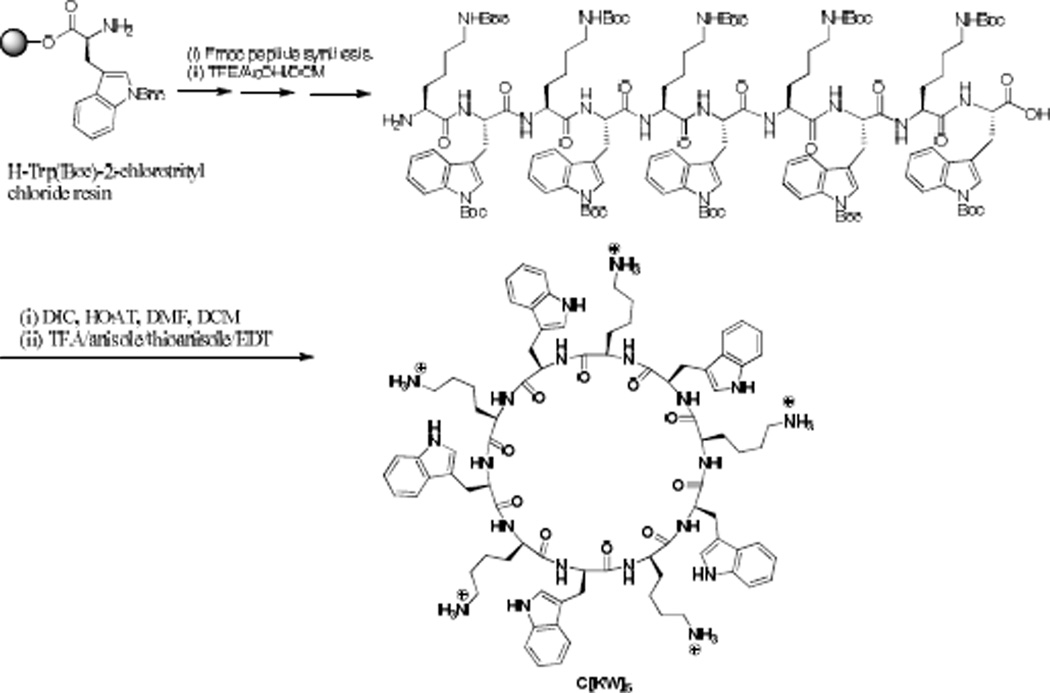
As a representative example, the synthesis of cyclic [KW]5 is depicted in Scheme 1. The linear peptide (KWKWKWKWKW) was assembled on the H-Trp(Boc)-2-chlorotrityl chloride resin. After the final deprotection of the N-terminal Fmoc group by piperidine in DMF (20% v/v), the side chain-protected peptide was cleaved from trityl resin in the presence of the cleavage cocktail acetic acid/TFE/DCM(1:2:7 v/v/v,) at room temperature to yield the side chain-protected linear peptide that was used directly for the cyclization reaction. The dried crude linear protected peptide was dissolved in DMF/DCM (5:1 v/v). HOAt (4 equiv) and DIC (4.5 equiv) were added to the mixture, and the solution was stirred for 4 h. After the cyclization reaction completed the solvents were removed under reduced pressure. Freshly prepared cleavage cocktail, reagent R, TFA/thioanisole/EDT/anisole (90:5:3:2 v/v/v/v) was added to the crude product. The mixture was stirred at room temperature for 2 h. After precipitation of crude peptide in cold diethyl ether (75 mL, Et2O), the crude peptide was lyophilized and purified by reversed-phase Hitachi HPLC (L-2455) on a Phenomenex Prodigy 10 µm ODS reversed-phase column (2.1 cm × 25 cm) using a gradient system to yield cyclic [KW]5. A similar procedure was employed for the synthesis of other cyclic peptides by using appropriate resins and protected amino acids.
Scheme 1.
