Abstract
Background
Promising data regarding the safety and efficacy of gastric bypass surgery (GBS) as an option to address obesity in the transplant population are emerging. The data lack on how GBS may alter the pharmacokinetics (PK) of modern immunosuppression. The objective of this study was to describe the alterations in the PK of modern immunosuppressants and the GBS population.
Methods
Data are presented on six subjects who participated in this trial – four were on dialysis and two were renal transplant recipients. Dialysisdependent bypass subjects received a single dose of 6 mg of sirolimus, two 4-mg doses of tacrolimus and two 1000-mg doses of mycophenolate mofetil (MMF) over the 24-h study period. Transplant recipients continued their current regimen. Maximum plasma concentration (Cmax), time to reach the maximum plasma concentration (Tmax) and the area under the plasma concentration vs. time curve (AUC0–12 and AUC0–∞ where appropriate) were calculated for tacrolimus, sirolimus, mycophenolic acid (MPA) and mycophenolic acid glucuronide (MPAG).
Results
Significant inter-patient variability in the Cmax, Tmax and AUC of tacrolimus, sirolimus MPA and MPAG was observed. A notable difference in the AUC:dose ratio for tacrolimus was seen when comparing data with published data in the non-bypass population. Similar differences in PK were seen with sirolimus, MPA and MPAG.
Conclusions
When comparing the PK of sirolimus, tacrolimus, MPA and MPAG to published PK data in the non-bypass population, significant differences are observed. It is likely that transplant recipients with GBS would need higher doses of tacrolimus, sirolimus and MMF to provide similar exposure to a non-bypass patient.
Keywords: gastric bypass, immunosuppression, pharmacokinetics
The development of new immunosuppressive agents, such as tacrolimus, sirolimus and mycophenolate mofetil (MMF), have led to a significant decline in the incidence of acute rejection seen in kidney-transplant recipients (1). The lower rejection rates have corresponded to an increase in the number of successful transplants performed over time (2). Despite the lower rejection rates, kidney-transplant recipients are still at a high risk of developing complications from their multiple co morbid disease states. A United Network for Organ Sharing (UNOS) report from 2005 identified cardiovascular disease as the leading cause of death in deceased donor transplant recipients (3). Two recent studies have evaluated the potential impact of obesity on outcomes in renal transplant recipients (4, 5). Both studies showed that increasing body mass index (BMI) is associated with an increased risk for graft loss and delayed graft function (DGF). The results of the two trials are conflicting as to whether or not death, with a functioning graft, is influenced by obesity. The recent analysis by Gore et al. constructed a multivariate model which adjusted for common co morbidities present in the dialysis population. This analysis found that BMI did not influence the incidence of death with a functioning graft. The two studies had conflicting results on whether or not BMI impacted the incidence of early rejection. Recently, the American Heart Association identified obesity as an independent risk factor for the development of coronary heart disease (6). With hopes to minimize post-transplant cardiovascular risk, surgical weight loss may become a more frequently utilized option to address obesity.
A recent study on current trends in bariatric surgery reported that the annual number of bariatric procedures has increased from an estimated 13 365 in 1993 to 72 177 in 2002. It is projected that the number of bariatric procedures performed in the United States in the year 2010 may be as high as 218 000. Close to 90% of the bariatric procedures performed in 2002 were gastric bypasses (7). The roux-en-y gastric bypass involves both a restrictive and malabsorptive process. The restrictive portion of the procedure involves creating a small 20- to 30-mL gastric pouch in order to limit food intake. A roux limb is then made by dividing the small intestine anywhere from 100 to 250 cm beyond the ligament of treitz. The distal (alimentary) limb is then attached to the gastric pouch creating a stoma 1.5 cm in diameter. The narrow opening to the small intestine slows the emptying of the stomach and produces a sensation of early satiety. Altering the available surface area of the stomach and small intestine may adversely impact the absorption and metabolism of drugs. The resulting gastric pouch of 20–30 mL does not produce as much gastric acid as a whole stomach. The resulting increase in stomach pH may adversely impact the absorption of drugs that are dependent on an acidic environment for solubility and absorption. The roux-en-y procedure bypasses a significant portion of the stomach and small intestine resulting in a significantly smaller surface area available for drug absorption (8). We hypothesize that the altered gastric pH, in addition to the reduced surface area of the stomach and proximal intestine, may lead to a decrease in drug absorption. Similarly, the reduction in the surface area of the proximal intestine may lead to a decrease in drug metabolism in the gut, thereby resulting in increased serum drug concentrations.
To date, there have only been a few published case reports providing data on the pharmacokinetic (PK) alterations seen in medications in the gastric bypass or jejunoileal bypass population (9–13). Significant alterations in PK occurred more often in patients who underwent jejunoileal bypass rather than those who underwent gastric bypass. More importantly, published guidelines on how to appropriately dose modern-day immunosuppressants are lacking. An initial report of the gastric bypass experience from Cincinnati demonstrated that the dose requirement of cyclosporine increased by 33% after the gastric bypass procedure (13). One may assume that similar dose adjustments may be necessary with the administration of tacrolimus, sirolimus or MMF.
The Cincinnati experience on gastric bypass in patients with renal failure and kidney transplant was updated and published in 2004 (14). A more recent update and follow-up includes 51 gastric bypass recipients (15). Improvements in co morbid conditions, such as blood pressure, cholesterol, triglycerides and diabetes control were seen with weight loss. Additionally, five patients had improvements or stabilization in their renal dysfunction as a result of weight loss. We herein report on a pilot study designed to specifically address potential changes in the PK of immunosuppressive medications after gastric bypass surgery (GBS).
Methods
Patient selection
Nineteen potential candidates were identified for enrollment into this protocol. Candidates for enrollment were renal-transplant recipients who had previously undergone gastric bypass, or gastric bypass patients with ESRD who were scheduled to undergo transplant. Candidates for this study were female or male of any race aged 18–70 yr. Study subjects were excluded if they were taking any medications known to interact with MMF, sirolimus or tacrolimus, or if they had an allergy to the aforementioned medications. Candidates were also excluded if they were physically or mentally unable to comply with the study protocol. Candidates eligible for enrollment into this study fell into one of two distinct categories; gastric bypass recipients with end-stage renal disease (ESRD) pretransplant (13) or post-transplant (6). Of the 19 potential candidates, 10 subjects consented to participate in the study. Of the 13 ESRD candidates, five consented to participate in the trial. Five of the six post-transplant candidates consented to participate in the trial. Five of the potential candidates were unavailable or declined participation. Data from four subjects who consented to the trial will not be presented for the following reasons: one ESRD subject with an ileal bypass performed peritoneal dialysis overnight on a cycler – this subject’s data were omitted owing to the presence of these confounding factors on the PK data; data from three post-transplant subjects are not presented as two of them only received cyclosporine and the third lost intravenous (IV) access four h into the study. The remaining six subjects in which PK data are presented all underwent traditional roux-en-y gastric bypass. Details of the operative technique used in all subjects enrolled in this study are published elsewhere (14). The study was approved by the local Institutional Review Board and all subjects provided written informed consent prior to enrollment.