Solid-phase synthesis of [KW]5.
All L-cyclic peptides were evaluated for their Src kinase inhibitory activity (Table 1). The effect of peptides on the activity of Src kinase was assessed by Transcreener® ADP2 Fluorescence Intensity (FI) Assay. The results of Src kinase inhibitory activity of cyclic peptides are shown in Table 1 (entries 1–7). Cyclic octapeptides [KF]4, [KA]4, [RFEF]2, and [KE]4 exhibited no Src kinase inhibition at the highest concentration 300 µM. C[RF]4, C[RW]4, and C[RE]4 exhibited modest inhibition of Src kinase with IC4 = 21.5–52.6 µM). Among these three peptides, C[RW]4 showed the highest inhibition of Src kinase with an IC50 value of 21.5 µM, suggesting that tryptophan residues contributes in Src kinase inhibition.
Table 1.
Src kinase inhibitory activity of peptides.
| Entry | Peptide | IC50a (µM) |
|---|---|---|
| 1 | Cyclic [KF]4 | 1567.6 |
| 2 | Cyclic [KA]4 | ≥300 |
| 3 | Cyclic [RFEF]2 | ≥300 |
| 4 | Cyclic [KE]4 | ≥300 |
| 5 | Cyclic [RE]4 | 52.6 |
| 6 | Cyclic [RF]4 | 31.3 |
| 7 | Cyclic [RW]4 | 21.5 |
| 8 | Cyclic [RW]3 | 25.0 |
| 9 | Cyclic [RW]5 | 2.8 |
| 10 | Cyclic [KW]5 | 46.9 |
| 11 | Linear (RW)5 | 7.1 |
| 12 | Linear (KW)5 | 69.1 |
| 13 | Staurosporine | 0.6 |
| 14 | PP2 | 0.5 |
The concentration of the compounds that inhibited the enzyme activity by 50%. All the experiments were carried out in triplicate.
To study the effect of the ring size on the Src kinase inhibitory activity, two other cyclic peptides namely C[RW]3 and C[RW]5 (Table 1, entries 8 and 9) containing six and ten alternative arginine and tryptophan residues were synthesized, and their Src kinase inhibitory activities were compared with C[RW]4. C[RW]3 exhibited slightly lower Src kinase inhibitory activity (IC50 = 25.0 µM) than C[RW]4. On the other hand, decapeptide C[RW]5 showed 9-fold higher Src kinase inhibitory activity (IC50 = 2.8 µM) versus C[RW]4, suggesting that the ring size was a critical factor in enhancing Src kinase inhibition in C[RW] series.
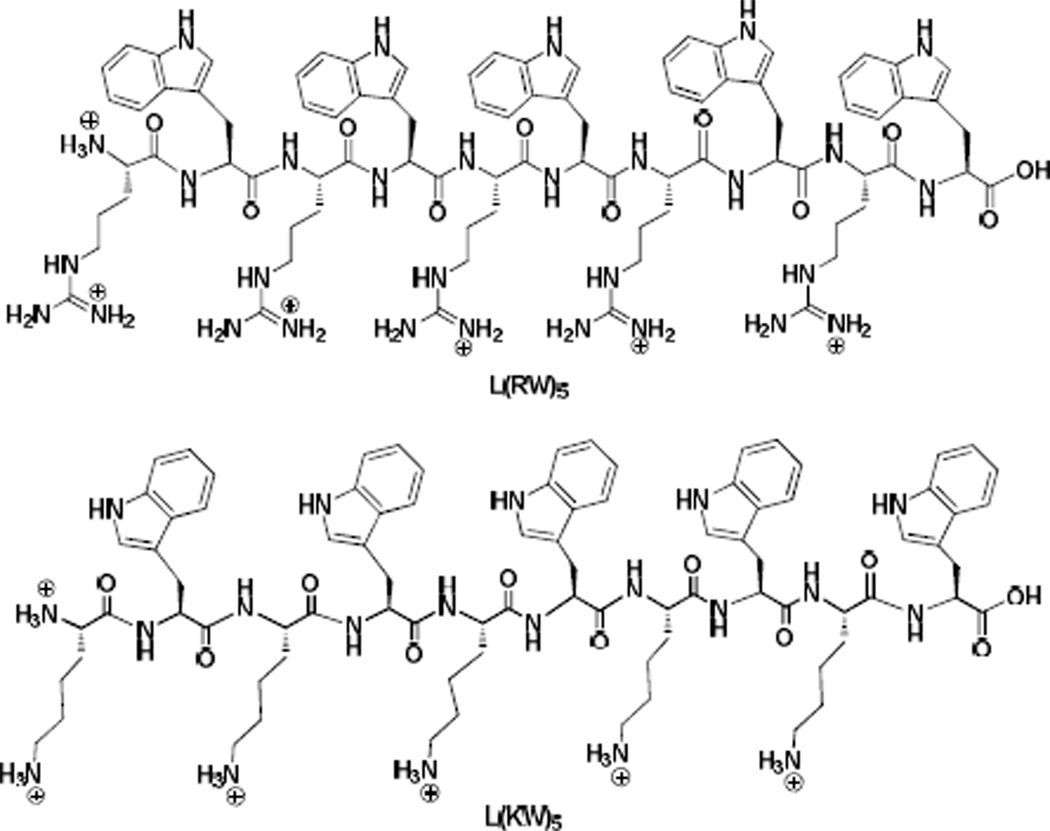