Immunosuppression
End-stage renal disease subjects, who were listed for transplant were given a 24-h regimen of immunosuppression consisting of sirolimus 6 mg orally at 08:00, MMF 1 g orally at 08:00 and at 20:00 and tacrolimus 4 mg orally at 08:00 and at 20:00. This regimen was chosen to resemble the current standard of care. Subjects who were transplant recipients remained on their maintenance regimen. Dosing regimens are detailed in Table 1.
Table 1.
Patient demographics
| Patient # | Age (yr) |
Time since bypass (yr) |
Type of bypass |
Wt (kg) |
BMI (kg/m2) |
BSA (m2) |
Scr (mg/dL) |
CrCl (mL/min) |
Immunosuppression |
|---|---|---|---|---|---|---|---|---|---|
| Pretransplant patients (dialysis dependent) | |||||||||
| 5 | 36.6 | 1.81 | roux-en-y | 99.0 | 32.0 | 2.1 | n/a | n/a | SRL 6 mg, MMF 1 g BID, FK 4 mg BID |
| 6 | 54.2 | 0.92 | roux-en-y | 93.0 | 33.1 | 2.0 | n/a | n/a | SRL 6 mg, MMF 1 g BID, FK 4 mg BID |
| 7 | 46.3 | 3.50 | roux-en-y | 92.2 | 27.9 | 2.1 | n/a | n/a | SRL 6 mg, MMF 1 g BID, FK 4 mg BID |
| 8 | 34.8 | 0.16 | roux-en-y | 91.0 | 31.5 | 2.0 | n/a | n/a | SRL 6 mg, MMF 1 g BID, FK 4 mg BID |
| Mean | 42.9 | 1.6 | 93.8 | 31.1 | 2.1 | ||||
| Post-transplant patients | |||||||||
| 2 | 47.6 | 2.94 | roux-en-y | 60.0 | 22.6 | 1.6 | 1.0 | 65.0 | MMF 1 gm BID/Pred 5 QD |
| 3 | 45.6 | 7.41 | roux-en-y | 138.6 | 51.0 | 2.4 | 2.6 | 70 | MMF 750 mg BID, FK 4 mg BID, Pred 7.5 QD |
| Mean | 46.6 | 5.2 | 99.3 | 36.8 | 2.0 | 1.8 | 67.5 | ||
BMI, body mass index; BSA, body surface area; MMF, mycophenolate mofetil; SRL, sirolimus; BID, twice daily; FK, tacrolimus; Pred., prednisone.
Study protocol
On the morning of the study, after an eight-h fast, subjects reported to the study site where a peripheral venous catheter was inserted. Each subject received the specific immunosuppressive medication as outlined at 08:00 and 20:00. Subjects continued to fast for an additional two h after their am and pm dosing. Two venous blood samples (6 mL) were drawn at 0.5, 1, 2, 3, 4, 6, 8, 12, 12.5, 13, 14, 15, 16, 18, 20 and 24 h postdose for sirolimus and 0.5, 1, 2, 3, 4, 6, 8 and 12 h postdose for each dose of MMF and tacrolimus.
Drug analysis
Tacrolimus and sirolimus concentrations in whole blood were determined using validated tandem mass-spectrometry (LC-MS/MS) assays (16). Plasma concentrations of mycophenolic acid (MPA) and mycophenolic acid glucuronide (MPAG) were determined with a validated HPLC assay according to a modification of the method of Shipkova et al. (17).
PK analysis
Pharmacokinetic analysis of concentration data for each administered dose of sirolimus, tacrolimus, mycophenolic mofetil (MPA and MPAG) was conducted using standard non-compartmental methods (WinNonlin Professional, Version 4.0.1; Pharsight Corp, Mountain View, CA, USA). Maximum concentration (Cmax) and time to reach the maximum concentration (Tmax) were determined by visual inspection of the plasma concentration time profiles. The area under the concentration vs. time curve (AUC0–12 or AUC0–24) was determined using the linear trapezoidal method up to the last measured concentration, and the extrapolated area was estimated by dividing the last measured concentration by the value of the elimination rate constant as calculated from the terminal portion of the concentration time curve (if estimable). For MPA, AUC0–∞ may not be well represented owing to the high inter-patient variability and enterohepatic recycling.
Results
Patient demographics
Data on six subjects who participated in the PK study will be presented. Four of the participants were ESRD subjects awaiting renal transplant and two were renal transplant recipients; all had undergone roux-en-y GBS. The mean age of the ESRD subjects was 42.9 yr compared with a mean age of 46.6 yr for the transplant recipients. The two groups were similar with regard to BMI and weight; the ESRD subjects had a mean BMI of 31.1 and a weight of 93.8 kg, and this was in comparison with a mean BMI of 36.8 and weight of 99.3 kg in the transplant recipients. The transplant recipients included in this analysis were on average 3.6 yr further out from their GBS than the ESRD subjects. Patient demographics are illustrated in Table 1.
Sirolimus PK
ESRD (single dose PK)
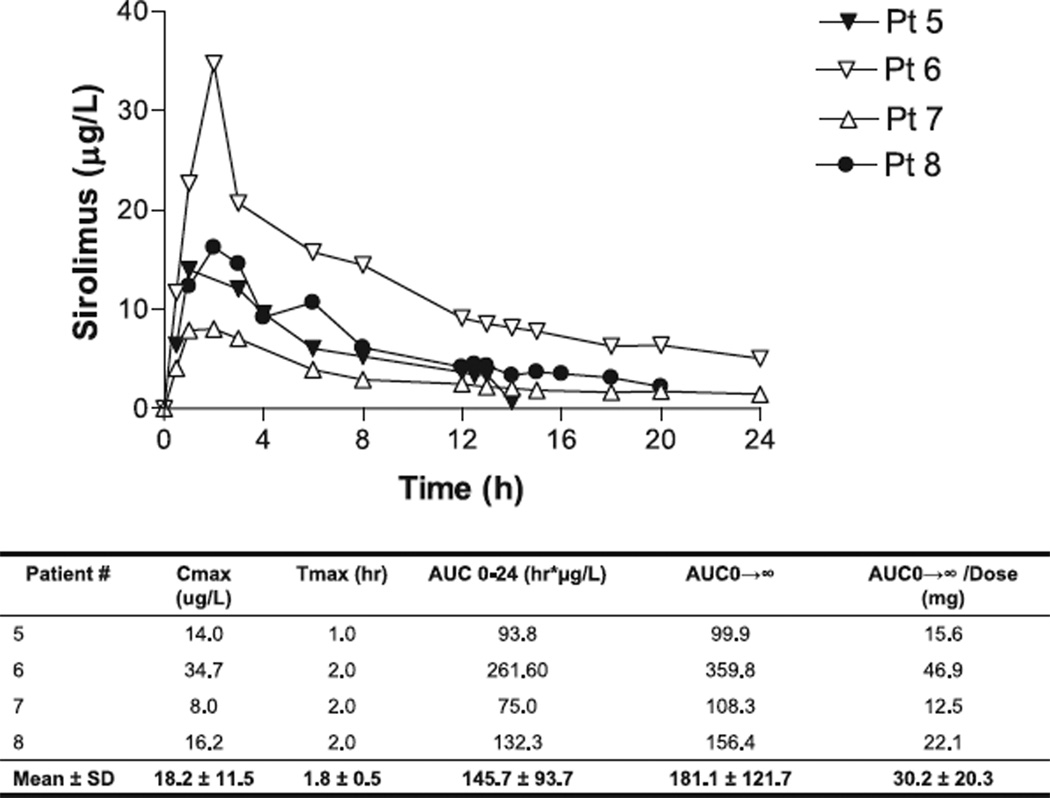
Four ESRD bypass recipients had PK profiles of sirolimus available for analysis. All subjects received a single dose of 6 mg of sirolimus (mean dose of 0.06 ± 0.002 mg/ kg).The mean Tmax was 1.8 h (range 1–2 h), mean Cmax was 18.2 µg/L (range 8.0–34.7), mean AUC0– 24 was 145.7 h µg/L (range 75.0–281.6), mean AUC0–∞ was 181.1 h µg/L (range 99.9–359.8) and the mean AUC0–∞:dose ratio was 30.2 (range 12.5– 46.9). Significant inter-patient variability was observed in the Cmax, AUC0–24, AUC0–∞ and the AUC0–∞:dose ratios. Concentration time curves of sirolimus are depicted in Fig. 1.
Fig. 1.
Concentration–time profile of sirolimus in end-stage renal disease gastric bypass recipients.
Tacrolimus PK
ESRD (single-dose PK)
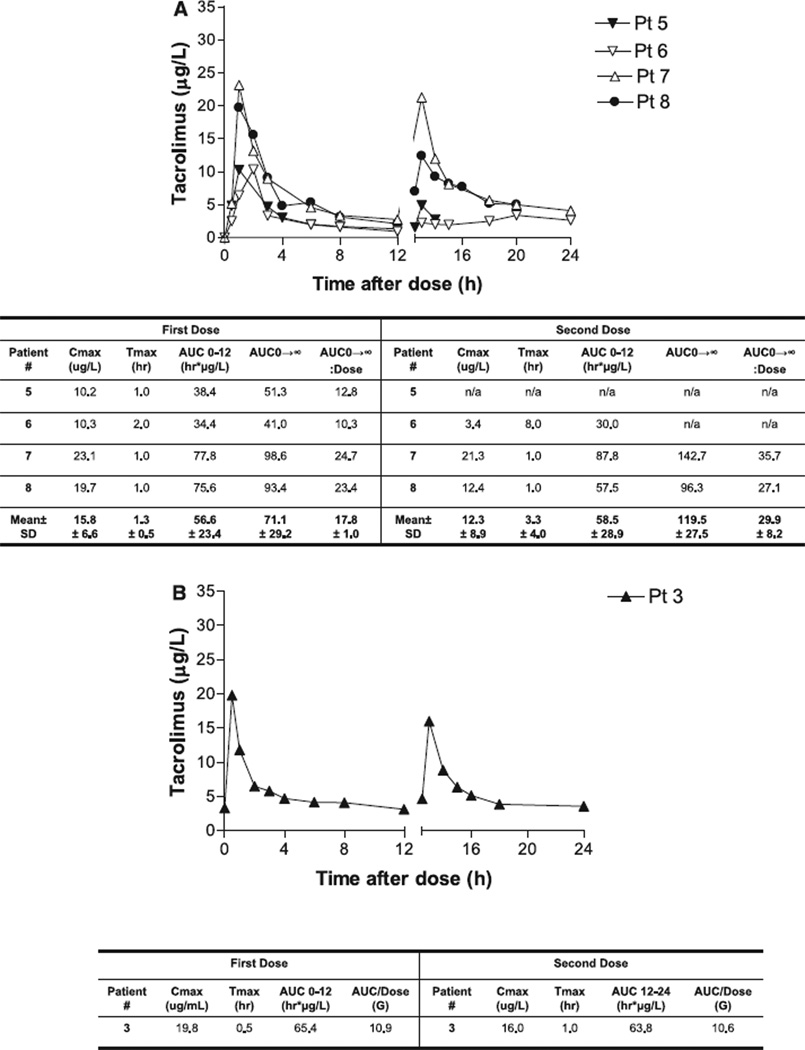
Four ESRD subjects received two 4-mg doses of tacrolimus and had PK profiles available for analysis. Subject number 5 lost IV access 1.5 h after the second dose of tacrolimus was administered. The mean total daily dose administered was 8.0 ± 0 mg or 0.085 ± 0.003 mg/kg. After the first tacrolimus dose, the mean Tmax was 1.3 h (range 1–2), the mean Cmax was 15.8 µg/L (range 10.2–23.1), the mean AUC0–12 was 56.6 h µg/L (range 34.4–77.8), the mean AUC0–∞ was 71.1 h µg/L (range 41.0– 98.6) and the mean AUC0–∞:dose ratio was 17.8 (range 10.3–24.7). The mean Tmax after the second tacrolimus dose was 3.3 h (range 1–8 h), the mean Cmax was 12.3 µg/L (range 3.4–21.3), the mean AUC0–12 was 58.5 µg/L (range 30.0–87.8), the mean AUC0–∞ was 119.5 h µg/L (range 96.3– 142.7) and the mean AUC0–∞:dose ratio was 29.9 (range 24.1–35.7). As in the non-bypass transplant population, we observed a wide inter-patient variability in tacrolimus PK and a lower Cmax with the second dose. Concentration time curves of ESRD subjects receiving tacrolimus are depicted in Fig. 2A.
Fig. 2.
(A) Concentration–time profile of tacrolimus in end-stage renal disease gastric bypass recipients. (B) Concentration– time profile of tacrolimus in post-transplant gastric bypass recipients.