To investigate the role of the constrained cyclic nature of the peptide, the corresponding linear (RW)5 (L(RW)5) (Figure 2) was designed and synthesized. C[RW]5 and L(RW)5 (Table 1, entries 9 and 11) share the same number and sequence of amino acids. C[RW]5 and L(RW)5 represent constrained and flexible linear peptides, respectively. L(RW)5 inhibited Src kinase with an IC50 value of 7.1 µM, showing 2.5-fold lower inhibition than C[RW]5 (IC50 = 2.8 µM). These data suggest that the cyclic nature of the peptide contributes to improving Src kinase inhibitory activity.
Figure 2.
Chemical structures of L(RW)5 and L(KW)5.
To get a better understanding of the effect of the arginine on the Src kinase inhibitory activity of C[RW]5 and L(RW)5, two homologues cyclic and linear peptides, C[KW]5 and L(KW)5 (Figure 2) were synthesized by replacing the arginine with lysine and their Src kinase inhibitory activities were evaluated. C[KW]5 and L(KW)5 inhibited the Src kinase activity with IC50 values of 46.9 µM and 69.1 µM (Table 1, entries 11 and 12), respectively, that are significantly lower than the corresponding C[RW]5 (IC50 = 2.8 µM)) and L(RW)5 (IC50 = 7.1 µM)). These data suggest that the presence of arginine is required for optimal Src inhibitory activity.
In order to determine the selectivity of C[RW]5 toward Src kinase, the IC50 values of the peptide were ascertained in two other divergent non-receptor protein tyrosine kinases, Abl and Csk in a radioactive assay using [ -32P]-ATP and polyE4Y as the substrate. Enzyme activity and inhibition were characterized using the following methods, adapted from previous work.14,15,21 Csk and Src activity levels were measured from incubations with polyE4Y (1 mg/mL). CrkL (0.5 mg/mL) was used as a substrate for Abl. C[RW]5 was dissolved in 10% DMSO/90% kinase buffer solution (KB10) and diluted in series as needed. IC50 measurement assay reactions varied in C[RW]5 concentration. Competition assays varied ATP concentration while maintaining constant concentrations of inhibitor, enzyme and substrate. Csk and Abl enzymes were incubated with fixed and excess concentration of substrate in a series of reactions in the presence of different concentrations of C[RW]5 to calculate the turnover. The measured activity was plotted versus the concentration of inhibitor to visualize graphically. The IC50 values of C[RW]5 were found to be 0.8 µM,, 00..88 µM, and 3.1 µM for Src, Csk, and Abl kinases, respectively, in the radioactive assay. These data suggested that the C[RW]5 is approximately 3.9-fold more selective towards Src and Csk versus Abl.
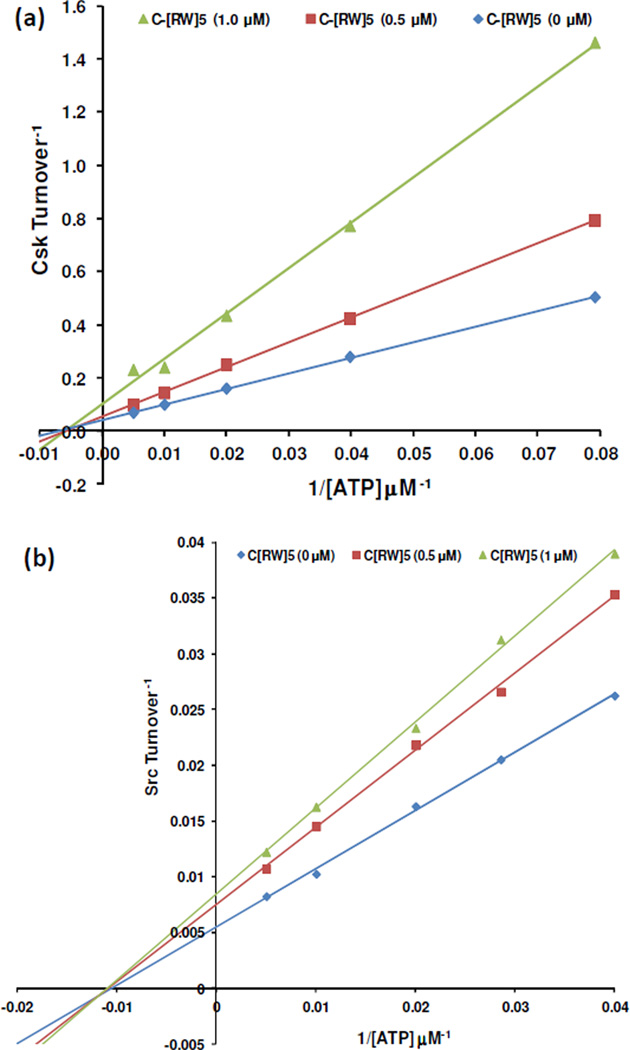
To evaluate whether the cyclic peptide was competing with ATP, further kinetic analysis was conducted. Csk and Src were incubated with a range of concentrations of ATP while substrate turnover was recorded in the presence or absence of the inhibitor. The plot showed that C[RW]5 followed a noncompetitive inhibition pattern against ATP for both Csk and Src (Figures 3). A double-reciprocal plot of the obtained data, followed by linear regression analysis (r2 ≥ 0.997) revealed that the Kcat/Km declined for both enzymes in the presence of the peptide. For example, Kcat/Km was 1.91, 1.44, and 1.34 min−1.µM−1 in the presence of 0, 0.5, and 1 µM of the inhibitor, respectively, suggesting that the catlytic efficiency of the enzyme was reduced in the presence of the peptide. The noncompetitive inhibition pattern indicated that Src and Csk retained a significant capacity to bind ATP. These data suggest that C[RW]5 is using other potential sites in the catalytic domain for generating kinase inhibitory activity possibly by binding to an area of the kinase domain with minimal overlap with the ATP binding site, such as ATP binding site adjacent domains. More mechanistic studies are required to determine the mode of inhibition and interaction of positively charged and hydrophobic amino acids in C[RW]5 with amino acids in the kinase domain.
Figure 3.