Post-transplant (steady-state PK)
One post-transplant subject who consented for enrollment was maintained on tacrolimus-based immunosuppression. This subject received two doses of 4 mg of tacrolimus (0.06 mg/kg), as this was his/her maintenance dose. After the first tacrolimus dose, the Tmax was 0.5 h, the Cmax was 19.8 µg/L, the AUC0–12 was 65.4 h µg/L and the AUC0–12:dose ratio was 10.9. The Tmax after the second tacrolimus dose was 1 h, the Cmax was 16.0 µg/L, the AUC was 63.8 h µg/L and the AUC0–12:dose ratio was 10.6. The concentration–time curve of the single post-transplant subject receiving tacrolimus is presented in Fig. 2B.
MPA and MPAG PK
ESRD (single-dose PK)
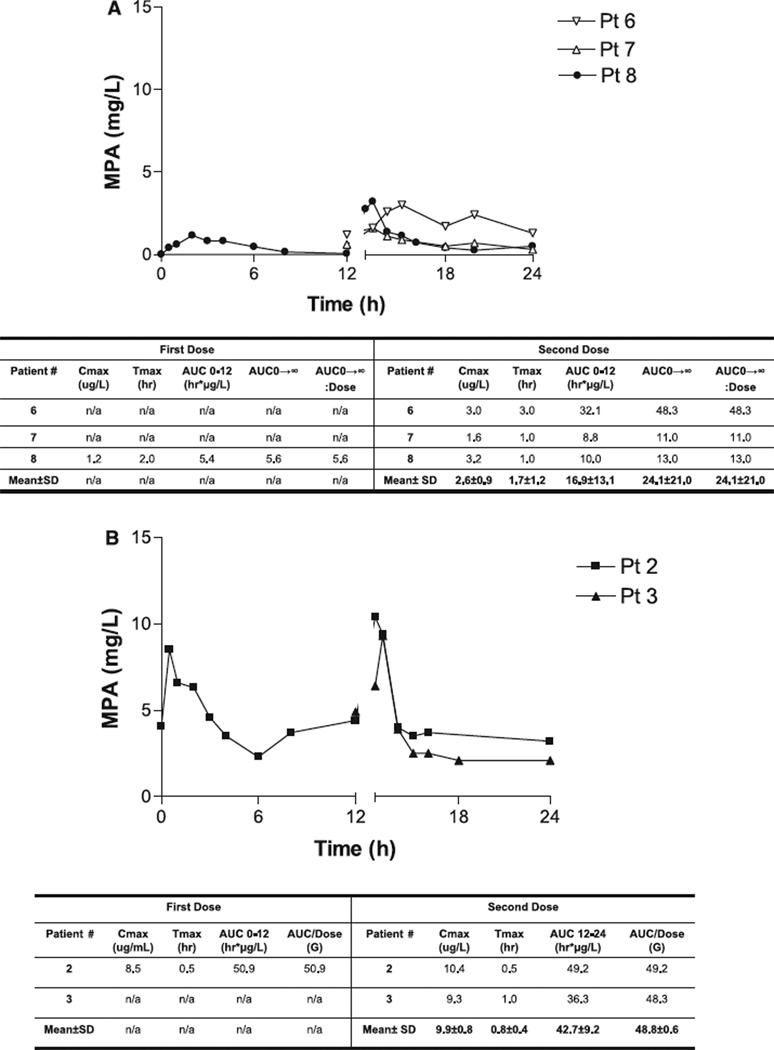
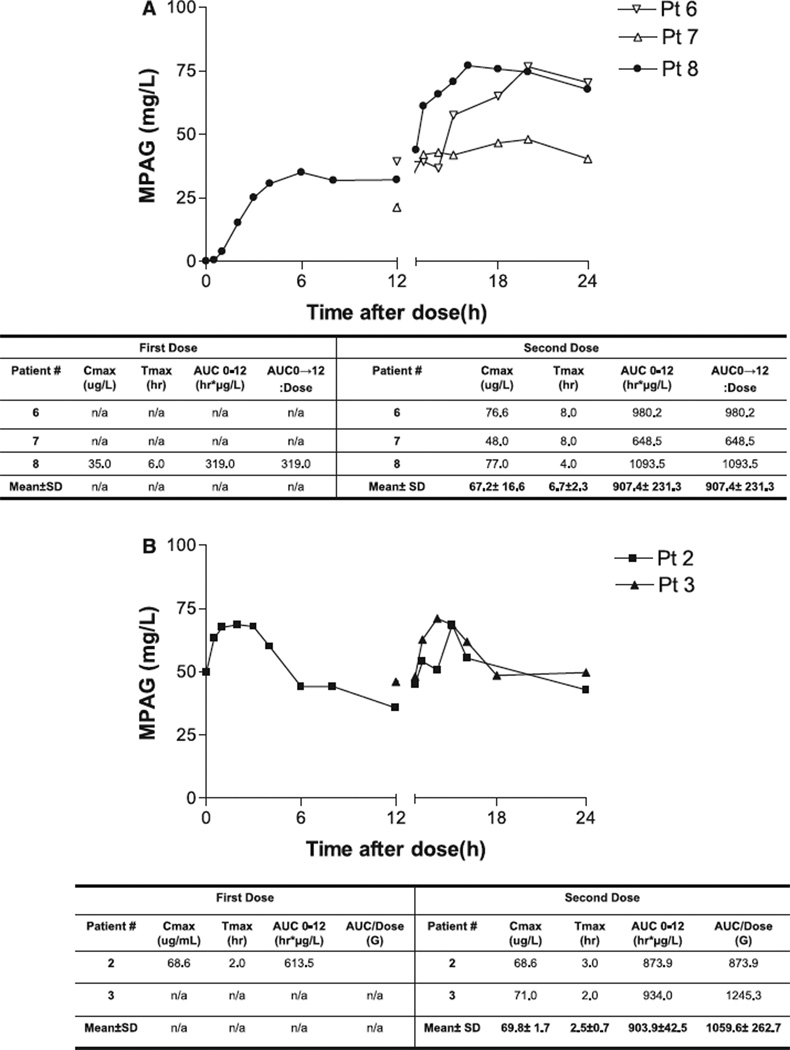
Four ESRD subjects received two 1000 mg doses of MMF and had PK profiles available for analysis. Two first-dose samples could not be analyzed owing to improper sample handling; additionally, all of patient number 5’s MPA samples had to be discarded owing to improper sample handling. Individual patient concentration– time profiles for the ESRD population of MPA are illustrated in Fig. 3A. Similar to our experience with tacrolimus and sirolimus, we observed substantial inter- and intra-patient variability within the profiles. First-dose kinetics was only available on patient number 8. The first-dose MPA Tmax was 2 h, MPA Cmax was 1.2 mg/L, the MPA AUC0–12 was 5.4 h mg/L, MPA AUC0–∞ was 5.6 h mg/L and the MPA AUC0–∞:dose ratio was 5.6. Second-dose kinetics was available in all three subjects. The mean second-dose MPA Tmax was 1.7 h (range 1–3), mean second-dose MPA Cmax was 2.6 mg/L (range 1.6–3.2), mean seconddose MPA AUC0–12 was 16.9 h mg/L (range 8.75– 32.05), the mean second-dose MPA AUC0–∞ was 24.1 (range 11.0–48.3) and the MPA AUC0–∞:dose ratio was 24.1. First-dose kinetics of MPAG was available only for patient number 8. The MPAG Tmax after the first dose was 6 h, MPAG Cmax was 35.0 mg/L and the MPAG AUC0–12 was 319.0 h mg/L. Second-dose MPAG kinetics demonstrated higher peaks and almost double the AUC0–12 seen with the first dose. The mean MPAG Tmax after the second dose was 6.7 h (range 4–8), mean MPAG Cmax was 67.2 mg/L (range 48–77) and the mean MPAG AUC0–12 was 907.4 h mg/L (range 648.5–1093.5). Individual concentration time profiles of MPAG are illustrated in Fig. 4A.