Pattern of inhibition of (a) Csk and (b) Src by C[RW]5; Lineweaver-Burk plot of 1/V versus 1/ATP with varying concentration of C[RW]5 shows linear noncompetitive inhibition.
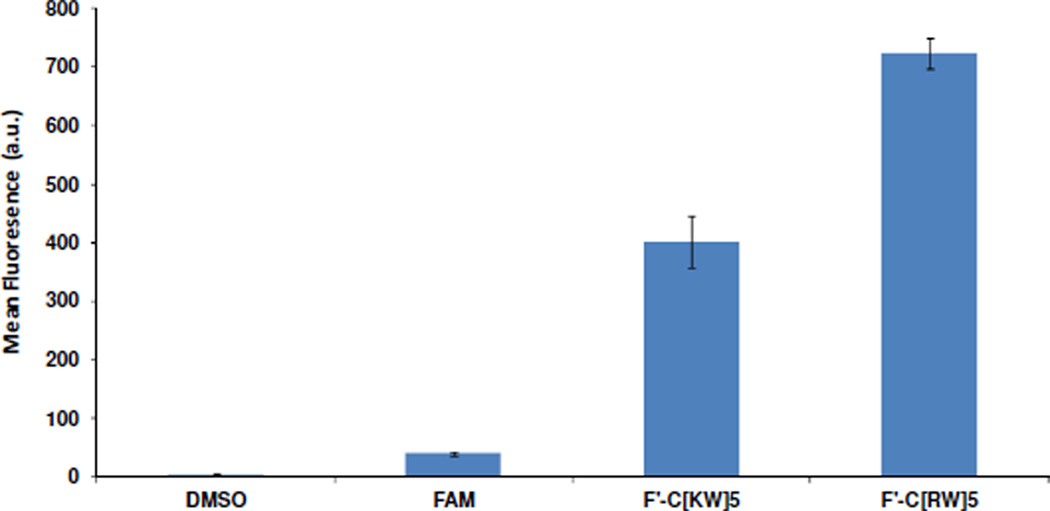
To evaluate the cellular permeability of these two peptides, a comparative intracellular uptake experiment was carried out by using synthesized fluorescently labeled C[RW]5 and C[KW]5 namely F•-C[RW]5 and F•-C[KW]5 and Fluorescence Activated Cell Sorter (FACS). The labeled compounds were incubated with human ovarian carcinoma (SK-OV-3) cells for 1 h at 37 °C. The cellular uptake of F•-C[WR]5 was found to be approximately 1.8-fold higher than that of F•-C[WK]5 (Figure 4). These results suggest that both C[RW]5 and C[KW]5 that contain similar number of amino acids have efficient cellular permeability, but the presence of the arginine in C[RW]5 improves the cellular uptake when compared to the corresponding peptide C[KW]5 containing lysine.
Figure 4.
Cellular uptake of fluorescently-labeled peptides F′-C[KW]5 and F′-C[RW]5 (5 µM) after 1 h incubation.
In conclusion, a new class of cyclic peptides containing different combinations of amino acids was evaluated for Src kinase inhibitory activity. To the best of our knowledge, this is the first report of cyclic peptides containing hydrophobic and positively charged residues as Src kinase inhibitors. The combination of tryptophan and arginine showed the highest potency to inhibit the Src kinase activity compared to other peptides. The cyclic nature, ring size, and the presence of arginine and tryptophan residues were found to contribute significantly to the Src inhibitory activity. C[RW]5 with an IC50 value of 2.8 µM exhibited a significantly higher inhibitory potency compared to C[RW]4, L[RW]5, L(KW)5, and C[KW]4. C[RW]5 was found to be selective to Src versus Abl kinase. The peptide found to be a noncompetitive inhibitor to ATP. These data suggest that C[RW]5 can be used as a scaffold for designing Src kinase inhibitors with further structural modifications.
Supplementary Material
Acknowledgments
We acknowledge the financial support from National Science Foundation, Grant Number CHE 0748555, and American Cancer Society grant number RSG-07-290-01-CDD. We thank the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8 P20 GM103430-12 for sponsoring the core facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data including cyclic and linear peptide synthetic procedures and additional supporting data including mass spectra can be found in the online version of this article.
References and notes
- 1.Sicheri F, Moarefi I, Kuriyan J. Nature. 1997;385:602. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 2.Thomas SM, Brugge JS. Annu. Rev. Cell Dev. Biol. 1997;13:513. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 3.Warmuth M, Damoiseaux R, Liu U, Fabbro D, Gray N. Curr. Pharm. Des. 2003;9:2043. doi: 10.2174/1381612033454126. [DOI] [PubMed] [Google Scholar]
- 4.Parang K, Sun G. Expert Opin. Ther. Pat. 2005;15:1183. [Google Scholar]
- 5.Parang K, Sun G. Curr. Opin. Drug Discovery Dev. 2004;7:630. [PubMed] [Google Scholar]
- 6.Toledo LM, Lydon NB, Elbaum D. Curr. Med. Chem. 1999;6:775. [PubMed] [Google Scholar]
- 7.Schenone S, Bruno O, Ranise A, Bondavalli F, Brullo C, Fossa P, Mosti L, Menozzi G, Carraro F, Naldini A, Bernini C, Manetti F, Botta M. Med. Chem. Lett. 2004;14:2511. doi: 10.1016/j.bmcl.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Budde RJ, Obeyesekere NU, Ke S, McMurray JS. Biochim. Biophys. Acta. 1995;1248:50. doi: 10.1016/0167-4838(94)00232-6. [DOI] [PubMed] [Google Scholar]
- 9.Kamath JR, Liu R, Enstrom AM, Lou Q, Lam KS. J. Pept. Res. 2003;62:260. doi: 10.1046/j.1399-3011.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 10.Lam KS, Wu J, Lou Q. Int. J. Pept. Protein Res. 1995;45:587. doi: 10.1111/j.1399-3011.1995.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari RK, Parang K. Curr. Pharm. Design. 2012;18:2852. doi: 10.2174/138161212800672714. [DOI] [PubMed] [Google Scholar]
- 12.Budde RJ, Ke S, Levin VA. Cancer Biochem. Biophys. 1994;14:171. [PubMed] [Google Scholar]
- 13.Belches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Oncogene. 2001;20:1465. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Wang Y, Kumar A, Ye G, Parang K, Sun G. J. Med. Chem. 2006;49:7532. doi: 10.1021/jm061058c. [DOI] [PubMed] [Google Scholar]
- 15.Huang K, Wang YH, Brown A, Sun G. J. Mol. Biol. 2009;386:1066. doi: 10.1016/j.jmb.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam NH, Ye G, Sun G, Parang K. J. Med. Chem. 2004;47:3131. doi: 10.1021/jm040008+. [DOI] [PubMed] [Google Scholar]
- 17.Pero SC, Oligino L, Daly RJ, Soden AL, Liu C, Roller PP, Li P, Krag DN. J. Biol. Chem. 2002;277:11918. doi: 10.1074/jbc.M111816200. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Ye G, Wang Y, Lin X, Sun G, Parang K. J. Med. Chem. 2006;49:3395. doi: 10.1021/jm060334k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Wang Y, Lin X, Sun G, Parang K. ChemMedChem. 2007;2:1346. doi: 10.1002/cmdc.200700074. [DOI] [PubMed] [Google Scholar]
- 20.Mandal D, Nasrolahi Shirazi A, Parang K. Angew. Chem. Int. Ed. 2011;50:9633. doi: 10.1002/anie.201102572. [DOI] [PubMed] [Google Scholar]
- 21.Kemble DJ, Wang YH, Sun G. Biochem. 2006;45:14749. doi: 10.1021/bi061664+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.