Fig. 3.
(A) Concentration–time profile of mycophenolic acid in end-stage renal disease gastric bypass recipients. (B) Concentration–time profile of mycophenolic acid in post-transplant gastric bypass recipients.
Fig. 4.
(A) Concentration–time profile of mycophenolic acid glucuronide in-end- stage renal disease gastric bypass recipients. (B) Concentration–time profile of mycophenolic acid glucuronide in post-transplant gastric bypass recipients.
Post-transplant (steady-state PK)
Steady-state PK of MPA and MPAG were evaluated in two subjects. Subject number 2 received two oral doses of 1000 mg and subject 3 received two oral doses of their maintenance dose of MMF of 750 mg. Subject number 3’s first-dose sample could not be analyzed owing to improper sample handling. Individual concentration time profiles of MPA for the post-transplant population are illustrated in Fig. 3B. First-dose kinetics for MPA was only available on subject number 2. The first-dose MPA Tmax was 0.5 h, MPA Cmax was 8.5 mg/L and the MPA AUC0–12 was 50.9 h mg/L. Second-dose kinetics was available in both subjects maintained on MMF. The mean second dose MPA Tmax was 0.8 h (range 0.5–1), mean second-dose MPA Cmax was 9.9 mg/L (range 9.30–10.40), the mean seconddose MPA AUC0–12 was 42.7 h mg/L (range 36.25–49.20) and the MPA AUC0–12:dose ratio was 48.8. First-dose kinetics of MPAG was only available for subject number 2. The MPAG Tmax after the first dose was 2.0 h, MPAG Cmax was 68.6 mg/L and the MPAG AUC0–12 was 613.5 h mg/L. Second-dose MPAG kinetics demonstrated similar peaks and a higher mean AUC0–12 than seen with the first dose. The mean MPAG Tmax after the second dose was 2.5 h (range 2–3), mean MPAG Cmax was 69.8 mg/L (range 68.6–71.0) and the mean MPAG AUC0–12 was 903.9 h mg/L (range 873.9–934.0). Individual concentration time profiles of MPAG are illustrated in Fig. 4B.
Discussion
Gastric bypass surgery has become an increasingly popular method to address obesity in the general population. Not until recently has GBS become an acceptable surgical option for the ESRD or transplant population. The reluctance to offer GBS as a weight-loss option for obese ESRD subjects or transplant recipients is multifactorial. The perceived surgical operative risks and the concern related to the effects of gastric bypass on absorption of immunosuppressants post-transplant are just two of many. The University of Cincinnati has the largest experience published to date on performing GBS in the ESRD and transplant population (15). This pilot study adds to this experience as the first report evaluating the changes seen in the PK of immunosuppression regimens, including tacrolimus, sirolimus and MMF when used in gastric bypass recipients.
The oral bioavailability of tacrolimus, sirolimus and MMF is governed by various mechanisms. Tacrolimus and sirolimus both demonstrate large inter-patient variation in their absorption-related PK parameters. This variability may be in part explained by the complex interactions that occur between drug transporters, such as P-glycoprotein (P-gp), Oatp2 and drug metabolism in the gut and liver (18). The majority of oral tacrolimus absorption occurs in the proximal duodenum; however, it has been shown that drug is absorbed from the intestine to the colon (19–21). In addition to the duodenum being the primary site for tacrolimus absorption, it is the primary site in the gut for tacrolimus metabolism by cytochrome P-450 (CYP) 3A4/5. Tacrolimus metabolite formation after oral administration is most highly concentrated in the duodenum and jejunum (22). Sirolimus is also metabolized by CYP 3A4/5 and has been shown to have similar absorptive and metabolic characteristics to tacrolimus. Both agents have also been shown to be substrates for P-gp, which is highly concentrated in the gut and has been shown to play a major role in the absorption and metabolism of its substrates (18). MMF is a pro-drug primarily absorbed in the stomach and rapidly metabolized by plasma esterases to its active form, MPA. MPA is then further metabolized to its inactive metabolite MPAG by uridine diphosphate (UDP)-glucuronosyltransferases both in the gastrointestinal tract, kidney and liver (23–25). The concentration time profile of MPA is unique in that it frequently demonstrates two distinct peaks when taken in combination with tacrolimus or sirolimus (26). The first peak is seen within one to three h of administration and a second is often seen around six to eight h later. The second peak is thought to be the result of the enterohepatic recirculation of MPAG back to MPA (27).
Unlike with tacrolimus and MMF, there is a limited body of literature available on single-dose PK with the sirolimus oral tablets. The vast majority of the PK data published is with the sirolimus oral solution. There is only one published study in renal transplant recipients receiving cyclosporine comparing the PK of sirolimus tablets to the oral solution on postoperative day 1. Significant differences in the AUC between the oral solution and tablet were not observed (28). Singledose healthy volunteer data are available utilizing the oral solution at single doses of 10 mg or 3 mg/ m2 (average dose 5.82 mg) (29, 30). We chose to compare our data with the single-dose oral solution healthy volunteer data as these patients closely resembled our population, in that, neither group was allowed to receive concomitant interacting medications. Patients in the transplant study utilizing the sirolimus tablets also received cyclosporine (28). There is a known drug interaction with cyclosporine and sirolimus when the two agents are administered together and 4-h apart (29). The subjects involved in our PK study were administered tacrolimus and MMF concomitantly with sirolimus, neither of which are known to alter the PK of sirolimus (31). As one would expect when comparing a tablet to an oral solution, we saw a longer Tmax and smaller Cmax with the bypass population (2.0 ± 0.7 h and 18.2 ± 11.5) than with the healthy volunteers [0.7 ± 0.3 and 32.2 ± 8.9 (27), 0.93 ± 0.38 and 46.0 ± 9.8 (28)]. A substantially lower AUC0–∞ and AUC:dose ratio were seen in the bypass population when compared with the healthy volunteers. The mean AUC0–∞ and AUC:dose ratio seen in the gastric bypass population were 181.1 ± 121.7 h µg/L and 30.2 ± 20.3, respectively. Healthy volunteers receiving a dose of 3 mg/m2 (average dose 5.82 mg) had an AUC of 335 ± 136 ng h/mL and an AUC:dose ratio of 57.5; healthy volunteers receiving a single dose of 10 mg had an AUC of 477 ± 135 ng h/mL and an AUC:dose ratio of 47.7. The lower AUC:dose ratio in the bypass population indicates that this population needs substantially more drug to achieve similar exposure to the non-bypass population. Potential explanations for the variations seen in the kinetics of the bypass population include the alterations in the absorptive function of the small intestine secondary to the gastric bypass procedure.
Pharmacokinetic data were available for analysis on five subjects receiving tacrolimus, four of which were pre-transplant (non–steady-state) and one was post-transplant (steady state). When the tacrolimus PK parameters in our gastric bypass population were compared with published reports of tacrolimus PK in the non-bypass population, significant differences were observed. The PK of tacrolimus in the four ESRD bypass subjects who received drug can be compared with the PK of tacrolimus in healthy volunteers who received a singles dose of 5 mg of tacrolimus in an anidulafungin PK study (32). Tacrolimus PK were evaluated after a single oral dose of 5 mg in 28 healthy male volunteers. The mean weight of the study population was lower than that of our bypass population at 75.5 ± 10.4 kg. The weight-based dose in this study was 0.66 mg/kg, whereas in our bypass population, it was 0.42 mg/kg. A lower Cmax (15.8 vs. 23.2 µg/L) and Tmax (1.3 vs. 2.0 h) were seen in the bypass population when compared with the healthy volunteers. Substantially lower AUC0–12 (56.6 vs. 107.8 h µg/L) and AUC0–∞ (71.1 vs. 269.6 h µg/L) were seen in the bypass population when compared with the healthy volunteers. The lower AUC0–∞:dose ratio seen with the bypass population (17.8 vs. 53.92) indicates that substantially more drug is needed in patients with a gastric bypass to achieve similar exposure to tacrolimus as those without a gastric bypass. Hardinger et al. published a study evaluating the PK of once vs. twice daily tacrolimus in stable transplant renal-transplant recipients (33). Participants in this study were maintained on a stable tacrolimus dose for six d. Twenty-four-hour PK profiles were drawn on day 7 on all 18 subjects to establish the baseline PK profile for the twice daily (BID) comparator arm of the study. The mean daily dose of tacrolimus administered in Hardinger’s group was slightly lower than that given to our single post-transplant bypass subject 4 mg (0.06 mg/kg) vs. 9 ± 1.7 mg (0.08 ± 0.01 mg/kg). Slight variations in the Tmax and Cmax were seen in the non-bypass and bypass subjects for the first (Tmax (0–12 h) 1.5 vs. 0.5, Cmax (0–12) 17.8 vs. 19.8) and second dose (Tmax (0–12 h), 3.0 vs. 1.0, Cmax (0–12h), 8.4 vs. 16.0) of tacrolimus. Both groups exhibited the diurnal variations that have been previously reported with tacrolimus administration (34). The most striking difference seen between the nonbypass and bypass subjects was in AUC0–12; a much lower AUC0–12 was seen in the bypass subjects (AUC0–12, 64.5 vs. 117 ± 40, AUC0–12, 63.8 vs. 97 ± 30) than in the non-bypass population. More importantly, a lower AUC:dose ratio (first dose AUC:dose0–12, 10.9 vs. 41.7; second dose AUC: dose0–12, 10.6 vs. 34.6) was seen in the bypass population, indicating that a higher dose is necessary in bypass patients to achieve similar exposure to that seen in a non-bypass patient population. It is not known exactly why the bypass patients require more tacrolimus to achieve similar concentrations than non-bypass patients; however, one may postulate that it may be attributed to a decreased absorptive capacity of the small intestine in patients with gastric bypass. The gastric bypass procedure involves the diversion of a significant portion of the duodenum, which plays a major role in the absorption and metabolism of drugs. As illustrated in the general transplant population, the presence of renal dysfunction in the bypass population did not further alter the PK of tacrolimus (35).
The PK profiles obtained for MPA and MPAG demonstrated dramatic inter- and intra-patient variability as illustrated in Figs 3A, B and 4A, B. Significant differences were seen in the kinetics of MPA and MPAG within both the ESRD bypass subjects and the stable post-transplant bypass subjects. The significantly higher MPA AUC0–12, seen in the stable post-transplant subjects is likely an effect of nonstationarity. Nonstationarity refers to a state in which drug exposure increases over time despite consistent dosage. MPA has been known to exhibit this property of decreased clearance over time as a function of several changes (e.g., improved renal function, changes in protein binding and reduced metabolism owing to steroid tapering) (36, 37). Lower Cmax that was seen in the ESRD population were also seen in a similar study published by MacPhee et al. (38). They postulated that the lower Cmax seen in the ESRD population may be attributed to altered absorption of MMF in the uremic state. The Cmax of MPA for both our transplanted and ESRD subjects was found to be lower than that in published MPA literature (36, 38). The lower Cmax in the bypass population may also be attributed to the decreased surface area of the stomach seen in gastric bypass recipients; the smaller surface area likely results in decreased drug absorption. A lower MPA AUC0–∞ was seen in the ESRD bypass population (24.1 ± 21.0 h µg/L) than what was seen in MacPhee’s hemodialysis (HD) MPA data (55.7 ± 32.6 mg/L h). The second-dose kinetics of our stable post-transplant bypass recipients can be compared with published PK data at steady state. The mean MPA AUC0–12 that is seen in our post-transplant gastric bypass population is lower than that seen in the general transplant population at >3 month post-transplant (65.3 ng h/mL vs. 42.7) (36). The alterations in the AUC0–12 may be attributed to the decreased absorption of MPA illustrated by the low Cmax and the possible blunting of the second peak of MPA that was seen in some of the gastric bypass recipients. It is unclear as to why the second peak of MPA is affected in this patient population. One possible explanation may be an alteration in the enterohepatic recirculation of MPA that may occur after gastric bypass. Significant differences in the MPA AUC between our ESRD and stable post-transplant subjects cannot be attributed to the differences in renal function or immunosuppressive regimens. Our post-transplant subjects were maintained on minimal doses of prednisone that were unlikely to impact MPA kinetics. The use of high-dose corticosteroids in the early postoperative period have been thought to be a contributing factor to the low MPA AUC seen during this time period. Significant differences in MPAG kinetics between our ESRD and post-transplant bypass subjects were not observed. This is somewhat surprising as MPAG is renally cleared and would be expected to be higher in the ESRD population (but a steady state for MPAG may not have been reached). The MPAG AUC seen in our bypass population (319 and 907 ± 231.3 h µg/L) were much lower than those seen in MacPhee’s experience (1565 ± 596 mg/L h) (38).
To date, there have only been a few papers published describing alterations in the PK of immunosuppressive medications in transplant recipients with bariatric surgery. Unfortunately, the majority of the current literature only describes alterations in cyclosporine PK and/or is in patients who have had jejunoileal bypass surgery (8, 39–41). These studies provide limited clinical relevance to contemporary immunosuppression management owing to the fact the tacrolimus has largely replaced the use of cyclosporine and that the roux-en-y procedure is now favored over jejunoileal bypass. Though somewhat outdated, these studies were consistent in reporting the fact that on average, a dose adjustment in cyclosporine or tacrolimus of at least 30–50% must be made in the setting of a new or reversed of jejunoileal bypass (11, 39). This would lead us to assume that in the setting of a roux-en-y gastric bypass, there may be a need for dose adjustment in the post-transplant setting. Additionally, three excellent reviews have been published describing the alterations of drug distribution seen following bariatric surgery. However, these articles do not provide any guidance on dosing of immunosuppressive agents in transplant recipients with GBS (8, 42, 43).
We were able to utilize the information obtained from our PK analysis of MPA to provide guidance for the appropriate dosing of MMF in the post-transplant setting for one of our subjects. Patient number 7, a Caucasian male, had a roux-en-y gastric bypass approximately 2.5 months after completing the PK analysis. Pretransplant PK parameters for MPA are as follows; MPA Tmax 1 h, Cmax 1.60 mg/L, AUC 8.75 h mg/L. Based on these values and Bayesian analysis, a dose of 1.5 g of MMF BID was recommended to achieve an AUC of 30–60 h mg/L. Abbreviated MPA AUC utilizing the technique published by Pawinski et al. (42) were performed at one, three and eight months post-transplant and remained low (36.5, 41.8 and 39.9 h mg/L) despite a total dose of 3 g/d of MMF and a tacrolimus-based regimen. Despite the low AUC and a higher dose of MMF than is typically used in combination with tacrolimus, this patient has been rejection and complication free.
One of the major limitations to this analysis is the limited sample size. It is difficult to draw any major conclusions from a sample size of only six subjects. The original design of the study involved utilizing subjects as their own control; however, during the course of the study, we were unable to obtain both a pre- and post-gastric bypass PK profile in an ESRD or transplanted candidate. The use of historical published data as a comparison is also a major limitation to the study as it is very difficult to identify published PK analyses that utilize similar dosing and sampling strategies.
Through this small single-center observational study, we were able to provide preliminary descriptive characteristics of alterations that may be seen in the immunosuppressants tacrolimus, sirolimus and MMF. We were able to apply our observations to one case and provide appropriate dose recommendations for post-transplant MMF dosing. Although we are unable to provide specific immunosuppression dosing guidelines in the post-transplant setting for gastric bypass patients, this study reveals that patients would likely need higher doses of tacrolimus, sirolimus and MMF in order to provide similar exposure to a non-bypass patient. Close monitoring of sirolimus and tacrolimus troughs in the early post-transplant or post-bypass setting is essential to assure appropriate exposure to both agents. Monitoring of MPA exposure, e.g., by abbreviated AUC estimation is warranted in the gastric bypass population to ensure adequate exposure of MPA is achieved.
Acknowledgement
This project was funded by the 2004 American College of Clinical Pharmacy (ACCP) Roche Transplant Research Fellowship Award.
Footnotes
There are no further conflicts of interest.
References
- 1.Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami RT, Dyke DB. Immunosuppression: practice and trends. Am J Transplant. 2004;4:38. doi: 10.1111/j.1600-6135.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Vazquez MA, Harmon WE, et al. for the American Society of Transplantation: Recommendations for the outpatient surveillance of renal transplant recipients. J Am Soc Nephrol. 2000;11:S1. [PubMed] [Google Scholar]
- 3.Adams PL. Long-term patient survival: strategies to improve overall health. Am J Kidney Dis. 2006;47:S65. doi: 10.1053/j.ajkd.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Gore JL, Pham PT, Danovitch GM, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Sharma AM. Obesity and cardiovascular risk. Growth Horm IGF Res. 2003;13:S10. doi: 10.1016/s1096-6374(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 8.Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health-Syst Pharm. 2006;63:1852. doi: 10.2146/ajhp060033. [DOI] [PubMed] [Google Scholar]
- 9.Blouin RA, Brouwer KL, Record KE, et al. Amikacin pharmacokinetics in morbidly obese patients undergoing gastric bypass surgery. Clin Pharm. 1985;4:70. [PubMed] [Google Scholar]
- 10.Fuller AK, Tingle D, DeVane CL, et al. Haloperidol pharmacokinetics following gastric bypass surgery. J Clin Psychopharmacol. 1986;6:376. [PubMed] [Google Scholar]
- 11.Knight GC, Macris MP, Peric M, et al. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with jejuno-ileal bypass. Transplant Proc. 1988;20:351. [PubMed] [Google Scholar]
- 12.Seaman JS, Bowers SP, Dixon P, Schindler L. Dissolution of common psychiatric medications in a roux-en-Y gastric bypass model. Phychosomatics. 2005;46:250. doi: 10.1176/appi.psy.46.3.250. [DOI] [PubMed] [Google Scholar]
- 13.Marterre WF, Hariharan S, First MR, Alexander JW. Gastric bypass in morbidly obese kidney transplant recipients. Clin Transplant. 1996;10:414. [PubMed] [Google Scholar]
- 14.Alexander JW, Goodman HR, Gersin K, et al. Gastric bypass in morbidly obese patients with chronic renal failure and kidney transplant. Transplantation. 2004;70:469. doi: 10.1097/01.tp.0000128858.84976.27. [DOI] [PubMed] [Google Scholar]
- 15.Alexander JW, Goodman H. Gastric bypass in chronic renal failure and renal transplant. Nutr Clin Pract. 2007;22:16. doi: 10.1177/011542650702200116. [DOI] [PubMed] [Google Scholar]
- 16.Koal T, Deters M, Casetta B, Kaever V. Simultaneous determination of four immunosuppressants by means of high speed and robust on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:215. doi: 10.1016/j.jchromb.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Shipkova M, Niedmann PD, Armstrong VW, et al. Simultaneous determination of mycophenolic acid and its glucuronide in human plasma using a simple high-performance liquid chromatography procedure. Clin Chem. 1998;44:1481. [PubMed] [Google Scholar]
- 18.Lo A, Burckart GL. P-glycoprotein and drug therapy in organ transplantation. J Clin Pharmacol. 1999;39:995. doi: 10.1177/00912709922011755. [DOI] [PubMed] [Google Scholar]
- 19.Wantanabe Y, Sato M, Abe Y, et al. Enteric absorption of FK 506: estimation by block liver perfusion technique in rate. Transplant Proc. 1998;30:3777. doi: 10.1016/s0041-1345(98)01233-0. [DOI] [PubMed] [Google Scholar]
- 20.Nishi K, Ishii T, Wada M, et al. The colon displays an absorptive capacity of tacrolimus. Transplant Proc. 2004;36:364. doi: 10.1016/j.transproceed.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Shimomura M, Masuda S, Hideyuki S, et al. Roles of the jejunum and ileum in the first-pass effect as absorptive barriers for orally administered tacrolimus. J Surg Res. 2002;103:215. doi: 10.1006/jsre.2002.6359. [DOI] [PubMed] [Google Scholar]
- 22.Lampen A, Christians U, Gonschior AK, et al. Metabolism of the macrolide immunosuppressant, tacrolimus, by the pig gut mucosa in the Ussing chamber. Br J Pharmacol. 1996;117:1730. doi: 10.1111/j.1476-5381.1996.tb15346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard O, Tojcic J, Journault K, Perusse L, Guillemette C. Influence of nonsynonymous polymorphisms of UGT1A8 and UGT2B7 metabolizing enzymes on the formation of phenolic and acyl glucuronides of mycophenolic acid. Drug Metab Dispos. 2006;34:1539. doi: 10.1124/dmd.106.010553. [DOI] [PubMed] [Google Scholar]
- 24.Shipkova M, Strassburg CP, Braun F, et al. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol. 2001;132:1027. doi: 10.1038/sj.bjp.0703898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the UDP-glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos. 2005;33:139. doi: 10.1124/dmd.104.001651. [DOI] [PubMed] [Google Scholar]
- 26.Shaw L, Korecka M, Van Breeman R, Nowak I, Brayman K. Analysis, pharmacokinetics and therapeutic drug monitoring of mycophenolic acid. Clin Biochem. 1998;31:323. doi: 10.1016/s0009-9120(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 27.Bullingham RES, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc. 1996;28:925. [PubMed] [Google Scholar]
- 28.Mathew TH, Van Buren C, Kahan BD, Butt K, Hariharan S, Zimmerman JJ. A comparative study of sirolimus tablet versus oral solution for prophylaxis of acute renal allograft rejection. J Clin Pharmacol. 2006;46:76. doi: 10.1177/0091270005282628. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman JJ, Harper D, Getsy J, Jusko WJ. Pharmacokinetic interactions between sirolimus and microemulsion cyclosporine when orally administered jointly and 4 hours apart in healthy volunteers. J Clin Pharmacol. 2003;43:1168. doi: 10.1177/0091270003257227. [DOI] [PubMed] [Google Scholar]
- 30.Brattstrom C, Sawe J, Jansson B, et al. Pharmacokinetics and safety of single oral doses of sirolimus (rapamycin) in healthy male volunteers. Ther Drug Monit. 2000;22:537. doi: 10.1097/00007691-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 31.McAlister VC, Mahalati K, Peltekian KM, Fraser A, MacDonald AS. A clinical pharmacokinetic study of tacrolimus and sirolimus combination immunosuppression comparing simultaneous to separated administration. Ther Drug Monit. 2002;24:346. doi: 10.1097/00007691-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Dowell JA, Stogniew M, Krause D, Henkel T, Damle B. Lack of a pharmacokinetic interaction between anidulafungin and tacrolimus. J Clin Pharmacol. 2007;47:305. doi: 10.1177/0091270006296764. [DOI] [PubMed] [Google Scholar]
- 33.Hardinger KL, Park JM, Schnitzler MA, et al. Pharmacokinetics of tacrolimus in kidney transplant recipients: twice daily versus once daily dosing. Am J Transplant. 2004;4:621. doi: 10.1111/j.1600-6143.2004.00383.x. [DOI] [PubMed] [Google Scholar]
- 34.Baraldo M, Furlanut M. Chronopharmacokinetics of cyclosporin and tacrolimus. Clin Pharmacokinet. 2006;45:775. doi: 10.2165/00003088-200645080-00002. [DOI] [PubMed] [Google Scholar]
- 35.Astellas pharmaceuticals. Prograf (tacrolimus) package insert. Deerfield, IL: 2005. [Google Scholar]
- 36.Roche pharmaceuticals. Cellcept (mycophenolate mofetil) package insert. Nutley, NJ: 2005. [Google Scholar]
- 37.Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther. 1998;64:672. doi: 10.1016/S0009-9236(98)90058-3. [DOI] [PubMed] [Google Scholar]
- 38.MacPhee IA, Spreafico S, Bewick M, et al. Pharmacokinetics of mycophenolate mofetil in patients with endstage renal failure. Kidney Int. 2000;57:1164. doi: 10.1046/j.1523-1755.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 39.Kelley M, Jain A, Kashyap R, et al. Change in oral absorption of tacrolimus in a liver transplant recipient after reversal of jejunoileal bypass: case report. Transplant Proc. 2005;37:3165. doi: 10.1016/j.transproceed.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Knight GC, Marcis MP, Peric M, Duncan JM, Frazier OH, Cooley DA. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with a jejuno-ileal bypass. Transplant Proc. 1988;20:351. [PubMed] [Google Scholar]
- 41.Chenhsu RY, Wu Y, Katz D, Rayhill S. Dose-adjusted cyclosporine C2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther Drug Monit. 2003;25:665. doi: 10.1097/00007691-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Macgregor AM, Bogs L. Drug distribution in obesity and following bariatric surgery: a literature review. Obes Surg. 1996;6:17. doi: 10.1381/096089296765557222. [DOI] [PubMed] [Google Scholar]
- 43.Malone M. Altered drug disposition in obesity and after bariatric surgery. Nutr Clin Pract. 2003;18:131. doi: 10.1177/0115426503018002131. [DOI] [PubMed] [Google Scholar]